Abstract
We have investigated metallothionein (MT) I and II mRNA and protein in B-cell lymphomas with particular reference to diffuse large B-cell lymphoma (DLBCL). The mRNA profiling was performed on Affymetrix arrays and showed up-regulated MT mRNA in 15 of 48 DLBCLs, including 12 of 23 activated B-cell (ABC) and 3 of 9 type-3 lesions. In contrast, MT mRNA was low to undetectable in 16 germinal center B-cell (GCB)-type DLBCLs. Only 1 of 15 patients with up-regulated MT mRNA achieved a sustained remission, suggesting that up-regulated MT mRNA constitutes a significant risk factor for treatment failure. This was confirmed in 2 independent series, which showed significantly shorter 5-year survival in DLBCL with high versus low MT-IIa levels. By immunohistology, MT was shown to be present in both macrophages and lymphoma cells. The proportion of MT-positive macrophages did not correlate with the survival. In contrast, in 115 DLBCLs, MT labeling of more than 20% lymphoma cells was associated with a significantly poorer 5-year survival, independent of the age, stage, or International Prognostic Index. Taken together, it is suggested that both increased MT mRNA and MT protein expression by more than 20% lymphoma cells constitute independent risk factors in DLBCL.
Introduction
The metallothioneins (MTs) are low-molecular-weight (6-7 kDa) nonenzymatic proteins consisting of 60 to 68 amino acids. Four subtypes are recognized (MT-I to MT-IV). Among these, MT-I and MT-II are the major forms. They are encoded by genes located on the same chromosome (no. 16 in humans), they are regulated and expressed coordinately, and their distribution and biologic functions are analogous.1,2 Both proteins contain 2 globular, cysteinrich domains, which can bind essential and toxic metals, such as zinc, copper, cadmium, and mercury. A main function is to act as an intracellular zinc reservoir.3 Furthermore, there are indications that the MTs are also involved in the protection against DNA damage, oxidative stress, and apoptosis.1,4-6
Several observations have indicated that altered MT expression is important for diseases in humans, including cancer, which often up-regulates MT compared with normal cells.5,7,8 Moreover, increased MT levels have been associated with high-grade histology and an aggressive behavior in several of types of cancer.5,9-11
So far, most studies of MT in malignant diseases have been performed in epithelial tumors. Very limited information is available about the expression of MT in lymphomas. In this study, we have investigated 141 B-cell lymphomas for MT protein expression by immunohistology. Furthermore, MT mRNA levels were investigated by expression profiling in 48 diffuse large B-cell lymphomas (DLBCLs) previously classified into 3 groups (germinal center B-cell [GCB], activated B-cell [ABC], and type-3) on Affymetrix arrays. The results indicate that increased MT mRNA and protein may constitute an independent risk factor in DLBCL.
Materials and methods
Biopsies
Formalin-fixed, paraffin-embedded specimens of 131 B-cell lymphomas sampled during the period 1982 to 2004 were collected from the archives at the Department of Pathology, Rigshospitalet, University of Copenhagen. An additional 10 cases were kindly made available from the Department of Pathology, Odense University Hospital. Only specimens obtained at diagnosis, prior to treatment, were included. All cases were reviewed by histology and immunohistology using standard panels of antibodies (ie, CD20, CD79a, CD10, CD21, CD23, CD3, CD5, Bcl-2, and cyclin D1). The samples were then classified in accordance with the World Health Organization (WHO)26 and comprised 115 DLBCLs, 11 follicular lymphomas (FLs), 2 mantle cell lymphomas (MCLs), 7 small lymphocytic lymphomas (SLLs), and 6 cases of multiple myeloma (MM). Of the DLBCLs, 86 were primary nodal and 29 were primary extranodal at presentation with involvement of the tonsil (9 cases), salivary glands (4 cases), thyroid (3 cases), testis (3 cases), bone (2 cases), nasal cavity (1 case), cervix uteri (1 case), liver (1 case), breast (1 case), kidney (1 case), pericardium (1 case), rhinopharynx (1 case), and skin (1 case).
Clinical data and survival
The clinical records were reviewed in all of the DLBCL patients with particular reference to age, site of initial involvement, stage at diagnosis, response to treatment, and survival. Information on the International Prognostic Index (IPI) could be retrieved in 74 of the cases. All patients were treated using anthracyclin-based regimens (CHOP [cyclophosphamide, vincristine, prednisone] or CHOP-like), supplemented in 6 patients with additional antibody therapy (eg, rituximab). Survival was calculated from the day of diagnosis until death or the date of last follow-up. Overall survival duration curves were plotted according to the Kaplan and Meier method.12 The log-rank test was used to assess the differences between the survival curves, and nominal P values were calculated.
Group comparisons were done using 2-sided, paired Student t test (equal variance assumed).
Immunohistology
Sections of formalin-fixed, paraffin-embedded biopsies were heated in a Milestone Micromed microwave oven (Sorisole, Italy) in citrate buffer (pH 6) for 15 minutes, incubated 60 minutes at room temperature with a 1:40 dilution of DAKO-MT (clone, E9; DAKO, Carpinteria, CA) reactive with MT-I and MT-II in routinely processed histologic samples,13 and stained in the Techmate 500 Immunostainer (DAKO, Glostrup, Denmark), using the DAKO Envision K5007 as a secondary antibody. Since the results suggested that some of the MT-positive cells represented macrophages, samples were also stained using CD68 (PGM1) on adjacent sections.
As a preliminary approach to scoring, all specimens were screened by 2 authors (C.B.P., E.R.) to obtain an overall impression of the reactivity patterns. Furthermore, in 20 randomly selected DLBCLs, the number of MT-positive tumor cells was counted in 10 representative areas at 400 × magnification. These results showed that 4 broad categories could be distinguished: no/occasional (< 5%); few (5%-20%); moderate (20%-50%); and many (> 50%) MT-positive lymphoma cells. In general, the intensity of the staining paralleled the number with weak to moderate staining reactions in cases with few MT-positive tumor cells and stronger reactions in cases with more MT-positive tumor cells. Similarly, for macrophages, 3 categories could be distinguished: few (+), moderate (++), and many (+++) MT-positive tumor-infiltrating macrophages. Therefore, for the scoring of the entire series, this semiquantitative scoring system was adopted. All samples were scored independently by 2 authors (C.B.P., E.R.) and were in case of disagreement reviewed in consensus.
Controls were included in all experiments and consisted of autopsy specimens of normal liver and kidney. In these controls, strong staining was seen of both nuclei and cytoplasm in proximal tubuli and hepatocytes.
mRNA expression profiling
Frozen tissue kept at -80°C was available from 48 of the DLBCLs and was subjected to expression profiling as described.14 Briefly, from each case, 5 μg purified RNA was used to generate biotin-labeled antisense cRNA. After fragmentation at 94°C for 35 minutes, the labeled cRNA samples were hybridized for 16 hours to Affymetrix HG-U133A arrays (Affymetrix, Santa Clara, CA), washed, and stained with phycoerythrin-conjugated streptavidin (SAPE) and scanned in the Affymetrix GeneArray 2500 scanner to generate fluorescent images. All procedures were as described in the Affymetrix GeneChip protocol. The image files (cel files) were imported into the software package DNA-Chip Analyzer (C. Li and W. H. Wong [www.dchip.org]). The array files were normalized and expression values calculated as described.14 Samples were categorized as being GCB, ABC, and type-3 by hierarchic clustering using a list of 78 classifier genes, as already published.15
For validation, data from 2 independent studies of 240 newly diagnosed DLBCLs analyzed on the Lymphochip platform16 and 176 newly diagnosed DLBCLs with available Affymetrix HU133 A + B data sets17 were downloaded from the Internet (Rosenwald et al16 ; http://llmpp.nih.gov/DLBCL/ and Monti et al17 ; http://www.broad.mit.edu/cgi-bin/cancer/publications/pub_paper.cgi?mode=view&paper_id=102) together with the relevant clinical information. Using this approach, data on MT-IIa mRNA levels and survival could be retrieved and analyzed from 238 patients in the Rosenwald et al study16 and from 130 patients in the study by Monti et al.17
Ethics
This study was approved by the local ethics committee (journal no. 01-226/02) and the Danish Data Protection Agency (journal no. 2002 111129A). Informed consent was obtained from participants in accordance with the Declaration of Helsinki.
Results
MT mRNA expression in DLBCL
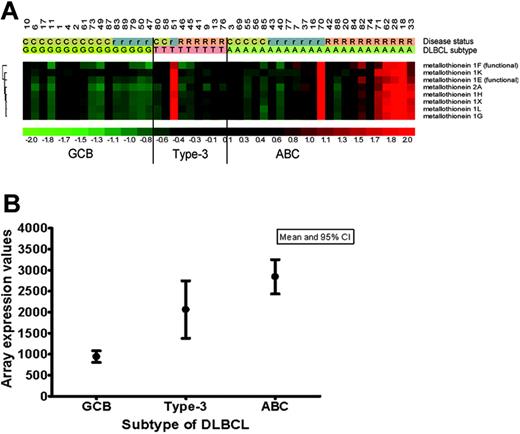
mRNA extracted from frozen tissues from 48 of the DLBCLs was examined by global expression profiling on Affymetrix arrays. Similar to other reports,18-20 the tumors could be grouped into 3 categories depending upon the differential expression of 78 genes as described in our previously developed classifier model: GCB (n = 16), ABC (n = 23), and type-3 (n = 9).15 A further analysis of other genes differentially expressed in these groups showed that the genes encoding mRNA MT-IIa and the different isoforms of MT-I (eg, MT-IL, G, H, X, G, F, E, K) were up-regulated (above mean) in 12 of 23 of the ABC tumors and in 3 of 9 type-3 tumors, but were low to undetectable in the 16 GCB lesions (Figure 1A-B). A correlation with the treatment outcome showed that only 1 of 15 patients with up-regulated MT mRNA achieved a sustained complete remission (CR). The remaining either showed only a temporary response (n = 4) or had refractory disease (n = 10). Conversely, in the 33 patients with low MT mRNA levels, 17 achieved a sustained CR, 9 achieved a temporary CR but relapsed, and 7 had refractory disease. These data suggested that up-regulated MT mRNA may identify a particular subgroup of ABC and type-3 DLBCL with a poor response to conventional chemotherapy and a potentially aggressive behavior.
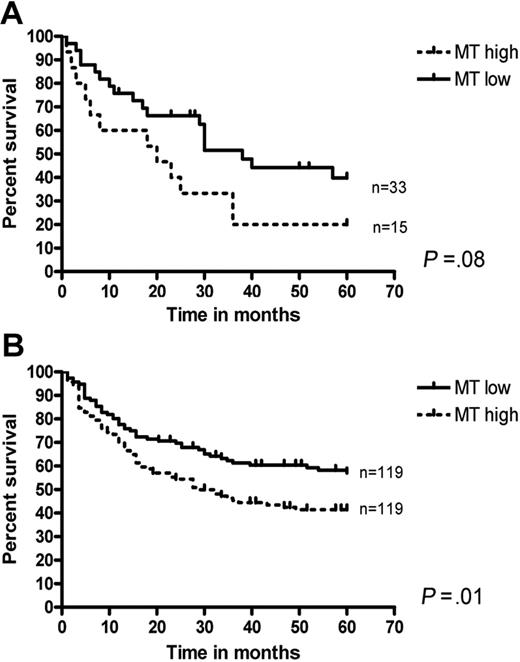
To address this possibility in more detail, the survival of patients with high versus low MT mRNA was investigated both in this series and in another 2 independent data sets (Rosenwald et al16 and Monti et al17 ). In our series of 48 patients, a tendency toward an inferior survival was seen in patients with high MT mRNA compared with those with low MT mRNA (Figure 2A). However, this difference did not reach statistical significance, presumably due to the relatively small number of patients. In contrast, among 238 patients downloaded from the publication of Rosenwald et al,16 those with high (above mean) MT-IIa mRNA showed a significantly poorer survival than those with lower MT-IIa levels (Figure 2B). A similar difference (P = .05) was observed when data from 130 patients from the study by Monti et al17 were analyzed (data not shown).
MT-I and MT-II expression in GCB, ACB, and type-3 subtypes of DLBCL. (A) MT-I and MT-II mRNA expression in 48 DLBCLs previously classified as GCB (n = 16), ABC (n = 23), and type-3 (n = 9) by expression profiling on Affymetrix arrays.15 For MT-I, the expression of 7 different isoforms represented on the Affymetrix 133A GeneChip is illustrated. C indicates complete remission; r, recurrent disease; and R, refractory disease. (B) MT-II mRNA expression values (arbitrary units) in the 3 different subtypes of DLBCL (GCB, ABC, type-3). CI indicates confidence interval.
MT-I and MT-II expression in GCB, ACB, and type-3 subtypes of DLBCL. (A) MT-I and MT-II mRNA expression in 48 DLBCLs previously classified as GCB (n = 16), ABC (n = 23), and type-3 (n = 9) by expression profiling on Affymetrix arrays.15 For MT-I, the expression of 7 different isoforms represented on the Affymetrix 133A GeneChip is illustrated. C indicates complete remission; r, recurrent disease; and R, refractory disease. (B) MT-II mRNA expression values (arbitrary units) in the 3 different subtypes of DLBCL (GCB, ABC, type-3). CI indicates confidence interval.
MT and survival in DLBCLs. Survival in DLBCL with high versus low MT mRNA in this series (A) and the series from the Rosenwald et al study17 (B).
MT and survival in DLBCLs. Survival in DLBCL with high versus low MT mRNA in this series (A) and the series from the Rosenwald et al study17 (B).
MT protein expression
To characterize MT expression at the protein level, an extended series of pretreatment, diagnostic biopsies from 141 B-cell lymphomas sampled during the period 1982 to 2004 were examined by immunohistology using anti-MT antibody (E9) reactive with MT-I and MT-II in formalin-fixed specimens.13 This series included the 48 DLBCLs analyzed on Affymetrix arrays as well as samples from an additional 67 DLBCLs. Furthermore, specimens from 20 small B-cell lymphomas (“Materials and methods”) and 6 multiple myelomas were investigated.
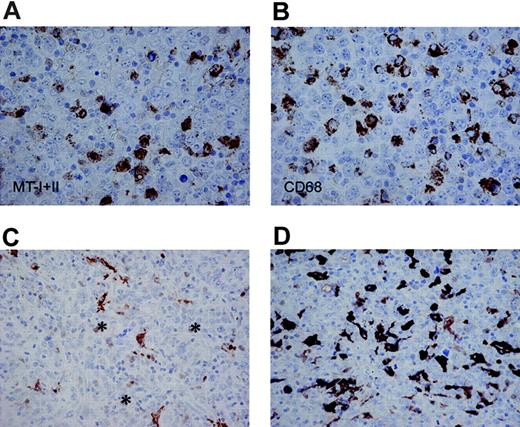
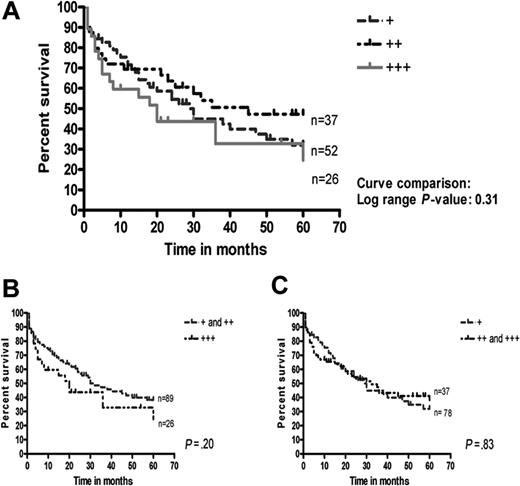
In all the B-cell lymphomas, the antibody stained a population of CD68+ dendritic cells, representing tumor-infiltrating macrophages (Figure 3). MT labeling of the macrophages was prominent in the cytoplasm, but the nuclei were also weakly stained in most cases. The number of MT-positive tumor-infiltrating macrophages was most abundant in DLBCLs (Table 1), but had no impact on the 5-year survival (Figure 4).
MT expression by macrophages in different types of B-cell lymphomas
. | No. patients . | . | . | . | |||
|---|---|---|---|---|---|---|---|
| Diagnoses . | + . | ++ . | +++ . | Total . | |||
| DLBCL | 52 | 37 | 26 | 115 | |||
| FL, grades I + II | 8 | 0 | 0 | 8 | |||
| FL, grade III | 3 | 0 | 0 | 3 | |||
| MCL | 2 | 0 | 0 | 2 | |||
| MM | 5 | 1 | 0 | 6 | |||
| SLL | 7 | 0 | 0 | 7 | |||
. | No. patients . | . | . | . | |||
|---|---|---|---|---|---|---|---|
| Diagnoses . | + . | ++ . | +++ . | Total . | |||
| DLBCL | 52 | 37 | 26 | 115 | |||
| FL, grades I + II | 8 | 0 | 0 | 8 | |||
| FL, grade III | 3 | 0 | 0 | 3 | |||
| MCL | 2 | 0 | 0 | 2 | |||
| MM | 5 | 1 | 0 | 6 | |||
| SLL | 7 | 0 | 0 | 7 | |||
+ indicates few; ++, moderate; +++, abundant numbers of MT-positive macrophages; DLBCL, diffuse large B-cell lymphoma; FL, follicular lymphoma; MCL, mantle cell lymphoma; MM, multiple myeloma; and SLL, small lymphocytic lymphoma
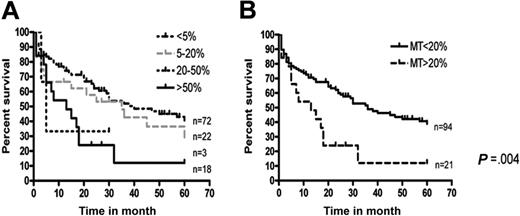
MT expression by the lymphoma cells was more restricted. As shown in Table 2, significant numbers of MT-positive tumor cells (> 20%) were observed in 21 of 115 of the DLBCLs. In the remaining B-cell lymphomas, no or only occasional MT-positive tumor cells were identified. Labeling of the lymphoma cells was mainly nuclear, although cytoplasmic reactivity was also present in some cases (Figure 5). No significant differences with respect to age, stage, or IPI at diagnosis were observed between DLBCLs with MT-positive tumor cells above or below 20% (Table 3). However, the survival at 5 years was significantly poorer in the former category (Figure 6), suggesting that more than 20% MT-positive lymphoma cells constitutes an independent risk factor in DLBCL. Small, reactive, tumor-infiltrating lymphocytes were MT negative (Figure 5).
MT expression in tumor cells from different types of B-cell lymphomas
. | MT-positive tumor cells, no. patients . | . | . | . | . | ||||
|---|---|---|---|---|---|---|---|---|---|
| Diagnosis . | Less than 5% . | 5% to 20% . | 20% to 50% . | More than 50% . | Total . | ||||
| DLBCL | 72 | 22 | 3 | 18 | 115 | ||||
| FL, grades I + II | 7 | 1 | 0 | 0 | 8 | ||||
| FL, grade III | 3 | 0 | 0 | 0 | 3 | ||||
| MCL | 2 | 0 | 0 | 0 | 2 | ||||
| MM | 6 | 0 | 0 | 0 | 6 | ||||
| SLL | 7 | 0 | 0 | 0 | 7 | ||||
. | MT-positive tumor cells, no. patients . | . | . | . | . | ||||
|---|---|---|---|---|---|---|---|---|---|
| Diagnosis . | Less than 5% . | 5% to 20% . | 20% to 50% . | More than 50% . | Total . | ||||
| DLBCL | 72 | 22 | 3 | 18 | 115 | ||||
| FL, grades I + II | 7 | 1 | 0 | 0 | 8 | ||||
| FL, grade III | 3 | 0 | 0 | 0 | 3 | ||||
| MCL | 2 | 0 | 0 | 0 | 2 | ||||
| MM | 6 | 0 | 0 | 0 | 6 | ||||
| SLL | 7 | 0 | 0 | 0 | 7 | ||||
DLBCL indicates diffuse large B-cell lymphoma; FL, follicular lymphoma; MCL, mantle cell lymphoma; MM, multiple myeloma; and SLL, small lymphocytic lymphoma
Clinical data in DLBCLs with low versus high MT-I and MT-II expression in the tumor cells
. | Low expression, less than 20% . | High expression, more than 20% . |
|---|---|---|
| Age, y (no. patients) | 64.2 (97) | 65.4 (18) |
| Stage (no. patients) | 2.58 (73) | 2.65 (17) |
| IPI (no. patients) | 2.31 (59) | 2.40 (15) |
| 5-year overall survival, % (no. patients) | 40.7 (97) | 12.0 (18) |
. | Low expression, less than 20% . | High expression, more than 20% . |
|---|---|---|
| Age, y (no. patients) | 64.2 (97) | 65.4 (18) |
| Stage (no. patients) | 2.58 (73) | 2.65 (17) |
| IPI (no. patients) | 2.31 (59) | 2.40 (15) |
| 5-year overall survival, % (no. patients) | 40.7 (97) | 12.0 (18) |
Correlation between the number of MT-positive tumor cells and macrophages in individual DLBCLs is summarized in Table 4. As shown, no specific correlation was identified. For example, among 18 DLBCLs with more than 50% MT-positive tumor cells, 6 contained few MT-positive macrophages, 6 contained an intermediate number, and 6 contained many MT-positive macrophages. Similarly, among 25 cases with many MT-positive macrophages, only 7 contained more than 20% positive lymphoma cells. Hence, it is less likely that phagocytosis of MT produced in lymphoma cells contributed significantly to the observed MT staining of macrophages.
MT expression in tumor cells versus macrophages in 115 DLBCLs
. | MT-positive tumor cells, no. patients . | . | . | . | |||
|---|---|---|---|---|---|---|---|
| Macrophage . | Less than 5% . | 5% to 20% . | 20% to 50% . | More than 50% . | |||
| + | 42 | 3 | 1 | 6 | |||
| ++ | 17 | 13 | 1 | 6 | |||
| +++ | 13 | 6 | 1 | 6 | |||
| Total | 72 | 22 | 3 | 18 | |||
. | MT-positive tumor cells, no. patients . | . | . | . | |||
|---|---|---|---|---|---|---|---|
| Macrophage . | Less than 5% . | 5% to 20% . | 20% to 50% . | More than 50% . | |||
| + | 42 | 3 | 1 | 6 | |||
| ++ | 17 | 13 | 1 | 6 | |||
| +++ | 13 | 6 | 1 | 6 | |||
| Total | 72 | 22 | 3 | 18 | |||
MT-I and MT-II staining of tumor-infiltrating macrophages. Adjacent sections of a DLBCL stained for MT-I and MT-II (A, DAKO-MT × 600) and CD68 (B, PGM1 × 600) showing numerous MT-positive stromal cells with a dendritic morphology (A), similar to the pattern with CD68 (B). In panels C and D, DLBCL with few (C) and abundant (D) numbers of MT-positive tumor-infiltrating macrophages is shown (DAKO-MT × 400). In these cases, only very occasional tumor cells (asterisks in C) are stained with a weak nuclear reactivity.
MT-I and MT-II staining of tumor-infiltrating macrophages. Adjacent sections of a DLBCL stained for MT-I and MT-II (A, DAKO-MT × 600) and CD68 (B, PGM1 × 600) showing numerous MT-positive stromal cells with a dendritic morphology (A), similar to the pattern with CD68 (B). In panels C and D, DLBCL with few (C) and abundant (D) numbers of MT-positive tumor-infiltrating macrophages is shown (DAKO-MT × 400). In these cases, only very occasional tumor cells (asterisks in C) are stained with a weak nuclear reactivity.
MT mRNA versus protein expression
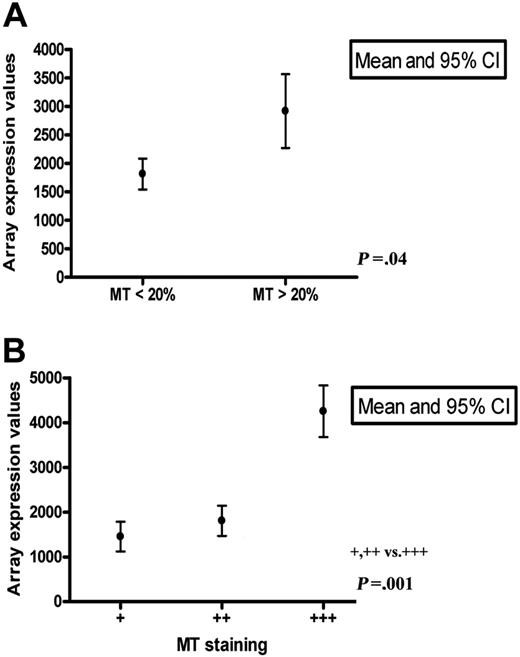
MT-II and the several different isoforms of MT-I are coordinately expressed under a number of conditions.1,21 This was also seen in our study, as illustrated in Figure 1A. For comparison between the MT mRNA and protein expression levels, we focused on MT-II. The results for 48 DLBCLs in which samples were available for both immunohistology and expression profiling on Affymetrix arrays are illustrated in Figure 7A-B and showed significantly increased MT mRNA (mean, 4253) in lymphomas with abundant (+++) numbers of MT-positive macrophages compared with those with few (+) or moderate (++) numbers (mean, 1598; P = .001). A similar correlation was seen between the MT mRNA expression level and the number of MT-positive tumor cells detected by immunohistology—that is, mean of 2737 in DLBCLs with more than 20% positive tumor cells versus mean of 1814 in DLBCLs with less than 20% positive tumor cells (P = .04). These data indicated that the MT mRNA level determined by expression profiling in crude extracts of DLBCLs correlated with, and was contributed to, by MT produced in both lymphoma cells and macrophages.
Survival in 115 DLBCLs in relation to the proportion of MT-positive tumor-infiltrating macrophages. No significant differences were seen between the 3 groups of few, moderate, and many (A), between few versus moderate/many (B), or between few/moderate versus many (C) MT-positive macrophages.
Survival in 115 DLBCLs in relation to the proportion of MT-positive tumor-infiltrating macrophages. No significant differences were seen between the 3 groups of few, moderate, and many (A), between few versus moderate/many (B), or between few/moderate versus many (C) MT-positive macrophages.
Discussion
The MTs are single-chain 6- to 7-kDa proteins with a high content of cystein, sulfur, and metal. Four subtypes are recognized in humans, MT-I and MT-II, which are widespread, and MT-III and MT-IV, which are more restricted in distribution. All the MTs contain 2 metal-binding domains, a C-terminal α and a N-terminal β domain. Both are rich in cysteins, which can bind essential and toxic metals. The MT genes are located on chromosome 16 in humans and can be activated by a variety of stimuli, including metal ions, oxidative stress, cytokines, glucocorticoids, growth factors, and hypoxia.2,8,22,23 The MTs have established roles in metal homeostasis and in the protection against reactive oxygen species. Furthermore, there are indications that the MTs are involved in cell proliferation, in the protections against DNA damage, in the prevention of apoptosis, and in the acquisition of resistance to a variety of commonly used cancer chemotherapies.8,21-24 Accordingly, it is conceivable that altered MT expression may play a role for neoplastic disorders.
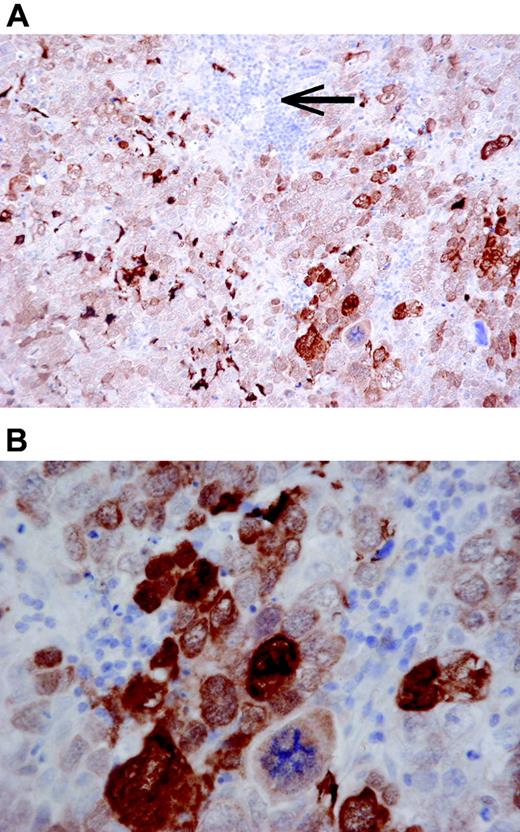
A DLBCL with strong MT staining of a majority of the tumor cells in both nuclei and cytoplasm (A, DAKO-MT × 200; B, DAKO-MT × 600). Note that small reactive lymphocytes (arrow) are MT negative.
A DLBCL with strong MT staining of a majority of the tumor cells in both nuclei and cytoplasm (A, DAKO-MT × 200; B, DAKO-MT × 600). Note that small reactive lymphocytes (arrow) are MT negative.
Expression of the MTs has been studied in considerable detail in human carcinomas, and these studies have shown that the MTs are often up-regulated in malignant compared with normal cells. Furthermore, it has been shown that up-regulated MT associates with high-grade histology and an aggressive behavior in many types of carcinoma.5 In contrast, very limited information is available about MT expression in lymphoma. Thus, to date, only 2 previous reports of a total of 12 lymphomas of different subtypes are available.7,25
In an attempt to investigate whether MTs have implications in lymphoma, we have examined MT mRNA and protein expression in mature B-cell lymphomas with particular reference to DLBCL, which constitutes the most frequent subtype of B-cell lymphoma in Western countries. Although classified as one disease by the REAL and WHO classifications,26 several more recent investigations have indicated that DLBCL is likely to encompass more than one biologic entity. Thus, it has been shown that different clinical subtypes can be recognized by phenotypic and genotypic investigations at the protein, mRNA, and DNA level.27-29 For example, it has been shown in this and other laboratories that, at the least, 3 subgroups with different outcomes can be distinguished depending upon the mRNA expression profiles. These include DLBCL with a GCB-like mRNA signature, DLBCL with an ABC-like signature, and an intermediate, so-called type-3 category. Whereas the GCB-like DLBCL generally shows an indolent course, the behavior of the ABC and type-3 lesions is more aggressive.15,18,19,30-32
Survival in 115 DLBCLs in relation to the proportion of MT-positive lymphoma cells. No difference was seen between tumors with no/occasional versus few (5%-20%) MT-positive tumor cells (A). In contrast, a significantly poorer survival was seen in patients with more than 20% MT-positive tumor cells compared with those with less than 20% MT-positive tumor cells (B).
Survival in 115 DLBCLs in relation to the proportion of MT-positive lymphoma cells. No difference was seen between tumors with no/occasional versus few (5%-20%) MT-positive tumor cells (A). In contrast, a significantly poorer survival was seen in patients with more than 20% MT-positive tumor cells compared with those with less than 20% MT-positive tumor cells (B).
Here, we show that mRNA encoding MT-I and MT-II is differentially up-regulated in half of DLBCLs with an ABC signature and in one third of type-3 tumors, but is low to undetectable in DLBCLs with a GCB signature. Furthermore, it is shown that up-regulated MT-I and MT-II mRNA in DLBCL correlates with treatment failure and a short survival. This was evident both in this series and in another 2 independent data sets published by Rosenwald et al (2002)16 and Monti et al (2005).17 Since the genes encoding MT-I and MT-II are not contained in either our or other previously reported classifier lists for distinction between ABC and GCB DLBCLs,15 they are clearly not merely markers of ABC versus GCB tumors, but rather represent additional markers for a particular subgroup of DLBCLs with a poor response to conventional chemotherapy and a short survival. This was supported by the results obtained by immunohistology, which showed that MT protein expression by the lymphoma cells in an extended series of 115 DLBCLs, 20 small B-cell lymphomas, and 6 multiple myelomas was confined to the DLBCLs. Furthermore, in the DLBCLs the presence of more than 20% MT-positive lymphoma cells was a significant risk factor associated with a short survival, independent of the age, stage, and IPI.
Very few previous reports have specifically addressed the MT mRNA and protein levels in lymphomas, and it is presently not clear how the MTs influence the behavior of DLBCLs at the molecular level. One obvious possibility is that enhanced MTs can confer resistance to conventional chemotherapies commonly used in treating DLBCL (eg, anthracyclins and cyclophosphamide).21,24 Indeed, studies have shown that MTs can inhibit anthracyclin-induced mitochondrial cytochrome c release and caspase-3 activation,33,34 and can mediate intracellular sequestration of cyclophosphamide.35,36 Thus, it is possible that up-regulated MT mRNA and protein may identify a particular subgroup of DLBCL, which could benefit from more intensive therapy and/or from other treatment modalities than conventional CHOP or CHOP-like regimens. Potentially interesting options in this respect are electron-affinic compounds such as motexafin gadolinium, which increases oxidative stress, enhances expression of metal response element-binding transcription factor-1-regulated genes, including MT, and can induce cell-cycle arrest and apoptosis in B-cell lines in vitro under appropriate conditions.37 Since cells may be more susceptible if already subjected to oxidative stress, both the MThigh DLBCL described in this report and the recently identified so-called OxPhos consensus cluster of DLBCL, characterized by increased levels of several genes associated with oxidative phosphorylation,17 could constitute interesting targets for future investigations in clinical trials.
MT-II mRNA expression levels in 48 DLBCLs. MT-II mRNA expression levels in 48 DLBCLs related to the number of MT-positive tumor cells (A) and macrophages (B) determined by immunohistology. As shown, increased MT mRNA in crude extracts correlated both with the number of MT-positive tumor cells and macrophages.
MT-II mRNA expression levels in 48 DLBCLs. MT-II mRNA expression levels in 48 DLBCLs related to the number of MT-positive tumor cells (A) and macrophages (B) determined by immunohistology. As shown, increased MT mRNA in crude extracts correlated both with the number of MT-positive tumor cells and macrophages.
In a previous study, it was suggested that MT may constitute a “malignancy marker” in B-cell lymphomas based on the observation that tumor-infiltrating lymphocytes in 11 gastrointestinal tract lymphomas were MT negative, unlike the lymphoma cells that were positive to varying extents.25 The results from this investigation support the view that tumor-infiltrating lymphocytes in B-cell lymphomas are generally MT negative. However, it is also shown that MT protein in these conditions is not confined to the lymphoma cells, but that a proportion of tumor-infiltrating macrophages is often MT positive, especially in DLBCL. Since no specific correlation was identified between the number of MT-positive macrophages and lymphoma cells in this series, the observed MT staining of macrophages presumably did not result from phagocytosis, but rather reflected endogenous synthesis. In keeping with this assumption are the observations that MTs can be induced in monocytes under in vitro conditions and that MT-positive macrophages are also recruited to mice brain tissue subjected to cryoinjury.38,39
The functional implications of the MT-positive tumor-infiltrating macrophages seen in this study and the stimuli that induce them are not known. Of interest, in this report the proportion of MT-positive macrophages in DLBCL did not seem to influence the survival, although they correlated with and presumably contributed to the MT mRNA levels measured in crude extracts. Hence, from a clinical perspective, it is recommended that MT measurements of crude extracts be supplemented with immunohistologic assessments, which permit distinction between MT-positive lymphoma cells and macrophages.
In conclusion, it is suggested that up-regulated MT-I and MT-II mRNA and increased MT-I and MT-II protein expression by the lymphoma cells constitute independent risk factors in DLBCL. How the MTs influence the behavior of DLBCL at the molecular level is unknown. However, it is tempting to assume that increased proliferation, decreased apoptosis, and increased acquisition of resistance to chemotherapy may be implicated. These issues will be important to address in future investigations.
Prepublished online as Blood First Edition Paper, July 25, 2006; DOI 10.1182/blood-2006-04-015305.
Supported by grants from Hovedstadens Sygehusfællesskab (HS) Research Foundation, the Novo Nordisk Foundation, the Danish Foundation for Cancer Research, the Toyota Foundation, the Danish Medical Research Council, the Gangsted Foundation, and the Svend Andersen Foundation.
The authors declare no competing financial interests.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal