Vascular endothelial growth factor (VEGF) is a key regulator of blood vessel formation during both vasculogenesis and angiogenesis. The prolonged expression of VEGF in the normoperfused skeletal muscles of adult rodents after gene transfer using AAV vectors induces the formation of a large set of new capillaries and small arteries. Notably, this process is accompanied by the massive infiltration by mononuclear cells. This observation raises the possibility that these cells might represent circulating progenitors that are eventually incorporated in the newly formed vessels. Here we explore this possibility by exploiting 4 different experimental models based on (a) the transplantation of male bone marrow into female recipients; (b) the transplantation of Tie2-GFP transgenic bone marrow; (c) the transplantation of bone marrow in the presence of erythropoietin (EPO), a mobilizer of endothelial progenitor cells (EPCs); and (d) the reimplantation of ex vivo–expanded EPCs. In all 4 models, VEGF acted as a powerful attractor of bone marrow–derived mononuclear cells, bearing different myeloid and endothelial markers proper of the EPCs to the sites of neovascularization. In no case, however, were the attracted cells incorporated in the newly formed vasculature. These observations indicate that new blood vessel formation induced by VEGF occurs through bona fide sprouting angiogenesis; the contribution of the infiltrating bone marrow–derived cells to this process still remains enigmatic.
Introduction
Over the past few years, a number of stimulating reports have challenged the notion that, contrary to vasculogenesis in the embryo, new blood vessel formation in postnatal life exclusively results from angiogenesis, a process triggered by the activation, proliferation, and migration of preexisting vascular endothelial cells, followed by the subsequent remodeling of the vessel wall (reviewed in Ferrara et al,1 and Losordo and Dimmeler2,3 ). Indeed, a complex functional relationship has been unveiled between cells of the vascular compartment and hematopoiesis. Bone marrow–derived circulating precursors expressing endothelial markers (endothelial progenitor cells [EPCs]) have been isolated from the peripheral blood of adult organisms,4 and mature endothelial cells have been obtained by in vitro expansion and differentiation of selected bone marrow populations.5-7 In the settings of myocardial or hind limb ischemia, the mobilization of these EPCs has been found to be significantly increased.8 Likewise, systemic administration of several cytokines and growth factors, such as GM-CSF, SDF-1, vascular endothelial growth factor (VEGF), and erythropoietin, has been shown to augment the EPC fraction.9-12 Finally, a large body of evidence indicates that the EPCs specifically home to sites of pathologic and physiologic neovascularization, probably attracted by local chemotactic stimuli. Different studies have also suggested that the EPCs participate in the repair of the vascular tissue following ischemic injury, as well as to the development of tumor vasculature and to the endothelialization of vascular grafts.8,13-18
These properties have recently been exploited for the treatment of patients suffering from myocardial and hind limb critical ischemia.19,20 These clinical studies have shown that the transplantation of both circulating and bone marrow autologous progenitors contributes to the enhancement of blood flow and to the recovery of ischemic tissue injury. However, these findings did not conclusively prove that these progenitor cells act by promoting vasculogenesis rather than by the paracrine secretion of factors and enzymes beneficial for vessel development and cell survival in the context of a genuine angiogenic process. Similarly, there is no general consensus about the extent and the significance of EPC incorporation into the endothelial layer of growing vessels as well as about the possibility that these cells might transdifferentiate into smooth muscle cells or pericytes.8,21-25
VEGF is a key regulator of blood vessel formation during both vasculogenesis and angiogenesis.26,27 During embryogenesis, VEGF acts an early determinant of hemangioblast differentiation and its action is fundamental to direct the development of both vascular and hematopoietic tissues. In adult life, the overexpression of different VEGF isoforms triggers a remarkable process of new blood vessel formation. In particular, the use of viral vectors based on the adeno-associated virus (AAV) has recently offered the possibility of exploring the effects of VEGF overexpression for prolonged periods of time (several months) in the adult organisms in the absence of any inflammatory or immune reactions. By exploiting this gene delivery system, we have recently observed that the prolonged expression of the 165–amino acid isoform of VEGF in the normoperfused skeletal muscles of adult rodents induces a powerful angiogenic response, with an increase in the number of capillaries and, most remarkably, with the formation of a network of newly formed arteries with a diameter of 20 to 120 μm, surrounded by cells positive for α-smooth muscle actin (α-SMA).28 Similar results have also been obtained by another laboratory by overexpressing VEGF from engineered myoblasts.29
Most notably, we observed that VEGF-induced neovascularization was accompanied by the massive infiltration of the sites of neovascularization by mononuclear cells of unknown derivation and function, several of which carried markers proper of endothelial progenitors, such as Flk-1, CD34, and Sca-1. These observations raised the possibility that the prolonged expression of VEGF might determine the recruitment of circulating progenitors that could eventually participate in blood vessel formation.
Here we investigate the actual role of circulating progenitor cells in the process of neovascularization induced by the local expression of VEGF. We demonstrate that this factor is a powerful attractor of bone marrow–derived progenitor cells to the sites of new blood vessel formation; however, in no instance could the direct incorporation of these cells be detected in the newly formed vasculature.
Materials and methods
Mice
Animal care and treatment were conducted in conformity with institutional guidelines in compliance with national and international laws and policies (European Economic Community [EEC] Council Directive 86/609; OJL 358; December 12, 1987). Balb/c mice were purchased from Charles River Laboratories Italia Srl (Milan, Italy) whereas FVB-NJ and FVB-TgN(Tie2GFP)287Sato30 mice were obtained from Jackson Laboratories (Bar Harbor, ME) and maintained under controlled environmental conditions.
Recombinant AAV vector preparation and characterization
The recombinant AAV (rAAV) vectors used in this study were prepared by the AAV Vector Unit at the International Centre for Genetic Engineering and Biotechnology (ICGEB) Trieste (http://www.icgeb.org/RESEARCH/TS/COREFACILITIES/AVU.htm), as already described.28 Animals received injections in the tibialis anterior muscle with 30 μL vector preparation, containing approximately 1 × 1010 viral genome particles.
Transplantation studies
Bone marrow was obtained from 6-week-old male Balb/c or FVB/N mice. Recipient, age-matched, syngenic female mice were lethally irradiated with a total dose of 8.5 Gy; 2 × 106 cells, resuspended in 0.2 mL medium, were transplanted via tail vein injection. After 4 weeks, blood counts and hematocrit values confirmed the full hematopoietic recovery.
Successful engraftment of the transplanted cells was further confirmed by the quantification of a specific mouse Y-chromosome sequence in DNA samples extracted from peripheral blood mononuclear cells (PBMCs) or bone marrow cells at 1, 2, and 6 months after transplantation, using primers Y-F (5′CATGCAAAATACAGAGATCA3′) and Y-R (5′TAAAATGCCACTCCTCTGTG3′) to produce a genomic segment of 181 bp.
As a cellular reference gene, mouse β-globin was amplified by primers BG-F (5′CAGCCTCAAGGGCACCTTTG3′) and BG-R (5′AGCAGCAATTCTGAATAGAG3′) to generate a 238-bp fragment. Exact quantification was obtained using an established competitive polymerase chain reaction (PCR) procedure28 that uses a synthetic DNA competitor for the quantification of both targets, as already detailed.28,31
Histology and flow cytometry
Paraffin sections were immunostained as described.28 For the staining of the whole animal vasculature with fluoresceinated lectin, 100 μL of Lycopersicum Esculentum lectin (Vector Laboratories, West Grove, PA) was injected into live mice through the jugular vein to ensure its systemic distribution. After 15 minutes, mice were anesthetized and in vivo perfused with 1% paraformaldehyde.
For immunofluorescence, frozen sections (5 μm thick) were fixed in cold acetone (20°C) and blocked for 30 minutes with 5% goat or 5% horse serum in PBS, depending on the secondary antibody. The following primary antibodies were used diluted 1:200 in blocking buffer: anti-CD11b (clone M1/70), anti-CD31 (clone 390), anti–Sca-1 (clone E13-161.7), anti-CD34 (RAM 34), anti–c-Kit (2B8; all from BD Pharmingen, Heidelberg, Germany); mouse monoclonal anti–Flk-1 (Santa Cruz Biotechnology, Santa Cruz, CA); anti-F4/80 (clone A3-1; Serotec, Raleigh, NC); Cy3-coniugated anti–α-SMA (clone 1A4; Sigma, St Louis, MO). For NG2 detection, a rabbit polyclonal antibody (Chemicon, Temecula, CA) was used on 4% paraformaldehyde-fixed frozen sections after overnight blocking with 5% horse serum.
For the detection of GFP in mice that received a transplant of donor Tie2-GFP transgenic marrow cells, anesthetized animals were perfused in vivo with PBS followed by 4% paraformaldehyde. Tibialis anterior muscles were excised and postfixed for 2 hours in paraformaldehyde, equilibrated for 24 hours in 30% sucrose in PBS, and frozen in liquid nitrogen. GFP expression was detected using a rabbit polyclonal anti-GFP antibody (Molecular Probes, Eugene, OR). The secondary antibodies used were Alexa Fluor 488– and AlexaFluor 555–conjugated goat antirat, Alexa Fluor 488–conjugated donkey antirabbit, and Alexa Fluor 594–conjugated donkey antimouse (all from Molecular Probes). Nuclei were counterstained with DAPI.
Microscopy and photography relative to images in Figures 2, 4, 5B, 6C, and 7D-E were performed using Leica MLB upright fluorescence microscope (Leica Microsystems, Wetzler, Germany) equipped with a Coolsnap CF CCD camera (Roper Scientific, Evry, France) using N PLAN 40 ×/0.40 NA and 63 ×/1.32 NA oil objectives and MetaView 4.6 software (Molecular Devices, Downingtown, PA). Images were assembled using Adobe Photoshop software, version 7.0 (Adobe Systems, San Jose, CA).
For flow cytometry analysis, cells were stained with FITC- or PE-conjugated anti-CD45, -CD11b, -CD11c, -CD14, -CD31, –Sca-1, –Flk-1, -CD34, and –c-Kit monoclonal antibodies (BD Pharmingen). Isotype-matched antibodies served as controls.
Immunofluorescence in situ hybridization (Immuno-FISH)
Following immunofluorescence detection of CD31 or α-SMA antigens, tissue sections were postfixed by protein cross-linking using EGS (Sigma) at 50 mM in PBS for 30 minutes at 37°C.32 The EGS stock solution was made in DMSO and the final dilution in PBS was prepared just before use. After washing, DNA was denatured in 70% formamide by placing slides at 72°C for 10 minutes; alcohol-dehydrated samples were than hybridized overnight at 42°C with a probe specific for mouse Y chromosome, labeled with FITC (Cambio, Newmarket, United Kingdom). After low-temperature washes, the tissue sections were mounted in an antifade medium containing DAPI as DNA counterstaining.
Transplantation of ex vivo–expanded EPCs
Adherent cells were obtained from Balb/c bone marrow and cultured for 2 weeks on vitronectin/gelatin-coated cell dishes in 2 different media: nondifferentiating medium (ND: DMEM 4.5 g/mL glucose supplemented by 0.5% chicken embryo extract [Gibco Life Science, Carlsbad, CA], 10% FCS, and 10% horse serum) and differentiating medium (D: EBM-2 supplemented with EGM-2 MV SingleQuots minus hydrocortisone [Clonetics-Cambrex, San Diego, CA]). Cells were then detached by nonenzymatic treatment and either analyzed by flow cytometry or labeled with PKH26 red fluorescence linker (Sigma). Eight Balb/c mice, which were given injections of the AAV-VEGF vector into the tibialis anterior muscle 20 days in advance, received 1 × 106 labeled cells each through tail vein injection. After 15 days, the animals were killed and the fluorescent cells were detected on 5 μm frozen sections of AAV-VEGF–treated tibialis anterior muscles.
Statistical analysis
One-way analysis of variance (ANOVA) and Benferroni/Dunn posthoc test were used to compare multiple groups. Pair-wise comparison between groups was performed using the Student t test. P less than .05 was considered statistically significant.
Results
AAV-VEGF induces pronounced angiogenesis and cell infiltration in normal skeletal muscle
To study the kinetics of the angiogenic response induced by VEGF gene expression, we gave mice injections of AAV-VEGF in the tibialis anterior muscle of one leg and with AAV-LacZ in the same muscle of the opposite leg and analyzed at different times after treatment. As shown in Figure 1A and quantified in Figure 1C by counting the number of α-SMA–positive arteriolae, a specific angiogenic effect started to be evident in the animals that had received injections of AAV-VEGF as early as 7 days after vector injection, increased at 14 days, was maximal at 1 month, and remained stable thereafter (> 8-fold increase compared with untreated controls; P < .001). Several cells in the VEGF-induced neovasculature were positive for NG2, a known marker of pericytes33,34 ; notably, NG2 was also present in the wall of the small arteries, which were thus surrounded by cells double-positive for both NG2 and α-SMA (Figure S1, available on the Blood website; see the Supplemental Figures link at the top of the online article). No obvious effect could be observed in the muscles that had received injections of AAV-LacZ, in agreement with our previous observations.28,35 The kinetics of vessel growth paralleled the progressive increase over time of the VEGF mRNA expression mediated by the AAV vector, as previously shown by real-time PCR analysis in similar experimental conditions.35
Mononuclear cells infiltrate the foci of VEGF-induced neovascularization. (A) Immunohistochemical time course analysis, by the visualization of small arteries using an antibody against smooth muscle actin (α-SMA), of the angiogenic response induced by AAV-mediated VEGF expression in normal skeletal muscle at different times after vector injection. Abundant mononuclear cell infiltrates are detectable in the perivascular spaces of the injected muscles (enlargements i-iii in panel B). Images were obtained using an Olympus CX40 microscope and 10 ×/0.30 NA and 40 × 0.75 UPlan FL objective lenses (Olympus, Tokyo, Japan). Images were captured using Olympus Camedia C-3030 digital camera and processed with Adobe Photoshop 7.0 software. (C) Quantification of the number of α-SMA–positive vessels over time. Shown are means and standard deviations of counts, expressed as number of α-SMA vessels per muscle fiber. The quantifications were carried out by 3 independent investigators who observed 10 different sections from 3 animals per time point. d indicates days; mo, months. (D) Quantification of the number of the infiltrating cells in the AAV-VEGF–treated muscles. Shown are means and standard deviations of counts, expressed as number of nuclei per muscle fiber. The quantifications were carried out by 3 independent investigators who observed 10 different sections from 3 animals per time point.
Mononuclear cells infiltrate the foci of VEGF-induced neovascularization. (A) Immunohistochemical time course analysis, by the visualization of small arteries using an antibody against smooth muscle actin (α-SMA), of the angiogenic response induced by AAV-mediated VEGF expression in normal skeletal muscle at different times after vector injection. Abundant mononuclear cell infiltrates are detectable in the perivascular spaces of the injected muscles (enlargements i-iii in panel B). Images were obtained using an Olympus CX40 microscope and 10 ×/0.30 NA and 40 × 0.75 UPlan FL objective lenses (Olympus, Tokyo, Japan). Images were captured using Olympus Camedia C-3030 digital camera and processed with Adobe Photoshop 7.0 software. (C) Quantification of the number of α-SMA–positive vessels over time. Shown are means and standard deviations of counts, expressed as number of α-SMA vessels per muscle fiber. The quantifications were carried out by 3 independent investigators who observed 10 different sections from 3 animals per time point. d indicates days; mo, months. (D) Quantification of the number of the infiltrating cells in the AAV-VEGF–treated muscles. Shown are means and standard deviations of counts, expressed as number of nuclei per muscle fiber. The quantifications were carried out by 3 independent investigators who observed 10 different sections from 3 animals per time point.
Remarkably, the foci of VEGF-induced neoangiogenesis were always found infiltrated by mononuclear cells (also see enlargements in Figure 1Bi-iii). A quantitative analysis performed on histologic images demonstrated that the infiltrates were already abundant in the perivascular spaces of the injected muscles at day 7 after transduction and progressively decreased afterward; cellular infiltration of the injected muscles was always found in close contact with the growing vasculature. The cellular infiltrates definitely arise as a consequence of VEGF expression, since we could never observe a similar effect using other AAV vectors expressing reporter genes (GFP, LacZ) or several other factors.
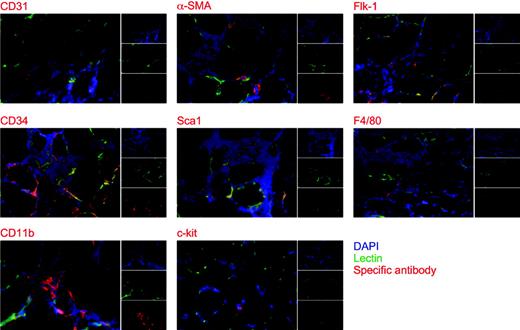
In order to define the origin of the cells infiltrating the VEGF-expressing muscles, we undertook their extensive immunologic characterization by immunofluorescence. These experiments were performed by the simultaneous visualization of the infiltrating cell nuclei with DAPI, of the vasculature by FITC-lectin, and of cell markers by using a series of specific antibodies. As shown in the representative pictures reported in Figure 2, approximately 30% of the infiltrating mononuclear cells were positive for CD31, whereas basically all the α-SMA–positive cells were incorporated into the wall of small arteries. Of interest, approximately 20% of the infiltrating cells scored positives for the broad myelocytic/monocytic marker CD11b, with significant positivity also for the macrophage marker F4/80. Positive signals were also detected for Flk-1, Sca-1, and CD34, which are expressed by differentiated endothelial cells and progenitor cells of both endothelial and muscle lineages.14,36,37 Finally, a careful analysis also allowed the identification of rare c-Kit–positive cells.
Taken together, these observations prompted us to hypothesize that the local expression of VEGF might trigger the recruitment and the subsequent differentiation of progenitor cells to the sites of new blood vessel formation.
VEGF recruits bone marrow cells to the sites of neovascularization in animals that received a bone marrow transplant
To evaluate whether adult bone marrow–derived cells actually contribute to the process of new blood vessel formation at the sites of VEGF expression, we transplanted unfractionated BM cells from male Balb/c donor mice into lethally irradiated syngenic female recipients, thus creating Y-Balb/c chimeric mice.
Immunologic characterization of the mononuclear cells infiltrating the foci of VEGF-induced neovascularization. Immunofluorescence staining was performed using CD31-, α-SMA–, Flk-1–, CD34-, Sca-1–, F4/80-, CD11b-, and c-Kit–specific antibodies on histologic sections of mouse tibialis anterior muscles perfused in vivo with FITC-lectin. The analysis was performed at 2 weeks after AAV-VEGF transduction. As shown in the 3 small subpanels, red indicates cells positive for the different antibodies; green, whole vasculature stained with FITC-lectin; and blue, nuclei stained with DAPI. The 3 stainings are merged in the large panels.
Immunologic characterization of the mononuclear cells infiltrating the foci of VEGF-induced neovascularization. Immunofluorescence staining was performed using CD31-, α-SMA–, Flk-1–, CD34-, Sca-1–, F4/80-, CD11b-, and c-Kit–specific antibodies on histologic sections of mouse tibialis anterior muscles perfused in vivo with FITC-lectin. The analysis was performed at 2 weeks after AAV-VEGF transduction. As shown in the 3 small subpanels, red indicates cells positive for the different antibodies; green, whole vasculature stained with FITC-lectin; and blue, nuclei stained with DAPI. The 3 stainings are merged in the large panels.
Animals that received a transplant had normal platelet counts, hematocrit values, and amounts of leukocytes in their peripheral blood 1 month after transplantation, indicating good hematopoietic recovery. To evaluate the efficiency of engraftment of donor cells in our experimental settings, we quantified the copies of donor-specific Y-chromosome sequences in the PBMCs and in the bone marrow of the animals at 4 and 12 weeks after transplantation, using quantitative competitive PCR and using the β-globin single-copy cellular gene as a reference. In all the animals used in this work, greater than 90% of bone marrow cells were found to be of donor origin, as exemplified for one sample in Figure 3C. Finally, an appropriate pool of hematopoietic progenitors was present in the bone marrow of the animals that received a transplant, as evaluated by colony-forming unit (CFU) assays (data not shown).
Sex-mismatch bone marrow transplantation model. (A) Flow chart of the experimental procedure. Unfractionated BM cells from male Balb/c donor mice were transplanted into lethally irradiated syngenic female recipients. After hematopoietic recovery, the efficiency of engraftment was evaluated by competitive PCR by quantifying the copies of donor-specific Y-chromosome sequences in the bone marrow of the animals that received a transplant, using β-globin as reference gene. (B) Schematic representation of the templates for quantitative PCR. Amplification of the Y-chromosome sequence and of the β-globin gene were obtained with primer pairs Y-F/Y-R and BG-F/BG-R, respectively. The multicompetitor used for competitive PCR contains a core sequence of 218 bp, corresponding to a 20-bp–deleted version of the mouse β-globin amplification fragment, flanked by Y-F and Y-R primer sequences. (C) Example of competitive PCR amplification. Fixed amounts of sample DNA from PBMCs were mixed with scalar amounts of the multicompetitor DNA and PCR amplified with the 2 primer pairs. After amplification, the gels were stained with ethidium bromide and the competitor (Comp), Y chromosome (Y chr), or β-globin DNA bands were quantified. According to the principles of competitive PCR, the ratio between the amplification products in each reaction is linearly correlated with the input DNA amounts for the 2 DNA species. Dashed red boxes indicate the point of equivalence. (D) Examples of FISH analysis on muscle sections from mice that received a transplant at 30 days after injection of AAV-VEGF, using a mouse-specific Y-chromosome probe labeled with FITC. The green dots, indicated by arrows in the enlargements, correspond to Y-chromosome–specific signals. Red indicates nuclei stained by propidium iodide; and M, muscle fibers. Images in panel D were obtained using a Zeiss LSM510 confocal microscope (Carl Zeiss, Göttingen, Germany), equipped with an Axiovert 100M reverse microscope and 40 ×/0.75 NA and 100 ×/1.30 NA oil objectives. LSM510 software 3.2 was used for image acquisition. Pictures were assembled with Adobe Photoshop 7.0 software.
Sex-mismatch bone marrow transplantation model. (A) Flow chart of the experimental procedure. Unfractionated BM cells from male Balb/c donor mice were transplanted into lethally irradiated syngenic female recipients. After hematopoietic recovery, the efficiency of engraftment was evaluated by competitive PCR by quantifying the copies of donor-specific Y-chromosome sequences in the bone marrow of the animals that received a transplant, using β-globin as reference gene. (B) Schematic representation of the templates for quantitative PCR. Amplification of the Y-chromosome sequence and of the β-globin gene were obtained with primer pairs Y-F/Y-R and BG-F/BG-R, respectively. The multicompetitor used for competitive PCR contains a core sequence of 218 bp, corresponding to a 20-bp–deleted version of the mouse β-globin amplification fragment, flanked by Y-F and Y-R primer sequences. (C) Example of competitive PCR amplification. Fixed amounts of sample DNA from PBMCs were mixed with scalar amounts of the multicompetitor DNA and PCR amplified with the 2 primer pairs. After amplification, the gels were stained with ethidium bromide and the competitor (Comp), Y chromosome (Y chr), or β-globin DNA bands were quantified. According to the principles of competitive PCR, the ratio between the amplification products in each reaction is linearly correlated with the input DNA amounts for the 2 DNA species. Dashed red boxes indicate the point of equivalence. (D) Examples of FISH analysis on muscle sections from mice that received a transplant at 30 days after injection of AAV-VEGF, using a mouse-specific Y-chromosome probe labeled with FITC. The green dots, indicated by arrows in the enlargements, correspond to Y-chromosome–specific signals. Red indicates nuclei stained by propidium iodide; and M, muscle fibers. Images in panel D were obtained using a Zeiss LSM510 confocal microscope (Carl Zeiss, Göttingen, Germany), equipped with an Axiovert 100M reverse microscope and 40 ×/0.75 NA and 100 ×/1.30 NA oil objectives. LSM510 software 3.2 was used for image acquisition. Pictures were assembled with Adobe Photoshop 7.0 software.
At 1 month after transplantation, mice received injections in the tibialis anterior muscle of the right leg with the AAV-VEGF vector, while the homologous muscle on the contralateral leg received injections of AAV-LacZ and was used as a matched control. Histologic examination was performed on muscle sections at different times (15 days, 1 month, and 6 months) after vector administration (n = 4 animals per group), followed by in situ hybridization to detect the Y chromosome using a FITC-labeled mouse DNA probe (Figure 3A flow chart). In our experimental settings, this procedure routinely detects approximately 80% Y-chromosome–positive cells when performed on tissues of male mice that had received AAV-VEGF injections (not shown). In the chimeric females that received a transplant, the number of infiltrating cells bearing the Y chromosome ranged between 55% and 80% of the total cells, thus unequivocally indicating their donor origin (Figure 3D). No significant difference in the number of Y-chromosome–positive cells was found between the groups of animals analyzed at the different time points after vector injection. Finally, no Y-chromosome signals were ever detected by the analysis of the muscles injected with AAV-LacZ (data not shown).
Taken together, these results clearly indicate that the vast majority of the cells infiltrating the muscles expressing VEGF originate from the bone marrow.
Bone marrow–derived cells do not transdifferentiate into mature blood vessel cells
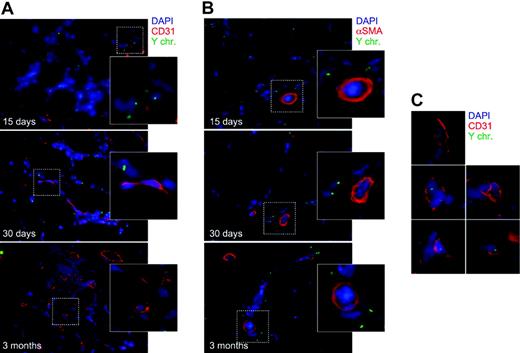
The presence of bone marrow–derived cells at the sites of VEGF expression suggests their possible direct participation in the process of new blood vessel formation. To explore the possibility that these cells might be directly incorporated into the wall of the newly formed vessels, we combined FISH analysis with immunofluorescence staining for endothelial (CD31) and smooth muscle (α-SMA) antigens. Serial sections throughout the whole VEGF-treated muscles of the animals that received a transplant were subjected to extensive fluorescent microscope examination. As shown in Figure 4A and 4B, the vast majority of cells expressing either of the 2 markers were not found to contain a Y chromosome, an observation undoubtedly indicating their origin from the recipient and not from the transplant. Accordingly, most of the infiltrating cells, which were positive for the Y chromosome, did not express either of the 2 differentiation markers. Indeed, after careful observation, a few cells displaying the CD31 antigen and bearing a Y chromosome were occasionally detected within the bulk of mononuclear infiltrating cells. However, these were definitely very rare events. Out of 1100 cells with a Y-chromosome–positive nucleus, less than 1% were found to also express the CD31 marker. Additionally, most of these CD31+/Y-positive cells did not appear to be incorporated into the endothelial layer of vessels and in several instances their appearance could indeed be compatible with the existence of small vessels traveled by Y-chromosome–positive leukocytes (a few examples of these structures are shown in Figure 4C). No cells of donor origin and positive for the α-SMA antigen were ever found in the tunica media of vessels after a serial examination of the treated muscles.
Immuno-FISH analysis of VEGF-treated muscles of mice that received a transplant at 15 days, 30 days, and 3 months after vector injection. Immunofluorescence staining for endothelial (CD31; A) and smooth muscle (α-SMA; B) antigens was combined with Y-chromosome fluorescent in situ hybridization. The vast majority of cells expressing either of the 2 markers were not found to contain a Y chromosome (enlargements in panels A and B). Very rare cells (< 1% of total Y-chromosome–positive nuclei) were positive for CD31 and Y-chromosome markers (shown in the enlargements in panel C); cells of donor origin and positive for the α-SMA antigen were never found in the tunica media of vessels. Blue indicates nuclei stained with DAPI; red, (A) CD31- and (B) α-SMA–positive cells; and yellow, Y chromosome.
Immuno-FISH analysis of VEGF-treated muscles of mice that received a transplant at 15 days, 30 days, and 3 months after vector injection. Immunofluorescence staining for endothelial (CD31; A) and smooth muscle (α-SMA; B) antigens was combined with Y-chromosome fluorescent in situ hybridization. The vast majority of cells expressing either of the 2 markers were not found to contain a Y chromosome (enlargements in panels A and B). Very rare cells (< 1% of total Y-chromosome–positive nuclei) were positive for CD31 and Y-chromosome markers (shown in the enlargements in panel C); cells of donor origin and positive for the α-SMA antigen were never found in the tunica media of vessels. Blue indicates nuclei stained with DAPI; red, (A) CD31- and (B) α-SMA–positive cells; and yellow, Y chromosome.
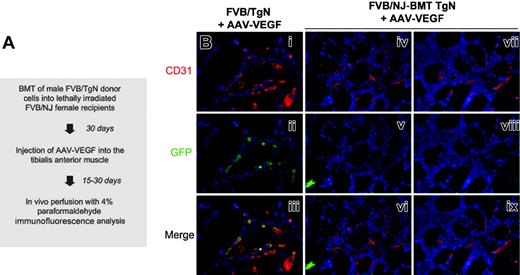
To definitely prove that bone marrow–derived mononuclear cells do not participate in VEGF-induced blood vessel formation through their direct incorporation in the newly formed vasculature, we also exploited a different chimeric mouse model (TgN-BMT) in which donor marrow cells from FVB/TgN transgenic mice, expressing GFP under the transcriptional regulation of the endothelial-specific Tie2 promoter,38 were transplanted into lethally irradiated syngenic FVB/NJ recipients, according to the flow chart shown in Figure 5A. In this model, it is expected that bone marrow precursors that differentiate into endothelial cells will express the GFP marker, which will then colocalize with other endothelial-specific markers such as CD31. The eventual histologic picture will therefore resemble the one observed in FVB/TgN muscles injected with VEGF, in which most of the endothelial cells also stain positive for GFP (Figure 5B left column).
Eight FVB/NJ recipient female mice were lethally irradiated and received transplants of FVB/TgN male bone marrow; 1 month after transplantation, more than 90% of bone marrow cells were found to be of donor origin. At this time, animals were injected with AAV-VEGF in the tibialis anterior muscle of one leg and with an equal amount of AAV-LacZ in the contralateral leg. Histologic samples from the treated muscles were prepared at 15 and 30 days from vector injection and extensively analyzed by double immunofluorescence using antibodies specific for CD31 and GFP. Serial tissue sections throughout the muscle samples revealed a pronounced angiogenic effect induced by the expression of VEGF. However, no Tie2-GFP–positive cells were ever found within the cellular infiltrates or incorporated in the wall of the newly formed vessels (Figure 5Biv-ix).
Taken together, these observations clearly rule out that the mononuclear cells infiltrating the muscles expressing VEGF significantly contribute to the process of new blood vessel formation through their direct differentiation to vascular cells. Thus, the VEGF-induced neovasculature essentially derives from the differentiation, proliferation, and migration of resident cells.
FVB/TgN transgenic mouse bone marrow transplantation model. (A) Flow chart of the experimental procedure. BM from FVB/TgN transgenic mice, expressing GFP under the transcriptional control of the endothelial specific Tie2 promoter, was transplanted into lethally irradiated FVB/NJ wild-type recipients 30 days before treatment with AAV-VEGF. (B) Immunostaining for the endothelial-specific CD31 and GFP markers in muscle sections of VEGF-treated mice at 1 month after vector injection. In the FVB/TgN transgenic control mouse, most of the endothelial cells also stained positive for GFP (i-iii). In contrast, no Tie2-GFP–positive cells were found within the cellular infiltrates or incorporated into the wall of the newly formed vessels in the FVB/NJ-BMT TgN chimeric mice (iv-ix). Red indicates CD31+ cells; green, GFP; and blue, nuclei stained with DAPI.
FVB/TgN transgenic mouse bone marrow transplantation model. (A) Flow chart of the experimental procedure. BM from FVB/TgN transgenic mice, expressing GFP under the transcriptional control of the endothelial specific Tie2 promoter, was transplanted into lethally irradiated FVB/NJ wild-type recipients 30 days before treatment with AAV-VEGF. (B) Immunostaining for the endothelial-specific CD31 and GFP markers in muscle sections of VEGF-treated mice at 1 month after vector injection. In the FVB/TgN transgenic control mouse, most of the endothelial cells also stained positive for GFP (i-iii). In contrast, no Tie2-GFP–positive cells were found within the cellular infiltrates or incorporated into the wall of the newly formed vessels in the FVB/NJ-BMT TgN chimeric mice (iv-ix). Red indicates CD31+ cells; green, GFP; and blue, nuclei stained with DAPI.
Effects of local VEGF expression on progenitor cell mobilization
The possible mobilization of bone marrow vascular precursor cells by the local expression of VEGF was further assessed by flow cytometry. Mice were injected with AVV-VEGF in the tibialis anterior muscle and blood samples were collected 30 days after treatment (n = 10 animals). The quantification of cell populations expressing markers characterizing early endothelial progenitors39 was performed in the monocyte-sized cell population from peripheral blood and spleen mononuclear cells of the treated animals. The results did not reveal any significant enrichment in the AAV-VEGF–treated animals with respect to normal controls for Flk-1, CD34, c-Kit, and Sca-1 (shown in Figure 6 for the first 2 markers; representative flow plots are shown in Figure S2). Similar negative results were also obtained for CD11b and CD31 (not shown).
Mononuclear cells from peripheral blood of mice injected with AAV-VEGF were also cultured for 4 days in order to obtain an adherent population of acLDil+/BS-1–lectin+ cells, matching the previously described EPC phenotype.15,40 In keeping with the results of the flow cytometry analysis, no increase was detected in the number of EPCs grown from the blood of the treated animals compared with untreated controls (data not shown).
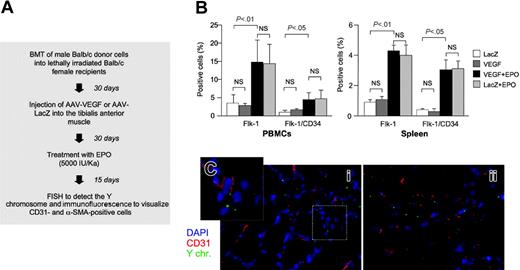
Erythropoietin (EPO) mobilizes bone marrow cells with an EPC phenotype that are recruited to the sites of neovascularization but are not incorporated in the neovessels. (A) Flow chart of the experimental procedure. Sex-mismatched AAV-VEGF–treated mice that received a transplant were treated with EPO in order to mobilize endothelial progenitor cells from the bone marrow. (B) Flow cytometry analysis of mononuclear cells from peripheral blood and spleen. Animals treated with EPO (but not those injected with AAV-VEGF or AAV-LacZ) showed a significant increase of Flk-1+ and Flk-1+/CD34+ cell populations, indicative of the mobilization of endothelial progenitors. Data (mean ± SD from 10 animals per group) represent the percentage of positive cells in the population of light scatter dot plots of monocyte-sized cells (see also Figure S2 and Asahara et al8 ). NS indicates not significant. (C) Immuno-FISH analysis of VEGF-treated muscle sections of animals treated with EPO. Despite the mobilization of EPCs, there was no increase in the number of Y-chromosome–positive cells expressing the CD31 endothelial markers at the sites of VEGF-induced neovascularization. Red indicates CD31+ cells; green, Y chromosome; and blue, nuclei stained with DAPI.
Erythropoietin (EPO) mobilizes bone marrow cells with an EPC phenotype that are recruited to the sites of neovascularization but are not incorporated in the neovessels. (A) Flow chart of the experimental procedure. Sex-mismatched AAV-VEGF–treated mice that received a transplant were treated with EPO in order to mobilize endothelial progenitor cells from the bone marrow. (B) Flow cytometry analysis of mononuclear cells from peripheral blood and spleen. Animals treated with EPO (but not those injected with AAV-VEGF or AAV-LacZ) showed a significant increase of Flk-1+ and Flk-1+/CD34+ cell populations, indicative of the mobilization of endothelial progenitors. Data (mean ± SD from 10 animals per group) represent the percentage of positive cells in the population of light scatter dot plots of monocyte-sized cells (see also Figure S2 and Asahara et al8 ). NS indicates not significant. (C) Immuno-FISH analysis of VEGF-treated muscle sections of animals treated with EPO. Despite the mobilization of EPCs, there was no increase in the number of Y-chromosome–positive cells expressing the CD31 endothelial markers at the sites of VEGF-induced neovascularization. Red indicates CD31+ cells; green, Y chromosome; and blue, nuclei stained with DAPI.
Bone marrow progenitor cells mobilized by erythropoietin home to the sites of neoangiogenesis but are not incorporated in the newly formed vessels
The above-described results indicate that AAV-mediated VEGF expression in muscle induces a strong local angiogenic response but is not sufficient to determine a significant mobilization of bone marrow precursors. Thus, higher dosages of the factor or the concomitant systemic administration of other cytokines might be necessary to induce precursor cell recruitment at the sites of neovascularization.
A cytokine that has been shown to effectively stimulate mobilization and migration of endothelial progenitors and to promote angiogenesis is erythropoietin (EPO).11,41 Therefore, we investigated the effects of systemic EPO administration combined with AAV-mediated VEGF gene expression in the skeletal muscle. Lethally irradiated female Balb/c mice (n = 20) received a transplant of male Balb/c bone marrow and, one month after transplantation, were injected with either AAV-VEGF (n = 10) or AAV-LacZ (n = 10) in the tibialis anterior muscle of one leg. After an additional 30 days, 6 mice per group were subjected to chronic treatment with EPO (5000 IU/kg for 2 weeks). At the end of the EPO treatment, the number of Flk-1–positive and Flk-1/CD34–double-positive cells was evaluated in peripheral blood and spleen (Figure 6B). Treatment with EPO was found to significantly increase (over 5-fold) the number of these precursor cells in both compartments, irrespective of the AAV vectors received. Accordingly, the number of acLDil+/BS-1–lectin+ cells grown from blood mononuclear cells of the EPO-treated animals was also found to be increased approximately 8 times (data not shown). Superimposable results were also observed in control animals, which did not receive transplants, that were treated for 14 days with the same amount of EPO (data not shown).
The AAV-VEGF–injected muscles were then analyzed by immuno-FISH in order to detect whether the increased precursor cell mobilization determined by EPO might eventually promote incorporation of bone marrow–derived cells into the newly formed vessels promoted by VEGF. As shown in Figure 6C, however, no significant increase in the number of Y-chromosome–positive/CD31+ cells was detected compared with untreated mice.
Ex vivo–expanded EPCs home to the sites of VEGF-induced neoangiogenesis but are not incorporated into newly formed vessels
We further explored the possibility that the in vitro expansion of bone marrow cells might promote maturation or activation of endothelial precursors able to integrate into the VEGF-induced vasculature once reinoculated into the recipient host.
The fraction of adherent cells from the bone marrow of Balb/c mice was expanded in vitro in 2 different media: one commonly used for EPC culture (D media) and the other one suitable for maintaining primary cells in a proliferation state (ND media; see “Transplantation of ex vivo–expanded EPCs”). After 2 weeks of culture, the cells were detached from the plate and their surface marker expression profile was determined by flow cytometry (Figure 7B). In both culture conditions, most cells were positive for the myelocytic/monocytic markers CD45 and CD11b; CD14 was expressed in over 60% and approximately 20% of cells grown in D and ND medium, respectively. Only a small fraction of cells showed positivity for the CD34 and c-Kit markers; less than 20% was positive for the endothelial-specific marker CD31. Finally, the positivity for Flk-1 was over 70% in cells grown in D medium and approximately 10% in cells grown in ND medium. Taken together, expression of these markers is consistent with those already described as displayed by bona fide EPCs.39,42,43
In vitro–cultured bone marrow cells with EPC phenotype home at sites of VEGF-induced angiogenesis but are not incorporated into the vessel wall.(A) Experimental design. Balb/c mice adherent bone marrow cells were cultured in vitro for 15 days in differentiating (D) and nondifferentiating medium (ND; see text) in order to enrich for endothelial progenitor cells. After 15 days, the EPCs were labeled with the PKH26 red fluorescent die and administered intravenously to syngenic recipient mice previously injected with AAV-VEGF in the right tibialis anterior muscle. (B) Flow cytometry phenotypic profile of the cell populations before injection into the animals (mean ± SD of 3 different experiments). (C) Number of PKH16-positive cells infiltrating the sites of VEGF-induced neovascularization at day 15 after cell injection. The cells expanded ex vivo using the ND medium were recruited approximately 4 times more efficiently than those cultivated in differentiating medium (mean ± SD of 3 different experiments). (D) Immunofluorescence analysis for the visualization of CD31 (green) and PKH26 (red). Colocalization of the 2 markers in the same cells was highly infrequent. (E) Immunofluorescence analysis for the visualization of CD11b (green) and PKH26 (red); arrows indicate 2 in vitro–labeled recruited cells. Most of the recruited cells were positive for the myelocytic/monocytic marker CD11b.
In vitro–cultured bone marrow cells with EPC phenotype home at sites of VEGF-induced angiogenesis but are not incorporated into the vessel wall.(A) Experimental design. Balb/c mice adherent bone marrow cells were cultured in vitro for 15 days in differentiating (D) and nondifferentiating medium (ND; see text) in order to enrich for endothelial progenitor cells. After 15 days, the EPCs were labeled with the PKH26 red fluorescent die and administered intravenously to syngenic recipient mice previously injected with AAV-VEGF in the right tibialis anterior muscle. (B) Flow cytometry phenotypic profile of the cell populations before injection into the animals (mean ± SD of 3 different experiments). (C) Number of PKH16-positive cells infiltrating the sites of VEGF-induced neovascularization at day 15 after cell injection. The cells expanded ex vivo using the ND medium were recruited approximately 4 times more efficiently than those cultivated in differentiating medium (mean ± SD of 3 different experiments). (D) Immunofluorescence analysis for the visualization of CD31 (green) and PKH26 (red). Colocalization of the 2 markers in the same cells was highly infrequent. (E) Immunofluorescence analysis for the visualization of CD11b (green) and PKH26 (red); arrows indicate 2 in vitro–labeled recruited cells. Most of the recruited cells were positive for the myelocytic/monocytic marker CD11b.
The cells were labeled by the PKH26 red fluorescent dye and injected into nonirradiated, syngenic mice that had previously received AAV-VEGF in the tibialis anterior muscle of one leg. Two weeks after injection, histologic examination of transduced and control AAV-LacZ–injected muscle specimens showed significant accumulation of red-labeled cells only at the sites of angiogenesis (Figure 7C-D). Quantification of the labeled cells in serial histologic muscle sections revealed that the recruitment of bone marrow cells was 4 times more efficient when the ND medium was used during the in vitro expansion (Figure 7C). Immunofluorescence staining revealed that the vast majority of the PKH26-labeled cells that had homed at the foci of VEGF-induced angiogenesis did not express the CD31 endothelial marker and were not incorporated into CD31+ vessels (Figure 7D); in contrast, most of these cells expressed the myeloid marker CD11b (Figure 7E).
These findings reinforce our previous results and highlight the tendency of mononuclear cells of bone marrow origin to home to the foci of neovascularization. Notably, we could not detect infiltrating fluorescent cells following the injection of freshly extracted, labeled total bone marrow (data not shown). This observation is in agreement with other published results,42 probably indicating that an activation step, such as that provided by the ex vivo culture, is required for the recruitment process to occur.
Discussion
Further to the work of Asahara et al4 in 1997, other investigators have subsequently confirmed the presence in adult organisms of circulating cells that express markers of both hematopoietic and endothelial lineages and behave as endothelial precursors. In particular, these cells have been shown to home to the sites of ischemia and to contribute to the formation of new blood vessels.5,8,19 However, the actual ability of these cells to be directly incorporated into the vascular structures is still a subject of open debate, since discordant results have been obtained by different laboratories in this respect.23,24
Here we investigated the possible contribution of bone marrow–derived progenitor cells to the process of neovascularization induced by the prolonged expression of VEGF, with special reference to the possibility that this factor might determine the recruitment of progenitors cells that might eventually be incorporated into the newly formed vessels. The results obtained, however, clearly ruled out this possibility.
The possible incorporation of bone marrow–derived cells into the VEGF-induced neovasculature was tested under 4 different experimental conditions. The first 2 models were based on the transplantation of male bone marrow into female mice or of Tie2-GFP transgenic bone marrow into normal recipients, followed by the detection of donor cells in the VEGF-induced vessels by their positivity for the Y chromosome or by their GFP fluorescence, respectively. The third experimental setting consisted of the treatment of the mice that received a transplant with EPO, a factor known to promote EPC mobilization. The fourth mouse model involved the ex vivo culture of bone marrow–derived EPCs, followed by their fluorescent labeling and reimplantation into the animals transduced with VEGF. Despite extensive histologic examination, a significant incorporation of bone marrow–derived cells was not detected in the VEGF-induced neovasculature in any of these experimental conditions. Occasional donor cells of male origin (Y+) positive for the CD31 endothelial marker were indeed present in some of the mononuclear infiltrates; however, we could not clearly establish whether these sporadic cells were adjacent or truly incorporated into small capillaries or whether they even represented leukocytes traveling inside small capillaries. Indeed, using the Tie2-GFP reporter system (in which activation of GFP expression only occurs in endothelial cells) we had never been able to confirm the presence of marrow-derived cells in the endothelium of small vessels or in larger collateral arteries.
These observations undisputedly indicate that the contribution of bone marrow precursor cells to the process of neovascularization induced by VEGF, if any, does not occur through the direct incorporation of these cells into the newly formed vasculature. Thus, the VEGF-driven neovascularization appears to be a bona fide angiogenic process, essentially depending on the proliferation and sprouting of cells resident in the treated tissues. In a strict manner, this conclusion only applies to our experimental conditions, in which VEGF is expressed at moderate levels for prolonged periods of time. After AAV transduction, the expression of the factor progressively increases over time in the treated muscles28 ; however, by using a standard enzyme-linked immunosorbent assay (ELISA), we have not been able to detect significant levels of VEGF secreted into the circulation (data not shown), an observation that correlates well with the absence of significant variations in the number of circulating cells with Flk-1, CD31, CD34, and c-Kit markers. Other investigators have reported that animal treatment with higher doses of VEGF, similar to other angiogenic cytokines and growth factors, including GM-CSF, statins, and EPO,11,21,44,45 promotes the mobilization of EPCs into the peripheral circulation. We cannot exclude that some of these conditions might also promote incorporations of these EPCs into the newly formed vasculature. In our case, however, this is clearly not the case for EPO, despite the remarkable mobilization of Flk-1+/CD34+ precursors induced by this cytokine and in contrast to what is described by other authors.11,21 Finally, we cannot exclude that the incorporation of bone marrow–derived EPCs into the vasculature might be favored in conditions of acute ischemia or after severe vascular injury.12,46,47 Despite these caveats, however, it seems important to point out that other laboratories, in agreement with our conclusions, have also found only occasional incorporation of bone marrow–derived precursors into the new vessels using other models of experimental angiogenesis.22-24,29,48
While the results reported in this manuscript clearly indicate that mononuclear cells of bone marrow origin are not incorporated in the VEGF-induced blood vessels, they also univocally show that VEGF determines a marked recruitment of bone marrow–derived cells to the sites of neoangiogenesis. Similarly, ex vivo–cultured adherent mononuclear cells of bone marrow origin, which stained positive for Dil-Ac-LDL and lectin but also expressed myeloid markers, were found to home to the sites of VEGF overexpression. These observations clearly raise the question of what might be the role of these mononuclear cells in the neoangiogenesis process. In this respect, it seems interesting to point out that T and B cells do not appear to play a direct role in VEGF-induced angiogenesis, since both new blood vessel formation and bone marrow cell recruitment were normal in severe combined immunodeficiency disease (SCID) mice that received a transplant (C.B.-17/IcrHsd-Prkdcscid) transduced with AAV-VEGF (data not shown). In addition, in normal mice, only occasional B and T cells are detectable in the muscle cell infiltrates.
As far as other types of circulating mononuclear cells are concerned, a vast proportion of the cells infiltrating the sites of VEGF neoangiogenesis displays broad myeloid markers, such as CD11b and CD45. In this respect, we have also analyzed by real-time PCR quantification the levels of expression of some chemokines and chemokine receptors that are known to act as chemoattractants for monocytic cells or to participate in the angiogenic process49,50 in the AAV-transduced tissues. The results obtained, which are shown in Figure S3, indicate that AAV-VEGF acts as a powerful inducer of the expression of MIP1β, RANTES, and MIG, as well as of the CCR1, CCR2, and CCR5 receptors. Obviously, it is unclear whether the increased expression of these genes is due to a direct effect of VEGF on the expressing tissues or occurs as a consequence of the infiltration by the mononuclear cells. In addition to chemokines, it should also be pointed out that VEGF itself is known to act as a chemotactic factor for monocytes and macrophages through the activation of the Flt-1 receptor.51 The exact identification of the phenotype of the infiltrating cells and, in particular, the discrimination between endothelial precursors, periendothelial myeloid cells, or dendritic cells—all of which have recently been described to participate in the angiogenesis process—will require the availability of specific markers defining each of these cell types.
While the exact phenotype and role of the bone marrow mononuclear cells still awaits to be defined, different indirect evidences suggest that their presence might be important for the process of new blood vessel formation. In particular, different experimental findings support the possibility that these cells of myeloid origin might act by exerting a local paracrine activity.52-56 The observation that the infusion of bone marrow–derived cells shows beneficial effects in various animal models of ischemia57 as well as in clinical settings58,59 is also consistent with this notion.
Prepublished online as Blood First Edition Paper, January 3, 2006; DOI 10.1182/blood-2005-08-3215.
Supported by grants from the Progetto Finalizzato “Genetica Molecolare” of the “Consiglio Nazionale delle Ricerche,” Italy; from the “Fondo per gli investimenti per la ricerca di base” (FIRB) program of the “Ministero dell'Istruzione, Università e Ricerca,” Italy; from the “Fondazione Cassa di Risparmio” of Trieste, Italy; and from the Telethon Foundation, Italy.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
The authors are grateful to Marina Dapas, Sara Tomasi, and Michela Zotti for their outstanding technical support and to Mauro Sturnega for his invaluable help in animal experimentation.




This feature is available to Subscribers Only
Sign In or Create an Account Close Modal