Overexpression of intracellular Notch plays an important role in the generation of human acute lymphoblastic T cell leukemia (T-ALL). In mouse models, it was shown that Notch-dependent T-ALL required pre-TCR signaling. Here we show that pre-TCR signaling is required to condition mice for Notch-dependent transformation but that it is not required to sustain malignant growth of T-ALL. In contrast to previous studies, we found that disease development does not require pre-TCR but that it can be accelerated in Rag2-/- mice by transient mimicking of pre-TCR signals. (Blood. 2006;108:305-310)
Introduction
Overexpression of intracellular Notch1 (ICN1) in lymphocyte precursors results in acute lymphoblastic T cell leukemia (T-ALL). The observation was initially made when a rare translocation of Notch1 into the Tcrb locus resulted in increased Notch-dependent transcription.1 Initially it was thought that elevated levels of ICN1 would only minimally contribute to human T-ALL, an incorrect assumption that was rectified when Notch1 was sequenced in T-ALL samples: more than 50% of human T-ALL exhibited mutations that were likely to result, and did result, in overexpression of ICN1. Thus, it is assumed that Notch1 plays a major role in the development of T-ALL.2
In the early days of Notch1-dependent T-ALL, a murine model was developed in which retroviral introduction of ICN1 into hemopoietic stem cells results in murine T-ALL with disease manifestations that resemble human T-ALL.3 In the course of studies on the murine model, it was proposed that ICN1 cooperates with pre-TCR in the generation of T-ALL because T-ALL did not arise in ICN1-transduced lymphocyte precursors deficient in pre-TCR expression or in pre-TCR signaling.4 This is of considerable interest given that recent studies show Notch1-pre-TCR cooperation in the physiologic process of generating α-β-lineage cells.5,6 A similar requirement for pre-TCR signaling in the generation of T-ALL in mice overexpressing Notch3 was noted when Notch3 transgenic mice7 were crossed onto the Ptcra-/- background8 : few Notch3 transgenic Ptcra-/- mice developed T-ALL, whereas the incidence of disease was much higher in Ptcra+/- Notch3 transgenic mice.9 The apparent pre-TCR signaling-independent T-ALL in this model made us reexamine the role of pre-TCR in the ICN1 model because these data suggested that perhaps pre-TCR signaling was not required to sustain malignant growth of Notch3-induced T-ALL. To this end we constructed ICN1-containing CMMP-EGFP recombinant retrovirus and analyzed early and late phases of T-ALL development in this murine model of disease. Results show that ICN1-dependent T-ALL is not essentially dependent on TCR signaling at the DN3 stage of T cell development but that pre-TCR signaling can facilitate the early onset of disease.
Materials and methods
Mice
BALB/c wt, BALB/c Rag2-/-, BALB/c nu/nu, and C57Bl/6 mice were all purchased from Taconic Farms (Germantown, NY) and were kept in specific pathogen-free animal facilities at the Dana-Farber Cancer Institute. All animal procedures were performed in compliance with the guidelines of the Dana-Farber Cancer Institute Animal Resources Facility.
Retrovirus and bone marrow transplantation
To generate recombinant retrovirus expressing constitutively active Notch1, a cassette encoding the entire intracellular domain of murine Notch1 (ICN1) was cloned upstream of an EGFP construct containing an internal ribosomal entry site (IRES; Clontech, Palo Alto, CA). The ICN1-IRES-EGFP fragment was inserted into the CMMP vector, a modified MMLV-based retroviral vector (kindly provided by R. Mulligan). The resultant ICN1 containing CMMP-IRES-EGFP retrovirus (mICN1) and the empty CMMP-IRES-EGFP relative control (empty) were used in all the transplantation experiments. Retroviral supernatants were produced by transient transfection of the retroviral vector and packaging plasmids10 into human embryonic kidney epithelial cell line 293T and then concentrated by ultracentrifugation.
Retroviral infection and cell transfer were conducted as previously described.11 Briefly, lineage marker-negative (Lin-) (CD3ϵ, TCRβ, Gr-1, Mac-1, Ter-119, DX5, and CD19-) bone marrow cells were sorted from 4- to 8-week-old female BALB/c, Rag2-/-, or nude mice. Lin- cells were cultured in the presence of IL-7, SCF, Flt3L, and IL-6 (all from R&D Systems, Minneapolis, MN). On days 2 and 4 after bone marrow harvest, cells were retrovirally transduced by “spin-infection.” On day 5, 1 to 2 × 106 cells were injected intravenously into lethally irradiated 4- to 8-week-old female syngeneic recipients.
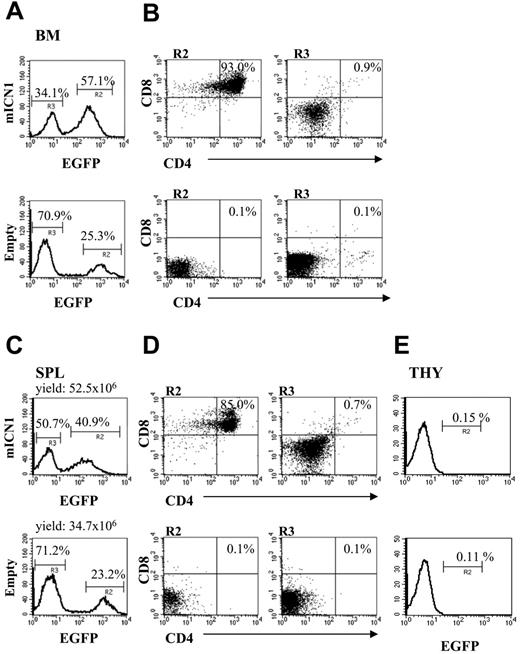
CD4+CD8+DP immature T cells appear 2 weeks after transplantation in bone marrow and spleen of BALB/c wt mice receiving syngeneic ICN1-transduced bone marrow cells. Cell suspensions from different organs were stained for the expression of CD4 and CD8 markers, and fluorescence-activated cell sorter (FACS) analysis was performed to determine EGFP+ and EGFP- population percentages in bone marrow (BM; A), spleen (SPL; C), and thymus (THY; E) of mice that underwent reconstitution with bone marrow wt cells transduced with mICN1-(containing top panels) or empty retroviruses (bottom panels). Percentages of CD4+CD8+ cells in the EGFP+ (B,D; R2, left panels) and EGFP- (B,D; R3, right panels) fractions from the same organs are indicated. Total yield of spleens is also included. Data are representative of at least 3 independent experiments.
CD4+CD8+DP immature T cells appear 2 weeks after transplantation in bone marrow and spleen of BALB/c wt mice receiving syngeneic ICN1-transduced bone marrow cells. Cell suspensions from different organs were stained for the expression of CD4 and CD8 markers, and fluorescence-activated cell sorter (FACS) analysis was performed to determine EGFP+ and EGFP- population percentages in bone marrow (BM; A), spleen (SPL; C), and thymus (THY; E) of mice that underwent reconstitution with bone marrow wt cells transduced with mICN1-(containing top panels) or empty retroviruses (bottom panels). Percentages of CD4+CD8+ cells in the EGFP+ (B,D; R2, left panels) and EGFP- (B,D; R3, right panels) fractions from the same organs are indicated. Total yield of spleens is also included. Data are representative of at least 3 independent experiments.
In a set of experiments, Rag2-/- hosts were intravenously injected with 50 μg purified anti-CD3ϵ (145-2C11; BD PharMingen, San Diego, CA), starting 10 days after bone marrow transplantation (BMT). In some cases, Rag2-/- recipients received triple injections of anti-CD3ϵ at 2-week intervals. All data related to the transplantation assay are representative of 3 independent experiments using at least 2 different preparations of the indicated retroviruses.
Analysis of animals after transplantation
After transplantation, animals were monitored by daily physical inspection and periodic peripheral blood sampling from retro-orbital sinuses. Two weeks after injection, and at weekly intervals thereafter, peripheral blood samples were stained for CD4 and CD8 expression and were analyzed by flow cytometry. Onset of leukemia was defined as the time after BMT when EGFP+CD4+CD8+ (double positive [DP]) immature T cells were identified in the peripheral blood, as previously described.12
Flow cytometry and cell sorting
Single-cell suspensions were prepared from peripheral blood, bone marrow, spleen, and thymus of ICN1-transplanted animals and related controls at the indicated time points. The following monoclonal antibodies were used: anti-CD4 (H129.19), anti-CD8 (53-6.7), anti-TCRβ (H57), anti-CD24 (M1/69), anti-CD3ϵ (145-2c11), anti-Gr-1 (RB6.782), anti-CD11b (M1/70), anti-erythroid cell marker (Ter-119), anti-pan NK cell marker (DX5), and anti-CD19 (1D3). These antibodies were directly coupled to phyco-erythrin (PE), fluorescein isothiocyanate (FITC), Cy-Chrome, or allophyco-cyanin (APC).
Cells were analyzed with the use of a FACSCalibur cytometer (Becton Dickinson, San Jose, CA) equipped with CellQuest Software (BD Biosciences) and FlowJo (Tree Star, San Carlos, CA) and were sorted by a MoFlo cell sorter (Cytomation, Fort Collins, CO).
Preparation and culture of DN cells
Double-negative (DN) cells were isolated from total thymocytes by staining cell suspensions with a biotinylated lineage-specific antibody cocktail followed by incubation with streptavidin-conjugated magnetic beads (Dynal, Oslo, Norway) and magnetic bead depletion of mature lineages. Enriched cell suspensions were surface stained with anti-CD44, anti-CD25, and streptavidin-APC. The DN3 or DN4 subsets were isolated by sorting Lin-CD44-CD25+ cells or Lin-CD44-CD25- cells, respectively. Cells were sorted using a FACSAria (Becton Dickinson, San Jose, CA); sorted cells were of 99% or greater purity, as determined by post-sort analysis.
OP9 cocultures
Before OP9 cocultures, sorted DN3 or DN4 cells were incubated with 1 μM CFSE (Molecular Probes, Eugene, OR) at 1 to 5 × 106 cells/mL PBS and 0.1% BSA for 10 minutes at 37°C. Cells were washed extensively with OP9-medium to eliminate the remaining CFSE and were plated onto subconfluent OP9-DL1 monolayers at 5 × 104 cells/well in a 24-well plate. All cocultures were performed in the presence of 1 ng/mL IL-7 and 5 ng/mL Flt3 ligand. Equal volumes of the γ-secretase inhibitor X (Calbiochem, San Diego, CA), serially diluted in dimethyl sulfoxide (DMSO) or 0.1% DMSO carrier alone, were added to selected wells on day 0 and were replaced together with the cytokines every 4 days. Cell division and developmental progression of CFSE-labeled cells were analyzed by flow cytometry. Contaminating OP9 cells were eliminated by filtering of the harvested cocultured cells through a 70-μM cell strainer before flow cytometric analysis. When total cellularity was indicated, cell counts were performed by trypan blue exclusion.
Cell lines
OP9 bone marrow stromal cells expressing the Notch ligand delta-like-1 (OP9-DL1) were maintained in α-MEM supplemented with 55 μM 2-mercaptoethanol, 10 mM HEPES (pH 7.5), 1 mM sodium pyruvate, 100 U/mL penicillin, 0.1 mg/mL streptomycin, 50 μg/mL gentamicin, and 20% heat-inactivated fetal bovine serum (FBS) and were passaged as described.13
Semiquantitative RT-PCR
Cells were sorted, and total RNA was extracted using the RNeasy kit (Qiagen, Valencia, CA) according to the manufacturer's instructions. To quantitate expression levels of the transcripts, PCR was performed on serial 1:5 dilutions of cDNA, and sample loading was monitored by a β-actin transcript that was subjected to the same treatment. Oligonucleotide primer sequences were: endogenous Notch1 forward, 5′-TGTTAATGAGTGCATCTCCAA-3′, and endogenous Notch1 reverse, 5′-CATTCGTAGCCATCAATCTTGTCC-3′; Notch3 forward, 5′-CCAGGGCTGCAACACTGAGGAATG-3′, and Notch3 reverse, 5′-TTGTGGCCAGCAGCTATGTCCTTG-3′; Deltex1 forward, 5′-CACTGGCCCTGTCCACCCAGCCTTGGCAG-3′, and Deltex1 reverse, 5′-ATGCGAATTCGGGAAGGCGGGCAACTCAG-3′; pTα forward, 5-CTACCATCAGGCATCGCT-3′, and pTα reverse, 5-CTATGTCCAAATTCTGTGGGTG-3′; β-actin forward, 5′-GTGGGCCGCTCTAGGCACCAA-3′, and β-actin reverse, 5-CTCTTTGATGTCACGCACGATTTC-3′.
Results
Extrathymic development of CD4+CD8+ cells with an abnormal phenotype
Overexpression of ICN1 with the newly constructed retrovirus that was introduced into lineage marker-negative (Lin-) bone marrow cells from BALB/c mice resulted in the presence of EGFP+CD4+CD8+ DP cells in the blood 2 weeks after reconstitution of lethally irradiated BALB/c mice with the retrovirally transduced bone marrow cells. In addition, bone marrow (Figure 1A-B) and spleen (Figure 1C-D) contained a high proportion (approximately 50%) of EGFP+CD4+CD8+ DP cells. At this stage, white blood cell (WBC) counts were only slightly (1.3 ×) elevated compared with those of control mice injected with empty vectors (14 × 109/L vs 11 × 109/L), and mice did not present with splenomegaly and lymphadenopathy (Figure 1). In agreement with earlier results obtained in a similar model,14 few EGFP+ cells could be detected at this stage in the thymus (Figure 1E), indicating that the development of CD4+CD8+ DP cells outside the thymus is much faster than the development of CD4+CD8+ DP cells that require immigration of lymphoid precursors into the thymus. This suggests that thymic immigrants are not represented by the immediate progeny of hematopoietic stem cells.
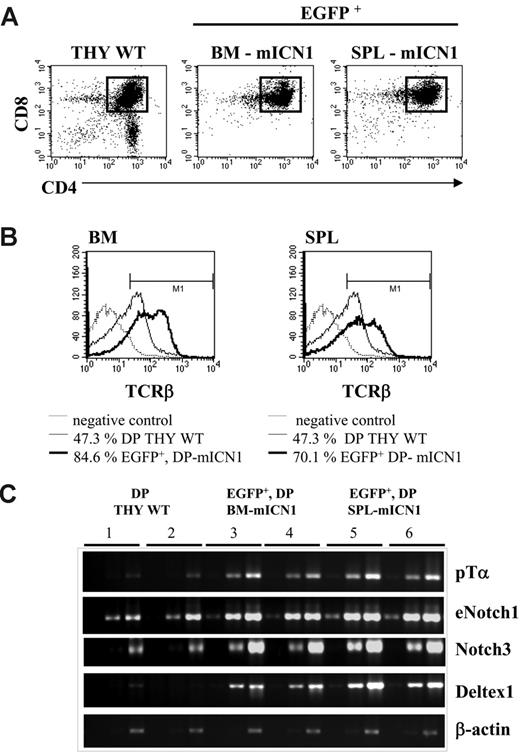
EGFP+CD4+CD8+DP cells at the early phase of ICN1-induced leukemia overexpress TCR-β at the cell surface and present enhanced expression of Notch-signaling-related genes. (A) Three BALB/c wt mice underwent transplantation with ICN1-transduced bone marrow cells. Two weeks after BMT, cells were harvested, pooled, and sorted, as indicated by the square marker, to obtain purified EGFP+CD4+CD8+ cells from bone marrow and spleen of mICN1 donors (BM-mICN1 and SPL-mICN1, respectively). DP thymic cells sorted from sex- and age-matched wt mice were used as controls (THY WT). (B) Cells sorted as in panel A were stained for TCRβ expression at the cell surface: number indicates the percentage of TCRβ+ cells; data are representative of at least 3 independent experiments. (C) Semiquantitative RT-PCR of Notch-signaling-related genes (endogenous Notch1, as eNotch1; Notch3; Deltex1, and Ptcra as pTα) was performed on serial 1:5 dilutions of cDNA derived from cell lysates of pools sorted as in panel A. Numbers indicate different pools derived from each population by 2 independent experiments. PCR were normalized according to β-actin expression.
EGFP+CD4+CD8+DP cells at the early phase of ICN1-induced leukemia overexpress TCR-β at the cell surface and present enhanced expression of Notch-signaling-related genes. (A) Three BALB/c wt mice underwent transplantation with ICN1-transduced bone marrow cells. Two weeks after BMT, cells were harvested, pooled, and sorted, as indicated by the square marker, to obtain purified EGFP+CD4+CD8+ cells from bone marrow and spleen of mICN1 donors (BM-mICN1 and SPL-mICN1, respectively). DP thymic cells sorted from sex- and age-matched wt mice were used as controls (THY WT). (B) Cells sorted as in panel A were stained for TCRβ expression at the cell surface: number indicates the percentage of TCRβ+ cells; data are representative of at least 3 independent experiments. (C) Semiquantitative RT-PCR of Notch-signaling-related genes (endogenous Notch1, as eNotch1; Notch3; Deltex1, and Ptcra as pTα) was performed on serial 1:5 dilutions of cDNA derived from cell lysates of pools sorted as in panel A. Numbers indicate different pools derived from each population by 2 independent experiments. PCR were normalized according to β-actin expression.
When compared with DP cells from a wild-type (wt) thymus (Figure 2A), the EGFP+CD4+CD8+ DP cells expressed higher levels of TCRβ chains at the cell surface (Figure 2B). Furthermore, at the RNA level, such cells expressed higher levels of Ptcra and other Notch1-regulated genes, such as endogenous Notch1, Notch3, and Deltex1 (Figure 2C).
All animals developed an aggressive form of leukemia, characterized by severe splenomegaly and lymphadenopathy, that resulted in death at approximately 9 weeks after transfer of retrovirally transferred bone marrow (Table 1).
Summary of results of bone marrow transplantation experiments
Adoptive transfer protocol . | No. of mice receiving transplants . | No. of mice with leukemia . | Survival time, d . | Terminal WBC count, × 109/L . | Spleen total yield, × 107 ± SD . | Leukemia onset, wk . |
|---|---|---|---|---|---|---|
| BALB/c wt | ||||||
| ICN1 | 16 | 16 | 58 | 158.5 | 88.0 ± 8.3 | 2 |
| Empty, killed | 11 | 0 | 58 | 10.2 | 4.5 ± 0.7 | NA |
| Rag2−/− | ||||||
| ICN1 | 13 | 12 | 80 | 27.6 | 23.2 ± 2.6 | 8 |
| ICN1 + 1 × CD3 | 15 | 15 | 77 | 28.1 | 35.7 ± 4.3 | 3 |
| ICN1 + 3 × CD3 | 13 | 13 | 62 | 180.2 | 91.8 ± 11.2 | 3 |
| Empty + 3 × CD3, killed | 12 | 0 | 76 | 9.9 | 1.7 ± 0.2 | NA |
Adoptive transfer protocol . | No. of mice receiving transplants . | No. of mice with leukemia . | Survival time, d . | Terminal WBC count, × 109/L . | Spleen total yield, × 107 ± SD . | Leukemia onset, wk . |
|---|---|---|---|---|---|---|
| BALB/c wt | ||||||
| ICN1 | 16 | 16 | 58 | 158.5 | 88.0 ± 8.3 | 2 |
| Empty, killed | 11 | 0 | 58 | 10.2 | 4.5 ± 0.7 | NA |
| Rag2−/− | ||||||
| ICN1 | 13 | 12 | 80 | 27.6 | 23.2 ± 2.6 | 8 |
| ICN1 + 1 × CD3 | 15 | 15 | 77 | 28.1 | 35.7 ± 4.3 | 3 |
| ICN1 + 3 × CD3 | 13 | 13 | 62 | 180.2 | 91.8 ± 11.2 | 3 |
| Empty + 3 × CD3, killed | 12 | 0 | 76 | 9.9 | 1.7 ± 0.2 | NA |
Retroviruses (ICN1 or empty) and number of anti-CD3 injections (1 ×, single; 3 ×, triple) used in the transplantation assay are indicated. Leukemia onset is defined as time (in weeks) after bone marrow transplantation when EGFP+CD4+CD8+ cells were identified in the peripheral blood.
NA indicates not applicable.
The early development of EGFP+CD4+CD8+ DP cells in various lymphoid organs and the later development of severe splenomegaly and lymphadenopathy were entirely thymus independent because they occurred with the same timing when retrovirally transduced bone marrow cells from BALB/c nu/nu mice were injected into BALB/c nu/nu recipients (Figure 3). Again, these results extend earlier results in which the development of EGFP+CD4+CD8+ DP cells was observed after injection of retrovirally transduced bone marrow cells into recipient mice after thymectomy.14
With progressive disease, the composition of phenotypes among EGFP+ cells shifted from a predominantly CD4+CD8+ DP population to a population of approximately equal proportions of CD4-CD8+ SP and CD4+CD8+ DP cells. The CD4-CD8+ SP cells all express CD24 on the cell surface, indicating that they comprised an immature T cell subset that could give rise to or be derived from CD4+CD8+ DP thymocytes (data not shown).
Pre-TCR signaling facilitates T-ALL generation but has no essential role in maintaining malignant growth
In previous experiments conducted with ICN1-overexpressing bone marrow cells, it was found that in the absence of pre-TCR signaling—that is, the absence of TCRs (Rag2-/- mice) or the absence of adapter protein (SLP76-/- mice)—mice did not develop EGFP+CD4+CD8+ DP cells.4 In contrast, results in Notch3-overexpressing mice showed that in the absence of Ptcra, mice would develop T-ALL, albeit with highly reduced frequency.9
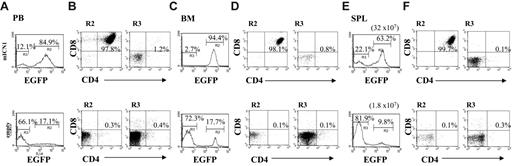
ICN1-induced leukemia is extrathymic in origin. Bone marrow cells from BALB/c nu/nu mice were transduced with mICN1 or empty retroviruses and injected into BALB/c nu/nu recipients. Cell suspensions were stained for the expression of CD4 and CD8 markers, and FACS analysis was performed to determine EGFP+ and EGFP- population percentages in peripheral blood (PB; E) 3 weeks after BMT and in bone marrow (BM; A) and spleen (SPL; C) 8 weeks after BMT of BALB/c nu/nu mice reconstituted with BALB/c nu/nu mice bone marrow transduced with mICN1-containing retroviruses. Percentage of CD4+CD8+ cells in the EGFP+ (B,D,F; R2, left panels) and EGFP- (B,D,F; R3, right panels) fractions from the same samples are indicated. Total yield of spleen is also included. EGFP+CD4+CD8+ cells were never detected in peripheral blood and organs from BALB/c nu/nu mice that underwent reconstitution with BALB/c nu/nu mice bone marrow transduced with empty-containing retroviruses (data not shown). Data are representative of at least 3 independent experiments.
ICN1-induced leukemia is extrathymic in origin. Bone marrow cells from BALB/c nu/nu mice were transduced with mICN1 or empty retroviruses and injected into BALB/c nu/nu recipients. Cell suspensions were stained for the expression of CD4 and CD8 markers, and FACS analysis was performed to determine EGFP+ and EGFP- population percentages in peripheral blood (PB; E) 3 weeks after BMT and in bone marrow (BM; A) and spleen (SPL; C) 8 weeks after BMT of BALB/c nu/nu mice reconstituted with BALB/c nu/nu mice bone marrow transduced with mICN1-containing retroviruses. Percentage of CD4+CD8+ cells in the EGFP+ (B,D,F; R2, left panels) and EGFP- (B,D,F; R3, right panels) fractions from the same samples are indicated. Total yield of spleen is also included. EGFP+CD4+CD8+ cells were never detected in peripheral blood and organs from BALB/c nu/nu mice that underwent reconstitution with BALB/c nu/nu mice bone marrow transduced with empty-containing retroviruses (data not shown). Data are representative of at least 3 independent experiments.
ICN1-induced T-ALL develops with delayed kinetics in Rag2-/- mice.Rag2-/- mice were reconstituted with Rag2-/- bone marrow transduced with mICN1-containing or empty retroviruses. Cell suspensions were stained for the expression of CD4 and CD8 markers, and FACS analysis was performed to determine EGFP+ percentages in peripheral blood (PB), bone marrow (BM), and thymus (THY) of Rag2-/- mice reconstituted with Rag2-/- bone marrow transduced with mICN1-containing retroviruses 2 weeks after BMT (A, top panels), 4 weeks after BMT (B, top panels), and 8 weeks after BMT (C, top panels). Percentages of EGFP+CD4+CD8+ cells from the same samples at 2, 4, and 8 weeks after BMT are also included (A-C, bottom panels). EGFP+CD4+CD8+ cells were never detected in peripheral blood and organs from Rag2-/- mice reconstituted with Rag2-/- bone marrow transduced with empty retroviruses (data not shown). Data are representative of at least 3 independent experiments.
ICN1-induced T-ALL develops with delayed kinetics in Rag2-/- mice.Rag2-/- mice were reconstituted with Rag2-/- bone marrow transduced with mICN1-containing or empty retroviruses. Cell suspensions were stained for the expression of CD4 and CD8 markers, and FACS analysis was performed to determine EGFP+ percentages in peripheral blood (PB), bone marrow (BM), and thymus (THY) of Rag2-/- mice reconstituted with Rag2-/- bone marrow transduced with mICN1-containing retroviruses 2 weeks after BMT (A, top panels), 4 weeks after BMT (B, top panels), and 8 weeks after BMT (C, top panels). Percentages of EGFP+CD4+CD8+ cells from the same samples at 2, 4, and 8 weeks after BMT are also included (A-C, bottom panels). EGFP+CD4+CD8+ cells were never detected in peripheral blood and organs from Rag2-/- mice reconstituted with Rag2-/- bone marrow transduced with empty retroviruses (data not shown). Data are representative of at least 3 independent experiments.
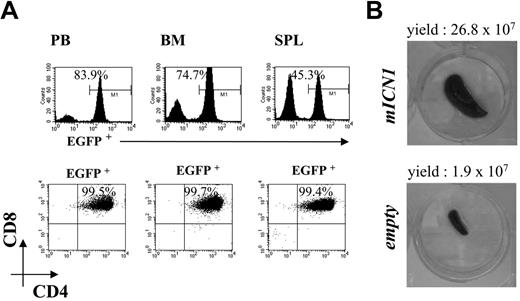
Phenotype o Rag2-/- mice at the late phase of ICN1-induced leukemia. (A) CD4 compared with CD8 FACS analysis was performed at 11 weeks after reconstitution for the detection of EGFP+ (top panels), and EGFP+CD4+CD8+ cells (bottom panels) in peripheral blood (PB), bone marrow (BM), and spleen (SPL) of Rag2-/- mice receiving syngeneic ICN1-transduced bone marrow. Rag2-/- control mice that underwent reconstitution with Rag2-/- bone marrow transduced with empty retrovirus and analyzed at the indicated time point do not contain EGFP+CD4+CD8+ cells in peripheral blood and organs (data not shown). (B) Macroscopic analysis and total yield of the spleen from the same animal described in panel A (top) and relative control mouse (bottom). Data are representative of at least 3 independent experiments.
Phenotype o Rag2-/- mice at the late phase of ICN1-induced leukemia. (A) CD4 compared with CD8 FACS analysis was performed at 11 weeks after reconstitution for the detection of EGFP+ (top panels), and EGFP+CD4+CD8+ cells (bottom panels) in peripheral blood (PB), bone marrow (BM), and spleen (SPL) of Rag2-/- mice receiving syngeneic ICN1-transduced bone marrow. Rag2-/- control mice that underwent reconstitution with Rag2-/- bone marrow transduced with empty retrovirus and analyzed at the indicated time point do not contain EGFP+CD4+CD8+ cells in peripheral blood and organs (data not shown). (B) Macroscopic analysis and total yield of the spleen from the same animal described in panel A (top) and relative control mouse (bottom). Data are representative of at least 3 independent experiments.
These findings raised the possibility that perhaps different expression levels of Notch could result in pre-TCR-dependent or pre-TCR-independent T-ALL, suggesting that pre-TCR may only be required at the early induction phase of T-ALL and not at later stages of disease. In fact, when the experiments in Rag2-/- mice were repeated with the retroviral construct used in this study, results differing from those of the previously published studies4 were obtained. Rag2-/- mice, after reconstitution with their retrovirally transduced bone marrow, developed disease with delayed kinetics. EGFP+CD4+CD8+ DP cells became visible in the bone marrow only 4 weeks after reconstitution (Figure 4B) and were detected in thymus and blood at 8 weeks (Figure 4C). Eventually these mice developed splenomegaly and lymphadenopathy and died approximately 11 weeks after bone marrow transplantation (Figure 5). To determine whether the onset of disease could be accelerated by signals that mimic pre-TCR signaling, the reconstituted Rag2-/- mice were injected with anti-CD3 antibodies that can relieve the developmental block at the DN3 stage of development by binding to CD3ϵ expressed on the cell surface of RAG-deficient mice in association with calnexin.15,16 As shown in Figure 6 and Table 1, a single injection of CD3 antibodies resulted in the early appearance of EGFP+CD4+CD8+ DP cells by 3 weeks in blood; mice succumbed to death 11 weeks after bone marrow reconstitution. Table 1 shows that triple injection with CD3 antibodies at 2-week intervals resulted in the early appearance of EGFP+CD4+CD8+ DP cells, followed by exaggerated WBC counts and splenomegaly and death by 9 weeks. Injection of CD3 antibodies into mice that underwent reconstitution with bone marrow transduced with empty vector had no impact. Thus, these data show that with the particular ICN1 retrovirus used, malignancy could develop in the absence of any TCR expression with cells normally arrested at the DN3 stage of development. By transiently mimicking pre-TCR signaling with the injection of CD3 antibodies, the development of disease in Rag2-/- mice could be accelerated and splenomegaly and lymphadenopathy could be increased, resulting in earlier death caused by T-ALL. These data show that pre-TCR signaling conditions mice to develop T-ALL but is no longer required once malignancy has developed.
Onset of ICN1-induced leukemia in RAG2-deficient mice is accelerated by treatment with anti-CD3ϵ antibodies.Rag2-/- mice were reconstituted with Rag2-/- bone marrow transduced with mICN1-containing or empty retroviruses. Ten days after transplantation, mice received single intravenous injections of anti-CD3ϵ. Cell suspensions from different organs were stained for the expression of CD4 and CD8 markers, and FACS analysis was performed to determine EGFP+ and EGFP- population percentages in peripheral blood (PB; A) 3 weeks after BMT and in bone marrow (BM; C) and spleen (SPL; E) 11 weeks after BMT of mice that underwent reconstitution with mICN1 (top panels) or empty-transduced bone marrow (bottom panels). Percentages of CD4+CD8+ cells in the EGFP+ (B,D,F; R2, left panels) and EGFP- (B,D,F; R3, right panels) fractions from the same organs are indicated. Total yield of spleens is also included. Data are representative of at least 3 independent experiments.
Onset of ICN1-induced leukemia in RAG2-deficient mice is accelerated by treatment with anti-CD3ϵ antibodies.Rag2-/- mice were reconstituted with Rag2-/- bone marrow transduced with mICN1-containing or empty retroviruses. Ten days after transplantation, mice received single intravenous injections of anti-CD3ϵ. Cell suspensions from different organs were stained for the expression of CD4 and CD8 markers, and FACS analysis was performed to determine EGFP+ and EGFP- population percentages in peripheral blood (PB; A) 3 weeks after BMT and in bone marrow (BM; C) and spleen (SPL; E) 11 weeks after BMT of mice that underwent reconstitution with mICN1 (top panels) or empty-transduced bone marrow (bottom panels). Percentages of CD4+CD8+ cells in the EGFP+ (B,D,F; R2, left panels) and EGFP- (B,D,F; R3, right panels) fractions from the same organs are indicated. Total yield of spleens is also included. Data are representative of at least 3 independent experiments.
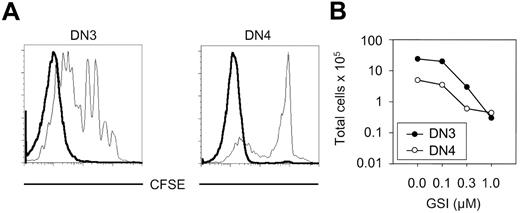
Analysis of proliferation and survival of DN3 and DN4 cells in the presence and absence of Notch signaling. (A) DN3 and DN4 cells derived from C57Bl/6 mice were labeled with CFSE and cocultured on OP9-DL1 monolayers with GSI (bold line) or without GSI (thin line) (1 μM). Proliferation was examined on day 7 by flow cytometric analysis of CFSE-labeled cells. (B) Cell counts for DN3 and DN4 cells derived from C57Bl/6 mice cocultured on OP9-DL1 monolayers with or without different concentrations of the GSI are shown. Cells were plated at an initial density of 5 × 104 cells/well, cultured in the presence of DMSO or increasing concentrations of GSI, and analyzed after 7 days of coculture. Total cellularity is plotted as a function of GSI concentration.
Analysis of proliferation and survival of DN3 and DN4 cells in the presence and absence of Notch signaling. (A) DN3 and DN4 cells derived from C57Bl/6 mice were labeled with CFSE and cocultured on OP9-DL1 monolayers with GSI (bold line) or without GSI (thin line) (1 μM). Proliferation was examined on day 7 by flow cytometric analysis of CFSE-labeled cells. (B) Cell counts for DN3 and DN4 cells derived from C57Bl/6 mice cocultured on OP9-DL1 monolayers with or without different concentrations of the GSI are shown. Cells were plated at an initial density of 5 × 104 cells/well, cultured in the presence of DMSO or increasing concentrations of GSI, and analyzed after 7 days of coculture. Total cellularity is plotted as a function of GSI concentration.
Notch signaling enhances proliferation of DN3 and DN4 cells
In the context of the experiments described, it is important to consider whether Notch signaling contributes to the survival of DN3 cells17 or whether, in concert with pre-TCR signaling, it contributes to the proliferation of DN3 and DN4 cells. This issue was addressed through analysis of the proliferation and expansion of CFSE-labeled DN3 and DN4 cells when cultured on OP9-DL1 cells in the absence or presence of various concentrations of the γ-secretase inhibitor (GSI) of Notch signaling,13 as shown in Figure 7.
Notch signaling contributes to the proliferation of immature thymocytes at both stages of differentiation such that the yield of DN3 decreases by 2 log and the yield of DN4 cells increases by 1 log in the presence of GSI (Figure 7B). Figure 7A shows that the decrease in cell numbers was actually caused by decreased proliferation reflected in the diminished dilution of the CFSE label. Thus, Notch signaling contributes significantly to the proliferation of cells before their acquisition of αβ lineage markers (CD4/CD8). Hence, Notch IC overexpression may lead to increased survival and proliferation of precursors of αβ lineage cells, thereby increasing genomic instability permissive for secondary genetic events that result in lymphoma.
Discussion
Here we report on the generation of T-ALL in an experimental model that uses ICN1-containing CMMP-EGFP recombinant retrovirus and faithfully reproduces observations made earlier of the thymus-independent generation of EGFP+CD4+CD8+ DP a few weeks after the reconstitution of irradiated animals with retrovirally transduced bone marrow.14 These cells are atypical in that they express increased TCRβ levels on the cell surface and exhibit increased RNA levels of several Notch target genes such as Ptcra, Notch1, Notch3, and Deltex1. This initial development of atypical T cell precursors is followed by the development of aggressive T-ALL associated with severe splenomegaly and lymphadenopathy resulting in death 8 to 9 weeks after bone marrow reconstitution. The course of events is similar in nu/nu mice that undergo reconstitution with retrovirally transduced bone marrow, indicating that not only the generation of atypical T cell precursors but, in fact, the generation of T-ALL can occur with the same kinetics as in wt mice in the absence of the thymus.
While investigating the role of pre-TCR signaling in the disease process, we were surprised to find that T-ALL developed even in Rag2-/- mice reconstituted with Rag2-/- bone marrow transduced with ICN1-containing retrovirus, albeit with delayed kinetics. Most likely these different findings were caused by different levels of ICN1 in the precursors of lymphocytes, resulting in passing of the pre-TCR-controlled checkpoint. This is of interest because it has recently become clear that the pre-TCR and Notch1 signaling synergize at this checkpoint in generating proliferating αβ lineage cells.5,6 Highly overexpressed ICN1 may have consequences similar to those of pre-TCR signaling because both pre-TCR and Notch signaling are thought to interfere with E2A activity, that is, with bHLH transcription factors thought to be involved in advancing differentiation and inhibiting proliferation.18 In fact, T-ALL does develop in E2A-deficient mice in the absence of pre-TCR.19 Earlier proposals suggested that Notch could interfere with E2A activity by enhancing pre-TCR signaling,20 but recently other pathways by which Notch can interfere with E2A in a pre-TCR-independent manner have been established.21 ICN1 overexpression may, at least in part, mimic pre-TCR signaling and allow for developmental progression of some DN3 cells by a pre-TCR-independent pathway. It is important to note in this context that Notch signaling contributes significantly to the proliferation of DN3 and DN4 cells (Figure 7); thus, overexpressed Notch may contribute to genomic instability at these stages of differentiation, facilitating secondary genetic events that result in malignant transformation. One could also envisage that the overexpression of intracellular ICN1, which results in elevated levels of Notch3 expression, could further T cell development in a Notch3-dependent manner because Notch3 overexpression can rescue T cell development in a pre-TCRα-deficient mice.9 Differences in the different retroviral systems may be attributed to the fact that in previous studies human ICN1 was used, whereas the retrovirus described here contains murine ICN1.
Whatever the reason for these discrepancies, our results clearly show that pre-TCR signaling plays no essential role in maintaining Notch1-dependent T-ALL. Not only can T-ALL develop in the absence of pre-TCR signaling in Rag2-/- mice, disease development in these mice can be accelerated such that it resembles T-ALL developing in wt mice by transiently mimicking pre-TCR signaling through the injection of CD3 antibodies. CD3 antibodies were previously shown to bind to CD3ϵ expressed at the surface of DN thymocytes in minute amounts in association with calnexin.15 Pre-TCR signaling conditions the mice to develop T-ALL with accelerated genetics, perhaps by inducing some genomic instability (proliferation, accessibility of TCR loci) that facilitates secondary genetic events, resulting in malignant transformation no longer requiring pre-TCR.
Prepublished online as Blood First Edition Paper, February 28, 2006; DOI 10.1182/blood-2006-01-0143.
Supported by the National Institutes of Health (grants P01CA 109901 and R01AI 45846) and by the Associazione Italiana per la Ricerca sul Cancro (AIRC). A.F.C. was supported by an Istituto Fondazione Italiana per la Ricerca sul Cancro (FIRC) di Oncologia Molecolare fellowship.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
We thank X. Li and F. Gounari for their support.



This feature is available to Subscribers Only
Sign In or Create an Account Close Modal