Plasmacytoid dendritic cell precursors (pDCs) are professional type I interferon-producing cells, a critical cell type in regulating innate and adaptive immune responses. By microarray gene expression analysis, we found that pDCs activated by virus or CpG-ODN preferentially express the ligand for the glucocorticoid-induced tumor necrosis factor receptor (GITRL), which was confirmed by reverse transcriptase-polymerase chain reaction (RT-PCR) and flow cytometry analysis. Using the same approaches, we found GITR is expressed by activated natural killer (NK) cells and T cells. We show that pDCs activated by CpG-ODN promote NK cell cytotoxicity and interferon (IFN)-γ production through type I IFNs and GITRL. Using a GITRL-transfected cell line, we further demonstrate that GITRL promotes NK cell cytotoxicity and IFN-γ production in synergy with interleukin-2 (IL-2), IFN-α, and NKG2D triggering. We also demonstrated that pDCs localized in close contact to NK cells in T-cell areas of the tonsils, and a subpopulation of the pDCs expressed GITRL. This study reveals a novel function of GITR/GITRL in pDC-mediated coactivation of NK cells.
Introduction
Plasmacytoid dendritic cell precursors (pDCs) are professional type I interferon (IFN)-producing cells (IPCs). Upon recognition of pathogens, pDCs rapidly produce a massive amount of type I IFNs as effector cytokines to activate the innate immune system and subsequently differentiate into DCs to trigger adaptive immune responses.1-4 Type I IFNs produced by pDCs not only directly inhibit viral replication in infected cells, but also play an essential role to link innate and adaptive immune system. Type I IFNs secreted by pDCs play a critical role in regulating the functions of various cell types of the immune system, including activating natural killer (NK) cells5,6 and myeloid dendritic cells,7,8 promoting the survival, memory, and IFN-γ production of T cells,9-11 and inducing B-cell differentiation into plasma cells.12
To further understand the molecular mechanisms by which pDCs regulate innate and adaptive immune responses, we performed Affymatrix microarray gene expression analyses of pDCs activated by HSV-1 or influenza A virus. We found that the activated pDCs preferentially express the ligand for the glucocorticoid-induced tumor necrosis factor receptor (GITR), which was further confirmed by reverse transcriptase-polymerase chain reaction (RT-PCR) and flow cytometry analysis. GITR is a member of the tumor necrosis family receptor (TNFR) superfamily13,14 and shares a significant homology with CD27, CD134 (OX40), and CD137 (4-1BB). Original studies suggested that GITR was selectively expressed by CD4+CD25+ regulatory T cells, and signaling through GITR by agonist anti-GITR antibody (Ab) could abrogate the suppressive function of CD25+CD4+ regulatory T cells.15,16 More recent studies show that GITR is expressed by CD4+ and CD8+ T cells, and both anti-GITR monoclonal (m) Ab and recombinant GITR ligand (GITRL) costimulate CD4+ and CD8+ T cells.13,14
To understand the function of GITRL expressed by activated pDCs, we performed extensive analyses of GITR mRNA expression by a large panel of human immune cells, including different DC subsets, B and T lymphocytes, NK cells, neutrophils, macrophages, and monocytes. We found that although human T cells and macrophages expressed GITR as previously reported in mice, resting NK cells expressed the highest level of GITR mRNA. We also show that the surface GITR expression by NK cells is further up-regulated by cytokines, suggesting a cross-talk between pDCs and NK cells through GITR-GITRL. We report here pDCs activated by toll-like receptor (TLR)-9 promote NK cell cytotoxic activity and IFN-γ production through GITRL.
Materials and methods
Isolation of blood pDCs and resting NK cells
pDCs and NK cells were isolated from adult blood buffy coats of healthy volunteers (Gulf Coast Regional Blood Center, Houston, TX). The blood from healthy donors was obtained in accordance with and by approval of the University of Texas, M. D. Anderson Cancer Center institutional review board for these studies. Informed consent was provided according to the Declaration of Helsinki. Briefly, the pDC- and NK-enriched population was obtained from peripheral blood mononuclear cells (PBMCs) by negative immune selection using a mixture of mAbs against lineage markers, CD3 (OKT3), CD14 (M5E2), CD15 (HB78), CD20 (L27), CD69 (FN50) and CD235a (10F7MN), followed by goat anti-mouse immunoglobulin G (IgG)-coated magnetic beads (M-450; Dynal, Oslo, Norway, and Miltenyi Biotec, Bergisch Gladbach, Germany). Each of the CD11c-/lineage-/BDCA-4+/CD4+ and CD56+/lineage- populations was isolated by a fluorescence-activated cell sorter (FACS) Aria (BD Biosciences, Sunnyvale, CA) using allophycocyanin (APC)-labeled anti-CD11c (B-ly6), phycoerythrin (PE)-labeled CD56 (B159), peridinin chlorophyll protein (Per-CP)-labeled BDCA-4 (Miltenyi Biotec), APC-Cy7-labeled CD4 (RPA-T4), and a mixture of fluorscein isothiocyanate (FITC)-labeled mAbs against lineage markers, CD3 (HIT3a), CD14 (MØP9), CD15 (HI98), and CD19 (HIB19) to reach greater than 98% purity. pDCs and NK cells were cultured in RPMI 1640 containing 5% human AB serum.
Transfectants
Human GITRL expressing CD32-L cells were generated by retroviral-mediated transduction. Briefly, full-length human GITRL coding sequence (accession no. NM_005 092; National Center for Biotechnology Information [NCBI]) was amplified by RT-PCR with RNA prepared from HSV-1-stimulated pDCs. The cDNA was subcloned into an murine stem cell virus (MSCV)-based retroviral vector pMIGW2. The resulting plasmid was verified by restriction enzyme digestion and DNA sequencing. To produce recombinant retrovirus, the vector was cotransfected with the packaging constructs pCL-gp (gag/pol) and pHCMV-VSVg (vesicular stomatitis virus [VSV] glycoprotein envelope) in HEK293T cells as described previously.17 Two days later, the virus containing culture supernatants were harvested and used to infect CD32 L cells at a multiplicity of infection (MOI) of 100. Under this condition more than 95% cells were productively transduced. Long-term and stable expression of GITRL in the established CD32 L-cell culture was confirmed by surface staining with an anti-GITRL mAb (R&D Systems, Minneapolis, MN).
Microarray analysis and bioinformatics
Total RNA from DCs was immediately isolated with the RNeasy kit from Qiagen (Valencia, CA), and used to generate cDNA and cRNA to hybridize on Human Genome U133 plus 2.0 array according to the manufacturer's protocol (Affymetrix, Santa Clara, CA). The scanned images were aligned and analyzed using the Affymetrix GeneChip software Microarray Suite 5.0. The signal intensities were normalized to the mean intensity of all the genes represented on the array, and global scaling was applied before comparison analysis. Freshly sorted peripheral blood subsets, before and after culture, were used for microarray analysis. Each population (greater than 99% purity) was isolated by cell-sorting peripheral blood cells as described.18
RT-PCR
To evaluate the expression of GITRL mRNA, RNA was isolated from freshly sorted pDCs, or pDCs activated for 20 hours with IL-3 (10 ng/mL), irradiated-inactivated HSV-1 (10 pfu/cells), influenza A virus (10 pfu/cells), and 5 μM CpG A (2216), CpG B (2006), or CpG C (C274). The sequences of primers were as follows: GITRL: forward, 5′-GCTGTGGCTCTTTTGCTCA-3′, reverse, 5′-ACCCCAGTATGTATTATTT-3′; and β-actin: forward, 5′-CTGGAACGGTGAAGGTGACA-3′, reverse, 5′-AAGGGACTTCCTGTAACAATGCA-3′.
Flow cytometric analysis
Freshly isolated pDCs, or pDCs activated with 10 pfu/cells HSV-1 and 5 μM CpG A, CpG B, or CpG C for 18 hours and 36 hours were stained with anti-GITRL mAb or isotype control mouse IgG1 (R&D Systems), followed by Arexa Fluor 647-conjugated goat anti-mouse IgG Ab (Molecular Probes, Carlsbad, CA). To analyze the expression of GITR on NK cells, purified NK cells were stimulated with IL-2 (200 U/mL; R&D Systems), IL-12 (10 ng/mL; R&D Systems), IL-15 (5 ng/mL; R&D Systems), IFN-α (1000 U/mL; PBL Biomedical Laboratories, Piscataway, NJ), and TNF-α (10 ng/mL; R&D Systems) for 0, 8, 16, 24, and 48 hours, and then activated NK cells were stained with FITC-conjugated mouse anti-GITR mAb (e-Bioscience, San Diego, CA).
Cytotoxic assay
pDCs were cultured with autologous NK cells (DC/NK ratio, 1:4) with or without CpG B in round-bottomed 96-well culture plates for 24 hours. In some experiments, pDCs were precultured with CpG B for 24 hours and washed with culture medium, and then cocultured with NK cells in the presence or absence of IFN-α (2000 U/mL) for 24 hours. In the Ab-neutralizing experiments, anti-GITRL mAb (30 μg/mL; R&D Systems), and/or a cocktail of rabbit polyclonal anti-IFN-α Abs (2000 neutralizing U/mL; PBL Biomedical Laboratories), rabbit polyclonal anti-IFN-β Abs (1000 neutralizing U/mL; PBL Biomedical Laboratories), and mouse anti-IFN-α/β receptor mAb (10 μg/mL, MMHAR-2; PBL Biomedical Laboratories) were added to the NK-DC culture. NK cells recovered from DC coculture were used as effector cells. Target cells were labeled with 3.7 MBq (100 μCi) Na2[51Cr]O4 for 1 hour at 37°C. 51Cr-labeled target cells (0.5-1 × 104 cells/well) and NK cells were mixed in a round-bottomed 96-well culture plate at the indicated effector-target (E/T) ratios. After 6 to 20 hours of incubation, radioactivity of cell-free supernatants was measured in a micro gamma counter. The spontaneous release was less than 20% of the maximum release. The percentage of specific 51Cr release was calculated according to the following formula: % specific lysis = (experimental - spontaneous) release/(maximal - spontaneous) release × 100.
Transwell experiments
Transwell experiments were performed in 24-well plates (Corning, Acton, MA). PDCs were plated in the lower wells and NK cells were added to the upper wells (DC/NK ratio; 1:4) in the presence or absence of anti-GITRL mAb and/or a cocktail of rabbit polyclonal anti-IFN-α Abs, rabbit polyclonal anti-IFN-β Abs, and mouse anti-IFN-α/β receptor mAbs. After culturing for 24 to 36 hours, cytotoxic activity of NK cells and IFN-γ production of culture supernatant were assessed.
Cytokine production
NK cells were cocultured for 24 hours with pDCs as described. In some experiments, freshly isolated NK cells were cultured with irradiated CD32-transfected L cells (L) or GITRL/CD32-transfected L cells (GITRL-L) in the presence or absence of 20 U/mL IL-2, 100 U/mL IFN-α, or 0.1 μg/mL anti-NKG2D mAb (R&D Systems) for 48 hours. The amount of IFN-γ in the culture supernatant was determined with Quantikine enzyme-linked immunosorbent assay (ELISA) kit (R&D Systems).
Immunohistology
For double staining of human CD56 with BDCA-2, the slides were incubated with mouse anti-human CD56 mAb (MEM 188; Southern Biotech, Birmingham, AL) and mouse anti-human BDCA-2 mAb (Miltenyi Biotec) followed by alkaline phosphatase-conjugated goat anti-mouse IgG2a or biotinylated goat anti-mouse IgG1 (Southern Biotech), then the avidin-peroxidase complex reagents (AK-5002; Vector Laboratories, Burlingame, CA) for 30 minutes. The slides were then incubated with the substrates SK-5300 (Vector Laboratories), which stained blue, and then incubated with SK-4200 (Vector Laboratories), which stained red. For immunoflurescence staining, the slides were incubated with biotinylated mouse anti-BDCA-2 mAb (Miltenyi Biotec) at room temperature for 1 hour. After washing, anti-BDCA-2 mAb-dependent tissue deposition of biotin was increased by using a tyramide signal biotin amplification system and visualized by Alexa Fluor 549-conjugated streptavidin (Molecular Probes). The slides were then stained with Alexa Fluor 488-conjugated anti-GITRL mAb (R&D Systems) for 1 hour. For imaging, a laser-scanning confocal device (Fluoview 300, Olympus, Hamburg, Germany) equipped with a 15-mW krypton/argon laser, and attached to an inverted microscope (Olympus 1 × 81) was used. Figures were compiled using Adobe Photoshop (Adobe Systems, San Jose, CA).
Results
GITRL and GITR expression by human blood cell types
In our microarray analyses of a large panel of human immune cells, including pDCs, myeloid DC subsets, B cells, T cells, NK cells, neutrophils, macrophages, and monocytes, we found that activated pDCs preferentially expressed human GITRL (Figure 1A). The expression of GITRL by pDCs activated with IL-3, HSV-1, influenza A virus, A-type CpG 2216 (CpG A), B-type CpG 2006 (CpG B), or C-type CpG C274 (CpG C) was further confirmed by RT-PCR and flow cytometry (Figure 1B-C).
To search for the cell types that express GITR and potentially interact with activated pDCs through GITRL, we analyzed the expression of GITR mRNA in our microarray database of human immune cells. Although CD4+CD45RA-CCR7+ central memory T cells (T4CM), CD4+CD45RA-CCR7- effector memory T cells (T4EM), and neutrophils all express significant levels of GITR mRNA, freshly isolated resting NK cells expressed the highest level of GITR mRNA compared with other immune cell types (Figure 1D). The surface expression of GITR on NK cells was then investigated by flow cytometry analysis using anti-GITR mAb (Figure 1E). A low level of GITR expression was detected on freshly isolated resting NK cells. Cytokines including IL-2, IL-12, and IL-15 strongly up-regulate GITR expression by NK cells in cultures. TNF-α moderately up-regulates GITR expression by NK cells. Although IFN-α strongly activates NK cells, it fails to up-regulate GITR expression on NK cells, as compared with GITR expression by NK cells cultured in medium only. These data suggest that GITRL expressed by activated pDCs may potentially interact with NK cells through GITR during immune responses.
Expression of human GITRL on pDCs and GITR on NK cells. (A,D) Quantification of GITRL (A) and GITR (D) mRNA in human primary cells was performed by human Affymatrix microarray gene expression analyses. Expression of GITRL or GITR in each population was analyzed at the 20-hour time point. (B) Isolated pDCs were stimulated with each stimuli for 20 hours. Following stimulation, total mRNA was prepared for GITRL mRNA evaluation by RT-PCR. (C) Purified pDCs were stimulated with each stimuli for 18 and 36 hours. Filled histograms represent isotype control; open histograms, staining of GITRL on pDCs. (E) Freshly isolated resting NK cells or NK cells stimulated with IL-2, IL-12, IL-15, IFN-α, or TNF-α for the indicated times were stained with anti-GITR mAb. Open histograms represent isotype control staining; filled histograms, GITR expression on NK cells. Numbers on histograms indicate the mean fluorescence intensity (MFI) of GITR expression. All data shown are representative of 4 experiments.
Expression of human GITRL on pDCs and GITR on NK cells. (A,D) Quantification of GITRL (A) and GITR (D) mRNA in human primary cells was performed by human Affymatrix microarray gene expression analyses. Expression of GITRL or GITR in each population was analyzed at the 20-hour time point. (B) Isolated pDCs were stimulated with each stimuli for 20 hours. Following stimulation, total mRNA was prepared for GITRL mRNA evaluation by RT-PCR. (C) Purified pDCs were stimulated with each stimuli for 18 and 36 hours. Filled histograms represent isotype control; open histograms, staining of GITRL on pDCs. (E) Freshly isolated resting NK cells or NK cells stimulated with IL-2, IL-12, IL-15, IFN-α, or TNF-α for the indicated times were stained with anti-GITR mAb. Open histograms represent isotype control staining; filled histograms, GITR expression on NK cells. Numbers on histograms indicate the mean fluorescence intensity (MFI) of GITR expression. All data shown are representative of 4 experiments.
pDCs activated by CpG-ODN promote NK cell cytotoxicity through GITRL
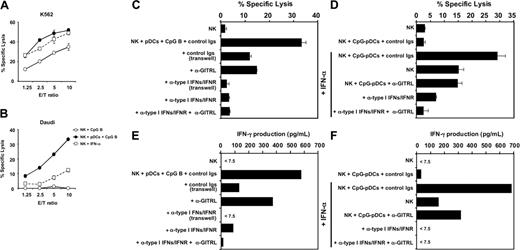
Previous studies demonstrated that pDCs activated by viruses or by TLR-9 ligand CpG-ODN could activate NK cells through type I IFNs. To investigate whether activated pDCs stimulate NK cells through GITRL, we selected CpG 2006 to activate pDCs. This is because CpG 2006 is a B-type CpG-ODN (CpG B), which has the ability to induce GITRL expression, but induces only a small amount of type I IFN production by pDCs. Freshly isolated resting NK cells were cocultured with autologous pDCs at a 1:4 ratio in the presence or absence of CpG B for 24 hours. The cytotoxic activity of NK cells recovered from pDCs cultures was tested against NK target cell line K562, which is sensitive to killing by both resting and activated NK cells, and target cell line Daudi, which is sensitive only to killing by activated NK cells. CpG-pDCs induced a strong cytotoxic activity of NK cells toward both K562 (Figure 2A) and Daudi (Figure 2B) compared with NK cells cultured with CpG B alone (Figure 2A-B) or pDCs without CpG B (data not shown). Interestingly, NK cells cultured with CpG-pDCs are much more potent in killing Daudi targets than NK cells stimulated by IFN-α alone (Figure 2B). These results suggest that CpG-pDCs may have additional mechanisms to augment NK cell cytotoxic activity. We therefore investigated whether surface molecules—in particular, GITRL expressed by CpG-pDCs—contributes to enhance NK cell cytotoxicity. We showed that the ability of CpG-pDCs to promote NK cell cytotoxicity to Daudi targets was partially blocked by separation of CpG-pDCs and NK cells by a transwell chamber system, suggesting that direct cell-to-cell contact through surface molecules was important for CpG-pDCs to promote NK cell cytotoxicity (Figure 2C). Indeed, addition of neutralizing anti-GITRL mAb in the beginning of the coculture of CpG-pDC and NK cells resulted in a partial inhibition of the ability of CpG-pDCs to promote NK cell cytotoxicity to Daudi targets (Figure 2C), indicating that GITRL expressed by CpG-pDCs may contribute partially to the promotion of NK cell cytotoxicity. Interestingly, blocking type I IFNs and type I IFN receptors by a cocktail of antibodies to IFN-α/β plus type I IFN receptors completely inhibited the ability of CpG-pDCs to stimulate NK cell cytotoxicity (Figure 2C). This suggests that the ability of GITRL expressed by CpG-pDCs to promote NK cell cytotoxicity may depend on the presence of type I IFN.
Cytotoxicity and IFN-γ production of NK cells in mixed culture with CpG-pDCs is dependent on type I IFNs and GITR. (A-B) NK cells were cultured with CpG B (○), pDCs plus CpG B (•), or IFN-α (2000 U/mL, □) for 24 hours. Cytotoxic activity of NK cells against K562 or Daudi was evaluated by 6 hours of 51Cr-release assay at different E/T ratios. Data are represented as the mean percentage of specific lysis ± SD and are representative of 5 experiments. (C) NK cells were cultured alone, together, or separately with pDCs plus CpG B in the presence or absence of control Igs, anti-type I IFNs/IFNR (a cocktail of Abs to IFN-α, IFN-β, and IFN-α/β receptor), and/or anti-GITRL in transwell chamber plates (0.4-μm porous membrane). After 36 hours of culture, cytotoxic activity of NK cells against Daudi was evaluated by 6 hours of 51Cr-release assay at an E/T ratio of 10. Data are represented as the mean percentage of specific lysis ± SD and are representative of 5 experiments. (D) NK cells were cultured with pDCs, which were preactivated with CpG B for 36 hours in the presence or absence of isotype Igs, anti-type I IFNs/IFNR, and/or anti-GITRL. Following this culture, the cytotoxic activity of NK cells against Daudi was evaluated by 6 hours of 51Cr-release assay at an E/T ratio of 10. Data are represented as the mean percentage of specific lysis ± SD and are representative of 5 experiments. (E-F) NK cells were cultured for 36 hours together or separately with pDCs plus CpG B in transwell chamber plates in the presence of isotype Igs, anti-type I IFNs/IFNR, and/or anti-GITRL (E) or cultured for 36 hours with pDCs that were preactivated for 36 hours with CpG B, with or without IFN-α (2000 U/mL) in the presence of isotype Igs, anti-type I IFNs/IFNR, and/or anti-GITRL (F). IFN-γ in the supernatant of NK-pDC culture was determined by ELISA. The data shown are representative of 5 experiments.
Cytotoxicity and IFN-γ production of NK cells in mixed culture with CpG-pDCs is dependent on type I IFNs and GITR. (A-B) NK cells were cultured with CpG B (○), pDCs plus CpG B (•), or IFN-α (2000 U/mL, □) for 24 hours. Cytotoxic activity of NK cells against K562 or Daudi was evaluated by 6 hours of 51Cr-release assay at different E/T ratios. Data are represented as the mean percentage of specific lysis ± SD and are representative of 5 experiments. (C) NK cells were cultured alone, together, or separately with pDCs plus CpG B in the presence or absence of control Igs, anti-type I IFNs/IFNR (a cocktail of Abs to IFN-α, IFN-β, and IFN-α/β receptor), and/or anti-GITRL in transwell chamber plates (0.4-μm porous membrane). After 36 hours of culture, cytotoxic activity of NK cells against Daudi was evaluated by 6 hours of 51Cr-release assay at an E/T ratio of 10. Data are represented as the mean percentage of specific lysis ± SD and are representative of 5 experiments. (D) NK cells were cultured with pDCs, which were preactivated with CpG B for 36 hours in the presence or absence of isotype Igs, anti-type I IFNs/IFNR, and/or anti-GITRL. Following this culture, the cytotoxic activity of NK cells against Daudi was evaluated by 6 hours of 51Cr-release assay at an E/T ratio of 10. Data are represented as the mean percentage of specific lysis ± SD and are representative of 5 experiments. (E-F) NK cells were cultured for 36 hours together or separately with pDCs plus CpG B in transwell chamber plates in the presence of isotype Igs, anti-type I IFNs/IFNR, and/or anti-GITRL (E) or cultured for 36 hours with pDCs that were preactivated for 36 hours with CpG B, with or without IFN-α (2000 U/mL) in the presence of isotype Igs, anti-type I IFNs/IFNR, and/or anti-GITRL (F). IFN-γ in the supernatant of NK-pDC culture was determined by ELISA. The data shown are representative of 5 experiments.
To confirm this hyptothesis, pDCs were preactivated with CpG B for 36 hours, washed, and then cultured with NK cells for 24 hours. Because pDCs rapidly produce large amounts of type I IFNs within the first 18 hours following activation and do not produce significant amounts of type I IFNs after that,1 the 36-hour preactivated and washed pDCs expressed high levels of GITRL but produced few type I IFNs. We showed that preactivated CpG-pDCs failed to promote NK cytotoxicity to Daudi targets (Figure 2D). However, addition of exogenous IFN-α allowed preactivated CpG-pDCs to strongly enhance the NK cell cytotoxicity, which is 2 times more potent than the NK cell cytotoxicity induced by IFN-α alone. The additive effect of IFN-α and preactivated CpG-pDCs was blocked by adding neutralizing anti-GITRL mAs. Thus, these results suggest that CpG-pDCs have 2 mechanisms to stimulate NK cell cytotoxicity: (1) the type I IFN-dependent pathway as previously reported5,6 ; and (2) the GITRL-dependent pathway as demonstrated here. Importantly, the ability of GITRL expressed by CpG-pDCs to stimulate NK cell cytotoxicity depends on the presence of type I IFNs.
pDCs activated by CpG-ODN promote IFN-γ production of NK cells through GITRL
During innate immune responses to virus, NK cells rapidly produce IFN-γ, which contributes to the activation of both innate and adaptive immune responses. Thus, in parallel to the experiments described that analyze NK cell cytotoxicity, the production of IFN-γ by NK cells in the supernatant from the same cultures was measured by ELISA. As shown in Figure 2E, CpG-pDCs induced NK cells to produce large amounts of IFN-γ. Transwell membrane or anti-GITRL partially inhibited the ability of CpG-pDCs to induce NK cells to produce IFN-γ. Blocking the function of type I IFNs by a cocktail of antibodies to type I IFNs and type I IFN receptors completely blocked the ability of CpG-pDCs to induce IFNγ production by NK cells (Figure 2E). Preactivated CpG-pDCs failed to induce NK cells to produce IFN-γ (Figure 2F). However, exogenous IFN-α and preactivated CpG-pDCs had an additive effect in stimulating NK cells to IFN-γ (Figure 2F). Blocking anti-GITRL partially inhibited this additive effect of IFN-α and preactivated CpG-pDCs. These data suggest that GITRL expressed by CpG-pDCs represent a critical molecule that costimulates NK cell cytotoxic function and IFN-γ production in the presence of type I IFNs.
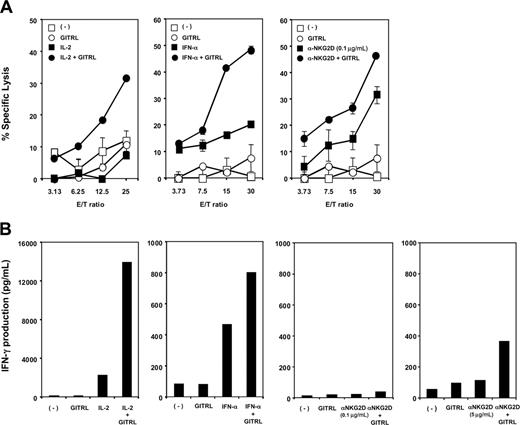
Engagement of GITR on NK cells augments IL-2-, IFN-α-, and NKG2D-dependent NK cytotoxicity and IFN-γ production. (A) Cytotoxic activity of NK cells against L cells (squares) or GITRL-L cells (circles) were evaluated at various E/T ratios in the presence (closed symbols) or absence (open symbols) of suboptimal doses of IL-2 (20 U/mL), IFN-α (100 U/mL), and anti-NKG2D (0.1 μg/mL). Data are represented as the mean percentage of specific lysis ± SD and are representative of 3 experiments. (B) NK cells were cultured with irradiated L cells or GITRL-L cells in the presence or absence of suboptimal doses of IL-2 (20 U/mL), IFN-α (100 U/mL), and anti-NKG2D (0.1 μg/mL or 5 μg/mL) for 48 hours. IFN-γ in the supernatant of cultures was determined by ELISA. The data shown are representative of 5 experiments.
Engagement of GITR on NK cells augments IL-2-, IFN-α-, and NKG2D-dependent NK cytotoxicity and IFN-γ production. (A) Cytotoxic activity of NK cells against L cells (squares) or GITRL-L cells (circles) were evaluated at various E/T ratios in the presence (closed symbols) or absence (open symbols) of suboptimal doses of IL-2 (20 U/mL), IFN-α (100 U/mL), and anti-NKG2D (0.1 μg/mL). Data are represented as the mean percentage of specific lysis ± SD and are representative of 3 experiments. (B) NK cells were cultured with irradiated L cells or GITRL-L cells in the presence or absence of suboptimal doses of IL-2 (20 U/mL), IFN-α (100 U/mL), and anti-NKG2D (0.1 μg/mL or 5 μg/mL) for 48 hours. IFN-γ in the supernatant of cultures was determined by ELISA. The data shown are representative of 5 experiments.
Recombinant GITRL costimulates NK cytotoxicity and IFN-γ production
To further confirm the function of GITRL in promoting NK cell cytotoxicity and IFN-γ production, we generated a stable CD32-L cell line expressing human GITRL, which were directly used as NK target cells. NK cells were cultured with 51Cr-labeled CD32-L (L) cells or GITRL transduced CD32-L (GITRL-L) cells in the absence or presence of suboptimal dose of IL-2, IFN-α, or anti-NKG2D mAb, and the cytotoxic activity of the cultured NK cells was analyzed by the 51Cr release assay. In parallel, NK cells were cultured with L cells or GITRL-L cells in the absence or presence of suboptimal doses of IL-2, IFN-α, or anti-NKG2D mAb for 48 hours, and IFN-γ production by NK cells was measured by ELISA analyses of the culture supernatants. Figure 3A and B shows that GITRL alone was insufficient to induce NK cells to kill target cells (Figure 3A) or to produce significant amounts of IFN-γ (Figure 3B). However, in the presence of the suboptimal dose of IL-2 or IFN-α, factors known to activate NK cells, GITRL strongly promoted NK-cell cytotoxic activity and IFN-γ production. GITRL promoted NK-cell cytotoxic activity but not IFN-γ production in the presence of low concentration of anti-NKG2D mAb (0.1 μg/mL). However, GITRL could moderately promote NK-cell IFN-γ production in the presence of higher concentrations of anti-NKG2D mAb (5 μg/mL). These results suggest that GITRL expressed by activated pDCs may represent an important molecule for costimulating NK-cell function.
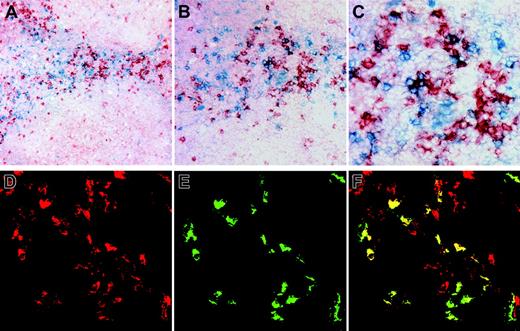
NK cells and pDCs colocalize in T-cell areas of normal human tonsils. (A-C) Double-staining of BDCA-2 (red) and CD56 (blue), showing CD56+ NK cells located in close association with BDCA-2+ pDCs in T-cell areas of human inflamed tonsils. Original magnifications, × 10 (A), × 20 (B), and × 40 (C). (D-F) Double-staining of BDCA-2 (red) and GITRL (green), showing a subpopulation of BDCA-2+ pDCs expressing GITRL. Original magnification, × 60 objective.
NK cells and pDCs colocalize in T-cell areas of normal human tonsils. (A-C) Double-staining of BDCA-2 (red) and CD56 (blue), showing CD56+ NK cells located in close association with BDCA-2+ pDCs in T-cell areas of human inflamed tonsils. Original magnifications, × 10 (A), × 20 (B), and × 40 (C). (D-F) Double-staining of BDCA-2 (red) and GITRL (green), showing a subpopulation of BDCA-2+ pDCs expressing GITRL. Original magnification, × 60 objective.
NK cells colocalize with pDCs in T-cell areas of human tonsil
To provide the anatomical evidence that pDCs may interact with NK cells in vivo, we investigated the localization of NK cells and pDCs in human inflamed tonsil by microscope analysis using specific antibodies for NK cells (CD56) and pDCs (BDCA-2). Both CD56+ NK cells and BDCA-2+ pDCs were found in T-cell areas and around the high endothelial venule (HEV) of the tonsil, and the pDCs were located in close contact to CD56+ NK cells (Figure 4A-C). CD56+CD3+ double-positive cells were rarely observed in sequential cryosections of human tonsils (data not shown). We also found that a subpopulation of pDCs expressed GITRL in human tonsils (Figure 4D-F). Thus, these results suggest that a cross-talk between pDCs and NK cells may occur in vivo, and GITRL expressed on pDCs may play a critical role in regulating the function of NK cells through direct interaction in human lymphoid tissue.
Discussion
Recent studies have shown that cross-talk between DCs and NK cells plays a critical role in regulating innate and adaptive immunity.19-22 In particular, it has been shown that pDCs play an important function in triggering NK cell activation through type I IFNs at an early stage of viral infection.1,5,20 The function of NK cells is also regulated by the integrating signals derived from various activating and inhibitory receptors as well as signals derived from the costimulatory molecules within the B7/CD28 family and TNF/TNFR family.23,24
GITR is a member of the TNFR superfamily originally cloned from dexametazone-treated mouse T cells.13,14 The murine GITR was identified on CD25+CD4+ T cells, CD4+ T cells, and CD8+ T cells, and was found to be critical for T-cell costimulation.13,14 It has also been recently reported that GITRL is constitutively expressed on freshly isolated B cells, macrophages, DCs, and endotherial cells in mice.13,14,25,26 However, the expression and function of human GITR and GITRL are largely unknown. By microarray gene expression analyses of more than 13 different cell types within the immune system at either resting or activated states in the human immune system, we found that GITRL is preferentially expressed by pDCs activated with viruses, but not by monocytes and myeloid DCs different from in those in the mouse system. We found that GITR is not only expressed by activated T cells, but is also expressed by resting NK cells, and its expression can be further up-regulated following NK cell activation by cytokines. In this study, we demonstrate that GITRL expressed on activated pDCs have the capacity to costimulate the cytotoxic activity and IFN-γ production of NK cells. This reveals a novel function of GITR/GITRL in the interaction between pDCs and NK cells.
The finding that the recombinant GITRL can promote NK cell cytotoxicity and IFN-γ production only in the presence of IL-2, type I IFNs, or anti-NKG2D mAb suggests that GITRL acts as a costimulatory molecule for NK cell activation. pDCs were found to express NKG2D ligand and MICA and MICB mRNA after virus activation (S.H., W.C., Y.-J.L., unpublished observation, January 2004). Therefore, GITRL and NKG2D receptor ligands expressed by activated pDCs may work synergistically to promote NK cell activation.
It has been shown that pDCs have the ability to produce a short burst of huge amounts of type I IFNs within the first 18 hours after activation.1 However, pDCs continue to express GITRL up to 36 hours after activation. These findings suggest that during viral infection, pDCs may first activate NK cells through type I IFNs, and then use GITRL to further potentiate and maintain NK cell activation. Martin-Fontecha et al have recently shown that NK cells migrate into the draining lymph nodes and provide an early source of IFN-γ that is necessary for T-helper 1 (Th1) polarization during immune responses.27 Because pDCs and lymph node resident NK cells are found to express the same homing molecules, including L-selectin, CXCR3, and CCR7,28,29 and we actually found that they both appeared to colocalize in the T-cell areas around the HEV (Figure 4), it is highly likely that pDCs may directly activate NK cells to produce IFN-γ through GITR-GITRL interaction in the T-cell-rich areas of human lymphoid tissues during antiviral immune responses.
In conclusion, we discovered a novel molecular mechanism by which human pDCs may costimulate NK cells through GITRL/GITR during viral infection.
Prepublished online as Blood First Edition Paper, January 5, 2006; DOI 10.1182/blood-2005-08-3419.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
We thank Karen Ramirez, Zhiwei He, and Eric Wieder for cell sorting and support.


This feature is available to Subscribers Only
Sign In or Create an Account Close Modal