Hydroxyurea is a cell-cycle-specific drug that has been used to treat myeloproliferative diseases and sickle cell anemia. We have recently shown that hydroxyurea, like nitric oxide (NO)-donor compounds, increased cGMP levels in human erythroid cells. We show now that hydroxyurea increases endothelial-cell production of NO; this induction of NO in human umbilical vein endothelial cells (HUVECs) and human bone marrow endothelial cell line (TrHBMEC) is blocked by competitive inhibitors of NO synthase (NOS), such as NG-nitro-l-arginine-methyl ester (L-NAME) and NG-nitro-l-arginine. It is dependent on cAMP-dependent protein kinase (PKA) and protein kinase B (PKB/Akt) activity. We found that hydroxyurea dose- and time-dependently induced rapid and transient phosphorylation of eNOS at Ser1177 in a PKA-dependent manner; inhibitors of PKB/Akt could partially abrogate this effect. In addition, hydroxyurea induced cAMP and cGMP levels in a dose-dependent manner, as well as levels of intracellular calcium in HUVECs. These studies established an additional mechanism by which rapid and sustained effects of hydroxyurea may affect cellular NO levels and perhaps enhance the effect of NO in myeloproliferative diseases. (Blood. 2006;108:184-191)
Introduction
Hydroxyurea is a cytostatic agent (S-phase inhibitor) that has been used to treat erythrocytosis and thrombocytosis in polycythemia vera and other myeloproliferative diseases.1 More recently, hydroxyurea demonstrated therapeutic benefit for sickle cell anemia by increasing fetal hemoglobin (HbF).2 In addition to the known inhibition of ribonucleotide reductase, hydroxyurea can be oxidized by heme groups to produce nitric oxide (NO) in vivo (in rats) and in humans.3,4 Hydroxyurea reacts with oxyHb and deoxyHb to form metHb, which then reacts with another hydroxyurea to form iron nitrosyl hemoglobin (HbNO).5 The formation of HbNO involves the specific transfer of NO from the -NHOH group of hydroxyurea by a series of intermediate reactions.3 Additionally, in vivo production of NO may result from the hydrolysis of hydroxyurea to hydroxylamine, followed by the rapid reactions of hydroxylamine with Hb.6 It has been reported that hydroxyurea administration significantly increased NOx (NO metabolites nitrite and nitrate) and cGMP levels in plasma of sickle cell patients.7 We have recently shown that hydroxyurea, like NO-donor compounds, increased cGMP levels contributing to gamma-globin gene expression in human erythroid cells.8
Hydroxyurea is completely absorbed and elimination is through both renal and nonrenal mechanisms.9 The metabolism of hydroxyurea to NO most likely occurs in the liver.3 Chemical oxidation of hydroxyurea with a variety of oxidants, including copper(II) ions, produces NO.9,10 Hydroxyurea-treated vascular endothelial cells showed significant morphologic changes such as an increase in apparent cell size, accompanied by an increase in cell Na and K contents.11 During hydroxyurea treatment, cells accumulate in the late G1 to early S phase of the cell cycle.12 Also, the levels of calcium (Ca2+) culminate at the G1/S border.13
It has been shown that the NO-forming reactions of hydroxyurea with hemoglobin or other blood constituents are too slow to account for NO production measured in vivo.14 Several mechanisms of NO production from hydroxyurea have been proposed: one of them suggested a catalase-mediated pathway as a potential source,15 another showed urease-dependent effects,14 and the third one showed that horseradish peroxidase catalyzed NO formation from hydroxyurea.16 It is known that l-arginine, as substrate of NO synthase (NOS), increased serum NO metabolite production in sickle cell patients only in combination with hydroxyurea.17 This could suggest NOS dependence in hydroxyurea induction of NO production. In accordance with this study, NOS inhibition prevented hydroxyurea-induced accumulation of DNA strand breaks.18
Several reports suggest that cytokines and interleukins, once thought to be explicit for the hematopoietic system, particularly granulocyte-macrophage colony-stimulating factor (GM-CSF), IL-3, IL-4, IL-6, and IL-8, are capable of affecting metabolism and function of endothelial cells.19-23 G-CSF, GM-CSF, vascular endothelial growth factor (VEGF), angiopoietin-1, sphingosine-1phosphate, and shear stress are known to activate endothelial NO production through the phosphatidylinositol 3′-kinase (PI 3-kinase)/protein kinase B (PKB/Akt) pathway and to induce endothelial-cell proliferation and migration.24-27 We also found an increase in eNOS expression in endothelial cells, as well as in NO and cGMP production in response to erythropoietin during hypoxia.28
To establish whether the action of NO in hematopoietic cells might involve paracrine NO mechanisms of endothelial cells, we have investigated the effects of hydroxyurea and other cytostatic drugs that increase HbF on these cells. We have studied in these experiments both primary endothelial cells (human umbilical vein endothelial cells [HUVECs]) and a human endothelial cell line (TrHBMEC). We find that hydroxyurea induces NO production in an eNOS-dependent manner. Inhibitors of cAMP-dependent protein kinase (PKA) or PI 3-kinase-mediated stimulation of PKB/Akt activity reduced this effect. However, hydroxyurea-induced phosphorylation of eNOS on serine 1177 is decreased by PKA inhibitors only. Hydroxyurea increases cAMP and cGMP, as well as intracellular calcium in these cells, which also supports NO production. Hydroxyurea increased NO production by enhancing the eNOS phosphorylation and hence eNOS activity in endothelial cells. These results demonstrate a mechanism by which hydroxyurea may affect NO levels in large (modeled by HUVECs) and small (modeled by TrHBMECs) blood vessels.
Materials and methods
Endothelial-cell cultures
HUVECs (BioWhittaker, Walkersville, MD) were cultured in endothelialcell growth medium (EGM) or EGM-2 (BioWhittaker) at 37°C in a humidified environment containing 5% CO2. Cells from the third and fourth passages were used in the present experiments. Transformed human bone marrow endothelial cells (TrHBMECs) are a continuous bone marrow endothelial cell line from an adult female donor's cells immortalized with the T antigen of simian virus 40.29 TrHBMECs were cultured in culture flasks and dishes on 0.2% gelatin (Sigma, St Louis, MO) at 37°C in Dulbecco modified essential medium-low glucose (DMEM; Invitrogen, Carlsbad, CA) supplemented with 10% fetal bovine serum (Gemini BioProducts, Woodland, CA), 100 U/mL penicillin, 100 μg/mL streptomycin, 1 μg/mL folic acid (Sigma), and 3 mM glutamine (Biofluids, Gaithersburg, MD). Experiments were done between passages 19 and 23. It is to be noted that the EGM for HUVECs and DMEM for TrHBMECs already contain 0.36 and 0.48 mM l-arginine, respectively. HUVECs and TrHBMECs were treated with hydroxyurea, butyric acid sodium salt, 5-azacytidine (Sigma-Aldrich, St Louis, MO), and bradykinin (Alexis, San Diego, CA) at concentrations indicated. Additives are left in throughout the periods of the culture. For signaling reactions, HUVECs and TrHBMECs were first incubated in endothelial-cell basal medium (EBM; BioWhittaker) and DMEM, respectively, for 6 hours, and pretreated for 1 hour with PI 3-K inhibitor 2-(4-morpholinyl)-8-phenyl-4H-1-benzopyran-4-one (LY294002) and for 30 minutes with PI 3-K inhibitor wortmannin and PKA inhibitor N-[2-(p-Bromocinnamylamino)ethyl]-5-isoquinolinesulfonamide, 2HCl (H-89; Calbiochem, San Diego, CA), followed by addition of hydroxyurea.
Ozone-based chemiluminescent determination of nitrite
For nitrite measurements, confluent HUVECs in 6-well plates were washed once with HEPES (N-2-hydroxyethylpiperazine-N′-2-ethanesulfonic acid) buffer (BioWhittaker) and EBM was added. After 3 hours, the medium was replaced with a new EBM and cells were treated with cytostatics. Confluent TrHBMECs in 6-well plates were washed once, and the medium was replaced with DMEM supplemented with 100 U/mL penicillin, 100 μg/mL streptomycin, 1 μg/mL folic acid, and 3 mM glutamine. The cells were left overnight at 37°C (glutamine was removed for eNOS inhibitor experiments). The next day, the medium was replaced with DMEM supplemented with 100 U/mL penicillin, 100 μg/mL streptomycin, and 1 μg/mL folic acid. For the eNOS inhibitors, TrHBMECs and HUVECs were pretreated for 30 minutes with NG-Nitro-l-arginine-methyl ester (L-NAME) and NG-Nitro-l-arginine (Alexis). The cells were exposed to cytostatic agents in the presence of l-arginine (Sigma). The supernatant of treated cells was immediately frozen on dry ice and stored at -80°C. Frozen samples were thawed and then the level of nitrite was determined with the Sievers Model 280 NO analyser (Sievers, Boulder, CO). Helium was bubbled through the reaction mixture. Nitrite was measured by reduction in acidified KI: 7 mL glacial acetic acid, 2 mL distilled water, 50 mg KI. A crystal of iodine was added to yield a concentration of 6 to 20 μM (Sigma-Aldrich).
Preparation of cell lysates
HUVECs were washed in ice-cold phosphate-buffered saline containing 1 mM sodium orthovanadate (Na3VO4; Sigma-Aldrich) and then lysed in ice-cold lysis buffer containing 20 mM Tris (tris(hydroxymethyl)aminomethane)/HCl (pH 7.4; Quality Biological, Gaithersburg, MD), 1 mM EDTA (ethylenediaminetetraacetic acid), 1 mM EGTA (ethyleneglycoltetraacetic acid), 1% Triton X-100, 1 mM Na3VO4 (Sigma-Aldrich), 1 mM Pefabloc SC, 10 μg/mL leupeptin, 10 μg/mL pepstatin A, and 5 μg/mL aprotinin (Roche Applied Science, Indianapolis, IN). After 20 minutes at 4°C, lysates were scraped from the dishes and precleared by centrifugation at 12 000g for 15 minutes at 4°C. Protein content of each sample was measured by using a BCA Protein Assay (Pierce, Rockford, IL).
Immunoprecipitation and immunoblotting
Anti-eNOS monoclonal antibody (1-2 μg/mL; BD Transduction Laboratories, San Jose, CA) was added to the precleared lysates and incubated overnight at 4°C, followed by incubation for 3 hours at 4°C with the protein A-agarose (Roche Applied Science) on a rocking platform. Immunoprecipitates were washed first with 1 mL ice-cold lysis buffer, followed by washing buffer 1 (50 mM Tris-HCl, pH 7.5, 500 mM NaCl, 0.05% sodium deoxycholate [Sigma-Aldrich], 0.1% Nonidet P40 [Roche Applied Science]) and washing buffer 2 (50 mM Tris-HCl, pH 7.5, 0.1% Nonidet P40, 0.05% sodium deoxycholate). Immunoprecipitated proteins were eluted from the agarose beads by heating to 90°C for 10 minutes in LDS sample buffer (Invitrogen). Proteins were then separated on 3% to 8% NuPAGE Tris Acetate gels, transferred onto polyvinylidene difluoride membranes (Invitrogen), and probed in immunoblots using eNOS, phospho-eNOS-specific (Ser1177 and Thr495), and Actin (Santa Cruz Biotechnology, Santa Cruz, CA) monoclonal antibodies according to the protocols provided by the suppliers. The membrane was incubated with a primary antibody overnight at 4°C, and then for 1 hour at room temperature with a horseradish peroxidase-conjugated sheep anti-mouse IgG. Proteins were visualized by chemiluminescence (ECL × Amersham Biosciences, Piscat-away, NJ). The intensities of immunoreactive bands in Western blots were analyzed by using the National Institutes of Health IMAGE program (FluorChem Imaging system; Alpha Innotech, San Leandro, CA).
Calcium measurements
HUVECs were preloaded with 1 μM Fura-2 acetoxymethyl ester (Fura-2/AM; Molecular Probes, Eugene, OR) for 60 minutes at room temperature in medium 199. After dye loading, cells were washed in the same medium and kept in the dark for at least 30 minutes before single-cell [Ca2+]i measurements. Coverslips with cells were mounted on the stage of an Axiovert 135 microscope (Carl Zeiss, Oberkochen, Germany) attached to the Attofluor Digital Fluorescence Microscopy System (Atto Instruments, Rockville, MD). The dynamic changes of [Ca2+]i were examined under a 40 × oil immersion objective during exposure to alternating 340- and 380-nm light beams, and the intensity of light emission at 520 nm was measured. The ratio of light intensities, F340/F380, which reflects changes in [Ca2+]i, was simultaneously followed in 15 to 30 single cells at a rate of about 1 point/second. The stimuli were prepared as follows: endothelin-1 (Peninsula Laboratories, Belmont, CA), ionomycin (Calbiochem), and thapsigargin (Calbiochem) were added by pipette in 1-mL volumes and final concentrations are indicated. All experiments were done at room temperature.
cGMP and cAMP immunoassays
HUVECs were washed with HEPES and left in serum-free EBM for 3 hours at 37°C in a humidified atmosphere with 5% CO2. HUVECs were pretreated for 30 minutes with 0.5 mM 3-isobutyl-1-methylxanthine (IBMX; Sigma) and 1 mM L-NAME, and then stimulated with hydroxyurea for 30 minutes for up to 2 hours. cGMP and cAMP levels were measured using the cGMP and cAMP immunoassays (R&D Systems, Minneapolis, MN) according to the protocol of the supplier.
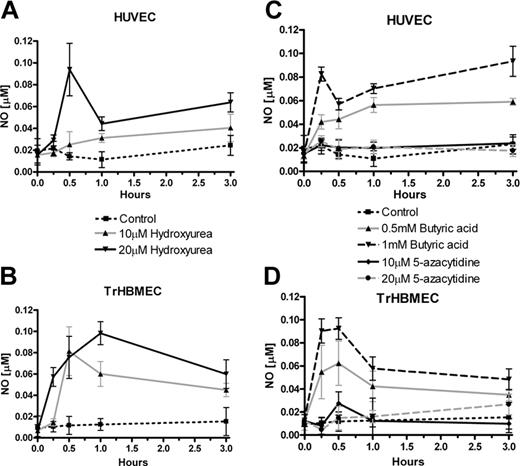
Measurement of NO production in endothelial cells during incubation with hydroxyurea and 2 other cytostatic agents. (A) HUVECs were treated with different concentrations of hydroxyurea (10 and 20 μM). (B) TrHBMECs, as well as HUVECs, were treated with different concentrations of hydroxyurea. (C) HUVECs were treated with different concentrations of butyric acid sodium salt (0.5 and 1 mM) and 5-azacytidine (10 and 20 μM). (D) TrHBMECs were treated with the same concentrations of butyric acid sodium salt and 5-azacytidine as HUVECs. Results are from 3 to 4 independent experiments: HUVECs are from different cryopreserved cells pooled from several donors, whereas TrHBMECs are thawed from a new cryovial for each experiment. Values are mean ± SEM (n = 3).
Measurement of NO production in endothelial cells during incubation with hydroxyurea and 2 other cytostatic agents. (A) HUVECs were treated with different concentrations of hydroxyurea (10 and 20 μM). (B) TrHBMECs, as well as HUVECs, were treated with different concentrations of hydroxyurea. (C) HUVECs were treated with different concentrations of butyric acid sodium salt (0.5 and 1 mM) and 5-azacytidine (10 and 20 μM). (D) TrHBMECs were treated with the same concentrations of butyric acid sodium salt and 5-azacytidine as HUVECs. Results are from 3 to 4 independent experiments: HUVECs are from different cryopreserved cells pooled from several donors, whereas TrHBMECs are thawed from a new cryovial for each experiment. Values are mean ± SEM (n = 3).
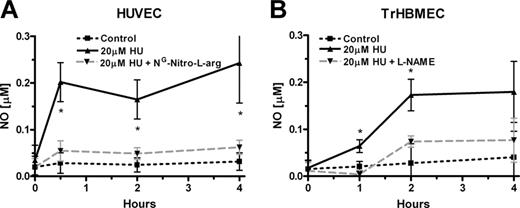
Measurement of NO levels during inhibition of eNOS in endothelial cells incubated with hydroxyurea (HU). (A) Hydroxyurea (20 μM) induction of NO is inhibited by competitive eNOS inhibitor NG-nitro-l-arginine (0.1 mM) in HUVECs in the presence of supplementary 0.1 mM l-arginine (*P < .05 versus cells treated with hydroxyurea and NG-nitro-l-arginine). (B) L-NAME (0.1 mM) also inhibited hydroxyurea (20 μM) induction of NO production in TrHBMECs in the presence of supplementary 0.1 mM l-arginine (*P < .05 versus cells treated with hydroxyurea and L-NAME). Values represent mean ± SEM (n = 3).
Measurement of NO levels during inhibition of eNOS in endothelial cells incubated with hydroxyurea (HU). (A) Hydroxyurea (20 μM) induction of NO is inhibited by competitive eNOS inhibitor NG-nitro-l-arginine (0.1 mM) in HUVECs in the presence of supplementary 0.1 mM l-arginine (*P < .05 versus cells treated with hydroxyurea and NG-nitro-l-arginine). (B) L-NAME (0.1 mM) also inhibited hydroxyurea (20 μM) induction of NO production in TrHBMECs in the presence of supplementary 0.1 mM l-arginine (*P < .05 versus cells treated with hydroxyurea and L-NAME). Values represent mean ± SEM (n = 3).
Statistical analysis
We transferred raw data from the Sievers program to the Origin program (OriginLab, Northampton, MA). The data were smoothed using the Savitzky-Golay filter method provided with the software. The data were analyzed by the one-way analysis of variance (ANOVA) and Dunnett posttests using Prism 4 software (GraphPad Software, San Diego, CA).
Results
Hydroxyurea increased NO production in endothelial cells
In accordance with our previously reported results that hydroxyurea augmented cGMP production in erythroid cells, we decided to examine hydroxyurea effects in endothelial cells that have high levels of the eNOS protein. Addition of hydroxyurea to HUVECs caused a dose- and time-dependent increase in NO production (Figure 1A). A more prominent elevation in NO levels, by hydroxyurea, is observed in TrHBMECs, a human bone marrow endothelial cell line (Figure 1B). Similar kinetics of NO production is observed during treatment of endothelial cells up to 50 μM hydroxyurea (data not shown).
To check the specificity of hydroxyurea as an inducer of NO, we incubated endothelial cells with other already known cytostatic drugs and HbF inducers, such as butyric acid and 5-azacytidine. Butyric acid stimulated an even more consistent dose- and time-dependent increase in NO production in HUVECs (Figure 1C), whereas 5-azacytidine, another cytostatic drug, failed to induce NO. Similar results were obtained during incubation of these drugs with TrHBMECs (Figure 1D). Applied concentrations of hydroxyurea and butyric acid did not inhibit, whereas 5-azacytidine slightly reduced, endothelial-cell viability during the time of incubation (data not shown).
Inhibition of eNOS prevented NO induction by hydroxyurea in endothelial cells
To test NOS dependence during hydroxyurea stimulation of NO production, experiments were also carried out in cells pretreated with the NOS inhibitors. Increased NO production in endothelial cells, during incubation with hydroxyurea, is blocked by competitive inhibitors of NOS such as L-NAME and NG-nitro-l-arginine. HUVECs, preincubated with NG-nitro-l-arginine markedly inhibited induction of NO by hydroxyurea (Figure 2A). Similar results were observed with L-NAME treatment of TrHBMECs. The effects of hydroxyurea stimulation on NO production were also diminished in TrHBMECs (Figure 2B). Bradykinin induction of NO production was also prevented by the eNOS inhibitors in both types of endothelial cells (data not shown).
PKA and PKB/Akt inhibitors prevented hydroxyurea induction of NO production in endothelial cells
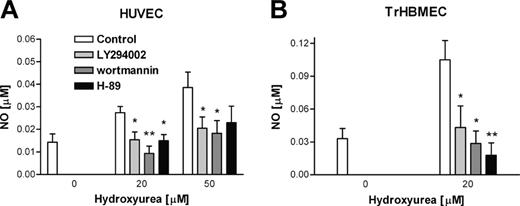
We measured endothelial-cell production of NO in the presence of hydroxyurea and inhibitors of PKA and PI 3-kinase. It has been reported that activation of PKB/Akt involves activation of PI 3-kinase,30 and that some hormones induced eNOS phosphorylation and NO production via the PI 3-kinase-PKB/Akt-dependent pathway.31,32 Hydroxyurea stimulation of NO production in HUVECs is inhibited by PKA (H-89) and PI 3-kinase (LY294002 and wortmannin) inhibitors (Figure 3A). Hydroxyurea induction of NO production in TrHBMECs is also inhibited by PKA and PI 3-kinase inhibitors (Figure 3B). Thus, hydroxyurea stimulation of NO production is regulated by mechanisms dependent on both PKA and PKB/Akt.
Phosphorylation of eNOS by hydroxyurea in HUVECs
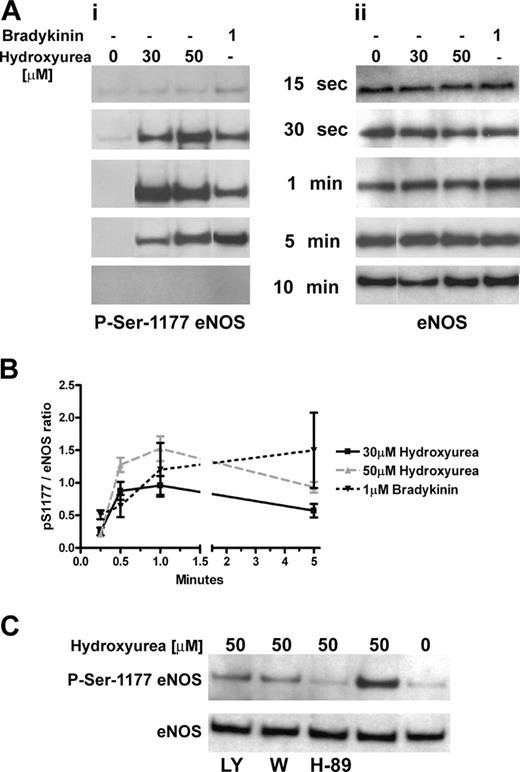
Bradykinin, a well-studied inducer of eNOS activity in endothelial cells, stimulated a transient phosphorylation of eNOS at Ser1179 that is correlated temporally with a transient dephosphorylation of eNOS at Thr497 in bovine aortic endothelial cells.33 It was also reported that cAMP-mediated eNOS activation is associated with phosphorylation of residue Ser1177 in HUVECs in a PI 3-kinase-independent manner.34 We found that hydroxyurea dose- and time-dependently induced rapid and transient phosphorylation of eNOS at Ser1177 in HUVECs, as does bradykinin. The transient phosphorylation appeared within 30 seconds, reached maximum after 1 minute of incubation, and was still present after 5 minutes. It disappeared after 10 minutes of treatment (Figure 4A). The densitometric analysis of the same samples revealed dose-dependent induction of eNOS phosphorylation at Ser1177 (Figure 4B). Phosphorylation of eNOS at Thr495 (the human counterpart to Thr497 in bovine cells) was more pronounced within the first 5 minutes of hydroxyurea treatment but declined steadily after 5 minutes and disappeared by one hour (data not shown).
Measurement of NO production in endothelial cells during incubation with hydroxyurea in presence or not of inhibitors of PKA and PKB/Akt. (A) After preincubation period with 20 μM H-89 (PKA inhibitor), 50 μM LY294002, and 0.1 μM wortmannin (PI 3-kinase inhibitors), HUVECs were treated with 2 different concentrations of hydroxyurea (20 and 50 μM), and aliquots were collected for measurement after 1 hour of incubation. (B) TrHBMECs were preincubated with PKA and PI 3-kinase inhibitors, and measurements were made after 1 hour of incubation with hydroxyurea. Values are mean ± SEM (n = 3). *P < .05 and **P < .01 compared with cells treated with only hydroxyurea.
Measurement of NO production in endothelial cells during incubation with hydroxyurea in presence or not of inhibitors of PKA and PKB/Akt. (A) After preincubation period with 20 μM H-89 (PKA inhibitor), 50 μM LY294002, and 0.1 μM wortmannin (PI 3-kinase inhibitors), HUVECs were treated with 2 different concentrations of hydroxyurea (20 and 50 μM), and aliquots were collected for measurement after 1 hour of incubation. (B) TrHBMECs were preincubated with PKA and PI 3-kinase inhibitors, and measurements were made after 1 hour of incubation with hydroxyurea. Values are mean ± SEM (n = 3). *P < .05 and **P < .01 compared with cells treated with only hydroxyurea.
Hydroxyurea phosphorylated eNOS in HUVECs. (A) Left panel: hydroxyurea (at concentrations of 30 and 50 μM) induced phosphorylation of eNOS at Ser1177 after 30 seconds of treatment and the signal remained for more than 1 minute; bradykinin (1 μM) induced similar phosphorylation. Right panel: corresponding total eNOS protein levels in HUVECs during incubation with hydroxyurea and bradykinin. Results are representative of 4 independent experiments. (B) Densitometric analysis of panel A during 5 minutes of incubation of HUVECs with hydroxyurea and bradykinin. Results are represented as the ratio of pSer1177 to total eNOS bands intensity. (C) PI 3-kinase inhibitors: LY294002 (LY, 50 μM) and wortmannin (W, 100 nM) demonstrated incomplete inhibition of eNOS phosphorylation at Ser1177 in HUVECs after 1 minute of incubation with hydroxyurea (50 μM) (upper bands). PKA inhibitor H-89 (20 μM) induced complete inhibition of eNOS phosphorylation at Ser1177 in HUVECs after 1 minute of incubation with hydroxyurea (50 μM) (upper band). Lower bands show total eNOS protein levels. Results are representative of 4 independent experiments.
Hydroxyurea phosphorylated eNOS in HUVECs. (A) Left panel: hydroxyurea (at concentrations of 30 and 50 μM) induced phosphorylation of eNOS at Ser1177 after 30 seconds of treatment and the signal remained for more than 1 minute; bradykinin (1 μM) induced similar phosphorylation. Right panel: corresponding total eNOS protein levels in HUVECs during incubation with hydroxyurea and bradykinin. Results are representative of 4 independent experiments. (B) Densitometric analysis of panel A during 5 minutes of incubation of HUVECs with hydroxyurea and bradykinin. Results are represented as the ratio of pSer1177 to total eNOS bands intensity. (C) PI 3-kinase inhibitors: LY294002 (LY, 50 μM) and wortmannin (W, 100 nM) demonstrated incomplete inhibition of eNOS phosphorylation at Ser1177 in HUVECs after 1 minute of incubation with hydroxyurea (50 μM) (upper bands). PKA inhibitor H-89 (20 μM) induced complete inhibition of eNOS phosphorylation at Ser1177 in HUVECs after 1 minute of incubation with hydroxyurea (50 μM) (upper band). Lower bands show total eNOS protein levels. Results are representative of 4 independent experiments.
To distinguish between a direct effect and activation of specific signaling pathways in phosphorylation of eNOS by hydroxyurea, we pretreated HUVECs with PKA and PI 3-kinase inhibitors. It has been reported that PKB/Akt is transiently activated in bradykininstimulated bovine endothelial cells downstream from PI 3-kinase. A rapid increase in NO production by bradykinin is mediated by the PKA-dependent phosphorylation of eNOS at Ser1179.35 The PKA inhibitor (H-89) induced almost complete inhibition of eNOS phosphorylation at Ser1177 activated by hydroxyurea (Figure 4C). The PI 3-kinase inhibitors (LY294003, wortmannin) induced partial inhibition (15%-35%) of eNOS phosphorylation at Ser1177 activated by hydroxyurea (Figure 4C), whereas H-89 reduction was 5- to 7-fold as calculated by densitometric analysis. Also, hydroxyurea did not increase phosphorylation of Akt at Ser475 (activation site) within the first hour of treatment (data not shown).
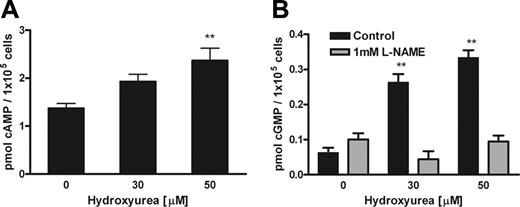
Hydroxyurea increased cAMP and cGMP levels in endothelial cells
Increased endothelial cAMP levels augmented agonist-induced production of NO.36 Our experiments demonstrated dose- and time-dependent effects of hydroxyurea on cAMP levels in HUVECs. Hydroxyurea increased cAMP levels and dose-dependent effects are detected within the first 30 minutes (Figure 5A). A relaxant response of rat aorta to cAMP-mediated vasodilators is regulated by NO production in endothelium and subsequent increase in cGMP in vascular smooth muscle cells.37 We demonstrated dose- and time-dependent effects of hydroxyurea on cGMP levels in HUVECs. Hydroxyurea induced cGMP levels within the first 30 minutes (Figure 5B). However, the effect is no longer detected at 2 hours, in contrast to cAMP levels (data not shown). The eNOS inhibitor L-NAME inhibited hydroxyurea stimulation of cGMP production in HUVECs after 30 minutes of treatment (Figure 5B). Hydroxyurea increases in cAMP and cGMP levels also occurred in TrHBMECs (data not shown). Moreover, sodium nitrite (0.001-10 mM) did not induce cGMP production of purified soluble guanylyl cyclase (sGC) as measured by radioimmunoassay (data not shown), indicating that the effects we are measuring are not due to addition of nitrite in the hydroxyurea solutions.
Hydroxyurea increased intracellular calcium concentration in endothelial cells
To clarify the mechanism by which hydroxyurea mediated signals for increases in eNOS activity, we examined the status of intracellular calcium in treated cells. As shown in Figure 6A, left panel, the addition of hydroxyurea induced a rise in [Ca2+]i in HUVECs. While the peak amplitude of [Ca2+]i response was comparable among individual cells, the time of initiation of response upon hydroxyurea application varied between 2 and 6 minutes. Medium 199, in which hydroxyurea was diluted, did not alter [Ca2+]i (Figure 6A right panel). The pattern of calcium signaling and the peak amplitude of [Ca2+]i responses induced by hydroxyurea (Figure 6B bottom trace) were comparable with that induced by endothelin-1, a native calcium-mobilizing agonist for these cells (Figure 6B upper trace). In contrast to hydroxyurea that induced a change in [Ca2+]i at 2 to 6 minutes following stimulation, in cultures treated with endothelin-1 the rise in [Ca2+]i was detectable within a few seconds after the agonist application. The calcium ionophore, ionomycin, which preferentially releases calcium from intracellular pools, induced some additional increase in [Ca2+]i in cells treated with hydroxyurea (Figure 6C bottom trace). The addition of thapsigargin, a blocker of SERCA-type (Ca2+)ATPase expressed in endoplasmic reticulum, induced a gradual depletion of intracellular calcium pool. After 20 minutes of treatment, hydroxyurea was unable to induce a rise in [Ca2+]i (Figure 6D). These results indicated that a hydroxyurea-induced rise in [Ca2+]i is probably dependent on an increase in the calcium leak from endoplasmic reticulum. The response was not unique for HUVECs, but was also observed in TrHBMECs (data not shown).
Hydroxyurea increased cAMP and cGMP levels in HUVECs. (A) Hydroxyurea showed dose-dependent induction of cAMP levels in HUVECs after 30 minutes of incubation. (B) Hydroxyurea showed dose-dependent induction of cGMP levels in HUVECs after 30 minutes of incubation, whereas pretreatment with L-NAME inhibited this effect. Values represent mean ± SEM (n = 3); **P < .01 compared with cells untreated with hydroxyurea.
Hydroxyurea increased cAMP and cGMP levels in HUVECs. (A) Hydroxyurea showed dose-dependent induction of cAMP levels in HUVECs after 30 minutes of incubation. (B) Hydroxyurea showed dose-dependent induction of cGMP levels in HUVECs after 30 minutes of incubation, whereas pretreatment with L-NAME inhibited this effect. Values represent mean ± SEM (n = 3); **P < .01 compared with cells untreated with hydroxyurea.
Effects of hydroxyurea on intracellular calcium concentration in HUVECs. (A) The effect of hydroxyurea on [Ca2+]i was independent of the time of its application (left panel vs right panel) and was not mimicked by the addition of medium 199 (right panel). Traces shown are representative from at least 3 experiments, each performed on 15 to 30 single cells during incubation with hydroxyurea. Gray areas indicate the duration of hydroxyurea treatment. Notice a variety in times of initiation of [Ca2+]i response after the addition of hydroxyurea. (B) Dependence of hydroxyurea-induced calcium response on calcium mobilization from intracellular stores. Comparison of [Ca2+]i signals induced by hydroxyurea (bottom trace) and endothelin-1, the native calcium-mobilizing agonist for these cells (upper panel). (C) Ionomycin-induced [Ca2+]i signals in controls (upper trace) and hydroxyurea-treated cells (bottom trace). (D) The lack of effects of hydroxyurea on [Ca2+]i response in cells with blocked endoplasmic reticulum calcium pump with thapsigargin. To avoid the UV damage of cells, the ratio of light intensities during thapsigargin treatment was followed at a rate of 1 point per 20 seconds.
Effects of hydroxyurea on intracellular calcium concentration in HUVECs. (A) The effect of hydroxyurea on [Ca2+]i was independent of the time of its application (left panel vs right panel) and was not mimicked by the addition of medium 199 (right panel). Traces shown are representative from at least 3 experiments, each performed on 15 to 30 single cells during incubation with hydroxyurea. Gray areas indicate the duration of hydroxyurea treatment. Notice a variety in times of initiation of [Ca2+]i response after the addition of hydroxyurea. (B) Dependence of hydroxyurea-induced calcium response on calcium mobilization from intracellular stores. Comparison of [Ca2+]i signals induced by hydroxyurea (bottom trace) and endothelin-1, the native calcium-mobilizing agonist for these cells (upper panel). (C) Ionomycin-induced [Ca2+]i signals in controls (upper trace) and hydroxyurea-treated cells (bottom trace). (D) The lack of effects of hydroxyurea on [Ca2+]i response in cells with blocked endoplasmic reticulum calcium pump with thapsigargin. To avoid the UV damage of cells, the ratio of light intensities during thapsigargin treatment was followed at a rate of 1 point per 20 seconds.
Discussion
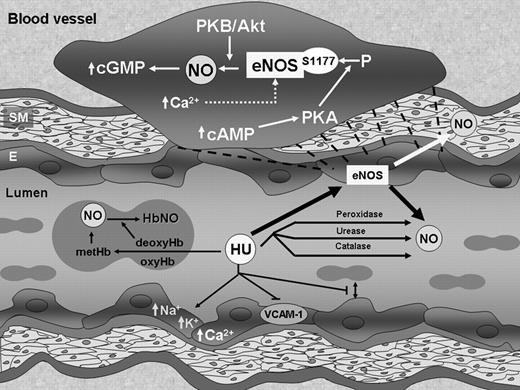
Hydroxyurea increased NO production in HUVECs and TrHB-MECs through eNOS phosphorylation and therefore eNOS activity. Cytostatic agents are used in the treatment of myeloproliferative disorders as well as to increase HbF in genetic diseases of hemoglobin. The increase in NO production occurred for up to several hours in endothelial cells during incubation with hydroxyurea. It is prevented by NOS inhibitors. Hydroxyurea stimulation of NO production is regulated by the mechanisms dependent on both PKA- and PI 3-kinase-induced stimulation of PKB/Akt. Involvement of eNOS, the major endothelial NOS enzyme, in hydroxyurea induction of NO is supported by the finding of phosphorylation of eNOS at Ser1177, which represents the activation site of the enzyme. Phosphorylation of Ser1177 is detected within 5 minutes of treatment of HUVECs with hydroxyurea and was completely PKA dependent and partially PKB/Akt dependent. Subsequently, hydroxyurea induction of eNOS is accompanied by rise of the intracellular calcium concentration. Increased [Ca2+]i was detected in individual cells between 2 and 6 minutes after hydroxyurea exposure and generally had a protracted effect. Further, we established that cAMP and cGMP levels are amplified in HUVECs during incubation with hydroxyurea, which is in accordance with our reported results that hydroxyurea induced cGMP levels in the erythroid progenitor cells.8 Furthermore, cGMP production demonstrated eNOS dependence during stimulation by hydroxyurea. Phosphorylation of eNOS and induction of NO levels, as well as of cGMP levels, suggest that eNOS contributes to hydroxyurea effects on endothelial cells (Figure 7). Although hydroxyurea can be metabolized directly to NO by several mechanisms including direct reaction with hemeproteins, systemic production of NO by cellular mechanism in endothelial cells can contribute to total body NO levels. Therefore, hydroxyurea could activate sGC by 3 autonomous mechanisms involving its degradation to NO, stimulation of eNOS activity, or direct interaction with sGC. Despite the failure of the reaction of hydroxyurea with blood to produce NO metabolites in vitro,14 patients taking hydroxyurea showed significant increases in plasma nitrite/nitrate and HbNO, suggesting in vivo conversion of hydroxyurea to NO.4 These levels do not appear to change overall circulatory status in those taking hydroxyurea; further cell-free hemoglobin or increased plasma xanthine oxidase activity inhibit the expected vasodilatory effects of NO donor administration to sickle cell disease patients.38,39 However, hydroxyurea may change the clinical symptoms of patients by inhibiting the degree of adherence of different blood cells and influencing the activity of the endothelium,11,40 which is in accordance with reported NO effects.41 Moreover, the weak relaxant effect of hydroxyurea that has been observed in aortic rings with endothelium might be due to production of NO from hydroxyurea.42
We found that butyric acid, another cytostatic agent used to increase HbF in genetic diseases of hemoglobin, increased NO production in endothelial cells. This is very consistent with the known vasorelaxation effect of butyrate. However, in contrast to hydroxyurea induction of NO, it has been reported that butyrateinduced vasorelaxation was unaffected by inhibition of NOS. Moreover, butyric acid suppressed eNOS protein and mRNA levels in HUVECs.43,44 5-azacytidine, an inhibitor of DNA methyltransferase activity, increased eNOS mRNA levels in nonendothelial cell lines (human aortic vascular smooth muscle cells and several human carcinoma cell lines). In contrast, the expression of eNOS mRNA levels in HUVECs was not increased by treatment with 5-azacytidine.45 These observations indicate that cytostatic agents do not generally induce NO or eNOS activity, as was demonstrated by the lack of NO production by 5-azacytidine.
Hydroxyurea effects on blood vessels. Hydroxyurea oxidizes oxy- and deoxy-hemoglobin to methemoglobin (metHb), which may react directly with other molecules of hydroxurea (or via hydroxylamine) to form nitrosylhemoglobin (HbNO), which can slowly release NO. Hydroxyurea may also chemically or enzymatically (peroxidase, urease, catalase) decompose to NO, as well as directly effect intracellular levels of cations, production of VCAM-1, and red-cell adhesion, among others. Our data show that hydroxyurea also stimulates the phosphorylation and thus activation of endothelial NOS (eNOS) with resultant production of NO. This effect is modulated by increased intracellular calcium levels and increases in cAMP, which stimulates phosphorylation at Ser1177 by PKA. NO production is regulated by both PKA and PKB/Akt. The increase in eNOS activity presumably leads to increases in cGMP in endothelial and surrounding cells; this increase results in many of the pleiotropic effects of endothelial-produced NO. SM indicates smooth muscle cells; E, endothelial cells.
Hydroxyurea effects on blood vessels. Hydroxyurea oxidizes oxy- and deoxy-hemoglobin to methemoglobin (metHb), which may react directly with other molecules of hydroxurea (or via hydroxylamine) to form nitrosylhemoglobin (HbNO), which can slowly release NO. Hydroxyurea may also chemically or enzymatically (peroxidase, urease, catalase) decompose to NO, as well as directly effect intracellular levels of cations, production of VCAM-1, and red-cell adhesion, among others. Our data show that hydroxyurea also stimulates the phosphorylation and thus activation of endothelial NOS (eNOS) with resultant production of NO. This effect is modulated by increased intracellular calcium levels and increases in cAMP, which stimulates phosphorylation at Ser1177 by PKA. NO production is regulated by both PKA and PKB/Akt. The increase in eNOS activity presumably leads to increases in cGMP in endothelial and surrounding cells; this increase results in many of the pleiotropic effects of endothelial-produced NO. SM indicates smooth muscle cells; E, endothelial cells.
It is known that agents that increase cAMP stimulate eNOS activity in HUVECs.46 A recent report revealed that a rapid increase in endothelial NO production by bradykinin is mediated exclusively by PKA signaling pathway.35 PKA signaling acts by increasing phosphorylation of Ser1177 and dephosphorylation of Thr495 to activate eNOS.47 Shear stress stimulates phosphorylation of bovine eNOS at the corresponding serine in a PKA-dependent, but PKB/Akt-independent, manner, whereas NO. production is regulated by the mechanisms dependent on both PKA and PKB/Akt.48 These results are in accord with our studies with hydroxyurea. Moreover, the cAMP-dependent pathway was activated in erythroleukemic cells in which the cGMP-dependent pathway was stimulated by hemin (also a gamma-globin inducer as hydroxyurea).49
It has been also demonstrated that eNOS is rapidly activated and phosphorylated on both Ser1177 and Thr495 in the presence of cGMP-dependent protein kinase II and the catalytic subunit of PKA in endothelial cells. These processes are more prominent in the presence of Ca2+/calmodulin.50 The transient rises of cGMP levels induced by bradykinin and endothelin-1, which both caused release of Ca2+ from internal stores, were similarly enhanced by activation of adenylyl cyclase and increased cAMP levels. The cAMP seems to enhance NO formation, which depends on Ca2+ release from internal stores.51 An elevated cGMP level attenuated the store-operated Ca2+ entry in vascular endothelial cells.52 The cGMP-mediated [Ca2+]i-reducing mechanisms may operate as a negative reaction to protect endothelial cells from the damaging effect of excessive [Ca2+]i. The main targets of cGMP are phosphodiesterases (PDEs), resulting in interference with the cAMP-signaling pathway.53 cAMP-hydrolyzing PDE isozymes in endothelial cells are represented by PDE2 and PDE4 as cGMP-stimulated and cGMP-insensitive PDE, respectively. In endothelial cells, PDE4 inhibition may up-regulate basal production of NO, being supported by PDE2 inhibition.54
It has been shown recently that a high proportion of patients with myeloproliferative disorders carry a dominant gain-of-function mutation of JAK2.55 Hydroxyurea is an established chemotherapeutic agent in the treatment of myeloproliferative disorders.56 Hydroxyurea targets ribonucleotide reductase involving the redox inactivation of a tyrosyl radical, and inhibits DNA synthesis, accounting for its cytostatic effects.57 This effect can be mediated by NO.58 NO is known to induce apoptosis and this can contribute to the therapeutic effects of hydroxyurea.59 The JAK2-STAT1 pathway has been shown to induce inducible NOS (iNOS) activity in several cell types.60-62 It would be interesting to see if eNOS activity is similarly affected such that hydroxyurea would increase an already elevated NO production in cells from individuals with myeloproliferative disorders. The resultant high level of NO could then contribute to increased cell death in these diseases.
A significant reduction of bone marrow fibrosis was noted in patients with myeloproliferative disorders after treatment with hydroxyurea.63 Hydroxyurea treatment seems to prevent and even resolves bone marrow fibrosis in chronic myelogenous leukemia.64 Other reports suggest that NO may attenuate cardiac fibrosis.65,66 Hepatic fibrotic changes were also marked in iNOS-deficient mice.67 These correlations between hydroxyurea and NO effects are consistent with our observation that hydroxyurea effects are mediated in part via NO production.
In summary, our experiments indicate that hydroxyurea enhances NO and cGMP production through PKA-mediated stimulation of eNOS in endothelial cells. Thus, in addition to a direct effect of hydroxyurea on hematopoietic/erythroid cells in bone marrow, we should consider also an indirect mechanism mediated via NO produced by endothelial stromal cells. Induction of HbF and inhibition of sickle cell adherence, as well as other NO effects on vascular endothelium, would all be potentially beneficial in hydroxyurea therapy of sickle cell anemia. The extent to which hydroxyurea-induced endothelial NO production and cytotoxicity are involved in other aspects of the therapeutic spectrum of NO, such as the treatment of polycythemia vera and other myeloproliferative diseases, requires further study. Other modulators of the eNOS-cGMP pathway may result in synergistic regimens that increase responsiveness and reduce morbidity associated with hydroxyurea therapy itself.
Prepublished online as Blood First Edition Paper, March 9, 2006; DOI 10.1182/blood-2005-11-4454.
Supported by the Serbian Ministry of Science and Environment (grant 145048B).
V.P.C. designed research, performed research, analyzed data, and wrote the paper; B.B.B.-C. performed research; M.T. performed research and analyzed data; S.S.S. analyzed data; C.T.N. analyzed data and wrote the paper; and A.N.S. designed research and wrote the paper.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
We are grateful to Babette B. Weksler from Cornell University Medical College, NY for providing the TrHBMEC line.

![Figure 6. Effects of hydroxyurea on intracellular calcium concentration in HUVECs. (A) The effect of hydroxyurea on [Ca2+]i was independent of the time of its application (left panel vs right panel) and was not mimicked by the addition of medium 199 (right panel). Traces shown are representative from at least 3 experiments, each performed on 15 to 30 single cells during incubation with hydroxyurea. Gray areas indicate the duration of hydroxyurea treatment. Notice a variety in times of initiation of [Ca2+]i response after the addition of hydroxyurea. (B) Dependence of hydroxyurea-induced calcium response on calcium mobilization from intracellular stores. Comparison of [Ca2+]i signals induced by hydroxyurea (bottom trace) and endothelin-1, the native calcium-mobilizing agonist for these cells (upper panel). (C) Ionomycin-induced [Ca2+]i signals in controls (upper trace) and hydroxyurea-treated cells (bottom trace). (D) The lack of effects of hydroxyurea on [Ca2+]i response in cells with blocked endoplasmic reticulum calcium pump with thapsigargin. To avoid the UV damage of cells, the ratio of light intensities during thapsigargin treatment was followed at a rate of 1 point per 20 seconds.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/108/1/10.1182_blood-2005-11-4454/2/m_zh80130697750006.jpeg?Expires=1765037758&Signature=3MF1Ogp-nvxrn~j12uTgc~1yBB1i5vhrXA1GKX6qK8GLb9gEQydwW6iLxV8Md5qlJWiyq6Oym~NhCXa1dcuZjm2vrkH7aIvCBM2H0PM5O6q1Y-1-V9KeBIrLQeckc3DGfKsqJd6S6ccivF~lsOed3-nLA0jTTGufzPRyFyylYfbTSmHq7jRoJUHBB-vWM1cnQDyt43oGhCdCYmBBT9VVvoquHO0uwo4Ra1OVfgDL0TOpB0FQO89dYgmzBt1~7uT693ACWm4afOsV56OMJRHWtvYLRHJWPVDzwuEemkAtVFMXli19SzHqNUEwNkcOT~5sotS61BMEqOsVWKlUUuhwnQ__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
This feature is available to Subscribers Only
Sign In or Create an Account Close Modal