A pilot study was conducted to evaluate safety and activity of nonmyeloablative allogeneic hematopoietic cell transplantation (HCT) in colorectal carcinoma (CRC) and to determine whether a T-cell response to a tumor-associated antigen (TAA) was induced. Fifteen patients with metastatic CRC underwent HCT from human leukocyte antigen (HLA)-matched siblings after a nonmyeloablative conditioning regimen. All patients engrafted with a median donor T-cell chimerism of 72% at day +56. Eight patients experienced grades II to IV acute graft-versus-host disease (GVHD). Despite progressive disease before HCT, partial remission and disease stabilization longer than 90 days were observed in 1 and 3 patients, respectively. Induction of TAA-specific T cells was evaluated with a carcinoembryonic antigen (CEA)-specific HLA-A*0201 pentamer in 6 patients with CRC. CEA-specific CD8+ T cells were detected in 3 of 3 patients concomitant with GHVD onset, but not in 3 of 3 patients without GVHD. They were also not detected in 9 of 9 control patients with GVHD who received transplants for diagnoses other than CRC. Antitumor activity of CEA-specific T cells was also validated in vitro. In one patient, the induction of CEA-specific T cells was associated with a decrease of serum CEA levels and a partial response. Thus, graft-versus-host reactions associated with allogeneic HCT can trigger the generation of T cells specific for CEA, and this may be associated with a clinical response.
Introduction
Allogeneic hematopoietic stem cell transplantation (HCT) is an effective treatment for many hematologic malignancies.1 The therapeutic effect of allogeneic HCT depends on an alloimmune response of donor T cells to recipient malignant cells, referred to as a graft-versus-tumor (GVT) effect.2,3 Nonmyeloablative conditioning regimens are an alternative for patients who are older or otherwise poor candidates for conventional allogeneic HCT since the toxicity of the procedure is decreased and a potent immunologic effect is retained.4-6 A GVT effect after nonmyeloablative allogeneic HCT had been reported in early clinical trials for renal cell carcinoma, breast cancer, and other solid tumors.7-12 Colorectal cancer (CRC) can be considered a candidate disease for this approach for several reasons. First, the prognosis of patients with metastatic unresectable CRC remains poor despite recent progress in chemotherapy and molecular-targeted therapy, with less than 5% of the patients surviving at 5 years.13-16 Second, gastrointestinal epithelium is one of the targets of graft-versus-host disease (GVHD),17 and therefore minor histocompatibility antigens (mHAg's)18 or tumor-associated antigens (TAAs)19,20 may be presented to donor T cells by CRC cells, leading to an immunologic response against the tumor. Finally, several clinical studies have shown that immunotherapy might represent a promising new treatment for CRC, but the vast majority of these studies were performed in an autologous setting, where patients' T-cell repertoire may often be severely affected by the tumor.21,22 With this rationale, a pilot study was conducted to evaluate the safety and feasibility of nonmyeloablative allogeneic HCT in patients with metastatic CRC resistant to conventional therapy. For this study, we used a nonmyeloablative conditioning regimen with “minimal intensity.” This allowed an evaluation of the GVT effect without the potential of being confounded by an effect associated with more aggressive pretransplantation treatment. To determine whether antigen-specific T cells with antitumor activity were induced in vivo following engraftment and/or GVHD onset, we analyzed the T-cell response directed to the carcinoembryonic antigen (CEA), a TAA that is overexpressed by CRC cells.
Patients, materials, and methods
Patients and eligibility
Eligible patients were younger than 70 years of age with metastatic CRC radiographically documented to be progressive despite prior standard therapies or to be resistant during first-line therapy. Only patients with a life expectancy greater than 4 months were enrolled in the study. Patients were also required to have a human leukocyte antigen (HLA)-identical sibling donor. A high tumor burden was not considered an exclusion criterion (Table 1). Patients were excluded if they had bone metastases only and/or active brain metastases. Any other medical comorbidity was not judged as an absolute contraindication to transplantation procedure. Performance status was scored according to the Karnofsky scale.23
Patient characteristics
. | Data . |
|---|---|
| No. patients | 15 |
| Median age, y (range) | 56 (36-66) |
| No. men/no. women | 9/6 |
| Prior hepatic metastasectomy, no. | 4 |
| Median no. prior therapies for metastatic disease (range) | 2 (1-5) |
| Median Karnofsky (range) | 70 (60-100) |
| High tumor burden, no.* | 8 |
| Median no. metastatic sites (range) | 2 (1-5) |
| Liver metastasis | 12 |
| Lung metastasis | 10 |
| Peritoneal carcinosis | 3 |
| PD status at transplantation | 15 |
| Patient/donor sex mismatch | 7 |
| Patient/donor ABO mismatch | 7 (3 minor and 4 major) |
| HLA-related donor | 15 |
| Median CD34+/Kg infused, × 106 (range) | 6.7 (2.5-11.3) |
| Patient/donor CMV status | |
| Negative/positive | 2/15 |
| Positive/positive | 13/15 |
| Hematologic toxicity | |
| ANC nadir, × 109/L (range) | 722 (519-2520) |
| Time to ANC nadir, d (range) | 9 (5-15) |
| Platelets nadir, × 109/L (range) | 119 (63-191) |
| Time to platelets nadir, d (range) | 12 (8-21) |
. | Data . |
|---|---|
| No. patients | 15 |
| Median age, y (range) | 56 (36-66) |
| No. men/no. women | 9/6 |
| Prior hepatic metastasectomy, no. | 4 |
| Median no. prior therapies for metastatic disease (range) | 2 (1-5) |
| Median Karnofsky (range) | 70 (60-100) |
| High tumor burden, no.* | 8 |
| Median no. metastatic sites (range) | 2 (1-5) |
| Liver metastasis | 12 |
| Lung metastasis | 10 |
| Peritoneal carcinosis | 3 |
| PD status at transplantation | 15 |
| Patient/donor sex mismatch | 7 |
| Patient/donor ABO mismatch | 7 (3 minor and 4 major) |
| HLA-related donor | 15 |
| Median CD34+/Kg infused, × 106 (range) | 6.7 (2.5-11.3) |
| Patient/donor CMV status | |
| Negative/positive | 2/15 |
| Positive/positive | 13/15 |
| Hematologic toxicity | |
| ANC nadir, × 109/L (range) | 722 (519-2520) |
| Time to ANC nadir, d (range) | 9 (5-15) |
| Platelets nadir, × 109/L (range) | 119 (63-191) |
| Time to platelets nadir, d (range) | 12 (8-21) |
A high tumor burden was defined by the presence of at least one of the following conditions: (1) More than 5 liver metastases, with the largest being > 5 cm or 1 single metastasis > 10 cm in diameter; (2) lung metastasis > 5 cm in diameter; (3) lymphoadenopathy > 5 cm in diameter; and (4) peritoneal carcinosis.
Study design
The protocol was approved by the Local Ethical Committees. All patients and donors had to sign a written informed consent. Patients were treated according to the Seattle regimen for nonmyeloablative HCT that included 30 mg/m2 fludarabine intravenously on days -4, -3, and -2, and 200 cGy of total-body irradiation (TBI) on day 0.4 Posttransplantation immunosuppression to prevent graft rejection and GVHD consisted of the combination of cyclosporine (CSA) and mycophenolate mofetil (MMF). CSA was started on day -3 and given at a dose of 6 mg/kg (oral) or 1.5 mg/kg (intravenous) every 12 hours. CSA levels were targeted to the upper therapeutic ranges (500 ng/L, as defined by the fluorescence polarization method from Abbott TDX, Abbott Park, IL) in the first 28 days, maintained in normal ranges until day +56, and then tapered at 25% per week to be discontinued on day +90. Tapering schedules were modified if GVHD developed and according to the disease status. MMF was started at a dose of 15 mg/kg (oral) every 12 hours on day 0 and stopped without tapering on day +27. All patients received prophylaxis against bacterial, viral, fungal, and Pneumocystis carinii infection according to previously published protocols.4
Selected donors were siblings and HLA-identical for classes I and II (A, B, C, and DRβ1) after testing with high-resolution molecular typing.24 Peripheral blood stem cells (PBSCs) were collected from donors by leukapheresis to obtain a target dose of more than 5 × 106 CD34+ cells/kg after treatment with granulocyte colony-stimulating factor (G-CSF; 16 μg/kg/d subcutaneously for 4 to 5 days).
Chimerism
The degree of donor chimerism was assessed at days +30, +56, +90, +180, and +360 after transplantation on circulating CD3+ lymphocytes and CD13+ myeloid cells as well as on bone marrow cells. In sex-matched recipient-donor pairs, chimerism was determined using the polymerase chain reaction (PCR) on a panel of informative variable number tandem repeat (VNTR) regions. Fluorescence in situ hybridization (FISH) on X and Y chromosomes was used for sex-mismatched transplantations. Mixed chimerism was defined as the presence of 1% to 95% donor CD3+ cells, whereas full chimerism was defined as more than 95% donor CD3+ cells.25
Treatment-related toxicities and GVHD
Treatment-related toxicities were graded according to National Cancer Institute-Common Toxicity Criteria 2.0 (http://ctep.cancer.gov/reporting/CTC-3.html). Severity of GVHD was graded according to the modified Seattle criteria.26 Grade II or greater acute GVHD was treated with CSAif occurring after discontinuation of the immunosuppressive therapy. If the patient was still on CSA, 1.0 to 2.0 mg/kg methylprednisolone was started. Steroid refractory GVHD was treated with infliximab (10 mg/kg/wk; Schering-Plough, Kenilworth, NJ).
Donor lymphocyte infusions
Patients who had loss of chimerism or progressive disease after they had discontinued immunosuppression in the absence of severe GVHD (ie, grades III-IV) were eligible for donor lymphocyte infusions (DLIs). When administered for disease progression, DLI was depleted of CD8+ cells by immunomagnetic selection to minimize GVHD risk27 (Miltenyi Biotec, Bergisch-Gladbach, Germany). DLI was administered at minimum intervals of 45 days starting at 1 × 107 CD3+ cells/kg. The dose was escalated with each subsequent infusion to 2.5 × 107, 5 × 107, and 1 × 108 CD3+ cells/kg. Patients with progressive disease but not candidates for additional DLIs were considered for treatment with low-dose interferon-α (IFN-α; 3 × 106 IU three times a week). When administered because of loss of chimerism, DLI was not CD8-depleted to facilitate donor engraftment.
Endpoints and assessment of response
The primary study endpoint was the achievement of stable mixed chimerism defined as between 1% and 95% PB donor CD3+ cells. Secondary endpoints were: (1) GVT and tumor response; (2) incidence of acute and chronic GVHD; and (3) treatment-related mortality.
Tumor response was scored according to the international RECIST (Response evaluation criteria in solid tumors) criteria.28 Tumor size was assessed by a spiral-computed tomography of chest and abdomen at days +30, +60, +90, +180, and +365, or when clinically indicated. A complete response (CR) was defined as complete disappearance of all measurable disease. A partial response (PR) indicated at least a 30% decrease in the sum of the longest diameters of metastatic lesions compared with previous measurement. Progressive disease (PD) was defined as a greater than 20% increase in the sum of the longest diameters of metastatic lesions compared with previous measurement or the occurrence of a new lesion. Stable disease (SD) was defined as changes not meeting the definitions of CR, PR, or PD.
HLA-pentamer staining
Blood samples were collected from HLA-A*0201-positive patients and their donors. Peripheral blood mononuclear cells (PBMCs) were isolated from heparinized blood by density gradient centrifugation using Lymphoprep (Nycomed, Oslo, Norway) and either used fresh or thawed in 90% fetal bovine serum and 10% dimethyl-sulfoxide. PBMCs were stained with an HLA-A*0201/CEA-Cap1 pentamer (YLSGANLNL; ProImmune, Oxford, United Kingdom). Cap1 is an HLA-A*0201-restricted, naturally processed epitope of CEA.29 Staining was performed according to the manufacturer's instruction after protocol optimization. Briefly, approximately 1 × 106 PBMCs were incubated with 1 μL of unlabeled pentamer for 15 minutes at room temperature (RT) in the dark, washed, and incubated with 6 μL phycoerythrin (PE)-labeled fluorotag for 15 minutes. Fluoroscein isothiocyanate (FITC)-conjugated anti-CD8 monoclonal antibody (mAb) (1 μL; Miltenyi Biotec) was added at this point for 3 minutes of additional incubation. Cells were then washed, propidium iodide (PI) was added, and samples were immediately acquired on a FACSCalibur (Becton Dickinson, San Jose, CA). A minimum of 500 000 total events was acquired per sample. Samples were gated on living lymphocytes as determined by forward- and side-scatter and by exclusion of PI-positive events, and the frequency of pentamer+ cells among CD8+bright living cells was determined. As a negative control, patients' T cells were similarly stained with an HLA-A*0201/Mart-1-A27L pentamer (ELAGIGILTV; ProImmune), provided that none of the patients and donors had active vitiligo. In selected cases, negative controls for pentamer staining included also PBMCs from HLA-A*0201- healthy controls; positive controls included PBMCs from HLA-A*0201+/cytomegalovirus (CMV)-seropositive patient-donor pairs stained with an HLA-A*0201/CMV-pp65 pentamer (NLVPMVATV; Proimmune). CEA-Cap1 pentamers were titrated against a peptide-specific T-cell line to minimize background staining while preserving the mean fluorescence intensity of positive cells, and then were used at a final dilution of 1/50 (ie, 10 μg/mL). To establish a threshold, background staining was assessed by testing PBMCs from HLA-A*0201+ healthy donors with the same CEA-Cap1 and Mart-1-A27L pentamers (n = 16), obtaining a mean of 0.15% ± 0.074% CD8+/pentamer+ for CEA-Cap1 and of 0.19% × 0.093% CD8+/pentamer+ for Mart-1-A27L (Table 2 and data not shown). Values above mean + 2 standard deviations were considered positive.
Development of GVHD after HCT and clinical response
ID no. . | Previous therapies . | GVHD grade . | GVHD site . | GVHD, d . | CSA stop, d . | GVHD treatment . | CEA before transplantation/at GVHD onset or d + 100, ng/mL . | CEA expression by tumor cells* . | Clinical response† . | HLA-A*0201 . | |
|---|---|---|---|---|---|---|---|---|---|---|---|
| GVHD involvement | |||||||||||
| 1 | De Gramont, Tomudex, FOLFOX, CPT-11, MMC | II | GI | 49 | 150 | PDN | 110/160 | ND | SD† | NEG | |
| 2 | 5FU-VP 16, liver surgery, FOLFOX | III | GI | 110 | 94 | CSA + PDN | 2/6† | ND | MR | NEG | |
| 4 | FOLFIRI, FOLFOX | II | Skin, GI, liver | 41 | 180 | PDN | 8/9 | ND | None | NEG | |
| 8 | FOLFOX, FOLFIRI, De Gramont, MMC | IV | GI, liver | 88 | 86 | CSA + PDN, infiximab | 2/3 | +++ | SD† | POS | |
| 10 | FOLFOX, liver surgery | III | Skin, GI | 121 | 110 | CSA + PDN, infliximab | 56/480 | ++ | None | NEG | |
| 11 | FOLFOX, liver surgery, FOLFIRI | II | GI, liver | 210 | 140 | CSA | 607/61 | +++ | PR | POS | |
| 12 | FOLFOX, surgery, FOLFIRI | III | Skin, GI | 120 | 100 | PDN + CSA | 18/36 | +++ | None | POS | |
| 15 | FOLFIRI, CPT-11, 5FU, MMC, cetuximab | II | Skin, GI | 51 | NA | PDN | 2112/1800 | ND | None | NEG | |
| No GVHD involvement | |||||||||||
| 3 | FOLFOX, liver surgery | 0 | NA | NA | 91 | NA | 90/100 | ND | None | POS | |
| 5 | FOLFOX | 0 | NA | NA | 118 | NA | 8/9 | ++ | SD† | NEG | |
| 6 | FOLFIRI, FOLFIRI | 0 | NA | NA | 85 | NA | 365/780 | ND | None | NEG | |
| 7 | FOLFIRI, FOLFOX | 0 | NA | NA | NA | NA | 90/170 | ND | None | POS | |
| 9 | FOLFOX | 0 | NA | NA | 90 | NA | 1/2 | ++ | None | POS | |
| 13 | FOLFOX, FOLFIRI, liver criosurgery, MMC | 0 | NA | NA | 88 | NA | 370/ND | ND | None | NEG | |
| 14 | FOLFOX, FOLFIRI, De Gramont | 0 | NA | NA | NA | NA | 2380/ND | ND | None | NEG | |
ID no. . | Previous therapies . | GVHD grade . | GVHD site . | GVHD, d . | CSA stop, d . | GVHD treatment . | CEA before transplantation/at GVHD onset or d + 100, ng/mL . | CEA expression by tumor cells* . | Clinical response† . | HLA-A*0201 . | |
|---|---|---|---|---|---|---|---|---|---|---|---|
| GVHD involvement | |||||||||||
| 1 | De Gramont, Tomudex, FOLFOX, CPT-11, MMC | II | GI | 49 | 150 | PDN | 110/160 | ND | SD† | NEG | |
| 2 | 5FU-VP 16, liver surgery, FOLFOX | III | GI | 110 | 94 | CSA + PDN | 2/6† | ND | MR | NEG | |
| 4 | FOLFIRI, FOLFOX | II | Skin, GI, liver | 41 | 180 | PDN | 8/9 | ND | None | NEG | |
| 8 | FOLFOX, FOLFIRI, De Gramont, MMC | IV | GI, liver | 88 | 86 | CSA + PDN, infiximab | 2/3 | +++ | SD† | POS | |
| 10 | FOLFOX, liver surgery | III | Skin, GI | 121 | 110 | CSA + PDN, infliximab | 56/480 | ++ | None | NEG | |
| 11 | FOLFOX, liver surgery, FOLFIRI | II | GI, liver | 210 | 140 | CSA | 607/61 | +++ | PR | POS | |
| 12 | FOLFOX, surgery, FOLFIRI | III | Skin, GI | 120 | 100 | PDN + CSA | 18/36 | +++ | None | POS | |
| 15 | FOLFIRI, CPT-11, 5FU, MMC, cetuximab | II | Skin, GI | 51 | NA | PDN | 2112/1800 | ND | None | NEG | |
| No GVHD involvement | |||||||||||
| 3 | FOLFOX, liver surgery | 0 | NA | NA | 91 | NA | 90/100 | ND | None | POS | |
| 5 | FOLFOX | 0 | NA | NA | 118 | NA | 8/9 | ++ | SD† | NEG | |
| 6 | FOLFIRI, FOLFIRI | 0 | NA | NA | 85 | NA | 365/780 | ND | None | NEG | |
| 7 | FOLFIRI, FOLFOX | 0 | NA | NA | NA | NA | 90/170 | ND | None | POS | |
| 9 | FOLFOX | 0 | NA | NA | 90 | NA | 1/2 | ++ | None | POS | |
| 13 | FOLFOX, FOLFIRI, liver criosurgery, MMC | 0 | NA | NA | 88 | NA | 370/ND | ND | None | NEG | |
| 14 | FOLFOX, FOLFIRI, De Gramont | 0 | NA | NA | NA | NA | 2380/ND | ND | None | NEG | |
De Gramont indicates 400 mg/m2 fluorouracil, 600 mg/m2 fluorouracil, and 200 mg/m2 leucovorin; Tomudex, 4 mg/m2; FOLFOX, 100 mg/m2 oxaliplatin, 400 mg/m2 fluorouracil, 600 mg/m2 fluorouracil, and 200 mg/m2 leucovorin; CPT-11, 100 mg/m2 Irinotecan; MMC, 10 mg/m2 mytomicin-C; 5FU/VP16, 500 mg/m2 fluorouracil, 200 mg/m2 leucovorin, and 100 mg/m2 VP 16; FOLFIRI, 180 mg/m2 irinotecan, 400 mg/m2 fluorouracil, 600 mg/m2 fluorouracil, and 200 mg/m2 leucovorin; cetuximab, 400 mg/m2 on day 1, 250 mg/m2 on days 8, 14, 21; PDN, prednisone; ND, not determined; and NA, not applicable.
CEA expression on tumor biopsies as detected by immunohistochemistry. ++ indicates moderately reactive; +++, strongly reactive tumor cells (“Patients, materials, and methods”)
SDs that lasted more than 90 days were considered as relevant clinical responses; short lasting stable disease were excluded
In vitro induction of CEA-specific T lymphocytes
CD8+ T cells from patients were stimulated in vitro with peptide-pulsed donor-derived dendritic cells (DCs) alternating with peptide pulsed T2 cells at weekly intervals. As a control, CD8+ cells from donor PBSCs were similarly stimulated with peptide-pulsed donor DCs or peptide-pulsed T2 cells (not shown). CD8+ cells were positively purified using magnetically labeled mAbs to CD8 (Miltenyi Biotec). DCs were generated from donor PBSCs or PBMCs as described by Dauer et al,30 with slight modifications. Briefly, CD14+ cells were positively purified with magnetically labeled mAbs to CD14 and cultured with granulocyte-macrophage-CSF (GM-CSF; 80 ng/mL) and interleukin-4 (IL-4; 40 ng/mL) for 40 hours. A cocktail of proinflammatory cytokines was then added for additional 24 hours (80 ng/mL GM-CSF, 40 ng/mL IL-4, 10 ng/mL IL-6, 10 ng/mL IL1-β, 20 ng/mL tumor necrosis factor-α [TNF-α], and 10 μM prostaglandin E2 [PGE2]) (GM-CSF, IL-4, IL1-β, IL-6, and TNF-α were obtained from Peprotech, London, United Kingdom; PGE2, Alexis Biochemicals, San Diego, CA). Finally, recombinant CD40 ligand at 250 ng/mL (rCD40L-FLAG-tag fusion protein plus enhancer was obtained from from Alexis Biochemicals) was added 30′ before peptide pulsing. The TAP (transporter associated with antigen processing)-deficient lymphoblastoid T2 cell line was used as an antigen-presenting cell because it is known to efficiently bind exogenously supplied peptides in the context of HLA-A*0201. Peptide pulsing and all T-cell cultures were performed in serum-free medium (AIM-V, Gibco BRL, Grand Island, NY; and X-Vivo15, BioWhittaker, Walkersville, MD). DCs and T2 cells were incubated in vitro with 50 μg/mL CEA-Cap1-6D (YLSGADLNL; Invitrogen, Carlsbad, CA) for 15 minutes at RT and for an additional 4 hours at 37°C, 5% CO2 in the presence of 2.5 μg/mL β2-microglobulin. Peptide Cap1-6D is an HLA-A*0201-restricted agonist epitope of CEA which has the same HLA class I-binding properties of its naturally processed counterpart Cap1, but with enhanced stimulatory activity on T cells.31,32 DCs and T2 cells were irradiated at 3000 cGy and used with a CD8+/DC ratio of 10:1 and a CD8+/T2 ratio of 4:1. Considering all cultures, a mean of 2.8 ± 1.6 × 106 CD8+ cells were used at the beginning and a mean of 0.7 ± 0.5 × 106 CD8+ cells were obtained after 3 cycles of stimulation. Recombinant IL-2 (20 U/mL) was added 48 hours after each stimulation. Seven days after the third cycle of stimulation, cultures were tested by IFN-γ enzyme-linked immunospot (ELISPOT). ELISPOT assay was carried out as previously described.33 Before developing the assay, T cells were routinely transferred from the ELISPOT plate to a normal 96-well round-bottom plate, and 20 U/mL IL-2 was added. In some cases, T cells recovered from specific wells (ie, wells in which T cells were tested with relevant tumor target) were retested 7 days later against Cap1-6D-pulsed T2 cells to confirm specificity of data obtained with tumor cells (not shown). Spots were counted by a computer-assisted ELISPOT reader (Bioline; AID, Turin, Italy). All cell lines were obtained from the American Type Culture Collection (ATTC; Manassas, VA). Two CRC cell lines (SW-403 and HT-29) were selected because they both express and secrete CEA, but only SW-403 cells express HLA-A2.31 These 2 CRC cell lines were checked by flow cytometry using specific mAbs to CEA (clone Col-1; Zymed, San Francisco, CA) and HLA-A2 (clone BB7.2; Becton Dickinson), confirming that SW-403 cells are CEA+/HLA-A2+ and HT-29 cells are CEA+/HLA-A2-. Immunohistochemical staining for CEA was performed on 5-μm-thick sections from paraffin-embedded tumor tissue using a mAb to CEA (DAKO, Glostrup, Denmark) by routine laboratory methods. For each slide, up to 10 high-power fields (× 40) were evaluated for the presence of tumor cells. The relative staining intensity was semiquanitatvely evaluated as follow: absent (-), mildly (+), moderately (++), or strongly (+++) reactive.
Ex vivo analysis of CEA-specific T lymphocytes
PBMCs from patients were thawed and rested overnight in AIM-V without any stimulation. The following day, 1 × 105 PBMCs/well were plated for ELISPOT either alone or together with 2.5 × 104 peptide-pulsed T2 cells/well or 5 × 103 CRC cells/well, as indicated. T2 cells were pulsed with a CEA-Cap1-6D, Mart-1-A27L, or CMV-pp65 peptide as described. All conditions were performed in quintuplicate. After 24 hours of incubation, ELISPOT assay was developed and spots were counted as already described.
Statistical analysis
Incidence of GVHD, infections, and mortality from various causes as well as the degree of donor chimerism were summarized using cumulative incidence estimates. Analyses were conducted by the Statistica software package (Statsoft, Tulsa, OK).34 Data were analyzed as of February 1, 2005. Statistical significance was assessed by the Fisher Exact Test for qualitative data, and by 2-tailed Student t test for quantitative data. Statistical significance was defined as a P value less than .05.
Results
Patient characteristics
Fifteen patients were enrolled in the study and underwent nonmyeloablative HCT between June 2001 and June 2004 (Candiolo n = 12; Milan n = 2; Pisa n = 1). The median time from diagnosis of metastatic disease to transplantation was 14 months (range, 6-34 months). All patients had previously failed treatment with chemotherapy regimens containing 5FU and either oxaliplatin or irinotecan (Tables 1, 2). All patients had progressive disease before transplantation. The median number of metastatic sites identified in each patient before HCT was 2.1-5 Eight patients had a high tumor burden, as defined by criteria described in Table 1.
Engraftment and chimerism
The patients received a median of 6.7 × 106 CD34+ cells/kg (range, 2.5-11.3 × 106 CD34+ cells/kg), 4.7 × 108 CD3+ cells/kg (range, 1.7-12.7 × 108 CD3+ cells/kg), and 0.44 × 108 CD56+ cells/kg (range, 0.15 × 108-1.1 × 108 CD56+ cells/kg). The median total nucleated cell count of the hematopoietic grafts was 7.9 × 108/kg (range, 5.2 × 108-13.3 × 108/kg). The median nadir of the neutrophil count after HCT was 0.722 × 109/L (range, 0.519 × 109-2.55 × 109/L). No patient experienced an absolute neutrophil count (ANC) below 0.5 × 109/L (500/μL) or a nadir of the platelet count below 60 × 109/L (median, 119 × 109/L; range, 63 × 109-191 × 109/L) (Table 1). No patient required platelet transfusions, and only 3 patients were transfused with red blood cells before day +100 after HCT.
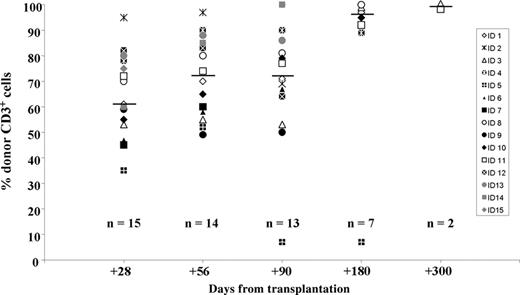
Based on the level of donor chimerism in the T-cell compartment, all patients had evidence of engraftment on days +28, +56, and +90. One patient had full hematopoietic chimerism (including T cells) at day +28. On day +180, 6 (86%) of 7 evaluable patients had full hematopoietic chimerism. The median percentage of T-cell-donor chimerism on days +28, +56, +90, and +180 was 60%, 72%, 71%, and 97% respectively (Figure 1). Higher levels of donor chimerism were achieved in bone marrow and in CD13+ cells from peripheral blood (not shown). One patient at day +220 after a CMV (day +192) and HHV-6 (day +210) infection had a decrease in the level of donor chimerism (from 52% to 7%). This patient became transfusion dependent and was treated with bulk DLIs. After 2 courses of DLI, the level of donor chimerism did not improve (day +256, data not shown). The patient died of disease progression on day +330.
Transplantation-related toxicity
One patient experienced a transient increase of creatinine (grade 3); otherwise, no grades 2 to 4 extrahematologic toxicities were observed. The most common adverse event reported during the first month after transplantation was nausea; however, no patient required parenteral nutrition as a consequence of the preparative regimen or the CSA administration. In the first 100 days, no patient experienced any bacterial or fungal infection requiring admission to the in-patient unit or intravenous antibiotic therapy. CMV reactivation occurred in 8 (53%) of 15 patients between days +45 and +157. All cases were successfully treated with ganciclovir. The median in-patient stay after allogeneic nonmyeloablative HCT was 5 days,3-12 and no patient was readmitted before day +100 because of transplantation-related morbidity.
Assessment of chimerism after transplantation. Percent of donor chimerism for peripheral CD3+ cells of each individual patient over time. Horizontal bars indicate median values.
Assessment of chimerism after transplantation. Percent of donor chimerism for peripheral CD3+ cells of each individual patient over time. Horizontal bars indicate median values.
Immunosuppression taper and GVHD
All patients discontinued MMF on day +27, while the median day for stopping CSA was 111 (range, 86-180 days). Early tapering of CSA in 1 patient occurred because of disease progression. Acute GVHD occurred in 8 patients at a median time of 99 days (range, 41-209 days) after transplantation (4 patients had grade II, 3 had grade III, and 1 had grade IV GVHD) (Table 2). In all but 3 patients, GVHD developed after CSA discontinuation. The gastrointestinal (GI) system was involved in all 8 patients, while skin was affected in 4 patients and liver in 3. 2 patients with steroid-refractory, grades III and IV lower GI-tract GVHD were successfully treated with infliximab (Table 2). The patient with grade IV GVHD experienced a flare while tapering immunosuppression because of disease progression, and this resulted in her death. Of the 8 patients who developed grades II to IV GVHD, 4 had T-cell chimerism of 70% or more on day +28 and 6 had T-cell chimerism of 80% or more on day +56. No chronic GVHD has been observed in the patients who survived more than 100 days.
Clinical outcome and causes of death
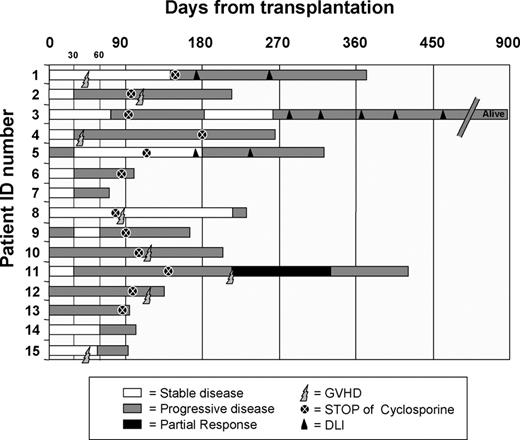
Following transplantation, 1 patient experienced a PR (pt no. 11) and 3 patients had SD that lasted more than 3 months (pt nos. 1, 5, and 8). The disease evaluation at days +28, +56, +90, +180, and +270 showed 10 progression-free (PF)/5 PD, 6 PF/9 PD, 3 PF/11 PD, 2 PF/6 PD, and 1 PR/3 PD, respectively (Figure 2). Among the 8 patients who developed acute GVHD, clinical responses included 1 PR, 2 SD lasting more than 90 days (pt nos. 1 and 8), and 1 mixed response (shrinkage of a pulmonary lesion concurrently with an increased cervical adenopathy, pt no. 2) (Table 2). Among the 7 patients without GVHD, the only notable clinical response was 1 SD (pt no. 5; Table 2). After a median follow-up of 240 days (range, 79-913 days), 14 patients have died and 1 is alive with PD. Progression was the cause of death in 13 of 14 patients, and 1 patient died of GVHD that developed during the tapering of immunosuppressive treatment because of disease progression.
Clinical course after transplantation. Disease status at different time points is indicated with bars of different colors. White bars indicate stable disease (SD); light gray bars, progressive disease (PD); and dark gray bars, partial response (PR). Discontinuation of immunosuppression is represented by a dark circle and the onset of GVHD by lighting. Donor lymphocyte infusions (DLIs) are described with a triangle.
Clinical course after transplantation. Disease status at different time points is indicated with bars of different colors. White bars indicate stable disease (SD); light gray bars, progressive disease (PD); and dark gray bars, partial response (PR). Discontinuation of immunosuppression is represented by a dark circle and the onset of GVHD by lighting. Donor lymphocyte infusions (DLIs) are described with a triangle.
In only 3 patients did the progression occur in organs not previously involved with the disease at the time of transplantation (central nervous system [CNS] involvement in 2 patients and adrenal lesion in 1 patient). Two patients with disease progression (pt nos. 1 and 3) were treated with CD8-depleted DLIs at days +176 and +280, respectively, after transplantation. They received 2 and 5 infusions, respectively, without any sign of acute GVHD and without clinical response.
Induction of CEA-specific T cells in patients with CRC
Among 15 patients, 6 were HLA-A*0201 positive and could be analyzed at different time points for the in vivo generation of CEA-specific T cells with pentamer staining (Table 2). Three of the 6 HLA-A*0201+ patients developed GVHD, and this was associated with the development of CEA-specific T cells (Table 3). Serum CEA levels were not increased in all patients before transplantation, but their tumor cells were expressing CEA as detected by immunohistochemistry on biopsy specimens (Table 2). In some cases, serum CEA levels increased significantly only later in the course of the disease (pt nos. 2, 4 and 8).
Pentamer staining in CRC patients and their donors
. | % CEA-Cap1+/CD8+ cells . | . | . | . | |||
|---|---|---|---|---|---|---|---|
| ID no. . | Patient* . | Sibling donor . | Before GVHD . | During GVHD . | |||
| 8 | 0.05 | 0.32 | 0.14 | 1.71 | |||
| 11 | 0.08 | 0.19 | 0.21 | 1.79 | |||
| 12 | 0.10 | 0.24 | 0.05 | 0.81 | |||
| 3 | 0.04 | 0.05 | 0.14 | NA | |||
| 7 | 0.25 | 0.11 | 0.33 | NA | |||
| 9 | 0.14 | 0.02 | 0.02 | NA | |||
. | % CEA-Cap1+/CD8+ cells . | . | . | . | |||
|---|---|---|---|---|---|---|---|
| ID no. . | Patient* . | Sibling donor . | Before GVHD . | During GVHD . | |||
| 8 | 0.05 | 0.32 | 0.14 | 1.71 | |||
| 11 | 0.08 | 0.19 | 0.21 | 1.79 | |||
| 12 | 0.10 | 0.24 | 0.05 | 0.81 | |||
| 3 | 0.04 | 0.05 | 0.14 | NA | |||
| 7 | 0.25 | 0.11 | 0.33 | NA | |||
| 9 | 0.14 | 0.02 | 0.02 | NA | |||
NA indicates not applicable (no GVHD).
Colon cancer patients analyzed before transplantation
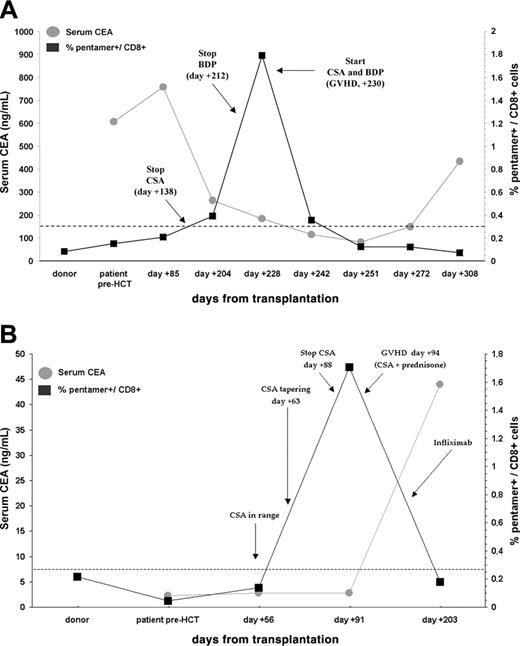
Patient no. 11 experienced a PR on day +210 (40% reduction of tumor size) that was associated with the discontinuation of immunosuppressive treatment and with grade II GVHD in the liver and in the upper GI tract. Concurrent with this clinical response, there was an increase in CEA-Cap1-specific CD8+ T cells and a significant decrease of serum CEA values (from 607 to 81 ng/dL) (Figure 3A). A rapid and progressive decline of pentamer+/CD8+ cells was observed after the initiation of immunosuppressive treatment with CSA. After 1 month of persistent negative pentamer values, CEA levels started to rise again associated with liver progression on day +330. The frequency of CEA-specific T cells before transplantation (both in the donor and in the patient) and before GVHD was below the positive threshold (Figure 3A). Donor chimerism on CD3+ cells was 92% at day +180 and 95% at day +260, suggesting that most of CEA-specific T cells detected at day +228 could be derived from the T-cell pool of the donor.
In patient no. 8, a peak of CEA-specific T cells was observed 3 days after CSA discontinuation, which preceded the onset of grade IV GVHD. The frequency of circulating CEA-specific T cells decreased after starting treatment with systemic steroids and CSA (Figure 3B). Donor T-cell chimerism was 82% at day +84 and 97% at day +180. Although CEA expression by tumor cells was documented on the tumor specimen at diagnosis (Table 2), serum CEA levels were in the normal range and increased only at disease progression, which occurred several weeks after GVHD therapy had been initiated and pentamer-positive cells had declined (Figure 3B). This patient remained stable for 220 days, which is the longest time interval recorded in our series (Figure 2).
In patient no. 12, circulating CEA-specific T cells were detected at day +107, just before grade III GVHD of skin and GI tract developed. The peak frequency of CEA-Cap1 pentamer-positive cells was much lower than patient nos. 8 and 11 (Table 3). CSA was started but, because of rapid tumor progression documented on day +130, the patient requested only supportive care at home. Patient nos. 3, 7, and 9 did not develop GVHD or a clinical response and had no significant increase of CEA-Cap1 pentamer+/CD8+ cells (Figure 2, and Table 3). The frequency of CEA-specific T cells before transplantation (in all 6 donors and patients) and before GVHD (in all 3 patients) was below the positive threshold (Table 3).
To determine whether the induction of CEA-specific CD8+ T cells was restricted to patients with CRC, we tested 9 patients with GVHD (with [n = 5] and without [n = 4] GI involvement) after allogeneic HCT for diseases other than CRC. All these patients had normal levels of serum CEA. CEA-specific T cells were not detected in any of the patients studied (Table 4, left column). This compares with the development of CEA-specific T-cell responses in 3 of 3 CRC patients with GVHD (P < .05, Fisher test).
Pentamer staining in patients with GVHD but without CRC
. | . | % CEA-Cap1+/CD8+ cells during GVHD . | . | |
|---|---|---|---|---|
| ID no. . | Disease . | GI . | Other sites . | |
| 1 | Myeloma | 0.09 | — | |
| 2 | Myeloma | 0.11 | — | |
| 3 | Myeloma | 0.19 | — | |
| 4 | Renal carcinoma | 0.12 | — | |
| 5 | Renal carcinoma | 0.13 | — | |
| 6 | Myeloma | — | 0.09 | |
| 7 | Myeloma | — | 0.15 | |
| 8 | Myeloma | — | 0.16 | |
| 9 | Renal carcinoma | — | 0.08 | |
. | . | % CEA-Cap1+/CD8+ cells during GVHD . | . | |
|---|---|---|---|---|
| ID no. . | Disease . | GI . | Other sites . | |
| 1 | Myeloma | 0.09 | — | |
| 2 | Myeloma | 0.11 | — | |
| 3 | Myeloma | 0.19 | — | |
| 4 | Renal carcinoma | 0.12 | — | |
| 5 | Renal carcinoma | 0.13 | — | |
| 6 | Myeloma | — | 0.09 | |
| 7 | Myeloma | — | 0.15 | |
| 8 | Myeloma | — | 0.16 | |
| 9 | Renal carcinoma | — | 0.08 | |
— indicates no GVHD involvement in that site.
In vitro cultures and assessment of antitumor activity
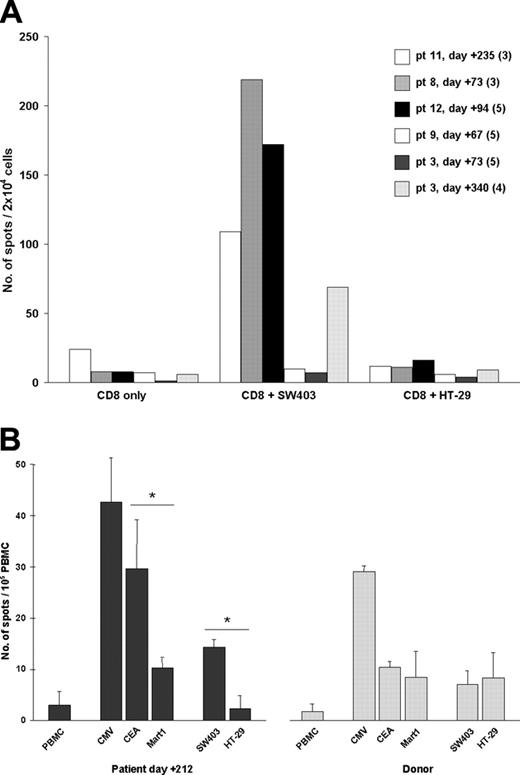
To determine whether CEA-specific T cells could effectively recognize CRC cells and to establish whether these cells could be present at a lower frequency in patients without GVHD, we chose to sensitize T cells in vitro prior to functional analysis with IFN-γ ELISPOT. This is a more sensitive technique for the detection of CEA-specific T cells than that observed with pentamer staining.35 PBMCs from patients were obtained at different time points after transplantation (as indicated in Figure 4A) and purified CD8+ lymphocytes were stimulated weekly in vitro with CEA-Cap1-6D-pulsed antigen-presenting cells (“Patients, materials, and methods”). T cells from patient nos. 11, 8, and 12 displayed specific activity against a CEA+/HLA-A2+ CRC cell line (SW-403), thus confirming the existence of circulating CEA-specific T cells. Activity of T cells from patient nos. 11 and 8 was readily detected after 3 cycles of stimulation, whereas activity of T cells from patient no. 12 was detected only after 5 cycles of stimulation, reflecting the lower frequency of CEA-pentamer+ cells observed in this patient. T cells taken during CSA tapering from patient nos. 3 (day +72) and 9 (day +67) who did not develop GVHD did not display any antitumor activity, even after 5 cycles of stimulation. However, after patient no. 3 had discontinued immunosuppression and had achieved complete donor chimerism, T cells taken on day +340 were analyzed and CEA-specific T cells were detected (Figure 4A). In selected cases (patient nos. 3, 8, and 11), specificity for the selected HLA-A2-restricted CEA-Cap1 epitope was further documented by ELISPOT data obtained with peptide-pulsed T2 cells as target (not shown; “Patients, materials, and methods”). This data suggests that tumor-specific T cells can be induced in vitro also in patients without GVHD after discontinuation of immunosuppression.
In vivo expansion of CD8+ T cells specific for CEA-Cap1 is associated in time with GVHD onset. PBMCs obtained at different time points before and after transplantation were stained with HLA-A*0201/CEA-Cap1 pentamer as described in “Patients, materials, and methods.” The percent of CD8+ HLA-A*0201/CEA pentamer+ cells within the CD8+ population is shown (black line). Arrows indicate time points in which immunosuppressive treatment was modified (CSA indicates cyclosporin A; BDP, beclomethasone diproprionate; and PDn, prednisone). The dashed line indicates background threshold of pentamer staining. Concomitant with pentamer staining, serum CEA levels were determined by carbonyl metallo-immunoassay (gray line). (A) Time course of patient no. 11. (B) Time course of patient no. 8.
In vivo expansion of CD8+ T cells specific for CEA-Cap1 is associated in time with GVHD onset. PBMCs obtained at different time points before and after transplantation were stained with HLA-A*0201/CEA-Cap1 pentamer as described in “Patients, materials, and methods.” The percent of CD8+ HLA-A*0201/CEA pentamer+ cells within the CD8+ population is shown (black line). Arrows indicate time points in which immunosuppressive treatment was modified (CSA indicates cyclosporin A; BDP, beclomethasone diproprionate; and PDn, prednisone). The dashed line indicates background threshold of pentamer staining. Concomitant with pentamer staining, serum CEA levels were determined by carbonyl metallo-immunoassay (gray line). (A) Time course of patient no. 11. (B) Time course of patient no. 8.
In vitro assessment of the antitumor activity of CEA-specific T cells. (A) Purified CD8+ cells from transplant-recipient patients were sensitized in vitro and then tested in ELISPOT for IFN-γ secretion, after a minimum of 3 stimulation cycles (numbers in parentheses indicate the number of cycles after which the ELISPOT assay was performed). T cells (2 × 104) were plated in the ELISPOT plate either alone (CD8+ only) or together with 5 × 103 CRC cells, as indicated. (B) Unselected PBMCs were similarly tested, but without any prior in vitro stimulation (patient no. 11). PBMCs (1 × 105) were plated in the ELISPOT plate either alone or together with 2.5 × 104 peptide-pulsed T2 cells (CMV-pp65, CEA-Cap1-6D, or Mart1-A27L) or 5 × 103 colon cancer cells, as indicated. Results represent mean ± SD of 5 replicates. *P < .05 as determined by a 2-tailed t test for unpaired samples. SW403 and HT29 are 2 CRC cell lines: although both cell lines express CEA, only SW403 expresses HLA-A*0201.
In vitro assessment of the antitumor activity of CEA-specific T cells. (A) Purified CD8+ cells from transplant-recipient patients were sensitized in vitro and then tested in ELISPOT for IFN-γ secretion, after a minimum of 3 stimulation cycles (numbers in parentheses indicate the number of cycles after which the ELISPOT assay was performed). T cells (2 × 104) were plated in the ELISPOT plate either alone (CD8+ only) or together with 5 × 103 CRC cells, as indicated. (B) Unselected PBMCs were similarly tested, but without any prior in vitro stimulation (patient no. 11). PBMCs (1 × 105) were plated in the ELISPOT plate either alone or together with 2.5 × 104 peptide-pulsed T2 cells (CMV-pp65, CEA-Cap1-6D, or Mart1-A27L) or 5 × 103 colon cancer cells, as indicated. Results represent mean ± SD of 5 replicates. *P < .05 as determined by a 2-tailed t test for unpaired samples. SW403 and HT29 are 2 CRC cell lines: although both cell lines express CEA, only SW403 expresses HLA-A*0201.
Ex vivo assessment of antitumor activity
To exclude the possibility that in vitro cultures allowed for priming of new CEA-specific T cells different from those detected with pentamer staining, an ELISPOT assay was also performed using unstimulated PBMCs in selected cases. As shown in Figure 4B, PBMCs of patient no. 11 taken at day +212 were effectively recognizing CEA-Cap1-6D-pulsed T2 cells but not T2 cells pulsed with an irrelevant peptide (Mart-1). Also, CEA+/HLA-A*0201+ CRC cells (SW-403) were specifically recognized, albeit to a lesser extent. CD8+ T cells from the sibling donor recognized T2 cells pulsed with CMV-pp65 peptide, but not T2 cells pulsed with CEA or Mart-1, or tumor cells. Two additional patients without GVHD were similarly tested (pt nos. 3 and 9) and only T cells reactive to CMV, but not to CEA, were detected in both donor and patient (not shown). Thus, circulating CEA-specific T cells detected by pentamer staining are functionally competent, also without prior in vitro stimulation.
Discussion
This clinical protocol for CRC was a translation of the experience with nonmyeloablative allogeneic HCT as a platform for adoptive, T-cell-mediated immunotherapy for hematologic diseases.4 Durable mixed chimerism was achieved in all but 1 case, and patients did not experience any relevant hematologic toxicity. Conversely, we did not observe any early significant nonhematologic toxicity other than infections, which resolved with standard therapies. Management of most patients after transplantation was conducted in the outpatient department. Transplantation-related mortality was 7%, and the patient who died from acute GVHD had CSA tapered because of tumor progression. The incidence and severity of acute GVHD compared favorably with previous studies in which the same conditioning regimen was used.4,36 Despite immunosuppressive therapy and progressive disease at baseline, the patients experienced limited tumor progression in the first 30 days. We conclude that nonmyeloablative allogeneic HCT was feasible and safe in patients with advanced CRC, although the incidence of late GVHD has to be determined with a larger series of patients and longer follow-up.
Metastatic CRC is still an incurable disease and responses to treatments beyond second-line are anecdotal. Since all patients were resistant to conventional therapy and had progressive metastatic disease involving multiple sites, the reported clinical responses (1 PR and 3 SDs that lasted more than 90 days) were encouraging. A more selective patient recruitment may be required to achieve a better response rate with HCT. It has been shown that a small tumor burden and/or a slowly progressing disease positively affects the clinical outcome after HCT.10,37,38 Moreover, to minimize the risk of acute GVHD in the current study, the taper of immunosuppressive therapy was cautious, potentially inhibiting the GVT reaction and limiting the number of responses.
GVT reactions—achieved after either conventional or nonmyeloablative HCT—have improved the survival of patients with many hematologic diseases. In patients with renal cell carcinoma or breast cancer, clinical evidence of a GVT effect has been provided,7-10,12 and T cells directed against mHAg's have been isolated ex vivo and characterized in vitro in few cases.39,40 Among other solid tumors, small case-series suggested the clinical existence of a GVT effect in CRC, ovarian cancer, and sarcomas, but no evidence of a direct and tumor-specific T-cell activity was provided.9,10,41,42 Most of the latter studies were conducted in very advanced-stage patients, in whom clinical evidence of a GVT effect may be difficult to detect. Given this limitation on the clinical side, we performed ex vivo studies aimed at determining whether antigen-specific T cells with potential antitumor activity were induced in vivo. Two categories of T-cell antigens were considered to be candidate targets for GVT: polymorphic mHAg's and TAAs. In the field of colon cancer, the only polymorphic mHAg known to be expressed by colon carcinoma cells is HA-1H, which is HLA-A*0201 restricted.43,44 Unfortunately, none of the 6 HLA-A*0201+ patients of our series expressed the HA-1H allele in the GVT direction (patient HA-1H+, donor HA-1R+) (not shown). We therefore focused our attention on TAAs, since a donor-derived T-cell response driven by GVHD and targeting TAAs has been documented in an in vivo mouse model.20 Moreover, in chronic myelogenous leukemia patients, TAAs can be effectively targeted by donor-derived antigen-specific T cells.19
Among the few characterized TAAs associated with CRC that have proven to be immunogenic and for which HLA pentamers have been validated, we chose to focus on CEA as the prototype antigen. We observed that when GVHD occurs, it also triggers a T-cell response directed to CEA. The in vivo induction of the anti-CEA response required the presence of GVHD, but also the presence of CEA-expressing tumor cells. CEA-specific T cells appeared concurrent with the development of GVHD in 3 of 3 CRC patients and in 0 of 9 control patients. This indicates that the observed CEA responses are specific, rather than being a general phenomenon associated with GVHD (ie, antigen-driven stimulation of T cells). In the patient who achieved a PR, the presence of CEA-specific T cells was clearly correlated in time with a decrease of serum CEA values and with size reduction of liver metastasis. Clinical benefits may not have been durable because of systemic immunosuppression, which was started for control of GVHD and was associated with a decrease in CEA-specific T cells. Considering also the long-lasting (220 days) SD achieved in patient no. 8, a correlation between clinical response and CEA response was shown in 2 of 3 cases. In the third patient, a clinical response was not achieved, but this patient had a lower frequency of CEA-reactive T cells, which was documented both by pentamer staining and in vitro sensitization experiments. Taken together, these data are not enough to clearly support a correlation between clinical response and the presence of CEA-specific T cells, but they strongly suggest that when the frequency of tumor-specific T cells is high enough a clinical benefit might ensue. A larger series of patients should be studied to determine the real clinical impact of CEA responses (or T-cell responses directed to other TAAs) after HCT.
The existence of a GVT effect directed to TAAs is a novel observation in the field of nonmyeloablative HCT applied to solid tumors, and could be more relevant than responses directed to mHAg's. In the case of TAAs, in fact, the antigenic targets of GVHD and GVT would be distinct: TAAs are overexpressed by tumor cells and minimally by normal epithelial cells, whereas mHAg's are ubiquitous or tissue restricted at best (ie, are expressed at equal levels by normal and malignant cells). These findings lay the ground for developing new therapeutic strategies that will exploit allogeneic HCT as a platform to make T-cell-based immunotherapy more specific, effective, and safe. In this context, 2 questions arise for future developments: (1) how to induce tumor-reactive T cells when they are not detectable; and (2) how to sustain tumor-reactive T cells when GVHD treatment has to be initiated. Vaccination of the donor (before harvest of the hematopoietic cell graft) and then of the engrafted patient with multiple TAAs is 1 potential strategy which addresses both questions.45-48 Several CEA-based vaccine formulations have proven to be safe in humans and not associated with autoimmune reactions, making vaccination of healthy individuals a potential approach for increasing the frequency of CEA-specific T cells in the donor hematopoietic cell graft.31,49-52 As for the vaccination of engrafted patients, timing of vaccine delivery could be critical for efficacy. Data obtained at different timepoints after transplantation suggest that full donor engraftment and discontinuation of immunosuppression will likely be important requirements for success. Tumor vaccination could be done after GVHD treatment, which may need to be limited to avoid tumor progression.
Alternatively, an adoptive cell transfer approach could be used, based on the ex vivo selection, activation, and expansion of CEA-specific lymphocytes. The source of these antitumor T cells could be peripheral blood from engrafted patients at the time of their appearance after HCT, as determined by monitoring with specific HLA pentamers. In vitro-expanded tumor-reactive T cells of donor origin could also be used. CEA-specific T cells from sibling donors, in fact, could be generated with the same in vitro sensitization protocol described for patients (not shown). Although we do not have formal proof that CEA-specific T cells generated in vivo are of donor origin, their development along with GVHD onset and the high degree of donor chimerism at the time of their detection (> 90%) suggest a donor prevalence in the repertoire of CEA-pentamer+ T cells.
In conclusion, the results of the present study suggest that a GVT effect can occur after HCT in patients with CRC, it correlates with GVHD and is directed against TAAs. Tumor vaccination to induce or to sustain TAA-specific T cells after transplantation is the next clinical application. Alternatively, adoptive immunotherapy with ex vivo-expanded TAA-specific T cells is a potential future line of investigation to dissociate GVT and GVHD. Finally, improved response rates may be observed if future studies include only patients without bulky disease.
Prepublished online as Blood First Edition Paper, January 10, 2006; DOI 10.1182/blood-2005-10-3945.
Supported by grants from “Associazione Italiana per la Ricerca sul Cancro” (AIRC, 2004), Milan, from “Ministero dell'Istruzione, dell'Università e della Ricerca,” Rome (MIUR-FIRB 2001), and from “Regione Piemonte” (Ricerca Scientifica Applicata 2003 and Ricerca Sanitaria Finalizzata 2004), Italy. A.V. is a fellowship recipient from AIRC.
F.C.-S. and A.C. contributed equally to this study.
F.C.-S. had the idea and projected the clinical trial, took care of patients, analyzed clinical data, and wrote the report. A. Cignetti conceived, coordinated, and analyzed experimental data, and wrote the report. A. Capaldi contributed to study design and day-to day clinical management, and participated in data analysis. K.V., A.V., and D.S. were involved in pentamer study and functional activity of T lymphocytes. A.R. took care of patients, designed the database for patients who received transplants, and analyzed results. E.S. and R.F. contributed to patient accrual, projected colorectal database, and contributed to survival analysis. G.G. and D.R.-S. contributed to patients' clinical care and to protocol design. M. Geuna and M.F. obtained peripheral blood stem cells and lymphocytes and contributed to chimerism evaluation. G.D.R. obtained and performed immunohistochemistry on tumor samples. A.S. was responsible for analysis and collection of patients' blood cells and monitoring of the clinical trial. M. Gatti set up and performed total body irradiation. G.L.-D. and E.B. took care of patients transplanted in Milan and Pisa, respectively, and collected clinical data for analysis. R.N. had an important role in the design of the study, in the critical analysis of the results, and in the revision of the paper. M.A. had the idea for the study, wrote the protocol, obtained funding, analyzed results, and revised the paper.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
The authors thank the personnel of the Units of Radiodiagnostic, Radiation Therapy, Pathology, and Pharmacy, and all medical, nursing, laboratory, and clinical staffs for their daily help and support in the conduction of the present study. A special thanks goes to the personnel of the Radiation Therapy Unit for taking care of cell irradiation. We also wish to thank Licia Rivoltini for helpful discussion. Above all we are indebted to the patients and their caregivers for the courage and dedication shown during the study.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal