Heparanase is an endoglycosidase that cleaves heparan sulfate (HS), the main polysaccharide of the basement membrane (BM). HS is responsible for BM integrity and barrier function. Hence, enzymatic degradation of HS in the vascular subendothelial BM is a prerequisite for extravasation of immune cells and plasma components during inflammation. Here, we demonstrate a highly coordinated local heparanase induction upon elicitation of delayed-type hypersensitivity (DTH) reaction in the mouse ear. By monitoring in vivo activation of luciferase gene driven by the heparanase promoter, we demonstrate activation of heparanase transcription at an early stage of DTH. We report that heparanase is produced locally by the endothelium at the site of DTH-associated inflammation. Key DTH mediators, tumor necrosis factor-α and interferon-γ, were found to induce heparanase in cultured endothelial cells. Endothelium emerges as an essential cellular source of heparanase enzymatic activity that, in turn, allows for remodeling of the vascular BM, increased vessel permeability, and extravasation of leukocytes and plasma proteins. In vivo administration of antiheparanase siRNA or an inhibitor of heparanase enzymatic activity effectively halted DTH inflammatory response. Collectively, our results highlight the decisive role of endothelial heparanase in DTH inflammation and its potential as a promising target for anti-inflammatory drug development.
Introduction
Delayed-type hypersensitivity (DTH) is an important in vivo manifestation of cell-mediated immune responses.1-3 The development of DTH involves recruitment and activation of antigen-specific T cells, synthesis of a cascade of chemotactic and activating cytokines, recruitment of antigen-nonspecific effector cells, fibrin deposition, and increased vascular permeability. This is followed, similar to other types of inflammatory responses, by translocation of leukocytes, including monocytes, neutrophils, and T lymphocytes, from the vascular system, through extracellular tissue barriers, into the site of inflammation.2-4 Subendothelial basement membrane (BM) represents the major physical obstacle for leukocyte extravasation and entry into inflammatory sites. BM is a specialized type of the extracellular matrix (ECM), underlying endothelial and epithelial cell layers in all tissues and organs. In the blood vessel wall, BM functions as a scaffold for cellular architecture and integrity of the endothelium. Enzymatic remodeling of the BM barrier is a prerequisite for leukocyte extravasation during inflammation. In addition, BM remodeling allows for the extravasation of plasma macromolecules.3
The BM is organized as a structural lattice of characteristic protein and polysaccharide constituents. Heparan sulfate glycosaminoglycan represents the principal polysaccharide participating in BM formation.5-7 Heparan sulfate is composed of repeating disaccharide units that form linear chains covalently bound to a core protein.8,9 These chains interact through specific attachment sites with the main protein components of BMs, such as collagen IV, laminin, and fibronectin. Such interactions make heparan sulfate an essential molecule responsible for the BM barrier function.10 The mammalian endoglycosidase heparanase is the predominant enzyme that degrades heparan sulfate.11-14 Enzymatic cleavage of heparan sulfate results in disassembly of extracellular barriers for cell movement and thus allows for migratory behavior of different cell types in a variety of pathophysiologic conditions, such as morphogenesis, angiogenesis, and cancer metastasis.11,15-20 Possible involvement of heparanase in inflammation also has been addressed,4,16,21 emphasizing the contribution of heparanase residing in activated cells of the immune system.16,21-24 The exact role of heparanase in the inflammatory process remains unclear. Prior to cloning of the heparanase gene, it has been shown that inhibition of T-lymphocyte–derived heparanase by species of heparin inhibits T-cell migration and T-cell–mediated immunity.21,22,25 The causative involvement of heparanase in this system was questionable, however, because of the multiple biological activities of heparin.26,27 At the same time, it was reported that degradation products, reportedly released by heparanase from the ECM, inhibit DTH reactivity in mice.28
Our research was undertaken to elucidate the source and biological significance of heparanase in inflammation. We applied DTH inflammatory model, as well as recently developed in vivo systems for heparanase overexpression,29 gene silencing,30 and monitoring heparanase promoter activation.31,32 Our results reveal that induction of heparanase gene expression in the vascular endothelium is an important parameter of the inflammatory response. Timely action of endothelial heparanase in the course of inflammation emerges as an essential step, allowing for remodeling of the vascular BM, increased vessel permeability, and extravasation of leukocytes and plasma proteins. A marked decrease in DTH was obtained upon local delivery of antiheparanase siRNA. To our knowledge, this study represents the first successful application of anti-inflammatory therapy based on electroporation-assisted heparanase siRNA delivery in vivo. Given the critical role of heparanase in inflammation and other mechanistically related pathologic processes (ie, tumor progression, angiogenesis),11,30,33 the anti-heparanase siRNA delivery approach, developed in our study, might be highly relevant to the design of future therapeutic interventions in these conditions.
Materials and methods
Cell culture
Human vascular endothelial EA.Hy926 cells34,35 were maintained in Dulbecco modified Eagle medium (DMEM) supplemented with 10% fetal calf serum (FCS) and antibiotics at 37°C and 8.5% CO2. Interferon-γ (IFN-γ) and tumor necrosis factor α (TNFα) were obtained from Sigma (St Louis, MO) and dissolved in water. Prior to treatment with cytokines, cells were maintained for 8 hours in serum-free medium. Then, IFN-γ or TNFα were added for additional 16 hours. Control cultures were treated with the vehicle alone.
RNA isolation and semiquantitative RT-PCR analysis
RNA was isolated with TRIzol (Life Technologies, Grand Island, NY) and quantitated by ultraviolet absorption. Oligo (dT)–primed reverse transcription was performed using 1 μg total RNA in a final volume of 20 μL, and the resulting cDNA was further diluted to 100 μL. Comparative semiquantitative polymerase chain reaction (PCR) was performed as follows: L19 cDNA was first amplified at low cycle number (human L19 primer sequences: L-19-U [5′-ATGCCAACTCTCGTCAACAG-3′] and L-19-L [5′-GCGCTTTCGTGCTTCCTT-3′]). The resulting PCR products were visualized by electrophoresis and ethidium bromide staining, and the intensity of each band was quantified using Scion Image software (Scion, Frederick, MD). If needed, cDNA dilutions were adjusted, and L19 RT-PCR products were re-amplified in order to obtain similar intensities for L19 signals with all the samples. The adjusted amounts of cDNA were used for PCR with primers HPU-355 (TTCGATCCCAAGAAGGAATCAAC) and HPL-229 (GTAGTGATGCCATGTAACTGAATC), designed to amplify a 564-bp PCR product specific for human heparanase.11 Only RNA samples that gave completely negative results in PCR without reverse transcriptase were further analyzed, to rule out the presence of genomic DNA contamination. Results are expressed as band intensity relative to that of L19 and represent the mean plus or minus standard deviation (SD, indicated by error bars) of 3 independent experiments.
Experimental animals
Female BALB/c mice were purchased from Harlan Laboratories (Jerusalem, Israel). Heparanase-overexpressing transgenic (hpa-tg) mice in a C57BL/6J genetic background29 were bred at the animal facility of the Hadassah-Hebrew University Medical Center. Background genes were tested using C57BL/6J genetic markers. The heparanase transgene was introduced to hpa-tg mice under the actin promoter, to drive overexpression of the heparanase gene in most tissues.29
Delayed-type hypersensitivity (DTH) assay
DTH reactions were induced in the ear skin of 5- to 6-week-old female BALB/c mice or in hpa-transgenic (hpa-tg) mice and their wild-type counterparts. Mice were sensitized on the shaved abdominal skin with 100 μL of 2% oxazolone dissolved in acetone/olive oil [4:1 (vol/vol)] applied topically.28 DTH sensitivity was elicited 5 days later by challenging the mice with 20 μL of 0.5% oxazolone in acetone/olive oil, 10 μL administered topically to each side of the ear. Thickness of a constant area (1 cm2) of the ear was measured with Mitutoyo engineer's micrometer, immediately before challenging, 24 hours after challenge, and every other day thereafter for 5 days. Five mice were used per each experimental condition and time point, and statistical analysis was performed using the unpaired Student t test. The increase in ear thickness over baseline levels (thickness of the ears treated with vehicle alone) was used as a parameter for the extent of inflammation. All experiments were repeated at least twice with similar results.
Immunohistochemistry
Immunohistochemical staining was performed as described11,32 with minor modifications. Briefly, 5 μm ear tissue sections were incubated in 3% H2O2, denatured by boiling (3 minute) in a microwave oven in citrate buffer (0.01 M, pH 6.0), and blocked with 10% goat serum in phosphate-buffered saline (PBS). Sections were incubated with specific polyclonal rabbit antiheparanase antibody (Ab-p3) that was raised against a peptide (R273KTAKMLKSFLKAGGEVI290) corresponding to an internal region of the heparanase 50-kDa subunit36 and was kindly provided by Dr Robert L. Heinrikson (Pfizer, Kalamazoo, MI). This antibody is cross-reactive with human and mouse heparanase.32,36 We have also used polyclonal rabbit antiheparanase antibody (733) directed against a synthetic peptide (158KKFKNSTYRSSSVD171) corresponding to the N-terminus of the 50-kDa subunit of the heparanase enzyme.37 Similar immunostaining pattern was obtained with the 2 antibodies. Color was developed by using the Zymed AEC substrate kit (Zymed Laboratories, San Francisco, CA) for 10 minutes, followed by counterstaining with Mayer hematoxylin. Controls without addition of primary antibody showed low or no background staining in all cases. Slides were visualized with a Zeiss Axioskop 50 microscope (Carl Zeiss, Oberkochen, Germany).
Plasmid constructs and in vivo electroporation
The 1.9-kb human heparanase promoter region (HPSE [–1791/+109]-LUC) was subcloned upstream of the luciferase (LUC) gene in a pGL2 basic reporter plasmid (Promega, Madison, WI), as described.31 Mut-HPSE-LUC construct, encoding for the LUC gene under a mutated heparanase promoter sequence, in which deletion was inserted between base pairs –1791/–337, was used as a control. The plasmid containing the LUC gene driven by a cytomegalovirus (CMV) enhancer/promoter (CMV-LUC) was kindly provided by Dr A. Oppenheim (Hadassah Medical Center, Jerusalem, Israel). Antiheparanase siRNA expression vectors were generated as described.30 The empty pSUPER vector was used as a control.
For in vivo electroporation, mice were anesthetized and plasmid DNA was intradermally injected with a 0.3-mL syringe and 30-gauge needle into the mouse ear (20 μg per site in 25 μL PBS). To keep variability to a minimum, the same skilled operator performed all injections. A 30-second time interval lapsed between injection and initiation of electroporation. The in vivo electroporation system (Genetronics, San Diego, CA) consisted of a square wave pulse generator (ECM 830) and a caliper electrode, applied topically. The caliper electrode (modes 384; BTX/Harvard Apparatus, Holliston, MA) consists of two 1-cm2 brass plate electrodes. The electroporation was performed by squeezing the ear between the 2 plates and applying 6 pulses of 75 V with a pulse length of 20 msec and interval of 1 second, and polarity reversal after 3 pulses.
Administration of heparanase enzymatic inhibitor ST1514 in vivo
Heparanase inhibitor ST1514 (52% glycol split nonanticoagulant heparin, H,52 gs, MW = 11 200)38-40 was kindly provided by Drs Claudio Pisano and Sergio Penco (Sigma-Tau, Pomezia, Rome, Italy). ST1514, or vehicle alone (PBS), were administered intraperitoneally (50 μg/mouse, n = 4 mice/ group X 2 ears/mice) 1 minute prior to challenge with oxazolone and every hour during the following 8 hours of the experiment.
Increased DTH reactivity in heparanase overexpressing transgenic mice. DTH reactions were elicited in the left ear skin of hpa-tg mice and their wild-type counterparts using oxazolone. Right ears of the same animals were treated with vehicle alone. Swelling of the challenged ears is expressed in mm as the increase over the baseline thickness measured in ears treated with vehicle alone. Challenged ears in hpa-tg mice (▵) showed a 3.5-fold increase in swelling over the baseline (▪), as compared to only 2-fold increase in wild-type mice (○), 24 hours after challenge with oxazolone. The differences between the 2 groups remained statistically significant for 3 days (n = 5 per experimental condition and time point). Data are expressed as mean ± SD. The experiment was repeated twice with similar results.
Increased DTH reactivity in heparanase overexpressing transgenic mice. DTH reactions were elicited in the left ear skin of hpa-tg mice and their wild-type counterparts using oxazolone. Right ears of the same animals were treated with vehicle alone. Swelling of the challenged ears is expressed in mm as the increase over the baseline thickness measured in ears treated with vehicle alone. Challenged ears in hpa-tg mice (▵) showed a 3.5-fold increase in swelling over the baseline (▪), as compared to only 2-fold increase in wild-type mice (○), 24 hours after challenge with oxazolone. The differences between the 2 groups remained statistically significant for 3 days (n = 5 per experimental condition and time point). Data are expressed as mean ± SD. The experiment was repeated twice with similar results.
Permeability assay
DTH challenged (n = 5) and untreated (n = 8) mice received intravenous injections of 100 μL Evans blue dye (30 mg/kg in 100 μL PBS σ) at 16 hours after oxazolone challenge. The intensity of vascular permeability was analyzed macroscopically.
Heparanase activity
Equal protein aliquots of cell lysates from 1 × 106 cells were incubated (3 hours, 37°C, pH 6.6) in dishes coated with 35S-labeled ECM, prepared as described.11,41 Sulfate-labeled material released into the incubation medium was analyzed by gel filtration on a sepharose 6B column.41 Nearly intact heparan sulfate proteoglycans are eluted just after the void volume (peak I, Kav < 0.2, fractions 1-10). Heparan sulfate degradation fragments produced by heparanase are eluted later with 0.5 < Kav < 0.8 (peak II, fractions 15-35).41 Each experiment was performed at least 3 times, and the variation in elution positions (Kav values) did not exceed 15% of the mean. Reaction buffer with or without recombinant human heparanase (1 ng/mL) was routinely used as a positive or negative control, respectively.
Luciferase assay
Mice ears were removed just before or 48 hours after the DTH challenge with oxazolone. The ears were snap frozen in liquid nitrogen and pulverized to a fine powder with a liquid nitrogen–cooled pestle. The powder was resuspended in 100 μL ice-cold reporter lysis buffer (Promega, Madison, WI), frozen and thawed 3 times, and centrifuged for 20 minutes at 4°C at 20 000g. Supernatant was transferred to a new tube, protein content was determined, and 25-μL samples were assayed for LUC activity using the luciferase reporter assay system (Promega). LUC activity was calculated as light units/unit protein, which yields values similar to those based on internal beta-galactosidase transfection standards.42 Data are presented as the means of at least 3 determinations, and all experiments were repeated at least twice with similar results.
Results
Association between DTH reactivity and heparanase levels
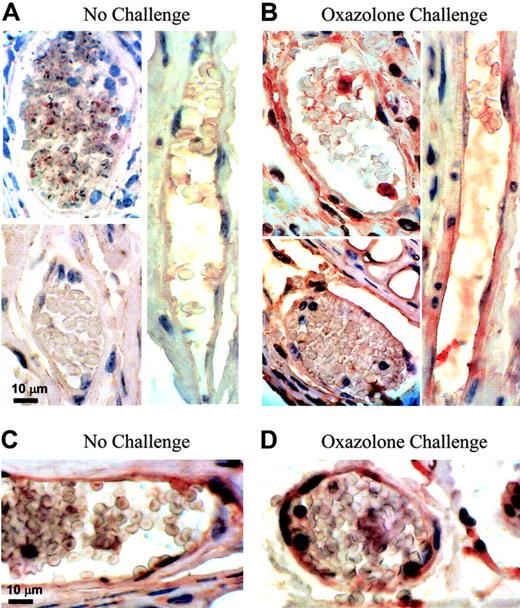
We first studied the DTH reactivity in homozygous transgenic (hpa-tg) mice overexpressing human heparanase in all tissues.29 Hpa-tg mice and their wild-type counterparts were sensitized with the hapten oxazolone, as described in “Materials and methods,” and the DTH reaction was elicited 5 days later by applying oxazolone onto the ears. Twenty-four hours after the oxazolone challenge, a markedly enhanced inflammatory response and edema formation were detected in hpa-tg mice in comparison with wild-type mice, as reflected by a 3.5-fold increase in ear thickness in the hpa-tg mice versus a 2-fold increase in wild-type mice (Figure 1). The differences in the extent of edema formation between the 2 groups of mice remained statistically significant for 3 days after challenge (Figure 1). These results prompted us to determine the levels of endogenous heparanase during DTH induction in wild-type mouse ears. As shown in Figure 2B, high levels of the heparanase protein were detected by immunostaining in the ears in which inflammation has been elicited by oxazolone, as compared with low levels or absence of heparanase in control, unchallenged ears (Figure 2A). Notably, a greater part of tissue elements expressing elevated levels of heparanase in the dermis of DTH-affected ears was represented by capillary vascular endothelium (Figure 2B). In the ears of hpa-tg mice, both prior to and after challenge, immunostaining revealed elevated heparanase protein content in epithelial keratinocytes (not shown) and vascular endothelial cells (Figure 2C,D). The intensity of heparanase staining in both unchallenged and challenged hpa-tg ears was similar and comparable to that observed in wild-type animals following the oxazolone challenge (Figure 2B).
Heparanase expression in vivo upon DTH induction. (A, B) Endogenous heparanase: 5 days after sensitization, left ear (B) of female BALB/c mice (n = 4) was treated with oxazolone and the right ear (A) with vehicle alone. Ear tissues were harvested 24 hours after challenge and processed for immunohistochemical analysis of heparanase expression (reddish staining). Vascular structures were recognized as luminal or slit-like structures that occasionally contained blood cells and were delineated by flattened endothelial cells. This experiment was repeated 3 times, and a similar immunostaining pattern was obtained with 2 different antiheparanase antibodies. Representative microphotographs are shown. (A) Nonchallenged ear: capillary endothelial cells in the ear skin dermis are negative for heparanase staining (magnification × 1000). (B) Oxazolone-challenged ear: heparanase-expressing capillary endothelial cells are easily detected (× 1000). Control sections stained using secondary antibody alone showed no staining. (C, D) When DTH reaction was elicited in hpa-tg mice, positive staining was detected in capillary endothelium both prior to (C) and after (D) the challenge. Images were captured with a Zeiss Axioskop 50 microscope (Zeiss, Oberkochen, Germany) equipped with 100 ×/1.30 oil objective or 20 ×/0.50 objective lenses. Images were captured with a Kodak DC290 digital camera (Kodak, Rochester, NY).
Heparanase expression in vivo upon DTH induction. (A, B) Endogenous heparanase: 5 days after sensitization, left ear (B) of female BALB/c mice (n = 4) was treated with oxazolone and the right ear (A) with vehicle alone. Ear tissues were harvested 24 hours after challenge and processed for immunohistochemical analysis of heparanase expression (reddish staining). Vascular structures were recognized as luminal or slit-like structures that occasionally contained blood cells and were delineated by flattened endothelial cells. This experiment was repeated 3 times, and a similar immunostaining pattern was obtained with 2 different antiheparanase antibodies. Representative microphotographs are shown. (A) Nonchallenged ear: capillary endothelial cells in the ear skin dermis are negative for heparanase staining (magnification × 1000). (B) Oxazolone-challenged ear: heparanase-expressing capillary endothelial cells are easily detected (× 1000). Control sections stained using secondary antibody alone showed no staining. (C, D) When DTH reaction was elicited in hpa-tg mice, positive staining was detected in capillary endothelium both prior to (C) and after (D) the challenge. Images were captured with a Zeiss Axioskop 50 microscope (Zeiss, Oberkochen, Germany) equipped with 100 ×/1.30 oil objective or 20 ×/0.50 objective lenses. Images were captured with a Kodak DC290 digital camera (Kodak, Rochester, NY).
Induction of heparanase expression in endothelial cells in vitro by DTH mediator cytokines
Tumor necrosis factor α (TNFα) and interferon γ (IFN-γ) are regarded as key mediators of the DTH reaction.3,43,44 Stimulatory effect of TNFα on heparanase expression and secretion by the vascular endothelium recently has been reported.45 We investigated the effect of IFN-γ on endothelial heparanase expression. For this purpose, we used one of the best characterized vascular endothelial cell lines, EA.hy926.34,35 EA.hy926 cells were treated or untreated with IFN-γ for 24 hours and then tested for heparanase mRNA expression. Semiquantitative reverse transcriptase–polymerase chain reaction (RT-PCR) revealed that IFN-γ treatment yielded a 3-fold increase in heparanase mRNA content, as compared to untreated cells (Figure 3A). Treatment with TNFα yielded a 2-fold increase in heparanase expression by EA.hy926 cells (not shown), in agreement with the previously reported ability of TNFα to augment heparanase expression in other types of endothelial cells.45 We next determined the levels of heparanase enzymatic activity in EA.hy926 endothelial cells, untreated or treated with IFN-γ, to verify the RT-PCR observations. Heparanase activity was tested by incubation (3 hours, 37°C) of cell lysate samples with a metabolically sulfate-labeled ECM. Sulfate-labeled degradation products released into the incubation medium were subjected to gel filtration on sepharose 6B columns.11,41 The substrate alone consisted almost entirely of nearly intact, high-molecular-weight material eluted just after the void volume (peak I, fractions 1-10, Kav < 0.2). This material (peak I) has been previously shown to be generated by a proteolytic activity residing in the ECM itself and/or expressed by the cells.16 The elution pattern of labeled material released during incubation of lysed untreated cells with sulfate-labeled ECM showed little or no heparanase enzymatic activity (Figure 3B). In contrast, a high heparanase activity was detected in lysates of IFN-γ–treated cells, as indicated by a 3- to 4-fold increase in release from ECM of low-molecular-weight sulfate-labeled fragments (peak II, fractions 20-35, 0.5 < Kav < 0.8)11,41 (Figure 3B). These fragments were shown to be degradation products of heparan sulfate, as they were 5- to 6-fold smaller than intact heparan sulfate side chains, resistant to further digestion with papain and chondroitinase ABC, and susceptible to deamination by nitrous acid.41
Effects of IFN-γ on heparanase expression in endothelial cells. (A) Semiquantitative RT-PCR. EA.hy926 cells were incubated (16 hours) in triplicate in the absence or presence of 80 ng/mL IFN-γ. RNA was then isolated from the cells, and comparative semiquantitative PCR was performed as described in “Materials and methods.” Aliquots (10 μL) of the PCR products were separated by 1.5% agarose gel electrophoresis and visualized (top). The intensity of each band was quantitated using Scion Image software, and the results are expressed as band intensity relative to that of the housekeeping gene L19. The bars represent the mean ± SD (error bars) of 3 independent experiments (bottom). (B) Heparanase activity. EA.hy926 cells were incubated (16 hours) in the absence (□), or presence (♦) of 80 ng/mL IFN-γ. Cell lysates were normalized for equal protein and incubated (3 hours, pH 6.0, 37°C) with sulfate-labeled ECM. Labeled degradation fragments released into the incubation medium were analyzed by gel filtration on sepharose 6B.
Effects of IFN-γ on heparanase expression in endothelial cells. (A) Semiquantitative RT-PCR. EA.hy926 cells were incubated (16 hours) in triplicate in the absence or presence of 80 ng/mL IFN-γ. RNA was then isolated from the cells, and comparative semiquantitative PCR was performed as described in “Materials and methods.” Aliquots (10 μL) of the PCR products were separated by 1.5% agarose gel electrophoresis and visualized (top). The intensity of each band was quantitated using Scion Image software, and the results are expressed as band intensity relative to that of the housekeeping gene L19. The bars represent the mean ± SD (error bars) of 3 independent experiments (bottom). (B) Heparanase activity. EA.hy926 cells were incubated (16 hours) in the absence (□), or presence (♦) of 80 ng/mL IFN-γ. Cell lysates were normalized for equal protein and incubated (3 hours, pH 6.0, 37°C) with sulfate-labeled ECM. Labeled degradation fragments released into the incubation medium were analyzed by gel filtration on sepharose 6B.
Heparanase promoter activation during DTH inflammation
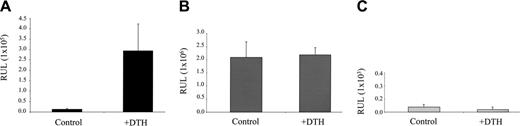
In order to test whether heparanase induction during inflammation occurs due to a transcriptional activation of the heparanase gene, we next applied the in vivo electroporation technique, based on injection of the expression vector into the ear, followed by application of an electric field32,46 to deliver the LUC reporter gene driven by the heparanase promoter (Hpse-LUC)31 into the ear, prior to DTH elicitation. Four days following sensitization with oxazolone, the ears of BALB/c mice in the experimental group were electroporated with the HPSE-LUC construct. Ears of mice in the control group were electroporated with construct containing the LUC gene under a constitutive CMV promoter (CMV-LUC).32 In an additional control group, ears of mice were electroporated with a construct containing the LUC gene driven by a mutated heparanase promoter, bearing a deletion between base pairs –1791 and –337 (Mut-HPSE-LUC). Twenty-four hours later, left ears of the mice in experimental and both control groups were challenged with oxazolone, while the right ears were left untreated. Forty-eight hours after challenge, when a strong DTH-associated swelling was readily detected in all ears challenged with oxazolone, but not in nonchallenged ears (not shown), the mice were killed and the ears removed, snap frozen, and lysed. Samples were normalized for total protein content and luciferase activity was measured as described in “Materials and methods.” As shown in Figure 4A, DTH induction in left ears provoked a marked activation of the heparanase promoter, yielding a 23-fold increase (P < .003) in LUC activity measured in left (DTH) versus right (control) ears of mice from the experimental group. In contrast, in the ears of mice electroporated with a CMV-LUC construct, DTH induction did not result in any statistically significant change in LUC activity (Figure 4B), while no LUC activity was detected in either left (DTH) or right (control) ears of mice electroporated with the Mut-HPSE-LUC construct (Figure 4C), verifying that the difference observed in the experimental group was heparanase promoter–specific and not due to variation in transfection efficiency. These data indicate that the increase in heparanase levels in DTH inflammation occurs through specific activation of the heparanase gene promoter.
Local silencing of heparanase profoundly decreases inflammatory response in vivo
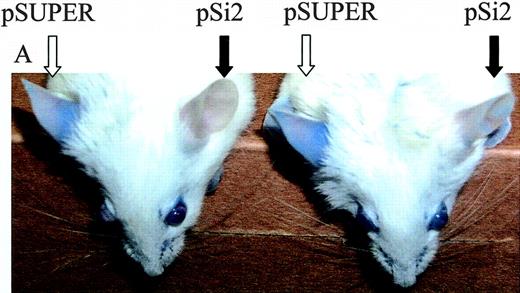
To explore the effect of local heparanase silencing on DTH reactivity, we delivered anti–mouse heparanase siRNA (pSi2) expression vector30 to BALB/c mouse ears 24 hours prior to challenge with the hapten. The design of the pSi2 vector and demonstration of its knock-down effect on mouse heparanase gene expression (80% inhibition) in cultured cells have been previously described by us.30 In the present study, we applied an in vivo electroporation technique, as described in “Materials and methods.” To demonstrate that this technique ensures actual delivery of the electroporated DNA and its uniform expression in the ear tissue, we first electroporated the ears of male BALB/c mice with a CMV-LUC construct, encoding for luciferase gene under the constitutive CMV promoter, and visualized the expression of luciferase in the mouse ears in vivo (Figure 5C), using a cooled charged coupled device (CCCD) camera.32
Heparanase promoter is activated upon DTH elicitation. The ears of oxazolone-sensitized BALB/c mice (n = 3) were electroporated with Hpse-LUC (A, experimental group), CMV-LUC (B, control group), or Mut-Hpse-LUC (C, control group) reporter constructs. Left ears in both the experimental and control groups were challenged 24 hours later, while right ears remained untreated. Forty-eight hours after challenge, when a pronounced DTH reaction was noted in the left, but not right, ears of all mice (as judged by ear swelling and edema formation), the ears were resected, snap frozen, and lysed. Lysates were normalized for total protein content. Luciferase activity was determined as described in “Materials and methods” and expressed in relative units of light (RUL). Two independent experiments were performed, 3 mice per treatment.
Heparanase promoter is activated upon DTH elicitation. The ears of oxazolone-sensitized BALB/c mice (n = 3) were electroporated with Hpse-LUC (A, experimental group), CMV-LUC (B, control group), or Mut-Hpse-LUC (C, control group) reporter constructs. Left ears in both the experimental and control groups were challenged 24 hours later, while right ears remained untreated. Forty-eight hours after challenge, when a pronounced DTH reaction was noted in the left, but not right, ears of all mice (as judged by ear swelling and edema formation), the ears were resected, snap frozen, and lysed. Lysates were normalized for total protein content. Luciferase activity was determined as described in “Materials and methods” and expressed in relative units of light (RUL). Two independent experiments were performed, 3 mice per treatment.
In the subsequent set of experiments, 6-week-old male BALB/c mice were sensitized with oxazolone and divided into 3 groups (n = 5 mice per group) 4 days after sensitization. The first and second groups were electroporated with antiheparanase siRNA expression vector (pSi2) and with empty vector (pSUPER),47 respectively; mice in the third group were not subjected to electroporation. Twenty-four hours later, ears in all 3 groups were challenged with the hapten. Hapten also was applied onto the ears of an additional 5 mice, which had not been previously sensitized or electroporated, serving as a negative control group. The ear thickness was monitored for 5 consecutive days (Figure 5A). Twenty-four hours after challenge, a marked inflammatory response was detected in both the pSUPER-electroporated and nonelectroporated ears, reflected by more than a 2-fold increase in ear thickness, as compared with the control group. In contrast, in the ears electroporated with the antiheparanase siRNA encoding vector pSi2, the inflammatory response was significantly inhibited, as noted by a 77% decrease in ear swelling and edema formation, compared to ears electroporated with the pSUPER vector alone (Figure 5A). We previously have demonstrated the persistence of heparanase silencing in murine cells in vitro following pSi2 transfection (75% inhibition at 48 hours after transfection and 50% inhibition at 96 hours after transfection). To test inhibitory effect of siRNA silencing in endothelial cells, we determined heparanase activity in human EA.hy926 vascular endothelial cells at various time points after electroporation with the anti–human heparanase siRNA encoding pSH1 vector, analogous to pSi2. Maximal effect of siRNA silencing (80% inhibition) was observed during the first 72 hours after electroporation and was still pronounced by 96 hours after electroporation (50% inhibition, not shown). To test the local heparanase expression in siRNA-treated skin and to ensure that electroporation of pSi2 resulted in heparanase silencing throughout the in vivo experiment, we compared heparanase immunostaining in tissue sections of the ears in which DTH was induced following electroporation with the pSi2 or pSUPER vectors. Intense heparanase staining was observed in pSUPER-electroporated ears, 48 hours after the challenge (that is, 72 hours after electroporation, Figure 5B, right), versus a very weak or no heparanase staining in pSi2-electroporated ears (Figure 5B, left), similar to heparanase levels observed in normal untreated ears (Figure 2A). Decrease in a number of positively stained cells other than endothelial cells (ie, epidermal and hair follicle keratinocytes, several dermisresiding cells that may express basal levels of heparanase32,39 ) also was found in pSi2-electroporated ears (Figure 5B, left). Altogether, these results demonstrate that heparanase gene silencing in pSi2-electroporated ears persisted at least for 2 days following the challenge.
Effect of antiheparanase siRNA on DTH reactivity in vivo. Ears of oxazolone-sensitized BALB/c mice were electroporated with anti–mouse heparanase siRNA expression vector pSi2 (▪); empty vector pSUPER (▴); or received no plasmid or electroporation (♦), followed by challenge with the hapten 24 hours later. Hapten also was applied on the ears of 5 additional mice, which have not been previously sensitized or electroporated (▪). Three independent experiments were performed, and 5 mice per treatment were used. (A) Ear thickness was measured for 5 consecutive days after challenge. Inset. Effect of treatment with an inhibitor (ST1514) of heparanase enzymatic activity on DTH reactivity. ST1514 or vehicle alone was administered intraperitoneally prior to challenge and every hour thereafter (50 μg/injection) during the following 8 hours of the experiment. Filled bars: ear thickness in ST1514-treated mice; empty bars: ear thickness in vehicle-treated mice. (B) The ears in which DTH was induced following electroporation with pSi2 (left) or pSUPER (right) vectors were harvested 48 hours after challenge and processed for immunohistochemical analysis of heparanase expression (reddish staining; sebaceous glands are positively stained in all preparates, due to nonspecific absorption, as previously reported.32 Top: magnification × 200; bottom: × 1000. Positively stained capillary endothelium is noted in the dermis of pSUPER but not pSi2-electroporated ear skin. (C) To demonstrate that electroporation ensures the actual delivery of plasmid DNA and its uniform expression in the ear tissue, the ears of male BALB/c mice were electroporated with a CMV-LUC construct, encoding for luciferase gene under the constitutive CMV promoter. Expression of luciferase in the mouse ears in vivo was monitored as described in “Materials and methods.”32
Effect of antiheparanase siRNA on DTH reactivity in vivo. Ears of oxazolone-sensitized BALB/c mice were electroporated with anti–mouse heparanase siRNA expression vector pSi2 (▪); empty vector pSUPER (▴); or received no plasmid or electroporation (♦), followed by challenge with the hapten 24 hours later. Hapten also was applied on the ears of 5 additional mice, which have not been previously sensitized or electroporated (▪). Three independent experiments were performed, and 5 mice per treatment were used. (A) Ear thickness was measured for 5 consecutive days after challenge. Inset. Effect of treatment with an inhibitor (ST1514) of heparanase enzymatic activity on DTH reactivity. ST1514 or vehicle alone was administered intraperitoneally prior to challenge and every hour thereafter (50 μg/injection) during the following 8 hours of the experiment. Filled bars: ear thickness in ST1514-treated mice; empty bars: ear thickness in vehicle-treated mice. (B) The ears in which DTH was induced following electroporation with pSi2 (left) or pSUPER (right) vectors were harvested 48 hours after challenge and processed for immunohistochemical analysis of heparanase expression (reddish staining; sebaceous glands are positively stained in all preparates, due to nonspecific absorption, as previously reported.32 Top: magnification × 200; bottom: × 1000. Positively stained capillary endothelium is noted in the dermis of pSUPER but not pSi2-electroporated ear skin. (C) To demonstrate that electroporation ensures the actual delivery of plasmid DNA and its uniform expression in the ear tissue, the ears of male BALB/c mice were electroporated with a CMV-LUC construct, encoding for luciferase gene under the constitutive CMV promoter. Expression of luciferase in the mouse ears in vivo was monitored as described in “Materials and methods.”32
We next tested the effect of the potent heparanase enzymatic inhibitor ST1514 (glycol split, nonanticoagulant heparin38-40 ) on DTH reactivity. Six-week-old male BALB/c mice were sensitized with oxazolone and divided into 2 groups (n = 4 mice per group × 2 ears) 4 days after sensitization. The first group was treated with ST1514 immediately prior to challenge and every hour thereafter during the following 8 hours of the experiment, as described in “Materials and methods.” The second group was treated with vehicle alone. Treatment with ST1514 resulted in a significant decrease of ear swelling and edema formation, as compared to treatment with the vehicle (Figure 5A, inset), further verifying the involvement of heparanase in DTH and the relevance of heparanase inhibition as an anti-inflammatory approach.
Heparanase silencing inhibits vessel permeability during DTH
Since we found reduced inflammatory response (reflected by a very limited ear swelling) following heparanase silencing in wild-type mice, as well as increased edema formation in hpa-tg mice, we next investigated whether heparanase directly affects vascular leakage, a hallmark of the early phase of inflammation. The ears of oxazolone-sensitized BALB/c mice were electroporated with pSi2-(left ear) or pSUPER-(right ear) vectors on day 4 after sensitization. Twenty-four hours later, both the right and left ears were challenged with oxazolone, and after an additional 16 hours mice received intravenous injections of Evans blue. As shown in Figure 6, 16.5 hours after DTH elicitation by oxazolone challenge, vascular leakage was significantly higher in pSUPER-than in pSi2-electroporated ears, as reflected by a marked difference in Evans blue extravasation. Macroscopically, a strong DTH-associated swelling was readily detected in all pSUPER-, but not in pSi2-electroporated ears (not shown). These findings indicate that increased heparanase activity expressed by activated endothelial cells at the site of inflammation enables vessel leakage during inflammation, most likely due to damage and disruption of the subendothelial BM.
Effect of local heparanase silencing on vascular leakage. Ears of 5 oxazolone-sensitized BALB/c mice were electroporated with either antiheparanase siRNA pSi2 (filled arrows) or empty pSUPER (empty arrows) vectors, 24 hours prior to induction of DTH reaction by application of oxazolone onto the ears of both sides. Evans blue dye was injected intravenously 16 hours later. Unlike the massive Evans blue extravasation observed in pSUPER-electroporated ears, pSi2 electroporation halted vascular leakage, as visualized by the near absence of extravasated Evans blue dye.
Effect of local heparanase silencing on vascular leakage. Ears of 5 oxazolone-sensitized BALB/c mice were electroporated with either antiheparanase siRNA pSi2 (filled arrows) or empty pSUPER (empty arrows) vectors, 24 hours prior to induction of DTH reaction by application of oxazolone onto the ears of both sides. Evans blue dye was injected intravenously 16 hours later. Unlike the massive Evans blue extravasation observed in pSUPER-electroporated ears, pSi2 electroporation halted vascular leakage, as visualized by the near absence of extravasated Evans blue dye.
Discussion
Contrary to early considerations, endothelial cells are now recognized as active participants in DTH reactivity and other types of inflammatory processes.3,48,49 Following alterations induced by pro-inflammatory cytokines (ie, TNFα, IFN-γ) acting in concert, endothelial cells become activated and synthesize numerous adhesion molecules involved in leukocyte-endothelium interactions.3 Endothelial cells also are capable of secreting different molecules (ie, cytokines, chemokines) that attract various types of immune cells into the site of inflammation and increase the motility of adherent leukocytes from the peripheral blood. Moreover, endothelial cells were proposed to contribute to local vessel hyperpermeability by remodeling the subendothelial BM and thus allowing the extravasation of plasma macromolecules (eg, fibrinogen) and immunocytes. However, attempts to identify the molecular mechanism responsible for increased vascular permeability in DTH inflammation were met with limited success. The data presented in this study directly implicate the heparanase enzyme, locally expressed by the vascular endothelium at the site of inflammation, in degradation of the subendothelial BM and subsequent vascular leakage—a hallmark of delayed hypersensitivity skin reactions.1
Mammalian heparanase cleaves heparan sulfate glycosaminoglycans in the BM and other types of ECM.11,50 Given the essential role of heparan sulfate chains in preserving the integrity and barrier properties of the BM and ECM,8-10,51 heparanase may adversely affect tissue architecture and play an important role in processes that involve ECM disintegration, such as implantation, morphogenesis, angiogenesis, and cancer metastasis.10,11,29,33,50,52,53
Less is known about the role of heparanase in inflammation. It was reported that polyanionic compounds known to inhibit heparanase enzymatic activity (eg, heparin) also inhibit inflammatory responses.21,25,54,55 Activated T lymphocytes have been viewed as a cellular source of the enzyme in inflammation.16,21,22,24,55 On the other hand, it was found that the ability of lymphocytes to degrade ECM is inhibited by key inflammatory cytokines.56
Here, we demonstrated the induction of locally expressed heparanase at the site of inflammation in vivo and established its mechanistic involvement in DTH inflammatory reaction. Overexpression of heparanase in a mouse transgenic model significantly enhanced DTH reactivity. Unlike the intensity of the DTH reaction, its time course remained the same in both transgenic and wild-type animals (Figure 1). This suggests that heparanase is involved primarily in the initial stages of the inflammatory response, that is, increase in vascular permeability, most likely through disruption of the subendothelial BM. By monitoring in vivo activity of luciferase driven by the heparanase gene regulatory sequence, we demonstrated that heparanase promoter activation occurs in the inflammation site upon the onset of a DTH response. Studying expression and cellular distribution of endogenous heparanase in BALB/c mice, we found that endothelial cells are the primary source of the enzyme at the early stages of DTH inflammation. Furthermore, TNFα and IFN-γ, key mediators of DTH inflammation,3,43,44 up-regulate heparanase gene expression and increase heparanase enzymatic activity in cultured endothelial cells. Our data on heparanase induction by TNFα are in agreement with the previously reported ability of TNFα to augment ECM degradation by endothelial cells56 and are consistent with a recent report by Chen et al.45 In the latter study, caspase 8 was identified as a part of a molecular pathway that underlies TNFα-induced heparanase secretion by endothelial cells. The molecular mechanism through which IFNγ augments heparanase expression remains to be further investigated. Computerized analysis of the heparanase gene 1.9-kb regulatory sequence using MatInspector software57 revealed 2 interferon-stimulated response elements (ISREs) consensus sequences in the promoter region that specifically bind transcription factors activated by interferon (not shown). A more refined analysis of the heparanase regulatory sequence will enable researchers to locate the precise binding site(s) in the heparanase promoter responsible for the IFN-γ–induced transcription.
Local in vivo electroporation of antiheparanase siRNA into the ear skin markedly inhibited DTH reactivity, demonstrating the decisive involvement of heparanase in inflammation. In order to distinguish between heparanase expressed by local cellular elements at the site of inflammation versus the enzyme expressed by circulating immunocytes, the in vivo siRNA experiments were designed to achieve heparanase silencing 1 day prior to challenge with the hapten. Since T cells, known to mediate DTH response, attach to the vascular endothelium and extravasate toward the hapten only after the challenge,2 we did not expose the recruited T cells to antiheparanase siRNA administered by local electroporation executed prior to challenge. The same is correct for any other free circulating cells of the immune system. On the other hand, endothelial cells are present at the challenged site before application of the hapten. Thus, unlike systemic administration of heparanase-inhibiting compound ST1514, treatment with siRNA prior to challenge restricted heparanase silencing to the local (eg, endothelium), rather than circulating (eg, T lymphocytes) cellular compartment. Although not capable of fully discriminating between the heparanase producer cell types, this approach allowed us to specifically analyze the role of nonlymphocyte-derived heparanase in inflammation. The decrease in heparanase protein, observed in the endothelium of immunostained ear tissue derived from pSi2-treated ears (Figure 5B), demonstrated the effectiveness of heparanase silencing in vivo. This decrease correlated with the absence of vessel leakage (Figure 6), as compared to control pSUPER-treated ears, in which vessel hyperpermeability and ear swelling were clearly noted. In summary, induction of locally expressed heparanase emerges as an important step in the series of events involved in onset of the DTH inflammatory process. Our results suggest that following hapten challenge, induction of heparanase expression driven by inflammatory cytokines (TNFα and, later on, IFN-γ) may occur locally in the endothelial cells. Upon elicitation of DTH reaction, TNFα (ie, released via mast cells' degranulation in response to hapten challenge44 ) may induce initial increase in production and secretion of heparanase by endothelial cells (this study and Chen et al45 ). At this stage, heparanase, known to promote cell adhesion,58 can improve T-cell adherence to the endothelium, as well as facilitate extravasation, through degradation of the subendothelial basement membrane. Then, IFN-γ released by the extravasated T cells may contribute to preservation and even further amplification of heparanase expression in the endothelium. Continuous increase in heparanase levels is likely to cause vascular leakage through degradation of HS chains, responsible for the structural integrity of the subendothelial BM. Such degradation results in disruption of the BM permeaselective properties and subsequent plasma and immunocyte extravasation. Disruption of the BM due to elevated heparanase levels has been demonstrated in hpa-tg mouse mammary epithelium.29 HS proteoglycans (ie, syndecans) also are present on the surface of endothelial cells and have been shown to modulate vascular permeability and leukocyte trafficking in inflammation.59 Thus, the observed effects of heparanase may, in addition to cleavage of BM-residing HS, be due to degradation of endothelial cell surface HS chains, impairing their barrier function. To our knowledge, these data represent the first successful in vivo application of heparanase siRNA-based anti-inflammatory therapy. Heparanase inhibition may be relevant in the development of future therapeutic tools in several disorders considered to be the consequence of DTH reactions such as rheumatoid arthritis, psoriasis, and inflammatory bowel disease (IBD). In particular, preferential expression of heparanase in the intestinal tissue of IBD patients, as well as increased susceptibility of hpa-tg mice to chemically-induced colitis (Y. Sherman, I.L., M.E., I.V., unpublished results, May 2005), attest to heparanase as a likely target for the design of a novel anti-IBD treatment.
Previously, we showed that heparanase gene silencing approach applied in vitro specifically suppresses invasive and metastatic potential in various tumor models.30 Thus, in addition to the potential promise for an anti-inflammatory treatment, the specific heparanase siRNA delivery system described in this study will encourage development of novel heparanase-based therapeutic modalities, highly pertinent in other pathological conditions involving undesired heparanase activity, particularly cancer.60-63
Supported by grants from the United States Army (award no. W81XWH-04-1-0235); the Israel Cancer Association; the European Commission (Fifth Framework program, contract no. QLK3-CT-2002-02049); grant 532/02 from the Israel Science Foundation; and by United States Public Health Service Grant RO1 CA106456-01 from the National Cancer Institute, National Institutes of Health.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
Prepublished online as Blood First Edition Paper, December 29, 2005; DOI 10.1182/blood-2005-08-3301.
We would like to thank Dr T. San (Department of Oncology, Hadassah University Hospital) for the excellent technical support and Dr S. Frankenburg (Department of Dermatology, Hadassah University Hospital) for fruitful discussions and for help in establishing the DTH model. We thank Drs Claudio Pisano and Sergio Penco (Sigma-Tau Research Department, Pomezia, Rome, Italy) and Prof Benito Casu (“Ronzoni” Institute, Milan, Italy) for kindly providing the ST1514 glycol-split heparin and for their continuous support.




This feature is available to Subscribers Only
Sign In or Create an Account Close Modal