Bradykinin (BK) liberates nitric oxide, prostacyclin, and tissue plasminogen activator from endothelial cells. We hypothesized that BK B2 receptor knockout (KO) mice (BKB2R-/-) have increased thrombosis risk. Paradoxically, the BKB2R-/- mice have long bleeding times and delayed carotid artery thrombosis, 78 ± 6.7 minutes, versus 31 ± 2.7 minutes in controls. The mechanism(s) for thrombosis protection was sought. In BKB2R-/- plasma coagulation, fibrinolysis and anticoagulant proteins are normal except for an increased prekallikrein and decreased factor XI. BKB2R-/- mice have elevated BK 1-5 (160 ± 75 fmol/mL, vs 44 ± 29 fmol/mL in controls) and angiotensin II (182 ± 41 pg/mL, vs 49 ± 7 pg/mL in controls). Ramipril treatment shortens vessel occlusion time. BKB2R-/- mice have elevated plasma 6-keto-PGF1α (666 ± 232 ng/mL, vs 23 ± 5.3 ng/mL in controls) and serum nitrate (61 ± 5.3 μM, vs 24 ± 1.8 μMin controls). Treatment with L-NAME (NG-mono-methyl-l-arginine ester) or nimesulide shortens the thrombosis time. BKB2R-/- mice have increased angiotensin receptor 2 (AT2R) mRNA and protein expression. Treatment with an AT2R antagonist, PD123 319, normalizes the thrombosis time and nitrate and 6-keto-PGF1α. The long bleeding times in BKB2R-/- mice also correct with L-NAME and nimesulide therapy. In BKB2R-/- mice, angiotensin II binding to an overexpressed AT2R promotes thromboprotection by elevating nitric oxide and prostacyclin. These investigations indicate a pathway for thrombosis risk reduction via the plasma kallikrein/kinin and renin angiotensin systems. (Blood. 2006;108:192-199)
Introduction
When the proteins of the plasma kallikrein/kinin system (KKS) assemble on a multiprotein receptor complex on endothelial cells or matrix, the serine protease, prolylcarboxypeptidase (PRCP), activates prekallikrein (PK) bound to high-molecular-weight kininogen (HK) to form plasma kallikrein and bradykinin (BK).1-5 Prior to these studies, PRCP had only been known as a BK and angiotensin II (AngII) degrading enzyme.6,7 Formation of plasma kallikrein and BK is a physiologic pathway because mice deficient in C1 inhibitor, the major plasma kallikrein inhibitor, have constitutive BK-mediated angioedema.8 Formed plasma kallikrein in the intravascular compartment has 3 substrates: it autodigests its receptor HK to liberate BK and it activates factor XII and single chain urokinase.5,9,10 BK by binding to the constitutive bradykinin B2 receptor (BKB2R) in the intravascular compartment promotes blood flow through nitric oxide (NO) and prostacyclin formation and tissue plasminogen activator (tPA) liberation.11-13 Single chain urokinase activation promotes plasmin formation.10 Factor XII activation amplifies PK activation and BK liberation and modulates HK binding to endothelial cells.9,14 Since BK contributes to the antithrombotic nature of endothelium, we postulated that BKB2R KO (BKB2R-/-) mice would be prothrombotic.15 To our surprise we found that BKB2R-/- mice have long bleeding times and delayed thrombosis times due to an overexpressed angiotensin receptor 2 (AT2R) and elevated NO and prostacyclin levels. These investigations indicate a pathway for thrombosis risk reduction via the plasma kallikrein/kinin and renin angiotensin systems.
Materials and methods
Materials
Plasma prekallikrein activator (PKA) and chromogenic substrates H-D-Pro-Phe-Arg-pNA·2HCl (S2302), H-D-Val-Leu-Lys-pNA·2HCl (S2251), H-D-Phe-Pip-Arg-pNA · 2HCl (S2238), and pyroGlu-Pro-Arg-pNA · HCl (S2366) were purchased from DiaPharma (Franklin, OH). The bradykinin B2 receptor antagonist (HOE140, icatibant, D-Arg-Arg-Pro-Hyp-Gly-Thi-Ser-D-Tic-Oic-Arg) was purchased from Phoenix Pharmaceuticals (Belmont, CA). Single chain HK (molecular weight [Mr] = 120 kDa on reduced sodium dodecyl sulfate-polyacrylamide gel electrophoresis [SDS-PAGE]) with a specific activity of 13 U/mg and PK (Mr = 88 and 85 kDa on reduced SDS-PAGE) with a specific activity of 27 U/mg were purchased from Enzyme Research Laboratories (South Bend, IN). FXI with a specific activity of 360 U/mg was purchased from Haematologic Technologies (Essex Junction, VT).
Animals
All animal care and experimental procedures complied with the principles of Laboratory and Animal Care established by the National Society for Medical Research and were approved by the University of Michigan Committee on Use and Care of Animals. BKB2R-/- mice, strain name B6;129S7-Bdkrb2tm1Jfh,16 and their controls, B6129SF2/J mice, were purchased from Jackson Laboratories (Bar Harbor, ME). The BKB2R-/- mice colonies were maintained by brother/sister mating and all progeny were genotyped by polymerase chain reaction (PCR) using DNA purified from tail biopsies prior to use in experiments using primers recommended by Jackson Laboratories. All studies were performed on mice 8 to 12 weeks of age. BKB2R-/- mice from Jackson Laboratories were also backcrossed one generation against 129S1/svImJ mice to make heterozygous animals. These heterozygous animals were then bred to make homozygous and wild-type animals that were studied to determine if they had the same thromboprotection as seen in the B6129SF2/J KO mice. Tail bleeding times were performed as previously reported.17,18
Mouse carotid artery thrombosis studies
Mice for the Rose Bengal and ferric chloride carotid artery thrombosis models were anesthetized with 40 mg/kg sodium pentobarbital injected intraperitoneally, and immobilized on an operating table.18 The Rose Bengal carotid artery thrombosis model was performed as previously reported.18 After vessel occlusion, the carotid arteries of some control and KO mice were ligated distal to the thrombus. The entire animal was Zn-formalin fixed by cardiac injection for organ removal for histologic analysis.19 In the ferric chloride model, the exposed artery was treated with a 1.0 × 1.0 mm Whatman no. 1 filter paper saturated with 8% FeCl3 for 1 minute followed by saline-saturated filter paper treatment. The time to vessel occlusion was monitored as in the Rose Bengal assay.18
Preparation of mouse plasma and serum
Animals were anesthetized with 40 mg/kg sodium pentobarbital injected intraperitoneally. A celiotomy was performed to expose the inferior vena cava (IVC) and blood was obtained from mice in 3.8 gm% sodium citrate by venipuncture of the IVC.18 After collection, the whole blood was centrifuged at room temperature for 15 minutes at 1000g. The supernatant plasma was frozen in small aliquots at -70°C until assay. When tissue plasminogen activator was measured, the citrated whole blood was acidified with acetic acid before centrifugation. In certain instances, mouse serum was collected by withdrawing whole blood in a syringe not containing anticoagulant, followed by incubation for 1 hour at 37°C followed by 3 hours at 4°C. After clot formation and riming the tube with a wooden stirrer, the serum was carefully aspirated around the formed clot and frozen at -70°C in small aliquots, until assay.
Coagulation and fibrinolysis assays
All coagulant assays were performed in Amelung KC4 coagulation analyzer (Sigma, St Louis, MO) on frozen mouse plasma samples that were thawed once for the assay. Assays for coagulation Factors XI, VIII, IX, XII, and HK were performed with their respective human factor-deficient plasma in APTT-based assays using APTT reagent (Organon Teknika, Durham, NC) as previously reported.20 HK-deficient plasma was donated to this laboratory by the late Mayme Williams (Philadelphia, PA). Assays for coagulation Factors VII, X, II, and V were performed with their respective human factor-deficient plasma (George King, Overland Park, KS) in prothrombin time-based assays using a reagent containing thromboplastin (Simplastin; Organon Teknika).20 Standard curves for each of these coagulant factor assays were prepared with pooled normal human plasma. Amidolytic assays for antithrombin, plasminogen, and PK were performed as previously reported.20,21 Plasma tPA and plasminogen activator inhibitor (PAI-1) were assayed using a chromogenic assay (Biopool International, Ventura, CA) according to the manufacturer's procedures.
Histology and immunohistochemistry of tissue samples
Carotid artery and organ samples fixed in Zn-formalin were freshly processed or paraffin embedded. Sections were stained with hematoxylin and eosin (H&E) or periodic acid-Schiff (PAS), or underwent immunohisto-chemical staining for fibrin(ogen) using a polyclonal goat anti-mouse fibrin(ogen) antibody (Accurate Chemical and Scientific, Westbury, NY) followed by immunoperoxidase staining with the ABC methodology (Vector, Burlingame, CA).19 Histologic examination was performed by a board-certified anatomic pathologist (J.W.H.) blinded to genotype/treatment group.
Measurement of RPPGF levels in mouse blood
Samples (0.3 mL) for measurement of mouse blood RPPGF (bradykinin 1-5) were collected by venipuncture in the IVC into a tuberculin syringe. Immediately after drawing blood, the sample was mixed with 0.9 mL cold anhydrous ethanol. After standing on ice for 30 to 60 minutes, tubes were centrifuged at 250g for 15 minutes at 4°C and the ethanolic plasma supernatant stored at -70°C until analysis as previously reported.18,22 RPPGF in the samples was analyzed by liquid-chromatography tandem mass-spectrometry by the method of Murphey et al.18,22 Concentrations in ethanolic supernatant were corrected for dilution by blood and reported as fmol per milliliter of blood (pM).
Other assays
Angiotensin II (AngII) was determined from freshly drawn mouse whole blood containing 25 mM EDTA, 0.44 mM o-phenanthroline, 1 mM p-hydroxymercuribenzoic acid, and 0.12 mM pepstatin A. Plasma was prepared by centrifugation at 3000g for 20 minutes at 4°C and stored at -70°C. AngII was extracted from plasma by chromatography over phenyl columns (model no. 12 102 062; Varian, Palo Alto, CA) followed by elution with methanol. After evaporation to dryness, the peptide fraction was resuspended in assay buffer. An enzyme immunoassay for AngII was purchased from Cayman Chemical (Ann Arbor, MI). Angiotensin converting enzyme (ACE; EC3.4.15.1) was measured in mouse serum by a commercial procedure performed according to the manufacturer's instructions (ALPCO Diagnostics, Windham, NH). In this assay, the ability of ACE to hydrolyse 3H-hippuryl-glycylglycine, to form 3H-hippuric acid, was determined.23 The stable analog of 6-keto-prostaglandin F1α (6-keto-PGF1α), was measured in mouse plasma anticoagulated with 3.8% sodium citrate by enzyme-linked immunosorbent assay according to the manufacturer's specifications (Cayman Chemical). Serum nitrite/nitrate was determined by a colorimetric assay kit according to the manufacturer's specifications (Cayman Chemical).
Influence of ramipril on thrombosis risk in the BKB2R-/- mice
Control and BKB2R-/- mice were treated with ramipril at 1.25 or 2.5 mg/kg per day, dissolved in their drinking water prepared fresh daily for 10 days.24
Influence of L-NAME treatment on thrombosis
Wild-type (B6129SF2/J mice) or BKB2R-/- mice were fed water alone or water containing 0.5 mg/mL L-NAME (NG-mono-methyl-l-arginine ester; Calbiochem, San Diego, CA) made fresh each day for 7 days according to the procedure of Obst et al.25 At the end of the treatment period, the time to vessel occlusion on the Rose Bengal thrombosis assay was determined as described in “Mouse carotid artery thrombosis studies.”
Influence of nimesulide treatment on thrombosis
Wild-type (B6129SF2/J mice) or BKB2R-/- mice were treated with water or water containing 50 μg/mL nimesulide [N-(4-nitro-2-phenoxyphenyl)- methanesulfonamide; Cayman Chemical], a cyclooxygenase 2 inhibitor, made fresh each day for 10 days according to the procedure of Pratico et al.26 At the end of the treatment period, the time to vessel occlusion was determined on the Rose Bengal thrombosis assay. When treatments with L-NAME (0.5 mg/mL) and nimesulide (50 μg/mL) were combined, the animal received the agents for 10 days in their drinking water, changed daily with the addition of fresh inhibitors.
Influence of angiotensin receptor 2 antagonism on thrombosis
Wild-type (B6129SF2/J mice) or BKB2R-/- mice had an osmotic pump placed (ALZET model no. 2001; ALZET, Cupertino, CA) containing saline or 30 mg/kg per day, per 24 μL PD123 319 (Pfizer, Ann Arbor, MI) for 7 days according to the procedure of Tsutumi et al.27 The AT2R antagonist PD123 319 was generously provided by Dr Joan Kiser (Pfizer). At the end of the treatment period, serum or plasma was harvested for NO and prostacyclin assay, respectively, or the time to vessel occlusion on the Rose Bengal thrombosis assay was determined as described in “Mouse carotid artery thrombosis studies.”
RNA isolation
BKB2R-/- and B6129SF2/J control mice, 8 to 12 weeks old and weighing 15 g to 20 g, were anesthetized with sodium pentobarbital. The kidney, liver, and heart of each animal were removed and immediately frozen in liquid nitrogen and stored at -80°C. Total RNA was isolated from each organ using TRIZOL Reagent (Invitrogen, Carlsbad, CA) from 3 to 5 individual mice under resting conditions. The RNA was further purified using mini-RNeasy columns (Qiagen, Valencia, CA) according to the manufacturer's protocol. All RNA samples were incubated with 4 U DNase I (Invitrogen) for 15 minutes at 37°C to remove DNA template before reverse transcriptase (RT)-PCR.
cDNA synthesis
Synthesis of the first strand of cDNA was performed using 2 μg to 5 μg total RNA from heart, kidney, or liver of wild-type or BKB2R-/- mice as template and 1 μL oligo(dT)12-18 primer (500 μg/mL), following the protocol accompanying the SUPERScript Preamplification System for First Strand cDNA Synthesis (Invitrogen). A 5-μL aliquot of the cDNA synthesis reaction was amplified with 300 pmol of gene-specific oligonucleotide primers for the bradykinin B1 receptor (BKB1R; Gene Bank accession no. U44436), BKB2R (no. NM_009747), AT1R (no. NM_009642), AT2R (no. NM_007429), MAS protooncogene (MAS), the angiotensin 1-7 G-protein-coupled receptor28 (no. NM_145379.1), factor XI (FXI; no. AF356627), prekallikrein (PK; no. BC026555), or plasminogen activator inhibitor 1 (PAI-1; no. M33960) primers in 20 μL reaction volume (Table 1). After the mixture was heated to 65°C for 5 minutes and chilled on ice and centrifuged, 4 μL 5X first-strand buffer, 2 μL 0.1 M DTT, 1 μL40 U/μL recombinant ribonuclease inhibitor was added, mixed and incubated with 1 μL (200 U) Superscript II RT (Invitrogen) for 50 minutes at 42°C. Next, it was incubated with 2 U E coli RNAse H at 37°C for 20 minutes to remove RNA complementary to the cDNA. The prepared cDNA was then purified using the QIAquick PCR purification kit (Qiagen) for use as a template for conventional and real-time PCR.
Real-time PCR primers
Target . | Sense primer . | Position on cDNA . | Antisense primer . | Position on cDNA . | Probe . | Position on cDNA . |
|---|---|---|---|---|---|---|
| BKB1R | 5′-CACTTTGCAAGGATGGTGGAGTTG-3′ | 764-787 | 5′-GGAGGCCAGGATGTGATAGTTGAA-3′ | 830-853 | —* | — |
| BKB2R | 5′-CTGGGTGTTTGGAGAGGTGT-3′ | 479-499 | 5′-ACGAGCATCAGGAAGCAGAT-3′ | 565-545 | 56-FAM/GGGTGGTGAACACCATGATC/3BHQ-1 | 505-525 |
| AT1R | 5′-CAGTGGAGAAGAGCCCAGTC-3′ | 679-699 | 5′-AGCACTCTGGGCAGTTCAGT-3′ | 821-801 | 56-FAM/TTCCCCTCCTACCCCATAAC/3BHQ-1 | 768-788 |
| AT2R | 5′-CCCTAAAAAGGTGTCCAGCA-3′ | 395-415 | 5′-CACAGGTCCAAAAAGCCAAT-3′ | 518-498 | 56-FAM/ATCTGGCCTTGGCTGACTTA/3BHQ-1 | 427-447 |
| MAS | 5′-TCTACTTGGGGATCGACTGG-3′ | 909-928 | 5′-GCACTGCTGTTGATGCAGAT-3′ | 1008-989 | 56-FAM/CCCCGAGTACGTCACTGACT/3BHQ-1 | 964-983 |
| PK | 5′-GGCTACACGAAGGAACAAGG3′ | 1588-1607 | 5′-CACCGGAATCTCCCTTACAA-3′ | 1759-1740 | 56-FAM/GTGCTGGCTACAAAGAAGGG/3BHQ-1 | 1709-1727 |
| FXI | 5′-GCAAATACAATGGGGTCTGGC-3 | 1809-1829 | 5′-CCAGTTGAACTCTTTCAGACTGTTT3′ | 1988-1968 | 56-FAM/GCCAAGTACGTGGACTGGAT/3BHQ-1 | 1901-1920 |
| PAI-1 | 5′-AGTCTTTCCGACCAAGAGCA-3′ | 1122-1142 | 5′-GCCGAACCACAAAGAGAAAG-3′ | 1330-1310 | 56-FAM/GATCGAGGTAAACGAGAGCG/3BHQ-1 | 1186-1205 |
| γ-actin | 5′-CGAAAAGACCTGTATGCCAAT-3 | 910-927 | 5′-GGGCTGTGATCTCCTTCTGC-3′ | 997-978 | HEX/TACCACCATGTACCCAGGCATTGCTGA/3BHQ-1 | 945-971 |
Target . | Sense primer . | Position on cDNA . | Antisense primer . | Position on cDNA . | Probe . | Position on cDNA . |
|---|---|---|---|---|---|---|
| BKB1R | 5′-CACTTTGCAAGGATGGTGGAGTTG-3′ | 764-787 | 5′-GGAGGCCAGGATGTGATAGTTGAA-3′ | 830-853 | —* | — |
| BKB2R | 5′-CTGGGTGTTTGGAGAGGTGT-3′ | 479-499 | 5′-ACGAGCATCAGGAAGCAGAT-3′ | 565-545 | 56-FAM/GGGTGGTGAACACCATGATC/3BHQ-1 | 505-525 |
| AT1R | 5′-CAGTGGAGAAGAGCCCAGTC-3′ | 679-699 | 5′-AGCACTCTGGGCAGTTCAGT-3′ | 821-801 | 56-FAM/TTCCCCTCCTACCCCATAAC/3BHQ-1 | 768-788 |
| AT2R | 5′-CCCTAAAAAGGTGTCCAGCA-3′ | 395-415 | 5′-CACAGGTCCAAAAAGCCAAT-3′ | 518-498 | 56-FAM/ATCTGGCCTTGGCTGACTTA/3BHQ-1 | 427-447 |
| MAS | 5′-TCTACTTGGGGATCGACTGG-3′ | 909-928 | 5′-GCACTGCTGTTGATGCAGAT-3′ | 1008-989 | 56-FAM/CCCCGAGTACGTCACTGACT/3BHQ-1 | 964-983 |
| PK | 5′-GGCTACACGAAGGAACAAGG3′ | 1588-1607 | 5′-CACCGGAATCTCCCTTACAA-3′ | 1759-1740 | 56-FAM/GTGCTGGCTACAAAGAAGGG/3BHQ-1 | 1709-1727 |
| FXI | 5′-GCAAATACAATGGGGTCTGGC-3 | 1809-1829 | 5′-CCAGTTGAACTCTTTCAGACTGTTT3′ | 1988-1968 | 56-FAM/GCCAAGTACGTGGACTGGAT/3BHQ-1 | 1901-1920 |
| PAI-1 | 5′-AGTCTTTCCGACCAAGAGCA-3′ | 1122-1142 | 5′-GCCGAACCACAAAGAGAAAG-3′ | 1330-1310 | 56-FAM/GATCGAGGTAAACGAGAGCG/3BHQ-1 | 1186-1205 |
| γ-actin | 5′-CGAAAAGACCTGTATGCCAAT-3 | 910-927 | 5′-GGGCTGTGATCTCCTTCTGC-3′ | 997-978 | HEX/TACCACCATGTACCCAGGCATTGCTGA/3BHQ-1 | 945-971 |
BKB1R indicates bradykinin B1 receptor; BKB2R, bradykinin B2 receptor; AT1R, angiotensin receptor type 1; AT2R, angiotensin receptor type 2; MAS, mas protooncogene or the angiotensin 1-7 receptor; PK, prekallikrein; FXI, factor XI; PAI-1, plasminogen activator inhibitor-1.
Detection of the PCR product was with SYBR-490. See “Materials and methods.”
Real-time RT-PCR
Real-time quantitative PCR for FXI, PK, PAI-1, BKB2R, BKB1R, AT1R, AT2R, MAS, or γ-actin cDNA (0.25 μg-4 μg) were separately amplified using a fluorescent oligonucleotide probe with a 5′ reporter dye (FAM or HEX) and a downstream 3′ Black Hole quencher dye (BHQ-1; Integrated DNA Technologies, Coralville, IA; Table 1). The BKB1R real-time RT-PCR was detected by SYBR-490 (SYBR Green Supermix; BioRad, Hercules, CA). The hybridization of the fluorescence probe to the PCR products was quantified by measuring the fluorescence in the iCycler iQ real-time PCR detection system (BioRad). The PCR mixture (50 μL) consisted of 0.2 μM of each primer, 0.02 μM probe, and TaqMan Universal Master Mix (2x) or platinum Taq. Two-step or 3-step cycle conditions were used: 95°C for 5 minutes, followed by 40 cycles at 95°C for 30 seconds and 58°C or 60°C for 30 seconds, using the iCycler iQ real-time PCR detection system. For the 3-step cycle, the following condition was used: 35 cycles at 95°C for 30 seconds, 60°C for 30 seconds, and 72°C for 1 minute, using the iCycler iQ real-time PCR detection system.
Real-time PCR data for each target were compared by normalization with γ-actin according to the procedure of Schmittgen et al.29 Real-time quantitative PCR data were compared using the comparative threshold cycle (Ct) value, defined as the PCR amplification cycle in which the reporter signal is greater than the minimal detection level. Final data are expressed as the ratio of fold change of cDNA in the knockout animal versus the fold change of cDNA in control. Final ratio values greater than 1 are considered significant.
Determination of AT2R antigen in mouse kidney
Kidney from BKB2R-/- mice and control animals were harvested after celiotomy and rinsed in RNAlater (Qiagen) and weighed. For each gram of tissue, 15 mL to 20 mL of Tissue-PE LB (Geno Tech, St. Louis, MO) containing “Protease Arrest” (Geno Tech) was added to extract the protein. The tissue was then homogenized at 4°C in the presence of 2 mM DTT and EDTA, followed by centrifugation at 20 000g for 30 minutes at 4°C and the pellet was solubilized with RIPA (50 mM Tris-HCl, 150 mM NaCl, pH 7.5 containing 1% NP-40, 0.5% sodium deoxycholate, and 0.1% SDS) and incubated on ice for 30 minutes. Following incubation, the sample was centrifuged at 10 000g for 10 minutes at 4°C and the supernatant collected for assay. Protein estimation was performed using the dye-binding assay (BioRad). Western blot of equal total protein concentration of the kidney lysates from control and BKB2R-/- mice was performed.1 The primary antibody was a rabbit antihuman antibody to AT2R (H143; Santa Cruz Biotechnology, Santa Cruz, CA) at a 1:200 dilution, followed by a goat antirabbit antibody conjugated with horseradish peroxidase (Sigma). The specific reactivity of the antibody with the electroblotted sample was detected with the ECL system from Amersham Biosciences (Piscataway, NJ). The immunoblot was then scanned by densitometer (model GS 300; Hoefer Scientific Instruments, San Francisco, CA) in the transmittance mode to determine the band intensity and thus the relative amounts of protein present.
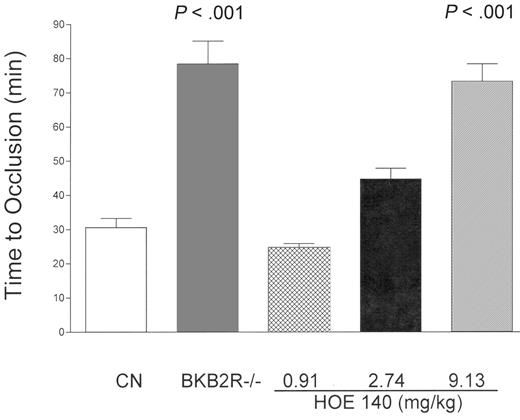
The influence of the absence of the BKB2R receptor on the time to thrombosis in the Rose Bengal model of carotid artery occlusion. Control mice (B6129SF2/J mice, CN, n = 19) and BKB2R-/- mice (n = 9) were examined for their time to carotid artery occlusion (see “Materials and methods”). Additionally, control mice were treated with increasing concentrations of icatibant (HOE140) (0.91 mg/kg to 9.13 mg/kg, given intraperitoneally) and the time to thrombosis was determined. These latter data are the mean plus or minus SD of 4 or more experiments at each experimental concentration of HOE140.
The influence of the absence of the BKB2R receptor on the time to thrombosis in the Rose Bengal model of carotid artery occlusion. Control mice (B6129SF2/J mice, CN, n = 19) and BKB2R-/- mice (n = 9) were examined for their time to carotid artery occlusion (see “Materials and methods”). Additionally, control mice were treated with increasing concentrations of icatibant (HOE140) (0.91 mg/kg to 9.13 mg/kg, given intraperitoneally) and the time to thrombosis was determined. These latter data are the mean plus or minus SD of 4 or more experiments at each experimental concentration of HOE140.
Data analysis
All data are presented as means of triplicate determinations and error bars indicate plus or minus one standard deviation (SD) or one standard error of the mean (SEM). Significance between groups is determined by the Student t test. P values less than .05 were considered significant. Concentrations of RPPGF were analyzed using the nonparametric Mann Whitney test.
Results
Determination of thrombotic risk in BKB2R-/- mice
The hypothesis that initiated these investigations was that since BK is a potent stimulator of NO, prostacyclin (PGI2), and tPA release from endothelial cells, BKB2R-/- mice would be prothrombotic.11-13 Contrary to that hypothesis, BKB2R-/- mice had delayed time to vessel occlusion (78 ± 6.7 minutes, n = 9 [mean ± SEM] vs 31 ± 2.7 minutes in B6129SF2/J controls, n = 19; P < .001) when carotid artery injury was instituted on the Rose Bengal model for thrombosis, an endothelial-cell photochemical injury model due to local free radicals release (Figure 1). Furthermore, BKB2R-/- mice had a mean tail bleeding time of 135 ± 11 seconds (mean ± SEM, n = 8) whereas control animals had a bleeding time of 81 ± 4 seconds (n = 8) (P < .001). Since the KO animals are prepared from brother/sister mating and the controls were not littermate wild-type animals, the B6129SF2/J BKB2R-/- mice were backcrossed with 129S1/svImJ mice from Jackson Laboratories to make heterozygous animals that were then mated to make homozygous and littermate wild-type mice. The mean times for thrombosis of 66 ± 2.8 minutes (n = 7) in the KO and 27 ± 3.4 minutes (n = 9) in the wild-type mice were not significantly different from the B6129SF2/J BKB2R-/- mice and B6129SF2/J controls. When control B6129SF2/J mice were treated with increasing concentrations (0.913 mg/kg, 2.74 mg/kg, and 9.13 mg/kg IP) of the BKB2R antagonist, icatibant, the mean time to thrombosis was also significantly delayed at the 2 higher concentrations of the antagonist to 45 ± 3.2 minutes and 73 ± 5.1 minutes, respectively (Figure 1). Alternatively, the BKB2R-/- mice on the ferric chloride model of arterial thrombosis, where FeCl3 is added onto the vessel serosa to induce outside-in acute toxicity, did not have prolonged thrombosis times (5.3 ± 0.4 minutes vs 6.3 ± 1.1 minutes in control mice). These data indicated that the absence or inhibition of the BKB2R in mice gave a phenotype that protected these animals from the apparent mild (Rose Bengal model) but not severe (ferric chloride model) thrombosis injury. Sections of the thrombosed carotid arteries stained with H&E or PAS demonstrated an occlusive or nearly occlusive thrombus that was composed of fibrin and platelets. The composition of the thrombus was not altered in BKB2R-/- mice.
Determination of the coagulation and fibrinolytic plasma protein phenotype of BKB2R-/- mice
The levels of plasma coagulation factors II, VII, IX, X, V, VIII, and fibrinogen were not significantly different between control and KO mice (data not shown). Similarly, plasma concentrations of HK, factor XII, antithrombin, and plasminogen did not significantly differ between the 2 groups of animals (Table 2). Samples of heart, lung, liver, kidney, and spleen were stained with H&E, PAS, and for fibrin(ogen). There was no evidence for significant extravascular fibrin(ogen) deposition in organs of either wild-type or the BKB2R-/- mice when compared with liver sections from a plasminogen-deficient mouse (ie, a mouse that has spontaneous fibrin(ogen) deposition). Plasma tPA levels also were not different between the control and BKB2R-/- mice (Table 2). Although mRNA for BK B1 receptor was slightly increased in kidney and heart in the BKB2R KO versus control on real-time PCR (Table 3), BKB2R-/- mice were not sensitized to a bradykinin B1 receptor agonist to liberate tPA. When the BKB2R-/- mice were treated with an intravenous infusion of the BKB1R agonist, DesArg9 bradykinin (1 μM), there was no increase in plasma tPA in the KO mice (0.16 ± 0.07 U/mL) when compared with salinetreated KO mice (0.22 ± 0.04 U/mL; P = .45). Further, DesArg9 Leu8 bradykinin, a BKB1R antagonist, like saline, had no influence on shortening the thrombosis time in BKB2R-/- mice (data not shown).
Plasma protein phenotype of BKB2R−/− mice
Assay . | Control, U/mL . | BKB2R−/−, U/mL . | P . |
|---|---|---|---|
| Antithrombin | 1.41 ± 11* | 1.44 ± 5 | .81 |
| Plasminogen | 1.52 ± 6.3 | 1.54 ± 8.7 | .85 |
| tPA | 0.25 ± 0.12 | 0.34 ± 0.33 | .64 |
| PAI-1 | 7.56 ± 0.98 | 10.7 ± 0.92 | <.04 |
| Factor XI | 0.39 ± 0.05 | 0.23 ± 0.03 | <.02 |
| Prekallikrein | 0.23 ± 0.015 | 0.29 ± 0.012 | <.006 |
| HK | 0.14 ± 0.02 | 0.12 ± 0.01 | .49 |
| Factor XII | 0.8 ± 0.17 | 1.14 ± 0.11 | .13 |
Assay . | Control, U/mL . | BKB2R−/−, U/mL . | P . |
|---|---|---|---|
| Antithrombin | 1.41 ± 11* | 1.44 ± 5 | .81 |
| Plasminogen | 1.52 ± 6.3 | 1.54 ± 8.7 | .85 |
| tPA | 0.25 ± 0.12 | 0.34 ± 0.33 | .64 |
| PAI-1 | 7.56 ± 0.98 | 10.7 ± 0.92 | <.04 |
| Factor XI | 0.39 ± 0.05 | 0.23 ± 0.03 | <.02 |
| Prekallikrein | 0.23 ± 0.015 | 0.29 ± 0.012 | <.006 |
| HK | 0.14 ± 0.02 | 0.12 ± 0.01 | .49 |
| Factor XII | 0.8 ± 0.17 | 1.14 ± 0.11 | .13 |
TPA indicates tissue plasminogen activator; PAI-1, plasminogen activator inhibitor-1; HK, high-molecular-weight kininogen.
These data are the mean plus or minus SD of 7 or more individual experiments.
Real-time PCR receptor mRNA expression in BKB2R−/− mice
. | Ratio of mean fold-change in gene expression* . | . | . | ||
|---|---|---|---|---|---|
| Receptor . | Heart . | Kidney . | Liver . | ||
| BKB1R | 1.9 | 1.7 | — | ||
| AT1R | 0.06 | 1.0 | 1.0 | ||
| AT2R | 8.0 | 16.0 | 4.0 | ||
| MAS | 0.93 | 0.57 | 1.62 | ||
. | Ratio of mean fold-change in gene expression* . | . | . | ||
|---|---|---|---|---|---|
| Receptor . | Heart . | Kidney . | Liver . | ||
| BKB1R | 1.9 | 1.7 | — | ||
| AT1R | 0.06 | 1.0 | 1.0 | ||
| AT2R | 8.0 | 16.0 | 4.0 | ||
| MAS | 0.93 | 0.57 | 1.62 | ||
Values for the Ct for each real-time PCR for each receptor were used to calculate the fold-change of the cDNA for the target gene compared with that of γ-actin by the formula 2−ΔΔCt, where ΔΔCt − (CtTarget − CtActin) Time X − (CtTarget − CtActin) Time 0 (see “Materials and methods”).29 Final values in the table are expressed as the ratio of the fold-change in the target gene in the knockout mouse over the fold-change in the target gene of the control mouse. These data are means of 3 to 5 individual experiments.
— indicates that the assay was not done.
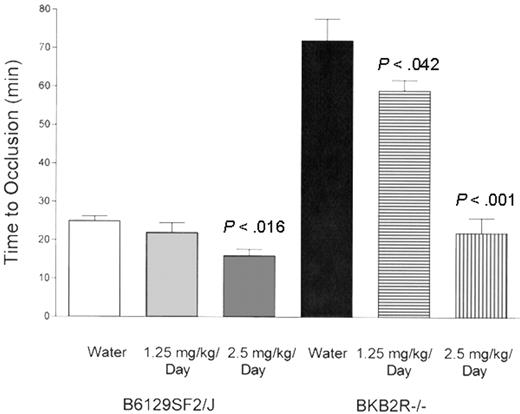
The influence of ramipril on thrombosis times in the BKB2R-/- mouse. The influence of ramipril on the time to occlusion in the Rose Bengal model of carotid artery thrombosis was performed with control mice (B6129SF2/J mice, n = 5 mice in each group) and BKB2R-/- mice (n = 5 mice in each group). In these experiments, both control and KO mice were treated with water alone (1.25 mg/kg per day), or with 2.5 mg/kg per day ramipril in their drinking water for 10 days. The data shown are the mean plus or minus SEM of the time to carotid artery occlusion in these animals under the 3 conditions.
The influence of ramipril on thrombosis times in the BKB2R-/- mouse. The influence of ramipril on the time to occlusion in the Rose Bengal model of carotid artery thrombosis was performed with control mice (B6129SF2/J mice, n = 5 mice in each group) and BKB2R-/- mice (n = 5 mice in each group). In these experiments, both control and KO mice were treated with water alone (1.25 mg/kg per day), or with 2.5 mg/kg per day ramipril in their drinking water for 10 days. The data shown are the mean plus or minus SEM of the time to carotid artery occlusion in these animals under the 3 conditions.
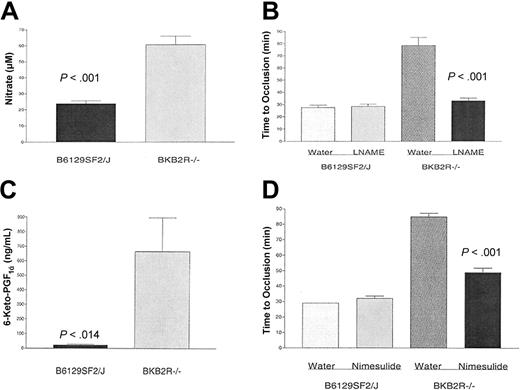
Measurement of nitric oxide and prostacyclin in BKB2R-/- mice. Mouse serum or plasma were collected from control (B6129SF2/J) or BKB2R-/- mice as indicated in “Materials and methods” for measurement of nitrate prepared from nitric oxide (A) or the stable prostaglandin I2 (prostacyclin) derivative, 6-keto-PGF1α (C), respectively. The data are the mean plus or minus SEM of 8 or more individual samples from BKB2R-/- and control mice. (B) BKB2R-/- mice and their controls were treated with 0.5 mg/mL L-NAME in their drinking water or with water alone for 7 days. After the treatment period, the time to vessel occlusion was determined using the Rose Bengal model for mouse carotid artery thrombosis. The data presented are the mean plus or minus SEM of 8 or more experiments with mice simultaneously treated with each of the conditions shown. (D) BKB2R-/- mice and their controls were treated with 50 μg/mL nimesulide in their drinking water or with water alone for 10 days. After the treatment period, the time to vessel occlusion was determined using the Rose Bengal model for mouse carotid artery thrombosis. The data presented are the mean plus or minus SEM of 5 or more experiments with mice simultaneously treated with each of the conditions shown.
Measurement of nitric oxide and prostacyclin in BKB2R-/- mice. Mouse serum or plasma were collected from control (B6129SF2/J) or BKB2R-/- mice as indicated in “Materials and methods” for measurement of nitrate prepared from nitric oxide (A) or the stable prostaglandin I2 (prostacyclin) derivative, 6-keto-PGF1α (C), respectively. The data are the mean plus or minus SEM of 8 or more individual samples from BKB2R-/- and control mice. (B) BKB2R-/- mice and their controls were treated with 0.5 mg/mL L-NAME in their drinking water or with water alone for 7 days. After the treatment period, the time to vessel occlusion was determined using the Rose Bengal model for mouse carotid artery thrombosis. The data presented are the mean plus or minus SEM of 8 or more experiments with mice simultaneously treated with each of the conditions shown. (D) BKB2R-/- mice and their controls were treated with 50 μg/mL nimesulide in their drinking water or with water alone for 10 days. After the treatment period, the time to vessel occlusion was determined using the Rose Bengal model for mouse carotid artery thrombosis. The data presented are the mean plus or minus SEM of 5 or more experiments with mice simultaneously treated with each of the conditions shown.
BKB2R-/- mice had a slightly increased level of PAI-1 in plasma (P < .04; Table 2). However, on real-time PCR, heart, kidney, and liver mRNA for PAI-1 was not increased. The BKB2R-/- mice were found to have reduced plasma factor XI coagulant activity (0.23 ± 0.03 U/mL, vs 0.39 ± 0.05 U/mL in controls; P < .02) and increased plasma PK amidolytic activity (0.29 ± 0.012 U/mL, vs 0.23 ± 0.015 U/mL in controls; P < .006; Table 2). However, on real-time PCR neither factor XI nor prekallikrein mRNA was decreased or increased, respectively, when compared with control animals.
Characterization of components of the renin-angiotensin system in BKB2R-/- mice
Since the BKB2R metabolizes BK, BKB2R-/- mice may have elevated BK that would lead to increased angiotensin converting enzyme (ACE) to degrade the peptide.30 Although there was a distinct difference in the plasma levels of ACE between male (268 ± 5.8 U) and female (167 ± 5.7 U) KO mice, there was no significant elevation of plasma ACE in BKB2R-/- mice over control mice for either males (267 ± 2.9 U, vs 294 ± 7.0Uin controls) or females (167 ± 4U, vs179 ± 1 U in controls).
Another measure of ACE activity is to determine if its products, bradykinin 1-5 (peptide RPPGF) and angiotensin II (AngII), were elevated in plasma.31 The median RPPGF concentration was 44 ± 29 fmol/mL in control animals versus 160 ± 75 fmol/mL in BKB2R-/- mice (P = .0029). RPPGF inhibits thrombin and thrombin activation of platelets in vitro at micromolar concentrations, but inhibits thrombosis in the mouse at lower concentrations.18,32-34 Additionally, studies determined if AngII was also elevated in the BKB2R-/- mice. In BKB2R-/- mice, AngII levels were increased from 49 ± 7 pg/mL in the controls to 182 ± 41 pg/mL in the KO mice (P < .003; n = 15). In order to determine if the elevated AngII levels were related to the delayed thrombosis, the BKB2R-/- mice were treated with the ACE inhibitor ramipril (Figure 2). At 1.25 mg/kg per day or 2.5 mg/kg per day ramipril, there was a significant (P < .042 or P < .001, respectively) shortening of the thrombosis time (59 ± 2.8 minutes or 23 ± 3.1 minutes [mean ± SEM], respectively) when compared with the untreated BKB2R-/- mice (72 ± 5.7 minutes; Figure 2). The correction in the time to thrombosis was associated with a fall in the plasma AngII level from 95 ± 13 pg/mL in the untreated BKB2R-/- mice to 29 ± 2.5 pg/mL (P < .008) or 47 ± 3.1 pg/mL (P < .05) in the mice treated with 1.25 mg or 2.5 mg ramipril/kg per day, respectively. Last, ramipril at 2.5 mg/kg per day also significantly (P < .016) shortened the time to thrombosis in control mice as well (25 ± 1.2 minutes in untreated control mice vs 16 ± 1.7 minutes in control mice treated with 2.5 mg/kg per day; Figure 2).
Further investigations determined how AngII elevation could lengthen bleeding times and delay stimulated vessel closure. Elevation of NO and prostacyclin could account for the prolonged bleeding time seen in these animals. Serum nitrate (61 ± 5.3 μM, n = 12, mean ± SEM) was significantly elevated (P < .001) in the BKB2R-/- mice over controls (24 ± 1.8 μM; n = 12; Figure 3A). When the BKB2R-/- mice were treated with the NO synthetase inhibitor, L-NAME, the KO mice corrected their time to thrombosis from 78 ± 7 minutes (mean ± SEM) in the untreated animals to 33 ± 2 minutes in the treated animals (P < .001; Figure 3B). L-NAME treatment had no influence on the time to thrombosis in control mice (Figure 3B).
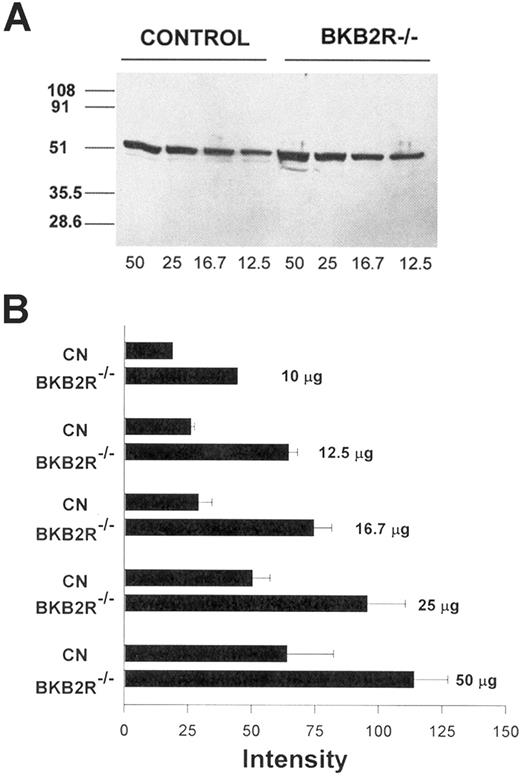
Angiotensin AT2 receptor antigen expression in the kidney. (A) Western blot of mouse kidney lysates for the AT2R receptor antigen. Kidney lysates from control and BKB2R-/- mice were prepared as indicated in “Materials and methods.” Equal amounts of total kidney lysate (12.5 μg to 50 μg) were added to each lane of the SDS-PAGE and, after electrophoresis, the gel contents were electroblotted onto nitrocellulose. An immunoblot was performed with anti-AT2R antisera followed by an antirabbit antibody conjugated with horseradish peroxidase. The protein was detected by the ECL system followed by an autoradiogram. The figure is a representative immunoblot experiment. (B) Densitometer scans were performed on multiple immunoblots of electrophoresed total kidney lysates from control and BKB2R-/- mice. The relative intensity of the bands was determined. The figure represents the mean plus or minus SEM of relative intensity of the bands of 3 or more experiments at each concentration of protein added. The data shown for 10 μg total kidney protein are the mean of only 2 experiments.
Angiotensin AT2 receptor antigen expression in the kidney. (A) Western blot of mouse kidney lysates for the AT2R receptor antigen. Kidney lysates from control and BKB2R-/- mice were prepared as indicated in “Materials and methods.” Equal amounts of total kidney lysate (12.5 μg to 50 μg) were added to each lane of the SDS-PAGE and, after electrophoresis, the gel contents were electroblotted onto nitrocellulose. An immunoblot was performed with anti-AT2R antisera followed by an antirabbit antibody conjugated with horseradish peroxidase. The protein was detected by the ECL system followed by an autoradiogram. The figure is a representative immunoblot experiment. (B) Densitometer scans were performed on multiple immunoblots of electrophoresed total kidney lysates from control and BKB2R-/- mice. The relative intensity of the bands was determined. The figure represents the mean plus or minus SEM of relative intensity of the bands of 3 or more experiments at each concentration of protein added. The data shown for 10 μg total kidney protein are the mean of only 2 experiments.
Similarly, 6-keto-prostaglandin F1α a stable derivative of prostacyclin, was significantly (P < .014) elevated (666 ± 232 ng/mL, n = 8, mean ± SEM) in the BKB2R-/- mice over controls (23 ± 5.3 ng/mL, n = 8; Figure 3C). When KO mice were treated with the cyclooxygenase 2 inhibitor, nimesulide, there was a normalization of the time to vessel occlusion from 85 ± 2 minutes (mean ± SEM) in the untreated mice to 49 ± 3 minutes in the nimesulide-treated KO mice (P < .001; Figure 3D). Treatment of control mice with nimesulide had no influence on the time to thrombosis (Figure 3D). These combined data indicated that both NO and prostacyclin contributed to the thrombosis protection seen in the BKB2R-/- mice.
Since stimulation of the angiotensin AT2 receptor (AT2R) or the angiotensin 1-7 receptor MAS can account for elevated NO and prostacyclin,34,35 investigations determined the expression of these receptors in BKB2R-/- mice (Table 3). On real-time PCR, the AT2R mRNA in BKB2R-/- mice was uniquely increased in relation to γ-actin and control mice in kidney (16×), heart (8×), and liver (4×) (Table 3). Alternatively, the mRNA on real-time PCR for the angiotensin AT1 receptor (AT1R), or MAS, the angiotensin 1-7 receptor, were not increased in the organs in the BKB2R-/- mice. The mRNA for the BKB1R was increased 1.7- to 1.9-fold in the BKB2R-/- mice.
Further, investigations determined if there was increased AT2R antigen expression in BKB2R-/- mice by immunoblot of total protein lysates of kidney from BKB2R-/- mice and controls using an antibody to the AT2R (Figure 4). At each concentration of total protein added to the gel (12.5 μg to 50 μg), there was increased AT2R antigen (Figure 4A). When the relative intensity of the immunoblot bands for each concentration of protein added in the control and BKB2R-/- mice lanes were scanned by densitometer, the relative amount of AT2R antigen was about twice as much in the kidney lysate from BKB2R-/- mice than in controls (Figure 4B).
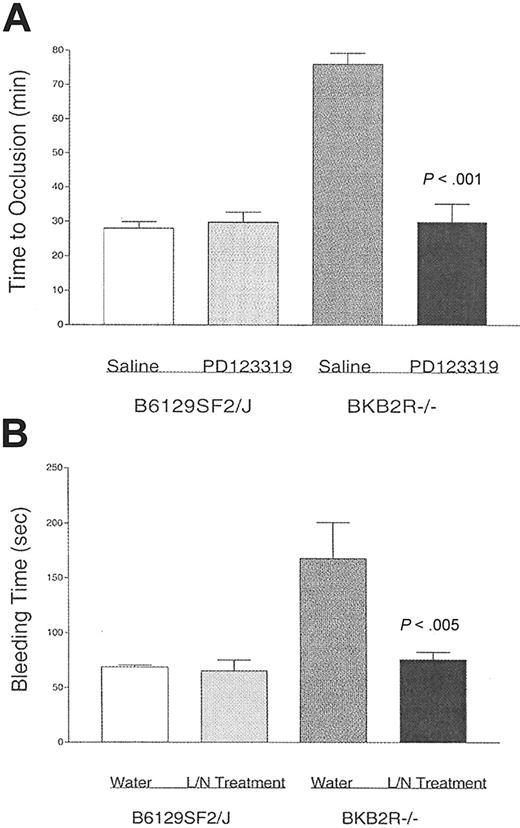
Studies next determined if the increased AT2R expression mediated the thrombosis protection in the BKB2R-/- mice (Figure 5). Control and BKB2R-/- mice were treated with the AT2R antagonist PD123 319 for 1 week by osmotic pump (Figure 5A). PD123 319 treatment had no influence on the time to thrombosis in control mice. However, PD123 319 treatment of the BKB2R-/- mice shortened their time to thrombosis back to control animals (Figure 5A). Untreated BKB2R-/- mice had a mean time to thrombosis of 76 ± 3 minutes (± SEM), whereas the PD123 319-treated BKB2R-/- mice had a mean time to thrombosis of 30 ± 5 minutes (P < .001; Figure 5A). Further studies showed that when the BKB2R-/- mice were treated with PD123 319, the serum nitrate levels fell from 45 ± 2.8 μM (mean ± SEM, n = 10) in untreated KO mice to 20 ± 6 μM (P < .001) in treated KO mice. Similarly, when the BKB2R-/- mice were treated with PD123 319, the plasma prostacyclin levels fell from 1166 ± 385 ng/mL (mean ± SEM, n = 5) in untreated KO mice to 14 ± 7.7 ng/mL (P < .001) in treated KO mice. Investigations next determined if treatment with L-NAME and nimesulide shortened the long bleeding times seen in these animals (Figure 5B). When the BKB2R-/- mice were treated with combined L-NAME and nimesulide, the bleeding time of the treated KO animals shortened to 76 ± 7 minutes (n = 5), a value similar to that of control mice (69 ± 2 seconds, n = 5) simultaneously obtained, and one significantly shorter than untreated BKB2R-/- mice (168 ± 33 seconds, n = 5; P < .005).
Discussion
These combined data indicate that AngII in BKB2R KO mice stimulates an overexpressed AT2R, producing increased NO and prostacyclin levels, prolongation of bleeding times, and thrombosis protection. The finding that BKB2R-/- mice are protected from thrombosis was not expected. We initially hypothesized that since BK is such a potent stimulator of NO formation and prostacyclin and tPA release, the absence of the constitutively expressed BK B2 receptor would make these animals prothrombotic.11-13 The finding that the BKB2R-/- mice were protected from thrombosis indicated that another overriding mechanism (or mechanisms) is occurring.
The notion that the BK B2 receptor contributes to the physiologic metabolism of bradykinin was helpful for our understanding of how these animals could, paradoxically, be protected from thrombosis.30 Although ACE activity levels are not absolutely elevated in KO mice, the products of its substrates (RPPGF, AngII) are. The finding that RPPGF (BK 1-5) is elevated indirectly indicates that there is increased BK and it was metabolized (Figure 5). RPPGF prevents thrombosis in mice at submicromolar concentrations in vivo.18 Further, RPPGF binds to the P4-P2 sites on protease activated receptor 1 and the P2 site on protease activated receptor 4 to prevent α-thrombin cleavage of these receptors.33,36 Although these investigations indicate for the first time that elevation of RPPGF occurs in vivo in thromboprotected mice, our present studies do not show causality. Measurement of plasma levels of RPPGF may not reflect its site of action on the membranes of platelets and endothelium.37 Further studies are needed to determine if this small plasma elevation of RPPGF contributes to the thrombosis protection. It should be noted that the delay in the time to thrombosis seen in the mice treated with icatibant, the BK B2 receptor antagonist, may be due to receptor antagonism or direct thrombin inhibition since the first 5 amino acids of this peptide are structurally similar to RPPGF.32,33
The influence of the angiotensin receptor 2 on thrombosis risk. (A) BKB2R-/- mice and their controls (B6129SF2/J) were treated with 30 mg/kg per day PD123 319 or saline by a subcutaneous osmotic pump for 7 days. After the treatment period, the time to vessel occlusion was determined using the Rose Bengal model for mouse carotid artery thrombosis. The data presented are the mean plus or minus SEM of 5 or more experiments with mice simultaneously treated with each of the conditions shown. (B) BKB2R-/- mice and their controls were treated with 0.5 mg/mL L-NAME and 50 μg/mL nimesulide (L/N treatment) added daily to fresh drinking water for 10 days. After the treatment period, the tail bleeding time was determined. The data presented are the mean plus or minus SEM of 5 experiments with mice simultaneously treated with each of the conditions shown.
The influence of the angiotensin receptor 2 on thrombosis risk. (A) BKB2R-/- mice and their controls (B6129SF2/J) were treated with 30 mg/kg per day PD123 319 or saline by a subcutaneous osmotic pump for 7 days. After the treatment period, the time to vessel occlusion was determined using the Rose Bengal model for mouse carotid artery thrombosis. The data presented are the mean plus or minus SEM of 5 or more experiments with mice simultaneously treated with each of the conditions shown. (B) BKB2R-/- mice and their controls were treated with 0.5 mg/mL L-NAME and 50 μg/mL nimesulide (L/N treatment) added daily to fresh drinking water for 10 days. After the treatment period, the tail bleeding time was determined. The data presented are the mean plus or minus SEM of 5 experiments with mice simultaneously treated with each of the conditions shown.
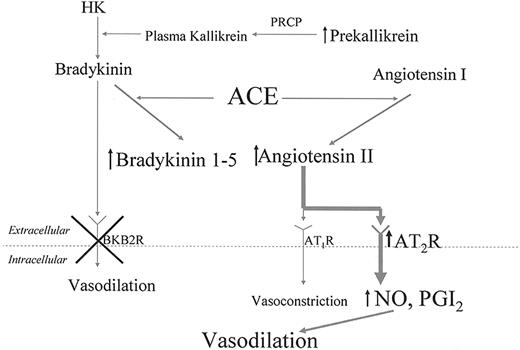
Mechanism for thromboprotection in BKB2R-/- mice. This figure shows that in the absence of the BKB2R-/- receptor, plasma prekallikrein, bradykinin 1-5, and angiotensin II are elevated. Angiotensin II interacts with an overexpressed AT2R receptor to liberate increased NO and prostacyclin. The increased production of these endothelial-cell products lengthens the animals' bleeding time and lengthens the time to thrombosis on the Rose Bengal model for carotid artery thrombosis. HK indicates high-molecular-weight kininogen; PRCP, prolylcarboxypeptidase; AT1R, angiotensin AT1 receptor; AT2R, angiotensin AT2 receptor; NO, nitric oxide; PGI2, prostaglandin I2 or prostacyclin.
Mechanism for thromboprotection in BKB2R-/- mice. This figure shows that in the absence of the BKB2R-/- receptor, plasma prekallikrein, bradykinin 1-5, and angiotensin II are elevated. Angiotensin II interacts with an overexpressed AT2R receptor to liberate increased NO and prostacyclin. The increased production of these endothelial-cell products lengthens the animals' bleeding time and lengthens the time to thrombosis on the Rose Bengal model for carotid artery thrombosis. HK indicates high-molecular-weight kininogen; PRCP, prolylcarboxypeptidase; AT1R, angiotensin AT1 receptor; AT2R, angiotensin AT2 receptor; NO, nitric oxide; PGI2, prostaglandin I2 or prostacyclin.
Demonstrating that plasma AngII is elevated could result from increased ACE activity due to the need to metabolize more bradykinin or reduced metabolism as result of the indirect effect of an absent BKB2R. Recent evidence suggests that the bradykinin receptors and the angiotensin receptors may interact and regulate each other at the level of the receptor.34,38,39 However, ascribing thromboprotection to AngII presents a paradox. Usually, elevations of AngII are associated with vasoconstriction and increased thrombosis risk due to elevated PAI-1 and tissue factor.40-42 However, the degree of elevation of plasma PAI-1 is not to the extent that one sees with endothelial-cell injury, suggesting that the slight increase is not clinically significant.40-42 If AngII interacts with an overexpressed AT2R, then it could result in delay in thrombosis since it could elevate prostacyclin and nitrate levels. Finding elevated 6-keto-PGF1α and NO levels in the BKB2R-/- mice suggests that the latter mechanism is taking place. Overexpression of the AT2R has been recognized to stimulate vascular BK formation and induce vasodilation.27,43 Stimulation of AT2R leads to BK and NO formation, preventing myocardial injury and also resulting in increased expression of the BK B2 receptor in vascular smooth muscle.34,38 Further, NO production in the kidney in the BKB2R-/- mice is mediated by the AT2 R.35
Our investigations indicate that in the absence of the BKB2R in mice, there is probable reduced BK uptake into cells and increased plasma prekallikrein, RPPGF, and AngII (Figure 6). The BKB1R is slightly overexpressed in BKB2R KO mice, as previously reported, but it does not contribute to the thrombosis protection seen since an agonist to it does not elevate tPA.38,44 The elevated AngII in the BKB2R KO mice probably binds to an overexpressed AT2R to contribute to thrombosis protection (Figure 6). What regulates AT2R expression in these mice is not known, but as already indicated, the bradykinin and AT receptors interact. Since the affinity of AngII for the AT2R and angiotensin receptor 1 are similar, increased expression of the AT2R allows for more AngII to bind, resulting in elevated NO and prostacyclin levels. The elevated NO and prostacyclin in the BKB2R KO mice results in bleeding time prolongation and thromboprotection (Figure 6). The thrombosis protection seen with the BKB2R-/- mice is mild. Using the more injurious model with ferric chloride to induce thrombosis, there is no thrombosis protection in the BKB2R-/- mice. This finding is consistent with the common understanding that plasma NO and prostacyclin levels are weaker protectors from thrombosis than antithrombin or protein C. The finding that antagonism of NO and prostacyclin synthesis, or of AT2R activity, shortens the thrombosis time and normalizes the bleeding time in these mice indicates that the AT2R overexpression is an effector of the delay in vessel occlusion. This notion is confirmed by showing that AT2R antagonism reduces NO and prostacyclin levels. These differences can be clinically significant. Since clinical thrombosis is the summation of risk factors, small changes in prostacyclin levels, as the result of treatment with cyclooxygenase 2 antagonists in systemic lupus erythematosis patients with other risk factors for thrombosis, result in increased risk for clinical thrombosis.45 Thus, the present investigations indicate that components of the plasma kallikrein/kinin system indirectly influence one's risk for thrombosis through its interaction with components of the renin angiotensin pathway. These findings broaden our notion of factors that modify thrombosis risk and suggest new targets for antithrombotic therapy.
Prepublished online as Blood First Edition Paper, March 2, 2006; DOI 10.1182/blood-2006-01-0094.
Supported by National Institutes of Health grants HL52 779, HL57 346, and HL65 194 (A.H.S.), an American Heart Association (AHA) Scientific Development Grant N004 313 (Z.S.-M.), and an AHA Fellow to Faculty Award 0 275 023N (J.W.H.).
An Inside Blood analysis of this article appears at the front of this issue.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
We would like to thank Dr Daniel Eitzman and Randy Westrick for showing us how to perform the Rose Bengal model for carotid artery thrombosis.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal