Comment on Valencia et al, page 253
In an article published in this issue of Blood, Valencia and colleagues provide strong evidence for a direct mechanistic link between 2 pathways, demonstrating that TNF can directly inhibit in vitro suppressive function of human Tregs via signaling through TNFRII, constituting direct negative feedback of TNF on CD25+CD4+ Tregs.
Tumor necrosis factor alpha (TNFα)isa key mediator in the pathogenesis of rheumatoid arthritis (RA),1 and its blockade provides substantial clinical benefit. More recently, a defect in the function of T-regulatory cells (Tregs) in patients with RA was found and is believed to be another crucial element in RA pathogenesis.
Intriguingly, Valencia and colleagues found that following anti-TNF therapy, patients with RA restored CD25+ Treg function that was associated with an increase of FoxP3 protein expression by these cells.
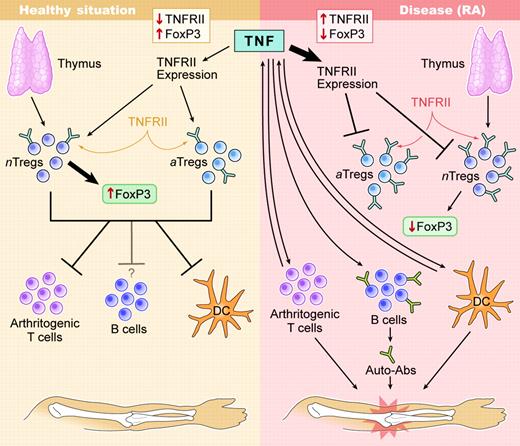
In the past several years, the concept that autoimmunity is caused by a disequilibrium in pathogenic and regulatory autoreactive responses has gained considerable momentum. Regulatory CD4+ T cells can be grouped into 2 major genres: one being intrinsic, thymus-generated Tregs characterized by constitutive CD25+ and FoxP3 expression,2 the other being adaptive Tregs generated after immunization with or exposure to (auto)antigens3 (see figure). In vivo effector functions exerted by Tregs are based on induction of cytokines such as IL-10, TGFβ, and IL-4/5; modulation of antigen-presenting cells (APCs); and, perhaps, direct suppressive effects exerted on pathogenic T cells. Therefore, all Tregs can act as “bystander suppressors,” because they suppress other effector T cells of different antigen specificities. This ability makes them the ideal physiologic and site-directed immunosuppressant, and they are therapeutically of high interest. Their antigen specificity is of major importance as well: polyclonal Treg populations will suppress ubiquitously (systemically), whereas autoantigen-induced specific Tregs can act locally, targeting the affected organ or draining lymph nodes directly.
Operational feedback loops involving direct effects of TNF on CD25+Tregs enhance diseased states in RA. Diseased states in RA are established and stabilized though negative feedback of TNF directly on TGFβ-induced CD25+Tregs. It is likely that in both populations intrinsic Tregs, as well as adaptively induced Tregs, can be affected. In many individuals, anti-TNF therapy can re-establish the equilibrium present in healthy individuals. Illustration by Kenneth Probst.
Operational feedback loops involving direct effects of TNF on CD25+Tregs enhance diseased states in RA. Diseased states in RA are established and stabilized though negative feedback of TNF directly on TGFβ-induced CD25+Tregs. It is likely that in both populations intrinsic Tregs, as well as adaptively induced Tregs, can be affected. In many individuals, anti-TNF therapy can re-establish the equilibrium present in healthy individuals. Illustration by Kenneth Probst.
In RA, defects in numbers and effector functions of CD4+CD25+ Tregs exist and can be reversed by anti-TNF therapy.4 In these earlier studies, it was unclear whether restoration of Treg function was a physiologic response due to overall dampening of inflammation after TNF blockade or whether Tregs were directly controlled by TNF. The new study by Valencia and colleagues provides a crucial piece of this puzzle in demonstrating that TNF can directly switch off Tregs by lowering FoxP3 expression, a crucial transcription factor associated with CD25+ Treg effector functions.5
What are the implications of these findings? As illustrated in the figure, this direct negative effect of TNF on Treg function will enhance the pathogenetic process leading to RA, setting in motion a vicious cycle that more rapidly will lead to a point of no return. Therapeutically, this explains the exquisite efficacy of anti-TNF therapy. The finding also implies that direct augmentation of Tregs or their induction might not be necessary in patients with RA who respond well to anti-TNF treatment. On the other hand, it should prompt us to further investigate whether TNF nonresponders have an irreversible defect in Treg function. In addition, establishing direct intracellular links between TNFRII signaling and FoxP3 expression could help to identify molecular signatures that could predict Treg-cell behavior on an individual basis.
An interesting task would be the exploration of the role of TNF on Treg function under physiologic situations. From the findings of Valencia et al and Ehrenstein et al,4 it appears that anti-TNF therapy does not act only on joint-specific, oligoclonal Tregs. This raises the important question of whether we could use anti-TNF therapy for restoration of Treg function in other autoimmune diseases where TNF is implicated.
Finally, integration of feedback loops and their disruption into our therapeutic thinking should involve bioinformatics approaches in the future that would cover not only cellular networks but also intracellular pathways. Such in silico models could help us identify crucial nodal points in transcription and predict under which circumstances and how rapid switches between healthy and diseased states occur. ▪

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal