To learn how plasma fibronectin stabilizes platelet-rich thrombi in injured mesenteric arterioles of mice, we studied the impact of plasma fibronectin on platelet thrombus formation ex vivo in a parallel flow chamber. Thrombi were greater on surfaces coated with fibrin cross-linked to fibronectin by activated factor XIII than on surfaces coated with fibrin lacking cross-linked fibronectin or with fibronectin alone. Platelet thrombi were even greater when plasma fibronectin was perfused with platelets, resulting in deposition of the perfused fibronectin in platelet thrombi. The effect of perfused fibronectin on thrombogenesis was lost if fibronectin deposition was blocked by coperfusion with the N-terminal 70-kDa fragment of fibronectin or a peptide based on the functional upstream domain of protein F1 of Streptococcus pyogenes. Increases in thrombus formation were dependent on a platelet activator such as lysophosphatidic acid, amount of fibronectin cross-linked to fibrin, and concentration of fibronectin in the perfusate. The dependency of fibronectin concentration extended into the range of fibronectin concentrations associated with increased risk of coronary artery disease. At such concentrations, the 2 mechanisms for insolubilization of plasma fibronectin—cross-linking to fibrin and assembly by adherent and aggregating platelets—synergize to result in many-fold enhancement of platelet thrombus formation.
Introduction
Fibronectin is a glycoprotein dimer of 230- to 250-kDa subunits that is present in a soluble form in plasma and other body fluids and in an insoluble form in an extracellular matrix.1,2 Because it is a ligand of platelet surface receptors, for nearly 3 decades fibronectin has been suspected of playing a role in formation of platelet thrombi.3,4 Mice with liver-specific conditional knockout of the fibronectin gene allowed the impact of plasma fibronectin on formation of stable thrombi to be evaluated in vivo.5,6 Absence of plasma fibronectin did not alter the bleeding time and in vitro clotting time.5 Intravital videomicroscopy, however, demonstrated that mesenteric arteriole thrombi detach and embolize more readily after injury of mesenteric arterioles of mice lacking plasma fibronectin.6 This result is reminiscent of the scarcity of platelet thrombi that formed when plasma fibronectin was removed in experiments in which platelets, red blood cells, and plasma were reconstituted and perfused through flow chambers coated with collagen or endothelial cells.7-9 These results indicate that plasma fibronectin interacts with platelets in important ways to form arterial thrombi.
Several mechanisms may underlie the observed effects of plasma fibronectin on platelet thrombus formation. α5β1, αIIbβ3, and αvβ3 integrins mediate adhesion of platelets to fibronectin-coated surfaces.10,11 As a consequence of activation of the blood coagulation cascade by tissue factor, either originating from the damaged vessel wall12 or hematopoietic cell–derived microparticles,13,14 plasma fibronectin becomes tethered to the α-chain of fibrin by ϵ-(γ-glutamyl)-lysyl cross-links in a reaction catalyzed by thrombin-activated coagulation factor XIII (FXIIIa).15,16 A second insolubilization pathway is assembly of plasma fibronectin into fibrillar extracellular matrix arrays by adherent platelets.11,17 Plasma fibronectin, therefore, could stabilize platelet thrombi by being cross-linked to fibrin clots and enhancing platelet adherence to fibrin or by polymerization on the surface of adherent and aggregated platelets, thus enhancing platelet cohesion. Plasma fibronectin also binds to thrombin-activated platelets in suspension,18 and monoclonal antifibronectin antibodies block platelet aggregation ex vivo.19,20 These latter results indicate that plasma fibronectin participates in platelet aggregation.
In the present study, we compared the effects of surfaces coated with a fibrin matrix alone with surfaces coated with a fibrin matrix cross-linked to plasma fibronectin by FXIIIa (fibronectin-fibrin) on formation of platelet thrombi under shear conditions. We then studied the impact of plasma fibronectin included in the perfusate, thus resulting in assembly of fibronectin by platelets in the developing thrombi. Our results indicate that plasma fibronectin insolubilized both by cross-linking to fibrin and assembly by developing platelet thrombi enhances thrombogenesis. When fibronectin concentrations for the cross-linking to fibrin and the perfusion extended into the range associated with increased risk of coronary artery disease,21,22 thrombogenesis was many-fold enhanced.
Materials and methods
Materials
The sources and preparations of many of the chemical and biologic materials were described previously.11 Purified human fibrinogen and fibronectin did not contain cross-contamination as detected by Western blotting with antibodies against human fibronectin or fibrinogen, respectively.11 The N-terminal 70-kDa fragment of fibronectin (70K) consists of type I modules 1-9 and type II modules 1-21,2 and lacks type III modules including the “synergy” and RGD-containing adhesive sequences in type III modules 9-10.23,24 Human FXIII was purchased from Enzyme Research Laboratories (South Bend, IN). ADP, bovine trypsin, and soybean trypsin inhibitor were obtained from Sigma (St Louis, MO). The functional upstream domain (FUD), a recombinant 49-residue derived from protein F1 of Streptococcus pyogenes, was prepared as described.25
Anti-70K fragment hybridoma antibodies were produced in the University of Alabama-Birmingham Epitope Recognition Core Facility.26 7E3 (anti-β3) and 10E5 (anti-αIIbβ3) were gifts from Barry Coller (Rockefeller University, New York, NY). LM609 (anti-αvβ3) and monoclonal antibody (mAb) 13 (anti-β1) were provided by David Cheresh (Scripps Research Institute, La Jolla, CA) and Steven Akiyama (National Institute of Environmental Health Sciences, Research Triangle Park, NC), respectively.
Washed human platelets were prepared as described previously.17 Red blood cells were washed 3 times with HEPES-Tyrode buffer including 0.1% fatty acid-free BSA and recovered by centrifugation at 2000g for 10 minutes. Approval to obtain blood samples was obtained from the University of Wisconsin-Madison review board. Informed consent was provided according to the Declaration of Helsinki. Before perfusion experiments, washed platelets and red blood cells were combined into a suspension that contained 2 × 108 platelets/mL plus 40% red blood cells.
Preparation of fibrin or fibronectin-fibrin matrices
Fibrin matrices were prepared by addition of 1 U/mL thrombin to human or mouse fibrinogen, 500 μg/mL; FXIII, 5 μg/mL; and 2 mM CaCl2 in Tris-buffered saline (TBS; 20 mM Tris-HCl, pH 7.4, and 150 mM NaCl).11 For fibronectin-fibrin matrices, 50 μg/mL fibronectin was added to the mixture prior to addition of thrombin unless otherwise noted, thus maintaining the physiologic 1:10 mass ratio of fibronectin to fibrinogen in plasma. In some experiments, 35 μg/mL (500 nM) 70K fragment or various concentrations of fibronectin were present. The mixture, 0.5 mL, was placed in wells of a 24-well plate with or without a 1.2-cm diameter coverslip or 3 mL was placed in 6-cm diameter Petri dishes with or without a 3.5-cm diameter coverslip. After incubation overnight at 4°C, all visible clot was aspirated. After 3 rinses, the matrices were postcoated with 1% fatty acid–free BSA. A thin layer of fibrin or fibronectin-fibrin matrix remained as assessed by phase microscopy (not shown). The matrices stained uniformly as expected for just fibrin or fibronectin and fibrin by double immunofluorescence (not shown).
To estimate amounts of fibronectin cross-linked to fibrin, the rinsed matrices were scraped and digested with 50 μL of 100 μg/mL trypsin for 5 minutes at room temperature. As a control, fibronectin or 70K fragment was digested with 1 μg/mL trypsin for 10 minutes at room temperature. Trypsin digestion was stopped by addition of soybean trypsin inhibitor (final concentration, 20 μg/mL). The solutions were mixed with 10 μL SDS lysis buffer (TBS, pH 7.4, including 6 M urea, 4% SDS, 1 mM EDTA, 1 mM EGTA, and cocktail of protease inhibitors), including 100 mM DTT, and electrophoresed (a 3.3% stacking gel and a 10% separating gel). Separated fragments were immunoblotted with an mAb selected to recognize an epitope in the N-terminal 27-kDa fragment that was generated by limited trypsin digest of fibronectin or 70K fragment.
Adhesion and aggregation of platelets, incorporation of soluble fibronectin or its 70K fragment into platelet thrombi, and inhibitory effects of FUD or 70K fragment under shear conditions
Adhesion and aggregation of platelets on fibrin or fibronectin-fibrin under shear conditions were studied using a parallel plate flow chamber (Glycotech, Rockville, MD) in which a silicone rubber gasket with a thickness of 0.127 mm and a flow path width of 2.5 mm were placed on a 3.5-cm diameter coverslip or 6-cm diameter Petri dish coated with fibrin or fibronectin-fibrin. The inlet of the flow chamber was connected to a buffer reservoir by silicone tubing, and the outlet was connected with a peristaltic pump to draw fluid through the flow chamber. In some experiments, HEPES-Tyrode buffer containing 50 μg/mL fibronectin with or without 5 μg/mL FXIII, 1 U/mL thrombin, and 2 mM CaCl2, was preperfused without platelets over fibrin- or fibronectin-fibrin–coated coverslips or Petri dishes for 15 minutes at a wall shear rate of 1250 s–1. Otherwise, the chamber was preperfused with HEPES-Tyrode buffer containing 0.1% fatty acid-free BSA.
The inlet was then connected with a prewarmed suspension of platelets and red blood cells in HEPES-Tyrode buffer including 5 μM 1-oleolylysophosphatidic acid (LPA), 2 mM CaCl2, and 0.1% fatty acid–free BSA. In various experiments, cells were coperfused with 10 to 600 μg/mL FITC-labeled fibronectin (FITC-fibronectin) or unlabeled fibronectin, or 7 μg/mL FITC-labeled 70K fragment (FITC-70K fragment) or unlabeled 70K fragment in the presence or absence of 1 μM FUD or 70K fragment. Perfusion was for 5 minutes at a wall shear rate of 300 or 1250 s–1 at 37 ± 1°C. The coverslip was then taken out, and adherent thrombi were washed, fixed, permeabilized, stained with rhodamine-conjugated phalloidin, and examined as described previously.17 Epifluorescence images were obtained using an Olympus BX60 microscope equipped with 10 ×/0.3 or 100 ×/1.3 objective lenses (Olympus, Melville, NY), a SPOT RT 2.3.1 camera (Diagnostic Instruments, Sterling Heights, MI), and SPOT RT 3.4 software. Confocal images were obtained using a Bio-Rad MRC-1024 laser scanning microscope equipped with a 10 ×/1.4 objective lens (Bio-Rad, Hercules, CA), a photomultiplier tube, and LaserSharp 2000Confocal software. Images were prepared for publication using Adobe Photoshop (Adobe Systems, San Jose, CA). Analyses of adherent and aggregated platelets were made on the area proximal to the inlet of the chamber. To detect secreted endogenous fibronectin or fibrinogen in platelet thrombi, platelets perfused over surfaces coated with mouse fibrin were fixed and immunostained with rabbit polyclonal anti–human fibronectin antibody and mouse monoclonal anti–human fibrinogen antibody that does not recognize mouse fibrinogen or fibrin, followed by incubation with FITC-conjugated anti–rabbit IgG and rhodamine-conjugated anti–mouse IgG antibody.
The surface coverage and thrombus volume in an area of 58 000 μm2 were calculated from confocal microscopic images of platelets stained by rhodamine-phalloidin, obtained at 1.0-μm intervals in the z-axis using an excitation wavelength of 585 nm, as described previously.27 Images were analyzed using Metamorph software (version 4.5; Universal Imaging, West Chester, PA). The area occupied by all thrombi in a given 1.0-μm cross-section was estimated, and the thrombus volume was calculated as the sum of these values.
The number of adherent platelets in thrombi in a flow field area of 38 mm2 was also estimated by an assay in which cellular ATP is detected by luminescence.11 Platelets adherent to a protein-coated Petri dish after 5 minutes of perfusion were washed twice. After removing extra solution around the marked flow field with Kimwipes, adherent platelets were lysed with 400 μL 50% luminescence reagent mixed with HEPES-Tyrode buffer. To calculate the number of adherent platelets, luminescence was measured by dilutions of standard suspensions of platelets. The comparison between platelet thrombi and the standard suspension took into account loss of ATP content due to secretion from dense granules,28 which we estimated as 25% based on the decrease in luminescence signals detected in platelets before and after aggregation in suspension stimulated by LPA.
Replication of results and statistical analysis
All experimental permutations were tested in 3 to 6 separate experiments carried out using blood of different volunteer blood donors. Data were expressed as mean plus or minus standard deviation (SD). The Student t test or ANOVA with the Dunnett test was used for comparison of 2 or many groups, respectively. Differences were considered significant at P below .05.
Results
Adhesion and aggregation of platelets are enhanced on fibronectin-fibrin compared with fibrin alone under shear conditions
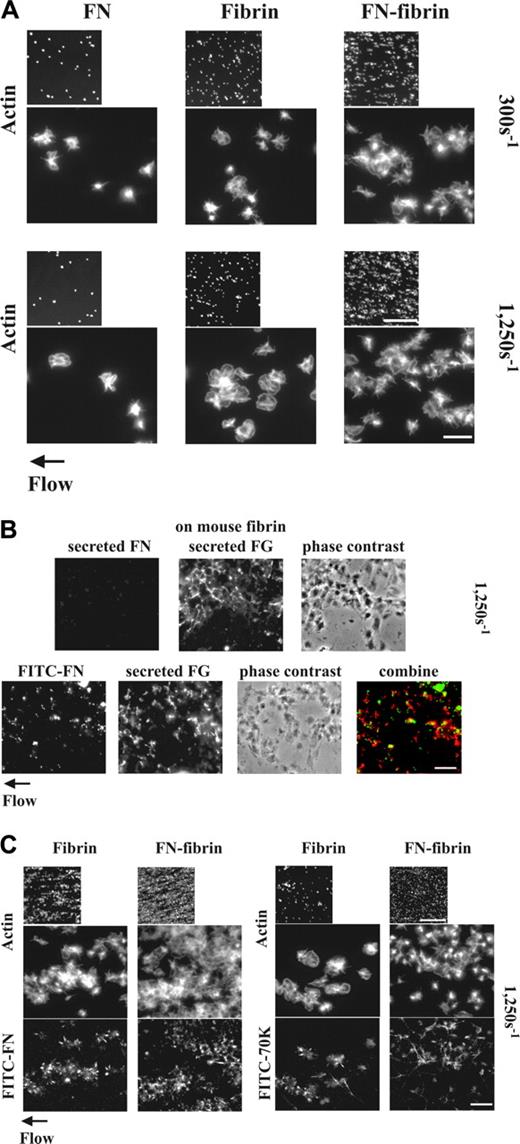
Platelets form thrombi more readily in a flow chamber on surfaces of fibrin than of adsorbed fibrinogen.11,29 Previous reports demonstrated that fibronectin-fibrin is a better substrate for adhesion and spreading of NIH3T3 cells than fibrin.30,31 We therefore hypothesized that fibronectin-fibrin might behave differently in supporting formation of platelet thrombi compared with fibrin alone. Indeed, after 5 minutes of perfusion at a wall shear rate of 300 or 1250 s–1 more LPA-activated platelets were adherent and aggregated on fibrin that had been formed in the presence of 50 μg/mL plasma fibronectin than on fibrin alone as detected by staining of filamentous actin cytoskeleton (Figure 1A). Surfaces coated with 50 μg/mL fibronectin alone supported platelet adhesion and aggregation less than fibrin alone (Figure 1A). The lack of platelet adhesion and aggregation on fibronectin-coated surfaces was consistent with previous results showing that surfaces coated with similar concentrations of fibronectin (10-20 μg/cm2) support platelet spreading poorly.32
Quantitative measurement of platelet thrombi supported the microscopic observations. Surface coverages of 5% and 20% were achieved by platelets adherent to fibrin and fibronectin-fibrin, respectively (Table 1). Morphometric quantification of thrombus volumes and luminescence quantification of numbers of adherent platelets both demonstrated an approximately 3-fold increase when fibronectin is cross-linked to the fibrin matrix (Table 1). Thrombus volumes and numbers of adherent platelets on each substrate were roughly the same at the 2 shear rates (not shown). Numbers of platelets adherent to surfaces coated with 50 μg/mL fibronectin alone were 2.5- and 6-fold less than numbers of adherent platelets to fibrin and fibronectin-fibrin, respectively, at the 2 shear rates (not shown).
Effect of plasma fibronectin or its N-terminal 70K fragment on surface coverage, thrombus volume, and number of adherent platelets at a wall shear rate of 1250 s–1
Substrate and perfusate . | Surface coverage, μm2 × 10-4 (%) . | Thrombus volume, μm3 × 10-4 . | Adherent platelets, × 10-6 . |
|---|---|---|---|
| Fibrin (+FXIII) | 0.3 ± 0.1 (5) | 1.4 ± 0.5 | 2.0 ± 0.8 |
| + FN | 1.9 ± 0.5 (33)* | 6.4 ± 1.2* | 7.8 ± 1.7* |
| + 70K | 0.3 ± 0.1 (5) | 1.7 ± 0.6 | 1.7 ± 0.8 |
| Fibrin (-FXIII) | 0.3 ± 0.1 (5) | 1.5 ± 0.6 | 1.8 ± 0.6 |
| + FN | 1.5 ± 0.7 (26)† | 5.6 ± 1.6† | 6.5 ± 1.9† |
| FN-fibrin (+FXIII) | 1.2 ± 0.3 (20)‡ | 3.5 ± 0.8‡ | 6.0 ± 1.4‡ |
| + FN | 4.2 ± 0.7 (72)*‡ | 11.3 ± 1.5*‡ | 18.0 ± 2.5*‡ |
| + 70K | 1.1 ± 0.3 (19)‡ | 5.0 ± 1.9‡ | 5.6 ± 1.9‡ |
| FN-fibrin (-FXIII) | 0.4 ± 0.1 (6) | 1.7 ± 0.6 | 1.9 ± 0.7 |
| + FN | 1.7 ± 0.6 (29)† | 5.9 ± 1.8† | 6.8 ± 1.6† |
Substrate and perfusate . | Surface coverage, μm2 × 10-4 (%) . | Thrombus volume, μm3 × 10-4 . | Adherent platelets, × 10-6 . |
|---|---|---|---|
| Fibrin (+FXIII) | 0.3 ± 0.1 (5) | 1.4 ± 0.5 | 2.0 ± 0.8 |
| + FN | 1.9 ± 0.5 (33)* | 6.4 ± 1.2* | 7.8 ± 1.7* |
| + 70K | 0.3 ± 0.1 (5) | 1.7 ± 0.6 | 1.7 ± 0.8 |
| Fibrin (-FXIII) | 0.3 ± 0.1 (5) | 1.5 ± 0.6 | 1.8 ± 0.6 |
| + FN | 1.5 ± 0.7 (26)† | 5.6 ± 1.6† | 6.5 ± 1.9† |
| FN-fibrin (+FXIII) | 1.2 ± 0.3 (20)‡ | 3.5 ± 0.8‡ | 6.0 ± 1.4‡ |
| + FN | 4.2 ± 0.7 (72)*‡ | 11.3 ± 1.5*‡ | 18.0 ± 2.5*‡ |
| + 70K | 1.1 ± 0.3 (19)‡ | 5.0 ± 1.9‡ | 5.6 ± 1.9‡ |
| FN-fibrin (-FXIII) | 0.4 ± 0.1 (6) | 1.7 ± 0.6 | 1.9 ± 0.7 |
| + FN | 1.7 ± 0.6 (29)† | 5.9 ± 1.8† | 6.8 ± 1.6† |
The surface coverage and thrombus volume were measured from confocal microscopic images, and the number of adherent platelets was calculated by luminescence signal as described in “Materials and methods.” Values represent the mean ± SD (n = 4-6).
FN indicates fibronectin; 70K, N-terminal 70 kDa region of FN.
P < .01 as compared with the absence of plasma FN on each substrate-coated surface.
n = 2-3 (statistics were not applied).
P < .01 as compared with a fibrin substrate.
Adhesion and aggregation of platelets on fibrin and fibronectin-fibrin incorporation of soluble fibronectin or 70K fragment into platelet thrombi and effect of perfused fibronectin on the formation of platelet thrombi under shear conditions. (A) A suspension of platelets and red blood cells was perfused through a flow chamber opposed to a coverslip coated with fibrin or fibronectin-fibrin (FN-fibrin) at shear rates of 300 or 1250 s–1 for 5 minutes. Coverslips were taken out of the chamber and washed. Platelets were fixed, permeabilized, stained with rhodamine-phalloidin, and observed by epifluorescence microscopy. The small and large pictures were taken with × 10 (bar = 100 μm) and × 100 objectives (bar = 10 μm), respectively. (B-C) A suspension of platelets and red blood cells without soluble proteins (B) or premixed with 100 nM FITC-fibronectin or FITC-70K fragment (B-C) was perfused through a flow chamber opposed to a coverslip coated with human fibrin or fibronectin-fibrin, or mouse fibrin at a shear rate of 1250 s–1 for 5 minutes. (B) Platelets adhered to mouse fibrin-coated surfaces in the absence (top panel) or presence of FITC-fibronectin (bottom panel). Adherent platelets were fixed and incubated with a rabbit polyclonal antibody against human fibronectin and a mouse mAb against human fibrinogen, followed by incubation with FITC-conjugated anti–rabbit IgG and rhodamine-conjugated anti–mouse IgG antibody (top panel) or incubated only with mouse antifibrinogen antibody and rhodamine-conjugated antimouse IgG (bottom panel). The images in the lower panel were obtained by confocal microscopy and are from a 1-μm slice taken 4 μm above the plane of the coverslip. Bar = 10 μm. (C) Coverslips were taken out of the chamber after perfusion with FITC-fibronectin or FITC-70K fragment, and platelets were stained with rhodamine-phalloidin. Microscopy was performed as described. The small and large pictures were taken with × 10 (bar = 100 μm) and × 100 × (bar = 10 μm), respectively. The images of phalloidin-stained platelets should be compared with the bottom sections of panel A.
Adhesion and aggregation of platelets on fibrin and fibronectin-fibrin incorporation of soluble fibronectin or 70K fragment into platelet thrombi and effect of perfused fibronectin on the formation of platelet thrombi under shear conditions. (A) A suspension of platelets and red blood cells was perfused through a flow chamber opposed to a coverslip coated with fibrin or fibronectin-fibrin (FN-fibrin) at shear rates of 300 or 1250 s–1 for 5 minutes. Coverslips were taken out of the chamber and washed. Platelets were fixed, permeabilized, stained with rhodamine-phalloidin, and observed by epifluorescence microscopy. The small and large pictures were taken with × 10 (bar = 100 μm) and × 100 objectives (bar = 10 μm), respectively. (B-C) A suspension of platelets and red blood cells without soluble proteins (B) or premixed with 100 nM FITC-fibronectin or FITC-70K fragment (B-C) was perfused through a flow chamber opposed to a coverslip coated with human fibrin or fibronectin-fibrin, or mouse fibrin at a shear rate of 1250 s–1 for 5 minutes. (B) Platelets adhered to mouse fibrin-coated surfaces in the absence (top panel) or presence of FITC-fibronectin (bottom panel). Adherent platelets were fixed and incubated with a rabbit polyclonal antibody against human fibronectin and a mouse mAb against human fibrinogen, followed by incubation with FITC-conjugated anti–rabbit IgG and rhodamine-conjugated anti–mouse IgG antibody (top panel) or incubated only with mouse antifibrinogen antibody and rhodamine-conjugated antimouse IgG (bottom panel). The images in the lower panel were obtained by confocal microscopy and are from a 1-μm slice taken 4 μm above the plane of the coverslip. Bar = 10 μm. (C) Coverslips were taken out of the chamber after perfusion with FITC-fibronectin or FITC-70K fragment, and platelets were stained with rhodamine-phalloidin. Microscopy was performed as described. The small and large pictures were taken with × 10 (bar = 100 μm) and × 100 × (bar = 10 μm), respectively. The images of phalloidin-stained platelets should be compared with the bottom sections of panel A.
Pretreatment of the fibrin matrices with an irreversible thrombin inhibitor, D-Phe-Pro-Arg-chloromethylketone, prior to connection with the flow chamber did not affect thrombus formation compared with nontreated matrices (not shown). A substrate of fibrin formed in the presence of fibronectin but the absence of FXIIIa behaved similarly to a substrate of fibrin alone (Table 1). Fibrin formed in the presence or absence of FXIIIa, however, did not show any difference in thrombogenesis (Table 1). These experiments indicate that cross-linking of plasma fibronectin to fibrin catalyzed by FXIIIa during formation of the fibrin matrix is required for the enhancement of platelet recruitment by the matrix.
To learn which integrins mediate platelet adhesion to fibrin or fibronectin-fibrin under shear conditions, anti-integrin antibody was preincubated with a suspension of platelets and red blood cells and coperfused at a shear rate of 1250 s–1. Deposition of platelets on fibrin or fibronectin-fibrin was inhibited by anti-αIIbβ3 and anti-αvβ3 but not anti-β1 (Table 2).
Effect of antibody against integrin on platelet accumulation on fibrin or fibronectin-fibrin in the absence of soluble fibronectin at a wall shear rate of 1250s–1
. | Adherent platelets, × 10-6 (% inhibition) . | . | |
|---|---|---|---|
| Antibody . | Fibrin . | FN-fibrin . | |
| Control | 1.8 ± 0.7 | 5.9 ± 1.2 | |
| With mouse IgG1 | 1.7 ± 0.6 (5) | 5.6 ± 1.0 (6) | |
| With rat IgG2 | 1.6 ± 0.7 (9) | 5.3 ± 0.9 (11) | |
| With anti-β3 (7E3) | 0.2 ± 0.1 (90)* | 0.7 ± 0.3 (88)* | |
| With anti-αIIbβ3 (10E5) | 0.2 ± 0.1 (90)* | 0.8 ± 0.3 (87)* | |
| With anti-αvβ3 (LM609) | 1.0 ± 0.5 (47)* | 3.0 ± 1.4 (50)* | |
| With anti-β1 (mAb13) | 1.6 ± 0.9 (12) | 4.6 ± 1.8 (24) | |
. | Adherent platelets, × 10-6 (% inhibition) . | . | |
|---|---|---|---|
| Antibody . | Fibrin . | FN-fibrin . | |
| Control | 1.8 ± 0.7 | 5.9 ± 1.2 | |
| With mouse IgG1 | 1.7 ± 0.6 (5) | 5.6 ± 1.0 (6) | |
| With rat IgG2 | 1.6 ± 0.7 (9) | 5.3 ± 0.9 (11) | |
| With anti-β3 (7E3) | 0.2 ± 0.1 (90)* | 0.7 ± 0.3 (88)* | |
| With anti-αIIbβ3 (10E5) | 0.2 ± 0.1 (90)* | 0.8 ± 0.3 (87)* | |
| With anti-αvβ3 (LM609) | 1.0 ± 0.5 (47)* | 3.0 ± 1.4 (50)* | |
| With anti-β1 (mAb13) | 1.6 ± 0.9 (12) | 4.6 ± 1.8 (24) | |
The luminescence signal was obtained as described in “Materials and methods.” Values represent the mean ± SD of 3 to 4 separate experiments.
P < .01 as compared with a control group.
Soluble fibronectin is deposited and increases adhesion and aggregation of platelets under shear conditions
In the flow experiment, the only source of fibrinogen, which is a major mediator of platelet aggregation,33 is from α-granules of adherent platelets. To localize this fibrinogen, platelet thrombi formed on surfaces coated with mouse fibrin were immunostained with mouse anti–human fibrinogen antibody. Platelets were not permeabilized to detect secreted fibrinogen. Fibrinogen was detected throughout the platelet thrombi (Figure 1B upper panel). Although fibronectin is also known to be secreted from α-granules of platelets activated by thrombin or collagen,34 released fibronectin was not detected by simultaneous immunostaining with rabbit polyclonal anti–human fibronectin antibodies (Figure 1B upper panel). However, when we added FITC-fibronectin at a concentration of 50 μg/mL (100 nM) to the perfusate and observed the localization of fibronectin and fibrinogen by dual fluorescent imaging in a confocal microscope, FITC-fibronectin was found in the same focal planes as secreted fibrinogen in platelet thrombi on mouse fibrin-coated surfaces but colocalized only partially with fibrinogen (Figure 1B lower panel).
Because platelets adhering to fibrin under shear conditions both form thrombi and assemble plasma fibronectin, whereas platelets adhering to adsorbed fibrinogen do neither,11 we hypothesized that fibronectin assembly is a postadhesive event that favors thrombogenesis. Thus, we studied effects of coperfusion of plasma fibronectin with platelets through fibrin- or fibronectin-fibrin–coated surfaces at a wall shear rate of 1250 s–1. More platelets adhered and aggregated on both substrates after 5 minutes of perfusion in the presence (Figure 1C) than the absence of 50 μg/mL FITC-fibronectin (Figure 1A). FITC-fibronectin localized at the periphery of adherent platelets and in the platelet aggregates on fibrin- or fibronectin-fibrin–coated surfaces (Figure 1C). As compared with surface coverages in the absence of plasma fibronectin, surface coverages were enhanced by platelets adherent to fibrin and fibronectin-fibrin, respectively, when 50 μg/mL plasma fibronectin was present (Table 1). Coperfusion of plasma fibronectin resulted in increases in thrombus volumes and numbers of adherent platelets by 3- to 4-fold (Table 1). The same fold enhancement by perfused fibronectin was observed at a shear rate of 300 s–1 (not shown).
We then tested effects of coperfusion with 70K fragment, which binds in the same distribution on spread platelets as intact fibronectin,11 and competes for assembly of intact fibronectin by binding to assembly initiation sites.17 Although 7 μg/mL (100 nM) FITC-70K fragment coperfused was incorporated into platelet thrombi, it did not enhance adhesion and aggregation of platelets (Figure 1C) and had no effect on surface coverage, thrombus volumes, or numbers of adherent platelets compared with the absence of soluble ligands (Table 1). The positive effect of perfused fibronectin on thrombogenesis on fibrin matrices formed in the absence of FXIII was similar to its effect on thrombogenesis on fibrin formed in the presence of FXIIIa (Table 1), demonstrating that the effect of perfused fibronectin on thrombus formation is not dependent on the presence of FXIIIa in the matrix. These results indicate that deposition of plasma fibronectin by adherent platelets enhances the ability of platelets to use fibrin or fibronectin-fibrin matrices to initiate and build platelet aggregates. The inability of the 70K fragment to enhance thrombogenesis indicates that the enhancement of platelets thrombus formation requires other regions of intact fibronectin in addition to the N-terminal region involved in cross-linking to fibrin and binding to assembly initiation sites.
The effect of soluble plasma fibronectin on thrombus formation under shear conditions is due to its deposition into platelet aggregates
To test whether the effect of perfused plasma fibronectin results from assembly within developing platelet thrombi or some other mechanisms, for example, additional incorporation into the fibrin coating, we performed 3 types of experiments.
First, we preperfused 50 μg/mL unlabeled fibronectin or FITC-fibronectin over fibrin or fibronectin-fibrin matrices, washed out unbound protein, and then perfused the cell suspension. Some preperfused fibronectin became associated with fibrin as ascertained by fluorescence microscopy, but there was no enhancement of platelet thrombus formation compared with the matrices not preperfused with plasma fibronectin (not shown).
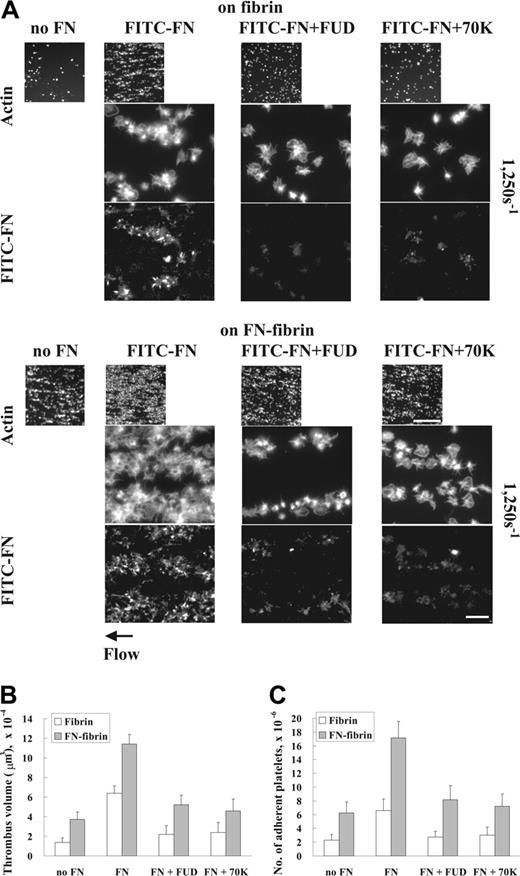
Second, we tested the effects of FUD, a peptide derived from protein F1 of S pyogenes, and 70K fragment, which are known to perturb fibronectin assembly by adherent fibroblasts and platelets under static conditions.17,35 Coperfusion of FUD or 70K fragment with FITC-fibronectin at a wall shear rate of 1250 s–1 diminished incorporation of FITC-fibronectin into platelet thrombi, concomitant with decreased formation of platelet thrombi on fibrin or fibronectin-fibrin (Figure 2A). Thrombus volumes and numbers of adherent platelets were decreased by 50% to 60% when unlabeled plasma fibronectin was coperfused with FUD or 70K fragment (Figure 2B-C). Perfusion of platelets with 1 μM FUD or 70K fragment did not inhibit adhesion and build-up of platelets on fibrin or fibronectin-fibrin when plasma fibronectin was not present in the perfusate (not shown).
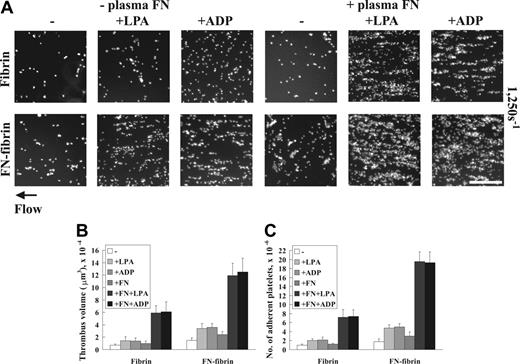
Third, we assessed the role of LPA, which enhances fibronectin assembly by adherent fibroblasts and platelets under static conditions.17,36 When we omitted LPA, plasma fibronectin alone did not enhance platelet thrombus formation (Figure 3). Coperfusion of 5 μM ADP, which like LPA enhances fibronectin assembly by adherent platelets under static conditions,17 substituted for LPA in enhancing thrombus formation under shear conditions (Figure 3). The positive effect of LPA or ADP on fibronectin-enhanced thrombus formation required a minimal concentration of 1 μM (not shown).
These results all indicate that direct assembly of plasma fibronectin by growing platelet thrombi is responsible for the enhancing effect of perfused plasma fibronectin on thrombogenesis.
Formation of platelet thrombi is dependent on amounts of fibronectin assembled by developing platelet thrombi and cross-linked into the fibrin matrix
The physiologic concentration of plasma fibronectin is 250 to 500 μg/mL,37,38 5- to 10-fold higher than the concentration used to make fibronectin-fibrin or included in the perfusate in Figures 1, 2, 3 and Table 1. We therefore tested whether higher concentrations of perfused plasma fibronectin positively influence thrombogenicity. Fibronectin, 10 to 600 μg/mL, was coperfused with platelets at a wall shear rate of 1250 s–1 over surfaces coated with fibrin that had been formed in the absence or presence of 50 μg/mL fibronectin. Platelets adhered and aggregated on fibrin or fibronectin-fibrin in a manner dependent on concentrations of perfused plasma fibronectin (Figure 4A). Both thrombus volumes and numbers of adherent platelets to fibrin or fibronectin-fibrin increased as the fibronectin concentration increased (Figure 4B). The increase of thrombus formation was maximum when concentrations of plasma fibronectin, 200 to 600 μg/mL, were perfused. These results indicate that plasma fibronectin enhances platelet thrombus formation more at physiologic concentrations than at lower concentrations.
Effect of inhibition of deposition of perfused fibronectin on platelet thrombus formation under shear conditions. A suspension of platelets and red blood cells was premixed with 100 nM FITC-fibronectin (A) or unlabeled fibronectin (B-C) in the presence or absence of 1 μM FUD or 70K fragment and perfused through a flow chamber opposed to a coverslip or culture dish coated with fibrin or fibronectin-fibrin for 5 minutes at a shear rate of 1250 s–1. (A) Coverslips were taken out of the chamber, and microscopy was performed as described in Figure 1. The small and large pictures were taken with × 10 (bar = 100 μm) and × 100 objectives (bar = 10 μm), respectively. (B-C) Thrombus volumes and platelet numbers were measured as described in Table 1. Values represent the mean ± SD (n = 3 experiments).
Effect of inhibition of deposition of perfused fibronectin on platelet thrombus formation under shear conditions. A suspension of platelets and red blood cells was premixed with 100 nM FITC-fibronectin (A) or unlabeled fibronectin (B-C) in the presence or absence of 1 μM FUD or 70K fragment and perfused through a flow chamber opposed to a coverslip or culture dish coated with fibrin or fibronectin-fibrin for 5 minutes at a shear rate of 1250 s–1. (A) Coverslips were taken out of the chamber, and microscopy was performed as described in Figure 1. The small and large pictures were taken with × 10 (bar = 100 μm) and × 100 objectives (bar = 10 μm), respectively. (B-C) Thrombus volumes and platelet numbers were measured as described in Table 1. Values represent the mean ± SD (n = 3 experiments).
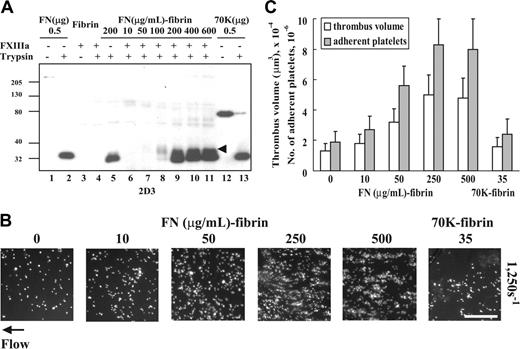
We also tested the effect of changing the amount of fibronectin cross-linked to fibrin during formation of fibrin clots. The N-terminal region of fibronectin is known to be cross-linked to the proteolytically sensitive C-terminal “tail” of the fibrin α-chain16 and thereby becomes bound to the series of cross-linked α-chain polymers. Cross-linking was quantified after digestion of these complexes with trypsin, that is, tryptic digests of cross-linked fibrin or fibronectin-fibrin were immunoblotted with 2D3, an mAb against an epitope that is in the N-terminal 27-kDa fragment of fibronectin. 2D3 detected the 27-kDa fragment of trypsin-digested fibronectin or 70K fragment as well as intact fibronectin and 70K fragment (Figure 5A lanes 1-2 and 12-13). The antibody also detected the 27-kDa fragment in a trypsin digest of fibrin that had been formed in the presence of 200 μg/mL fibronectin and absence of FXIIIa (Figure 5A lane 5). A larger fragment of 37 kDa, which presumably is the complex of the 27-kDa fragment and a piece excised from the fibrin α-chain, was detected in the trypsin digests of fibrin cross-linked to fibronectin by FXIIIa (Figure 5A lanes 7-11, arrowhead). The 37-kDa band predominated in the digest of fibrin cross-linked in the presence of 100 μg/mL fibronectin (lane 8). The 27-kDa fragment was also detected in trypsin digests of fibrin formed in the presence of FXIIIa and higher concentrations of fibronectin (200-600 μg/mL). We suspect that the uncross-linked fragment is derived from dimeric fibronectin that is cross-linked by only one of its subunits. The effects of increased amounts of fibronectin in the fibrin matrices on platelet thrombus formation were then tested. Platelets adhered and aggregated on fibronectin-fibrin in a manner dependent on amounts of fibronectin cross-linked to fibrin with the maximum effect on thrombus volumes and platelet adhesion at 250 to 500 μg/mL concentrations (Figure 5B-C). Fibrin cross-linked to 70K fragment, 35 μg/mL, did not support increased adhesion and aggregation of platelets compared with a fibrin matrix alone.
Effects of LPA or ADP on platelet thrombus formation under shear conditions. A suspension of platelets and red blood cells in the presence or absence of 100 nM plasma fibronectin (FN) or 5 μM LPA or ADP was perfused through a flow chamber opposed to a coverslip or culture dish coated with fibrin or fibronectin-fibrin for 5 minutes at a shear rate of 1250 s–1. (A) Coverslips were taken out of the chamber, and microscopy was performed as described in Figure 1. Bar = 100 μm. (B-C) Thrombus volumes and platelet numbers were measured as described in Table 1. Values represent the mean ± SD (n = 3 experiments).
Effects of LPA or ADP on platelet thrombus formation under shear conditions. A suspension of platelets and red blood cells in the presence or absence of 100 nM plasma fibronectin (FN) or 5 μM LPA or ADP was perfused through a flow chamber opposed to a coverslip or culture dish coated with fibrin or fibronectin-fibrin for 5 minutes at a shear rate of 1250 s–1. (A) Coverslips were taken out of the chamber, and microscopy was performed as described in Figure 1. Bar = 100 μm. (B-C) Thrombus volumes and platelet numbers were measured as described in Table 1. Values represent the mean ± SD (n = 3 experiments).
When the concentrations of plasma fibronectin present both during fibrin formation and in the perfusate were simultaneously changed, thrombogenicity gradually increased to a maximum of approximately 15-fold (Figure 6A). Thrombus formation at fibronectin concentrations of 400 to 600 μg/mL was significantly enhanced compared with thrombi formed when concentrations of 100 μg/mL were present (Figure 6B).
Effect of concentrations of plasma fibronectin in the perfusate on platelet thrombus formation under shear conditions. (A) A suspension of platelets and red blood cells was premixed with plasma fibronectin (FN), 10 to 600 μg/mL (20-1200 nM), and perfused through a flow chamber opposed to a coverslip or culture dish coated with fibrin or fibronectin-fibrin for 5 minutes at a shear rate of 1250 s–1. Coverslips were taken out of the chamber, and microscopy was performed as described in Figure 1. Bar = 100 μm. (B) Thrombus volumes (□, on fibrin or ▪ on fibronectin-fibrin) and platelet numbers (○, on fibrin or •, on fibronectin-fibrin) were measured as described in Table 1. Values represent the mean ± SD (n = 3-4 experiments).
Effect of concentrations of plasma fibronectin in the perfusate on platelet thrombus formation under shear conditions. (A) A suspension of platelets and red blood cells was premixed with plasma fibronectin (FN), 10 to 600 μg/mL (20-1200 nM), and perfused through a flow chamber opposed to a coverslip or culture dish coated with fibrin or fibronectin-fibrin for 5 minutes at a shear rate of 1250 s–1. Coverslips were taken out of the chamber, and microscopy was performed as described in Figure 1. Bar = 100 μm. (B) Thrombus volumes (□, on fibrin or ▪ on fibronectin-fibrin) and platelet numbers (○, on fibrin or •, on fibronectin-fibrin) were measured as described in Table 1. Values represent the mean ± SD (n = 3-4 experiments).
Discussion
Arterial thrombotic diseases are the most common causes of morbidity and mortality in western countries and are known to result from thrombosis secondary to the disruption of atheromatous plaque.39 Arterial thrombi are composed of aggregates of platelets interacting with subendothelial matrix proteins and small amounts of fibrin clots formed due to activation of the coagulation cascade.40 The relative content of platelet and fibrin in the thrombus and the distribution of platelets are considered important because platelet-rich thrombi are less sensitive to thrombolysis.40
Questions about roles of plasma fibronectin in formation of arterial thrombosis have assumed new importance based on recent studies of plasma fibronectin-knockout mice in which stable platelet-rich thrombi do not form in the injured mesenteric arterioles.6 We demonstrate here that a substrate of fibronectin-fibrin supports adhesion and formation of cohesive aggregates of platelets more than a substrate of fibrin alone and that plasma fibronectin is assembled by adherent and aggregating platelets and further enhances thrombogenesis under shear conditions. The effects of fibronectin incorporated in platelet thrombi and fibronectin cross-linked to fibrin on surface coverage, thrombus volume, and number of platelets were synergistic, that is, greater than additive effects of the 2 types of insolubilized fibronectin alone (Table 1). These results, therefore, define 2 complementary mechanisms to explain why plasma fibronectin-knockout mice are unable to form stable thrombi: lack of cross-linking of plasma fibronectin to fibrin and absence of assembled plasma fibronectin in developing platelet thrombi.
Effect of amounts of fibronectin cross-linked to fibrin on platelet thrombus formation under shear conditions. (A) Fibrin clots incubated with different concentrations of fibronectin (FN) in the presence or absence of FXIIIa were digested with trypsin. The solubilized clots were electrophoresed under reduced conditions and immunoblotted with the 2D3, a monoclonal antibody against an epitope within the N-terminal 27-kDa tryptic fragment of fibronectin. Lane 1, 0.5 μg fibronectin; lane 2, 0.5 μg trypsin-digested fibronectin; lane 3, cross-linked fibrin lacking fibronectin; lane 4, trypsin-digested cross-linked fibrin lacking fibronectin; lane 5, trypsin digest of fibrin formed in the presence of fibronectin, 200 μg/mL, and absence of FXIIIa; lanes 6-11, trypsin digests of fibrin formed in the presence of fibronectin, 10, 50, 100, 200, 400, or 600 μg/mL, and FXIIIa, 5 μg/mL; lane 12, 0.5 μg 70K fragment; and lane 13, 0.5 μg trypsin-digested 70K fragment. Arrowhead indicates a 37-kDa band immunoblotted only in trypsin digests of fibrin cross-linked to fibronectin. The 27- and 37-kDa bands were not detected by nonimmune mouse IgG (not shown). (B) Fibronectin, 0 to 500 μg/mL, or 70K fragment, 35 μg/mL, was added to the mixture of human fibrinogen, 500 μg/mL; FXIII, 5 μg/mL; 2 mM CaCl2. The clot was formed by addition of thrombin, 1 U/mL, and after overnight incubation at 4°C, the bulk fibrin clots was removed. A suspension of platelets and red blood cells was perfused through a flow chamber opposed to a coverslip or culture dish coated with fibrin or fibrin cross-linked to different amounts of fibronectin or 70K fragment for 5 minutes at a shear rate of 1250 s–1. Platelet thrombus was visualized with rhodamine-phalloidin as described in Table 1. Bar = 100 μm. (C) Thrombus volumes and platelet numbers were measured as described in Table 1. Numbers on x-axis represent the concentration (μg/mL) of fibronectin or 70K fragment present during formation of a fibrin matrix. Values represent the mean ± SD (n = 3 experiments).
Effect of amounts of fibronectin cross-linked to fibrin on platelet thrombus formation under shear conditions. (A) Fibrin clots incubated with different concentrations of fibronectin (FN) in the presence or absence of FXIIIa were digested with trypsin. The solubilized clots were electrophoresed under reduced conditions and immunoblotted with the 2D3, a monoclonal antibody against an epitope within the N-terminal 27-kDa tryptic fragment of fibronectin. Lane 1, 0.5 μg fibronectin; lane 2, 0.5 μg trypsin-digested fibronectin; lane 3, cross-linked fibrin lacking fibronectin; lane 4, trypsin-digested cross-linked fibrin lacking fibronectin; lane 5, trypsin digest of fibrin formed in the presence of fibronectin, 200 μg/mL, and absence of FXIIIa; lanes 6-11, trypsin digests of fibrin formed in the presence of fibronectin, 10, 50, 100, 200, 400, or 600 μg/mL, and FXIIIa, 5 μg/mL; lane 12, 0.5 μg 70K fragment; and lane 13, 0.5 μg trypsin-digested 70K fragment. Arrowhead indicates a 37-kDa band immunoblotted only in trypsin digests of fibrin cross-linked to fibronectin. The 27- and 37-kDa bands were not detected by nonimmune mouse IgG (not shown). (B) Fibronectin, 0 to 500 μg/mL, or 70K fragment, 35 μg/mL, was added to the mixture of human fibrinogen, 500 μg/mL; FXIII, 5 μg/mL; 2 mM CaCl2. The clot was formed by addition of thrombin, 1 U/mL, and after overnight incubation at 4°C, the bulk fibrin clots was removed. A suspension of platelets and red blood cells was perfused through a flow chamber opposed to a coverslip or culture dish coated with fibrin or fibrin cross-linked to different amounts of fibronectin or 70K fragment for 5 minutes at a shear rate of 1250 s–1. Platelet thrombus was visualized with rhodamine-phalloidin as described in Table 1. Bar = 100 μm. (C) Thrombus volumes and platelet numbers were measured as described in Table 1. Numbers on x-axis represent the concentration (μg/mL) of fibronectin or 70K fragment present during formation of a fibrin matrix. Values represent the mean ± SD (n = 3 experiments).
The enhancing effect of the fibronectin-fibrin matrix on thrombogenesis required cross-linking, even though fibronectin remained associated with the matrix when FXIIIa was omitted (Figure 5A). The N-terminus of fibronectin becomes cross-linked by FXIIIa to the C-terminus, residues 368-610, of the fibrin α-chain.16 Mice rendered genetically deficient in the catalytic A subunit of FXIII have spontaneous miscarriages due to severe uterine bleeding41 and hemostatic abnormalities due to impaired clot formation and reduced clot stability.42 Inherited deficiency of FXIII in humans results in a life-long, severe bleeding disorder.43 Death from intracranial hemorrhage is common in FXIII-deficient patients compared with patients deficient in other blood coagulation factors.43 Cross-linking of fibrin-fibronectin by FXIIIa during fibrin polymerization has been suggested to anchor the fibrin clot in the wound region and support normal hemostasis and wound healing in vivo.43 In support of this suggestion, fibronectin that has been mutated in N-terminal glutaminyl residues so that it cannot be cross-linked to fibrin is defective in supporting adhesion and spreading of NIH3T3 cells on fibrin matrices.44 Our findings suggest an additional mechanism by which FXIIIa and plasma fibronectin may interact to favorably influence hemostasis.
Effect of matched increases in the concentration of plasma fibronectin present during formation of fibrin matrices and in the perfusate on platelet thrombus formation under shear conditions. (A) Fibronectin (FN), 0 to 600 μg/mL, was present during formation of fibronectin-fibrin clots. The same concentration of plasma fibronectin was included in the perfusate. After 5 minutes, perfusion at a wall shear rate of 1250 s–1 coverslips were taken out of the chamber and washed. Microscopy was performed as described in Figure 1. Bar = 100 μm. (B) Thrombus volumes (□) and platelet numbers (○) were measured as described in Table 1. Values represent the mean ± SD (n = 4 experiments). The values at fibronectin concentrations of 400 and 600 μg/mL were significantly different from values at 100 μg/mL (P < .001 in the Dunnett test after ANOVA).
Effect of matched increases in the concentration of plasma fibronectin present during formation of fibrin matrices and in the perfusate on platelet thrombus formation under shear conditions. (A) Fibronectin (FN), 0 to 600 μg/mL, was present during formation of fibronectin-fibrin clots. The same concentration of plasma fibronectin was included in the perfusate. After 5 minutes, perfusion at a wall shear rate of 1250 s–1 coverslips were taken out of the chamber and washed. Microscopy was performed as described in Figure 1. Bar = 100 μm. (B) Thrombus volumes (□) and platelet numbers (○) were measured as described in Table 1. Values represent the mean ± SD (n = 4 experiments). The values at fibronectin concentrations of 400 and 600 μg/mL were significantly different from values at 100 μg/mL (P < .001 in the Dunnett test after ANOVA).
Self-assembly of fibronectin is a cell-mediated, multistep process.45,46 Fibronectin assembly is initiated by binding of its N-terminal 70K region to the surface of fibroblasts or platelets.11,46 Surface-associated fibronectin molecules then form deoxycholate-insoluble multimeric fibrils as the assembly progresses.45-47 Suspended platelets stimulated with thrombin also bind soluble fibronectin via αIIbβ3.18 Such binding is to the RGD containing III-10 module of fibronectin and blocked by mAbs against αIIbβ3.18 The observations that assembly of fibronectin by platelets aggregating under shear conditions was blocked by the N-terminal 70K fragment, which competes for binding sites for fibronectin on adherent platelets,11,17 and FUD, which binds to the 27-kDa region of fibronectin and inhibits binding of fibronectin to assembly sites of adherent fibroblasts,25,35 led us to conclude that the deposition of fibronectin in developing platelet thrombi is mediated by binding of its N-terminal region rather than the RGD-containing midpiece.
What then are the platelet receptors that explain the effects of fibronectin? This question evolves into 3 questions. (1) What receptors enhance platelet adhesion to fibronectin-fibrin versus fibrin? (2) What receptors bind the N-terminal 70K part of fibronectin to drive fibronectin assembly? (3) What receptors interact with assembled fibronectin to mediate cohesive aggregation of platelets?
Regarding the first question, effects of blocking antibody and peptides in static adhesion assays demonstrate that LPA-stimulated platelets adhere to fibronectin-fibrin via αvβ3 and α5β1 (not shown), to fibrin via αvβ3 and αIIbβ3,11 and to fibronectin via αIIbβ3, αvβ3, and α5β1.11 In the flow system, 10E5 (anti-αIIbβ3) and LM609 (anti-αvβ3) but not mAb13 (anti-β1) each block platelet adhesion to fibrin or fibronectin-fibrin (Table 2), indicating that the β3 integrins are the major receptors for platelet adhesion to both substrates. The need for cross-linking of intact fibronectin containing type III modules and involvement of αIIbβ3 are consistent with previous observation that type III modules 9-10 of fibronectin are important for platelet adhesion.48,49
Regarding the second question, the observation that FITC-70K fragment localizes within platelet thrombi just like FITC-fibronectin under shear conditions and in so doing blocks fibronectin assembly suggests the involvement of non–integrin receptors for binding of the N-terminal region of fibronectin.11,50-52
Regarding question 3, assembly of fibronectin enhanced platelet build-up even though there was abundant endogenous fibrinogen present in aggregating platelets to interact with αIIbβ3. Because intact fibronectin but not 70K fragment was required for enhancement of thrombus formation, it seems likely that the integrin-binding type III region of fibronectin mediates cohesion. Studies of the SP1 mammary carcinoma cells53 and CHO cells54 have defined experimental conditions under which assembled fibronectin is required for formation of compact cell aggregates. Such formation is mediated by α5β1.54,55 Our preliminary study, however, demonstrated that coperfusion of fibronectin with the mAb13, anti-β1 antibody did not inhibit build-up of platelets on fibrin matrices (not shown), and thus β3 rather than β1 integrins likely mediate cohesion of platelets by assembled fibronectin.
Coperfusion with a platelet activator such as LPA or ADP was required to demonstrate an effect of perfused fibronectin on platelet thrombus formation. ADP stimulates activation and aggregation of platelets via P2Y1 and P2Y12 receptors.56 Platelets possess LPA1 (Edg2), LPA2 (Edg4), and LPA3 (Edg7) receptors for LPA.57,58 Activated platelets generate LPA, which is the major component in the serum that enhances fibronectin assembly by fibroblasts.59 LPA is also present in the atherosclerotic lesion.60,61 Although LPA is a poor agonist of platelet aggregation,61 LPA was as effective as ADP in supporting plasma fibronectin-enhanced platelet thrombus formation in our system. Thus, our results suggest a pathway by which LPA has a thrombogenic action by enhanced assembly of plasma fibronectin by platelet thrombi formed at sites of the vessel injury.
Altered concentrations of plasma fibronectin have been reported in certain diseases. Concentrations are reduced in disseminated intravascular coagulation, sepsis, liver disease, and following surgery or major trauma.38,62 Concentrations are increased in men compared with women,38 in the aged,63 and in some patients with malignancy.64 A single family with deficiency in plasma fibronectin has been reported, in which the deficiency was related to abnormal wound healing but not bleeding.65 Especially pertinent to our results, plasma fibronectin levels have been found to be moderately but statistically elevated in patients with coronary artery disease selected from 2 separate populations.21,22 In these studies, mean concentrations in controls were 360 to 380 μg/mL versus 460 to 470 μg/mL in patients with disease (The units in Orem et al22 were given as mg/dL; that must be a mistake). Large amounts of fibronectin are detected in atherosclerotic lesions, especially in developing fibrous plaques.66 However, mechanisms to explain the association between plasma fibronectin concentration and coronary artery disease have not been elucidated. Our finding that thrombus formation on fibrin matrices increases as plasma fibronectin concentration increases above 100 μg/mL and a previous report that platelet thrombus formation on collagen-coated surfaces increases with increased concentrations of plasma fibronectin up to a level of 700 μg/mL9 suggest a potential mechanism, that is, insolubilization of plasma fibronectin within developing platelet thrombi, which helps form arterial thrombi. Further, our results may also explain why heterozygote fibronectin-knockout mice with the half level of normal plasma fibronectin have defects in formation of stable thrombi in the injured mesenteric arterioles compared with mice with normal fibronectin levels.67
Prepublished online as Blood First Edition Paper, January 3, 2006; DOI 10.1182/blood-2005-10-4168.
Supported by National Institutes of Health grant HL21644.
J.C. designed research, performed research, analyzed data, and wrote the paper; and D.F.M. designed research, analyzed data, and wrote the paper.
An Inside Blood analysis of this article appears at the front of this issue.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
We thank Doug Annis for characterization of monoclonal antibodies against 70K fragment.



This feature is available to Subscribers Only
Sign In or Create an Account Close Modal