The laminin receptor integrin α6 chain is ubiquitously expressed in human and mouse hematopoietic stem and progenitor cells. We have studied its role for homing of stem and progenitor cells to mouse hematopoietic tissues in vivo. A function-blocking anti–integrin α6 antibody significantly reduced progenitor cell homing to bone marrow (BM) of lethally irradiated mice, with a corresponding retention of progenitors in blood. Remarkably, the anti–integrin α6 antibody profoundly inhibited BM homing of long-term multilineage engrafting stem cells, studied by competitive repopulation assay and analysis of donor-derived lymphocytes and myeloid cells in blood 16 weeks after transplantation. A similar profound inhibition of long-term stem cell homing was obtained by using a function-blocking antibody against α4 integrin, studied in parallel. Furthermore, the anti–integrin α6 and α4 antibodies synergistically inhibited homing of short-term repopulating stem cells. Intravenous injection of anti–integrin α6 antibodies, in contrast to antibodies against α4 integrin, did not mobilize progenitors or enhance cytokine-induced mobilization by G-CSF. Our results provide the first evidence for a distinct functional role of integrin α6 receptor during hematopoietic stem and progenitor cell homing and collaboration of α6 integrin with α4 integrin receptors during homing of short-term stem cells.
Introduction
Hematopoietic stem cell (HSC) properties, like self-renewal, proliferation, differentiation, and migration are critically dependent on the regulatory signals from the surrounding bone marrow (BM) microenvironment. Cytokines, growth factors, stromal cell surface molecules, and extracellular matrix (ECM) molecules contribute to this regulation by binding to distinct cell surface receptors and thereby activating intracellular signal transduction pathways. During steady-state hematopoiesis, small numbers of HSCs are circulating in blood, indicating that their continuous mobilization into blood and homing into BM is a physiologic process.1 For clinical stem cell transplantation, HSCs are mobilized from BM by injection of growth factors and can be harvested from blood. After transplantation, HSCs selectively transmigrate through the sinusoidal walls into the BM extravascular spaces to engraft and reconstitute hematopoiesis. In leukemias, cell proliferation, differentiation, and migration are dysregulated, and consequently high numbers of immature malignant cells are released into blood and accumulate in the BM, and aberrantly in other tissues.
The molecular mechanisms involved in HSC homing and mobilization have been widely studied, but are still not fully understood.2,3 They are apparently multistep processes involving chemokines, growth factors, matrix-degrading enzymes, and cell adhesive interactions mediated by specific receptors on hematopoietic cells. Members of the integrin family of adhesion receptors are widely expressed in the hematopoietic system, and both in vitro and in vivo studies have indicated decisive roles for several integrins in these interactions. All integrins are heterodimeric transmembrane molecules consisting of α and β subunits. They mediate binding to ECM and cell surface ligands by their extracellular domains and provide a link with cytoskeletal proteins with the cytoplasmic domain, thereby acting as bidirectional signaling molecules.4 In vivo experiments using gene-deleted mice and function-blocking antibodies have shown that β1 integrins are required for HSC migration into fetal liver and adult BM.5-7 So far 12 β1 integrins, containing different α chains, have been characterized.8 Of these, the functional role of integrin α4 receptor during embryonic and postnatal hematopoiesis, including mobilization and homing of hematopoietic progenitor cells (HPCs), is well established.9-15 Although it is evident from several studies that other receptors and ligands participate in these processes,14,16-19 their roles remain less well defined.
We have previously shown that the integrin α6 chain is ubiquitously expressed in human HPCs,20 in line with its high expression in mouse primitive hematopoietic cells.21 The integrin α6 chain, assembled with integrin β1 or β4 chains, forms major receptors for ECM laminins.22,23 In BM, laminin isoforms, laminin-8 and laminin-10, are present at sites of hematopoietic cell development and trafficking,24-26 and might therefore regulate HSC functions. Our previous studies have shown that BM laminins promote adhesion and migration of human HPCs through interaction with integrin α6β1 in vitro.20 Herein, we have analyzed the role of integrin α6 receptor for homing and mobilization of mouse primitive hematopoietic cells in vivo by using function-blocking antibodies and a mouse transplantation model. In parallel, we also studied the role of integrin α4 receptor and a possible collaboration of integrin α6 and α4 receptors in these processes. Our results provide the first data showing that α6 integrins function in vivo as hematopoietic stem and progenitor cell homing receptors. Furthermore, we for the first time, show the role of integrin α4 receptor for homing of long-term multilineage reconstituting HSCs and collaboration of these 2 integrins in homing of short-term HSCs.
Materials and methods
Animals
C57BL/6 mice (CD45.1, CD45.2, and F1-CD45.1/CD45.2), matched for sex and age, were used. Mice were housed at Lund University animal facilities under pathogen-free conditions. The experiments were approved by the ethics committee at Lund University.
Hematopoietic growth factors
Human flt-3 ligand (FL) was a gift of Immunex (Seattle, WA). Human G-CSF and murine GM-CSF were from Amgen (Thousand Oaks, CA). Mouse IL-3 was from PeproTech (Rocky Hill, NJ) and human erythropoietin (EPO) from Boehringer Mannheim (Mannheim, Germany).
Proteins and antibodies
Recombinant laminin-827 and laminin-1028 were produced as described. Human placental laminin-10/11, was from Gibco (Täby, Sweden) or Chemicon (Chandlers Ford, United Kingdom). This laminin contains laminin-10 (α5β1γ1) and laminin-11 (α5β2γ1).29,30 However, based on amino acid sequencing, the major component is laminin-10.29 Human plasma fibronectin and mouse laminin-1 were from Gibco. The purified preservative-free monoclonal antibodies used for functional assays were GoH3 against integrin α6 (CD49f; Immunotech, Marseille, France), PS/2 against integrin α4 (CD49d; Chemicon, Temecula, CA), HA2/5 against integrin β1 (CD29), rat IgG2a, and hamster IgM (PharMingen, San Diego, CA).
Isolation and analysis of HSCs and HPCs
Primitive hematopoietic cells not expressing lineage markers, but expressing Sca-1 and c-kit (Lin–Sca-1+c-kit+; LSK) from BM were isolated as described.31 Lin– cells were selected by using antibodies against CD4 (H129.19), CD5 (53-7.3), CD8a (53-6.7), Mac-1 (M1/70); B220 (RA3-6B2), Ter119, Gr-1 (RB6-8C5) (PharMingen) and with sheep anti–rat IgG (Fc)–conjugated immunomagnetic beads (Dynal, Oslo, Norway) and stained with a goat anti–rat-Tricolor (Caltag, Burlingame, CA), anti-Sca-1-FITC (E13-161.7) and anti-c-kit-APC (2B8) antibodies (PharMingen). Cells with low viability were excluded from Lin– gate by staining with 7-amino-actinomycin D (7-AAD; Sigma-Aldrich, St Louis, MO). LSK cells were sorted on a FACSVantage or FACSDiva (Becton Dickinson, San Jose, CA). Reanalysis reproducibly showed a purity of 97% to 100%. Integrin α6 expression in LSKCD34– cells was analyzed by using PE-anti–integrin-α6 (GoH3), rat anti–mouse RAM-34 (CD34-FITC), biotin anti-Sca-1 and streptavidin-phycoerythrin-Texas red (PharMingen). Analyses were performed by FACSCalibur or FACSDiva and FlowJo (TreeStar, San Carlos, CA) or CellQuest program (Becton Dickinson).
Cell adhesion assay
Flat-bottom 96-well plates (Nunc, Roskilde, Denmark; Greiner, Frickenhausen, Germany) were coated with proteins in PBS (Gibco) at 10 to 30 μg/mL. The cell adhesion and antibody inhibition assays were performed as described.20 In some experiments, the cells were incubated with 5 ng/mL 12-tetradecanoyl phorbol-13-acetate (TPA; Sigma-Aldrich) for 1 hour before the adhesion assay. The antibodies were used at 25 μg/mL. Because LSK cell adhesion to laminin-10/11 and laminin 10 was similar at both protein concentrations, 10 μg/mL was used in antibody inhibition assay. Laminin-8 was used at 30 μg/mL. The adherent cells from the entire bottom area of the wells were counted by using an inverted Olympus IX70 microscope (Olympus, Tokyo, Japan).20
Assays for CFU-GM homing
BM cells from 6- to 8-week-old mice were incubated with anti-integrin or control antibodies (2 μg/106 cells) in IMDM, 5% FCS on ice for 30 to 40 minutes. Thereafter the cells (5 × 106 in 500 μL) were injected into tail veins of 8- to 10-week-old mice. The mice were irradiated 4 to 8 hours before transplantation with a single 975-cGy dose using a cesium 137 source (Instrument AB Scanditronix, Uppsala, Sweden). Three hours after injection of the cells, blood, BM, and spleen were harvested.
The mice were anesthetized with isoflurane and blood was harvested by retro-orbital sampling. Erythrocytes were depleted with 2% Dextran-T 500 (Amersham, Pharmacia Biotech, Uppsala, Sweden). BM cells were harvested by crushing femora in IMDM, 5% FCS. One femur of each mouse was used for the assays. Spleen cells were extracted by pushing through a 70-μm cell strainer. Ten percent of a femur and 2% of the spleen homogenate/dish (vol/vol) were used for CFU-GM assay. The cells were plated in duplicate in 1 mL IMDM, 20% FCS, 1% penicillin/streptomycin, 1% l-glutamine (BioWhittaker, East Rutherford, NJ), 1% 2-mercaptoethanol (Sigma-Aldrich), 1% methylcellulose M3134 (StemCell Technologies, Vancouver, BC, Canada), and cytokines (FL, GM-CSF, IL-3, and G-CSF) in 35-mm Petri dishes. In one experiment, EPO was also used. Cultures were incubated at 37°C in a humidified atmosphere, 5% CO2 in air for 7 days. The myeloid colonies containing more than 50 cells were scored using an inverted microscope. The mean value of each duplicate was calculated.
The number of recovered CFU-GMs was corrected to represent the whole BM, estimating that one femur represents 5.9% of the total BM.18 Donor cells incubated with antibodies were cultured to assess the number of CFU-GMs injected. Two mice in each experiment were irradiated but did not receive a transplant; to assess the number of residual host-derived CFU-GMs, 0 to 3 CFU-GMs were observed in BM and spleen samples, whereas no CFU-GMs were obtained in blood samples. The number of host-derived residual CFU-GMs was subtracted from CFU-GMs recovered in mice given transplants.
Competitive repopulation assay to assess homing of HSCs
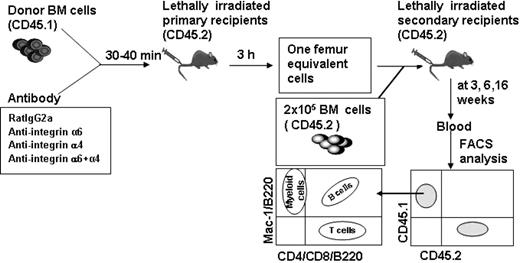
BM cells from 7- to 8-week-old CD45.1 donor mice (test cells) were incubated with antibodies (2 μg/106 BM cells) for 30 to 40 minutes on ice.18 Thereafter, the cells (10 × 106/mouse) were injected intravenously into lethally irradiated CD45.2 mice. Three hours after injection the BM cells were harvested and washed with 5 mL IMDM, 5% FCS to remove nonbound antibodies. The cells from one femur were injected together with 2 × 105 BM cells from CD45.2 mice (competitor cells) into lethally irradiated CD45.2 mice.
To analyze multilineage reconstituting HSCs, blood was harvested from the retro-orbital sinus at 3, 6, and 16 weeks after transplantation and analyzed for expression of donor (CD45.1) and for competitor/recipient (CD45.2) leukocyte antigens. Erythrocytes were lysed by ammonium chloride (StemCell Technologies). Lineage reconstitution was examined using fluorochrome-conjugated antibodies against CD4 and CD8 (T cells), B220 (B cells), Mac-1 (CD11b; myeloid cells), CD45.1 (A20), and CD45.2 (104; PharMingen).31 Mice that had more than 1% donor-derived (CD45.1) cells in both lymphoid (B220+) and myeloid (Mac-1+) subpopulations were considered to be repopulated by test cells.32,33
To study the effect anti-integrin antibodies on HSC engraftment and survival, 1 × 106 BM cells (CD 45.1) incubated with antibodies or control IgG were injected into lethally irradiated mice (F1-CD45.1/CD45.2). Three hours later, 3 × 106 CD45.2 BM competitor cells were injected, and engraftment was analyzed by the competitive repopulation assay.
Mobilization assays
Anti-integrin or control antibodies (2 mg/kg/d for 3 days) were injected intravenously into the mice (CD45.1).13 In some experiments, G-CSF (Neupogen, Amgen; 100 μg/kg subcutaneously, twice daily for 3 days) was injected in parallel.13 The following day the white blood cell counts and the CFU-GMs were analyzed in blood samples. Mobilization of long-term HSCs was studied by injecting 150 μL blood intravenously together with 200 000 CD45.2 BM competitor cells into lethally irradiated CD45.2 recipient mice. Multilineage reconstituting HSCs were analyzed as described (see “Competitive repopulation assay to assess homing of HSCs”).
Statistical analysis
Statistical significance was determined using the unpaired t test for parametric or Mann-Whitney t test for nonparametric comparisons.
Results
Expression of integrin α6 receptor on mouse BM stem and progenitor cells
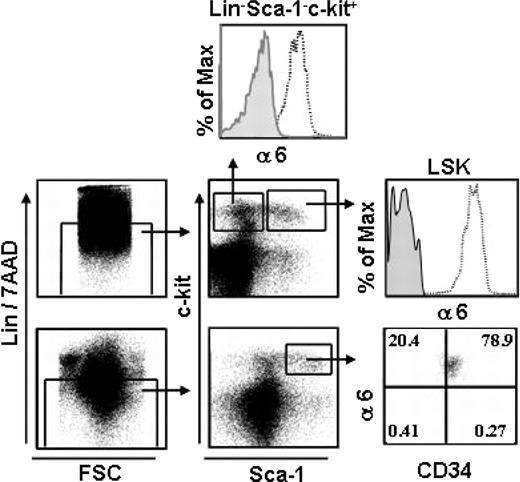
We analyzed integrin α6 receptor expression in mouse BM LSK cells, which is a highly enriched stem and progenitor cell population, representing only 0.05% to 0.1% of total BM cells.1 All (99.7%) LSK cells expressed integrin α6 chain (Figure 1). In the adult LSK cell population, the most primitive long-term HSCs reside in the CD34– fraction, whereas short-term HSCs have been shown to be CD34+.34,35 Flow cytometry analysis showed expression of α6 integrin at equally high intensities in both populations. Furthermore, more than 90% of committed myeloid progenitors, which have the phenotype Lin–Sca-1–c-Kit+,1 expressed the integrin α6 receptor. Consequently, integrin α6 receptor is ubiquitously expressed both in the most primitive mouse HSC identified by immunophenotype and in more differentiated stem and progenitor cell populations.
Integrin α6 β1 mediates stem and progenitor cell adhesion to laminins
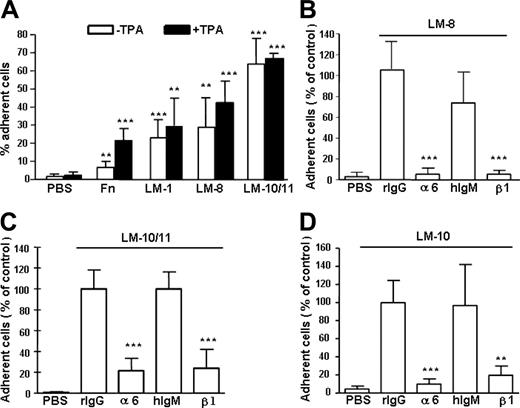
In a cell adhesion assay, 60% to 70% of LSK cells adhered to laminin-10/11 and 30% to 40% adhered to laminin-8 (Figure 2A), showing similar isoform-specific adhesion to laminins as human primitive hematopoietic CD34+CD38– cells.20 Activation of integrin receptors by a protein kinase C activator TPA36 did not increase LSK cell adhesion to laminins. In contrast, adhesion to fibronectin significantly increased after TPA treatment, indicating steady-state activation of laminin but not fibronectin receptors in LSK cells.
Integrin α6 is expressed in mouse BM HSCs. BM cells were stained and gated for Lin– and 7AAD– cells (left panels). Lin–/7AAD– cells were gated for Sca-1– C-Kit+ clonogenic progenitors and the more primitive Sca-1+ C-Kit+ (LSK) cells; 93% and 99% of the cells expressed integrin α6 receptor (α6), respectively. The top and middle right panels show staining with the integrin α6 antibody (open histograms) and isotype control antibody (gray histograms). The bottom right panel shows expression of integrin α6 receptor in LSKCD34– and LSKCD34+ cells. The vertical and horizontal bars were set on the basis of isotype-matched negative control profiles (> 99% negative). Shown is one representative analysis of 2.
Integrin α6 is expressed in mouse BM HSCs. BM cells were stained and gated for Lin– and 7AAD– cells (left panels). Lin–/7AAD– cells were gated for Sca-1– C-Kit+ clonogenic progenitors and the more primitive Sca-1+ C-Kit+ (LSK) cells; 93% and 99% of the cells expressed integrin α6 receptor (α6), respectively. The top and middle right panels show staining with the integrin α6 antibody (open histograms) and isotype control antibody (gray histograms). The bottom right panel shows expression of integrin α6 receptor in LSKCD34– and LSKCD34+ cells. The vertical and horizontal bars were set on the basis of isotype-matched negative control profiles (> 99% negative). Shown is one representative analysis of 2.
Adhesion of mouse BM Lin– Sca1+c-kit+ (LSK) cells to laminins and fibronectin and inhibition cell adhesion to laminins by antibodies against integrin α6 and β1 chain. (A) Adhesion of LSK cells to fibronectin (Fn), laminin-1 (LM-1), laminin-8 (LM-8), and laminin-10/11 (LM-10/11). PBS indicates cell adhesion to wells coated with PBS instead of proteins. The proteins were used at 30 μg/mL. □ indicates cell adhesion without treatment with TPA; ▪, cell adhesion after treatment with TPA. Asterisks indicate significant differences compared with cell adhesion on PBS-coated plates: *P < .05; **P < .01; ***P < .001. Results (mean ± SD) are from 2 experiments performed in triplicate. (B-D) Adhesion of LSK cells to wells coated with laminin-8 (30 μg/mL; B), laminin-10/11 (10 μg/mL; C), and laminin-10 (10 μg/mL; D) in the presence of antibodies GoH3 against integrin α6 chain (α6), Ha2/5 against integrin β1 chain (β1), or control rat or hamster monoclonal antibodies (rIgG and hIgM, respectively). The results are shown as percent cell adhesion of adhesion in the presence of the rIgG control antibody. The antibodies against integrin α6 and β1 chains significantly reduced cell adhesion to laminins, compared with respective isotype control antibodies. **P < .01; ***P < .001. Data represent mean ± SD of 2 experiments performed in triplicate.
Adhesion of mouse BM Lin– Sca1+c-kit+ (LSK) cells to laminins and fibronectin and inhibition cell adhesion to laminins by antibodies against integrin α6 and β1 chain. (A) Adhesion of LSK cells to fibronectin (Fn), laminin-1 (LM-1), laminin-8 (LM-8), and laminin-10/11 (LM-10/11). PBS indicates cell adhesion to wells coated with PBS instead of proteins. The proteins were used at 30 μg/mL. □ indicates cell adhesion without treatment with TPA; ▪, cell adhesion after treatment with TPA. Asterisks indicate significant differences compared with cell adhesion on PBS-coated plates: *P < .05; **P < .01; ***P < .001. Results (mean ± SD) are from 2 experiments performed in triplicate. (B-D) Adhesion of LSK cells to wells coated with laminin-8 (30 μg/mL; B), laminin-10/11 (10 μg/mL; C), and laminin-10 (10 μg/mL; D) in the presence of antibodies GoH3 against integrin α6 chain (α6), Ha2/5 against integrin β1 chain (β1), or control rat or hamster monoclonal antibodies (rIgG and hIgM, respectively). The results are shown as percent cell adhesion of adhesion in the presence of the rIgG control antibody. The antibodies against integrin α6 and β1 chains significantly reduced cell adhesion to laminins, compared with respective isotype control antibodies. **P < .01; ***P < .001. Data represent mean ± SD of 2 experiments performed in triplicate.
Integrin α6β1 receptor binds to most laminin isoforms, but other receptors, including integrins α6β4, α2β1, α3β1, α7β1, α-dystroglycan, and heparan sulfate proteoglycans, also bind to laminins.23 We therefore analyzed the contribution of integrin α6β1 receptor for LSK adhesion to laminins by using function-blocking antibodies against α6 and β1 chains. Adhesion to laminin-8 was almost completely abrogated, whereas adhesion to both laminin-10 and laminin-10/11 was inhibited by 80% to 90% with both antibodies (Figure 2B-D), showing that integrin α6β1 is the major receptor for both laminins in LSK cells.
Integrin α6 mediates CFU-GM homing to BM but not to spleen
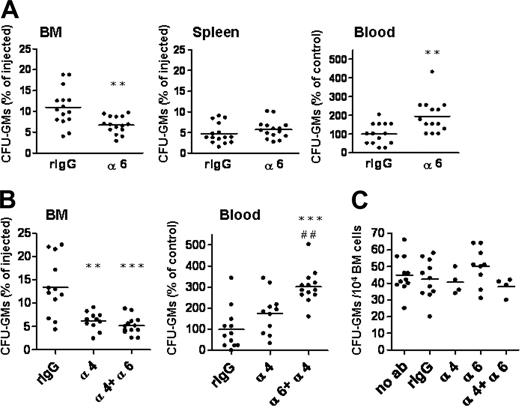
We studied the role of integrin α6 for progenitor cell homing in vivo by using mouse BM transplantation experiments. The anti–integrin α6 antibody GoH3 used has been shown to be functionally active in vivo by inhibiting transendothelial migration of neutrophils.37,38 BM cells were incubated with the anti-integrin or control antibodies and injected intravenously into recipient mice. It has been shown previously that most stem and progenitor cells that home into BM transmigrate rapidly after injection into blood.17,21,39 Therefore, in line with previous studies,10,16-18,21,32,39-41 homing was assayed after 3 hours. More than 10% of the injected CFU-GMs were recovered in BM after incubation with the control antibody, in agreement with published results14,18 (Figure 3). The anti–integrin α6 antibody inhibited homing of CFU-GMs into BM by 39% (Figure 3A). Accordingly, significantly more CFU-GMs were recovered in blood. Notably, homing of progenitors to spleen was not affected by inhibition of α6 integrin receptor.
The anti–integrin α6 antibody inhibits homing of progenitors to BM, but does not enhance inhibition of homing mediated by the integrin α4 antibody. BM cells were incubated with function-blocking antibodies against integrin α6 (α6), integrin α4 (α4), both against integrins α6 and α4 (α6 + α4) or rat IgG2a control antibody (rIgG), and injected into lethally irradiated recipient mice. Three hours after injection, cells from BM, blood, and spleen were collected for CFU-GM assay. Data are pooled from 2 to 4 independent experiments. (A) The anti–integrin α6 antibody inhibited homing of CFU-GMs into BM, but not into spleen, and increased the number of CFU-GMs in blood. (B) The anti–integrin α4 antibody inhibited homing of CFU-GMs into BM. Incubation with both antibodies against integrin α6 and α4 chains did not further inhibit CFU-GM homing but resulted in retention of progenitors in blood. *P < .05; **P < .01; and ***P < .001, compared with results obtained with rIgG control antibody. ##P < .01 compared with results obtained with anti–integrin α4 antibody. (C) Incubation of BM cells with anti–integrin α6, anti–integrin α4, both antibodies, or control antibody did not affect growth of CFU-GMs, compared with cells not incubated with antibodies (no ab). The horizontal bars show mean values.
The anti–integrin α6 antibody inhibits homing of progenitors to BM, but does not enhance inhibition of homing mediated by the integrin α4 antibody. BM cells were incubated with function-blocking antibodies against integrin α6 (α6), integrin α4 (α4), both against integrins α6 and α4 (α6 + α4) or rat IgG2a control antibody (rIgG), and injected into lethally irradiated recipient mice. Three hours after injection, cells from BM, blood, and spleen were collected for CFU-GM assay. Data are pooled from 2 to 4 independent experiments. (A) The anti–integrin α6 antibody inhibited homing of CFU-GMs into BM, but not into spleen, and increased the number of CFU-GMs in blood. (B) The anti–integrin α4 antibody inhibited homing of CFU-GMs into BM. Incubation with both antibodies against integrin α6 and α4 chains did not further inhibit CFU-GM homing but resulted in retention of progenitors in blood. *P < .05; **P < .01; and ***P < .001, compared with results obtained with rIgG control antibody. ##P < .01 compared with results obtained with anti–integrin α4 antibody. (C) Incubation of BM cells with anti–integrin α6, anti–integrin α4, both antibodies, or control antibody did not affect growth of CFU-GMs, compared with cells not incubated with antibodies (no ab). The horizontal bars show mean values.
Blocking of the integrin α4 receptor by the antibody also inhibited CFU-GM homing partially (Figure 3B), in agreement with published results.14,17,18 However, the combined inhibition of both α6 and α4 integrin receptors did not result in further inhibition of homing of progenitors into BM, although the number of CFU-GMs in blood was significantly increased compared with inhibition of integrin α4 receptor only (Figure 3B). This might indicate that inhibition of integrin α6 receptor interferes with migration of progenitors into other organs as well. Incubation of BM cells with anti–integrin α6, anti–integrin α4, both antibodies, or control antibody did not affect growth of CFU-GMs compared with cells not incubated with antibodies (Figure 3C).
Integrin α6 and α4 receptors mediate homing of long-term multilineage repopulating HSCs to BM
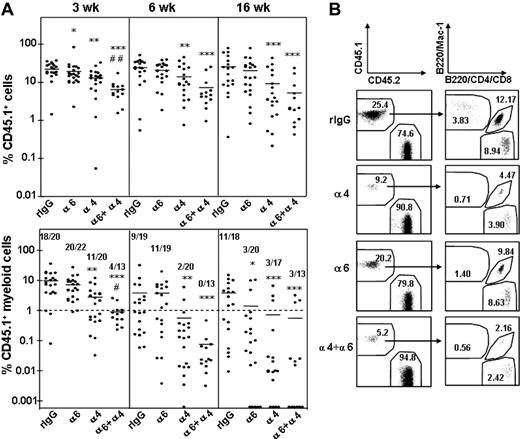
The role of integrin α6 and α4 receptor for homing of long-term multilineage repopulating HSCs into BM was assessed in a competitive reconstitution assay18,31 (Figure 4). BM cells from CD45.1 mice were incubated with anti-integrin antibodies or control antibody and injected into lethally irradiated congenic CD45.2 mice. BM cells were harvested from femurs 3 hours after injection and injected together with CD45.2 competitor BM cells into lethally irradiated CD45.2 secondary recipient mice. Blood samples were collected at 3, 6, and 16 weeks after transplantation, and the proportion of test cell-derived (CD45.1) total, myeloid (Mac-1), B (B220), and T (CD4 and CD8) cell reconstitution was determined (Figure 4). Because myeloid cells, in contrast to lymphocytes, are short lived and must be continuously generated from the HSCs, sustained myeloid reconstitution is the best indicator of HSC reconstitution.32,33
Homing of short-term HSCs was not dependent on integrin α6 receptor function, because myeloid reconstitution was achieved in 20 of 22 and 11 of 19 mice at 3 and 6 weeks, respectively, after anti–integrin α6 antibody treatment, compared with 18 of 20 and 9 of 19 of control mice (Figure 5A). In contrast, treatment with the anti–integrin α4 antibody reduced significantly homing of short-term HSCs, as shown by donor myeloid reconstitution in only 11 of 20 and 2 of 20 mice at 3 and 6 weeks after transplantation, respectively. Notably, the combined inhibition of both integrin α6 and α4 receptors significantly inhibited homing of short-term repopulating HSCs, beyond that observed with anti-α4 antibody alone. Specifically, only 4 of 13 mice were reconstituted with donor myeloid cells at 3 weeks. This result indicates a role for integrin α4 receptor and collaboration of the 2 receptor pathways in recruiting short-term repopulating cells into BM.
Recruitment of long-term multilineage reconstituting HSCs into BM was dependent on integrin α6 function, as shown by blood cell analysis at 16 weeks after transplantation. Only 3 of 20 mice were reconstituted with donor myeloid cells after transplantation with BM cells treated with the anti–integrin α6 antibody, compared with 11 of 18 mice that received cells treated with control IgG. Likewise, blocking of integrin α4 function inhibited homing of long-term HSCs because as only 3 of 17 mice showed myeloid reconstitution. These results show that integrin α6 and α4 receptors independently contribute to homing of long-term HSCs into BM.
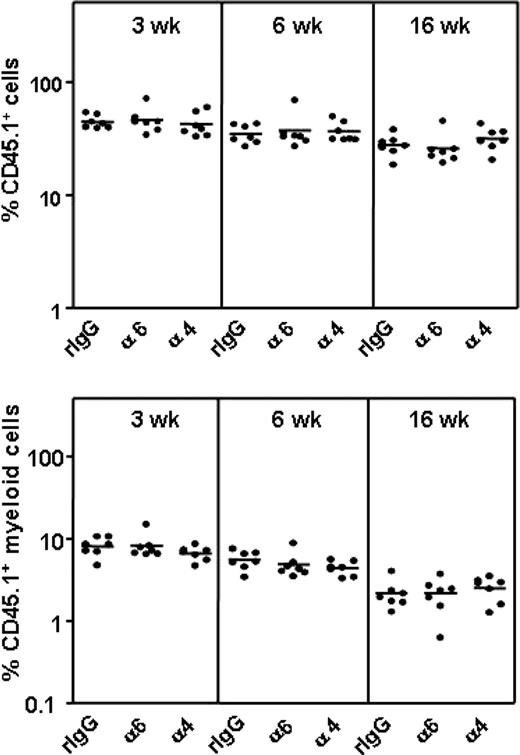
HSCs enter and engraft the BM after recovery of the integrin function
The function-blocking antibodies have a limited half-life on cell surface due to antibody dissociation42 or internalization of the antibody-receptor complex,43 contributing to a recovery of the receptor function over time. We therefore investigated whether the effect of anti–integrin α6 and α4 antibody treatment on HSC homing was transient. BM cells incubated with antibodies or control IgG were injected into lethally irradiated mice and analyzed in these primary recipients 3, 6, and 16 weeks later by the competitive repopulation assay (Figure 6). Homing and engraftment of multilineage repopulating HSCs was not inhibited in this experimental setting. This confirms that the observed low HSC frequencies in BM 3 hours after transplantation indeed are due to impaired homing of HSCs into BM after functional inhibition of the integrin receptors. These findings suggest that the antibody-treated HSCs enter into BM as soon as the integrin receptor function is recovered and emphasize the importance of performing functional assays by antibody perturbation within a limited time period. In agreement with this, no inhibition of cord blood CD34+ cell or engraftment by anti–integrin α6 antibodies was observed in NOD/SCID mice.44 Importantly, the results show that the function-blocking antibodies have no negative effects on HSC survival or development.
Competitive repopulation analysis to assess HSC homing. BM cells from CD45.1 mice were incubated with rat IgG2a antibody or antibodies against integrin α4, α6, or both α4 and α6, and were injected into lethally irradiated CD45.2 mice. BM cells were harvested 3 hours after injection and cells from one femur were injected together with CD45.2 competitor BM cells into lethally irradiated CD45.2 secondary recipient mice. Blood was harvested at 3, 6, and 16 weeks after transplantation and CD45.1+ and CD45.2+ cells as well as B-cell (B220), T-cell (CD4/CD8), and myeloid cell (Mac-1) reconstitution of CD 45.1 cells were analyzed by flow cytometry.
Competitive repopulation analysis to assess HSC homing. BM cells from CD45.1 mice were incubated with rat IgG2a antibody or antibodies against integrin α4, α6, or both α4 and α6, and were injected into lethally irradiated CD45.2 mice. BM cells were harvested 3 hours after injection and cells from one femur were injected together with CD45.2 competitor BM cells into lethally irradiated CD45.2 secondary recipient mice. Blood was harvested at 3, 6, and 16 weeks after transplantation and CD45.1+ and CD45.2+ cells as well as B-cell (B220), T-cell (CD4/CD8), and myeloid cell (Mac-1) reconstitution of CD 45.1 cells were analyzed by flow cytometry.
Inhibition of HSC homing by antibodies against integrin α6 and α4. (A) Peripheral blood reconstitution of CD 45.1 cells and CD 45.1 myeloid cells at 3, 6, and 16 weeks. The CD 45.1 BM cells were incubated with rat IgG2a antibody (rIgG) or antibodies against integrin α4 (α4), α6 (α6), or both α4 and α6 (α4 + α6) before the homing and competitive repopulation assay. Data are pooled from 3 independent experiments. *P < .05; **P < .01; and ***P < .001, compared with reconstitution from cells incubated with rIgG; #P < .05 and ##P < .01, compared with reconstitution from cells treated with anti–integrin α4 antibody. The horizontal bars show mean values for each measurement. The numbers in bottom panels show the number of mice with myeloid CD 45.1 cell reconstitution more than 1% and the number of mice analyzed. (B) Representative fluorescence-activated cell sorting (FACS) dot plots showing blood reconstitution 16 weeks after secondary transplantation. The left panels show reconstitution of CD45.1 BM cells and CD45.2 competitor/recipient cells. The right panels show blood reconstitution of myeloid (Mac-1) and lymphoid (B220, CD4, CD8) cells derived from CD45.1 cells. The numbers indicate the mean percentage of cells in each fraction (n = 13-22).
Inhibition of HSC homing by antibodies against integrin α6 and α4. (A) Peripheral blood reconstitution of CD 45.1 cells and CD 45.1 myeloid cells at 3, 6, and 16 weeks. The CD 45.1 BM cells were incubated with rat IgG2a antibody (rIgG) or antibodies against integrin α4 (α4), α6 (α6), or both α4 and α6 (α4 + α6) before the homing and competitive repopulation assay. Data are pooled from 3 independent experiments. *P < .05; **P < .01; and ***P < .001, compared with reconstitution from cells incubated with rIgG; #P < .05 and ##P < .01, compared with reconstitution from cells treated with anti–integrin α4 antibody. The horizontal bars show mean values for each measurement. The numbers in bottom panels show the number of mice with myeloid CD 45.1 cell reconstitution more than 1% and the number of mice analyzed. (B) Representative fluorescence-activated cell sorting (FACS) dot plots showing blood reconstitution 16 weeks after secondary transplantation. The left panels show reconstitution of CD45.1 BM cells and CD45.2 competitor/recipient cells. The right panels show blood reconstitution of myeloid (Mac-1) and lymphoid (B220, CD4, CD8) cells derived from CD45.1 cells. The numbers indicate the mean percentage of cells in each fraction (n = 13-22).
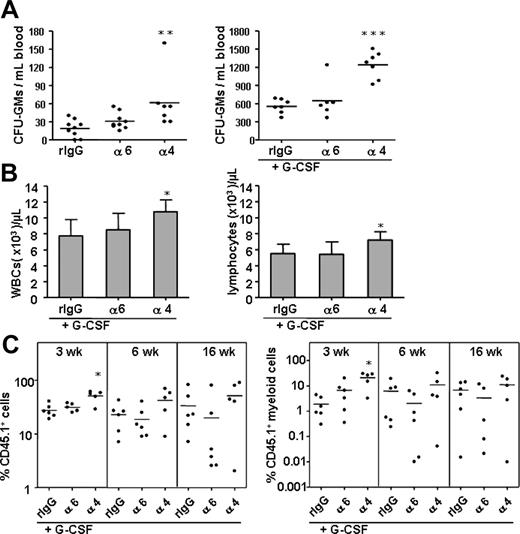
The anti–integrin α6 antibody does not mobilize progenitors
Previous studies have shown that perturbation of integrin α4 function by antibodies or by gene deletion mobilizes clonogenic progenitors and HSCs into blood.13,14 We assessed the effect of the anti–integrin α6 antibody on progenitor mobilization and compared it with mobilization induced by the anti–integrin α4 antibody (Figure 7). As expected, the anti–integrin α4 antibody significantly mobilized leukocytes, lymphocytes, and CFU-GMs into blood and enhanced the mobilizing effect of G-CSF.13 In contrast, injection of the anti–integrin α6 antibody did not promote progenitor mobilization alone or in the presence of G-CSF. Inhibition of integrin α6 receptor did not potentiate G-CSF–stimulated mobilization of any HSC population assayed by the competitive repopulation assay. In agreement with the results on CFU-S mobilization,13 the anti–integrin α4 antibody enhanced cytokine-stimulated mobilization of short-term HSCs. However, HSC reconstitution analysis later, at 6 and 16 weeks, did not show any synergistic mobilization with the G-CSF and anti–integrin α4 antibody.
Anti–integrin α6 or α4 antibodies do not affect HSC survival or engraftment. CD45.1 BM cells were incubated with rat IgG isotype control antibody (rIgG), anti–integrin α6 (α6) or α4 (α) antibodies and injected into lethally irradiated CD45.1/2 mice, followed by injection of CD45.2 competitor BM cells 3 hours later. The percentages of CD45.1 nucleated cells and CD45.1 myeloid cells in blood at 3, 6, and 16 weeks after transplantation are shown. The horizontal bars indicate mean values.
Anti–integrin α6 or α4 antibodies do not affect HSC survival or engraftment. CD45.1 BM cells were incubated with rat IgG isotype control antibody (rIgG), anti–integrin α6 (α6) or α4 (α) antibodies and injected into lethally irradiated CD45.1/2 mice, followed by injection of CD45.2 competitor BM cells 3 hours later. The percentages of CD45.1 nucleated cells and CD45.1 myeloid cells in blood at 3, 6, and 16 weeks after transplantation are shown. The horizontal bars indicate mean values.
The anti–integrin α6 antibody, in contrast to anti–integrin α4 antibody, does not mobilize progenitors or augment HSC or progenitor mobilization by G-CSF. CFU-GMs in blood were analyzed after injection of the anti–integrin α6 (α6), anti–integrin α4 (α4) antibody, and control rat IgG2a antibody (rIgG) without (A; left panel) or with simultaneous mobilization G-CSF (A; right panel). (B) Anti–integrin α4 but not anti–integrin α6 antibody enhanced G-CSF–stimulated mobilization of white blood cells (WBCs) and lymphocytes. (C) A synergistic effect of the anti–integrin α4 but not anti–integrin α6 antibody on mobilization by G-CSF of short-term HSCs analyzed by competitive repopulation assay. Reconstitution from CD45.1 mobilized blood cells expressed as percent of total nucleated cells; myeloid cell reconstitution from CD45.1 mobilized blood cells, as percent of total nucleated cells. *P < .05; ** P < .01; ***P < .001.
The anti–integrin α6 antibody, in contrast to anti–integrin α4 antibody, does not mobilize progenitors or augment HSC or progenitor mobilization by G-CSF. CFU-GMs in blood were analyzed after injection of the anti–integrin α6 (α6), anti–integrin α4 (α4) antibody, and control rat IgG2a antibody (rIgG) without (A; left panel) or with simultaneous mobilization G-CSF (A; right panel). (B) Anti–integrin α4 but not anti–integrin α6 antibody enhanced G-CSF–stimulated mobilization of white blood cells (WBCs) and lymphocytes. (C) A synergistic effect of the anti–integrin α4 but not anti–integrin α6 antibody on mobilization by G-CSF of short-term HSCs analyzed by competitive repopulation assay. Reconstitution from CD45.1 mobilized blood cells expressed as percent of total nucleated cells; myeloid cell reconstitution from CD45.1 mobilized blood cells, as percent of total nucleated cells. *P < .05; ** P < .01; ***P < .001.
Discussion
The adhesion-migration mechanisms governing homing of circulating HSCs into BM are accomplished by an interplay between chemokines, growth factors, and adhesion molecules,3,7,14,16,18,21,32,40,44-47 but the particular molecular interactions are still largely unclear. During transmigration through the sinusoidal walls, the extravasating cells traverse the endothelial cell monolayer and the underlying subendothelial basement membrane. Consequently, the transmigrating hematopoietic cells interact with endothelial cells and with the ECM molecules of the sinusoidal basement membranes.3 As shown before,20,21 and in the present study, the laminin receptor integrin α6 chain is expressed in both mouse and human primitive HSCs and committed HPCs. This ubiquitous expression and conservation across species suggests a functional role during hematopoiesis. Therefore, we here used mouse transplantation models to analyze the contribution of α6 integrin to homing and mobilization of defined stem and progenitor populations in vivo.
We show here that the function-blocking antibody against integrin α6 chain significantly inhibits homing of CFU-GMs into BM within 3 hours after intravenous injection and leads to retention of progenitors in blood. However, progenitor homing was only partially inhibited, and 60% of CFU-GMs were recovered in the BM after treatment with the antibody. A similar partial inhibition was obtained by a function-blocking antibody against integrin α4 receptor. This is in agreement with previous studies, in which an anti–integrin α4 antibody, integrin α4 gene-deleted BM cells, or recipients treated with antibodies against VCAM-1, the endothelial ligand for α4 integrins, were used.10,14 In these studies, a more effective inhibition of progenitor homing was achieved by combinatorial inhibition of several pairs of receptors on progenitors and their counterreceptors or ligands. Receptors that have been shown to collaborate with α4 integrin-VCAM-1–mediated adhesion include E- and P-selectins on endothelial cells and both β2 and β1 integrins on hematopoietic cells.10,14,16-18,40,48 However, as shown here, inhibition of integrin α6 function did not potentiate the inhibition of CFU-GM homing by the anti–integrin α4 antibody. The collaboration of other receptors than integrin α4 with integrin α6 during progenitor homing should be addressed in further studies.
We analyzed the role of integrin α6 and α4 receptors for homing of long-term multilineage reconstituting HSCs by competitive repopulation assay.34 In previous studies, where either gene-deleted hematopoietic cells14 or function-blocking antibodies have been used, integrin α4 receptors have been shown to promote homing of short-term HSCs, defined as CFU-S, and short-term radioprotective cells, representing primarily megakaryocyte-erythroid reconstitution potential.10,17,18,49 These studies have been considered good surrogate assays to evaluate homing of long-term HSCs, which, however, have not been studied.48 In one study, anti–integrin α4 antibodies profoundly inhibited homing of long-term competitive repopulating HSCs in E-selectin–deficient mice,18 but the role of integrin α4 receptor alone for homing of long-term multilineage repopulating HSCs has been so far unclear. Our present results show that inhibition of either α4 or α6 integrin function by antibodies profoundly inhibits homing of long-term multilineage repopulating HSCs, indicating that both α6 and α4 integrin receptor function is critical for homing of the most primitive functionally defined HSCs into BM. Inhibition of α4 integrin, in contrast to α6 integrin receptor, also inhibited homing of short-term HSCs assayed at 6 weeks. However, inhibition of both integrin α6 and α4 receptors synergistically inhibited homing of short-term repopulating cells. Whether this synergistic inhibition is due to direct inactivation of both receptors by antibodies, or mediated indirectly by modulation of their activation status,36 is so far unclear. Such modulation has been described, for instance, for CD31/PECAM-1 receptor, shown to regulate both cell adhesive and migratory functions of α4 or α6 integrins in hematopoietic cells.37,38,47
Integrin α4 chain may assemble with β1 or β7 chain to form receptors for ECM protein fibronectin and endothelial counterreceptors VCAM-1 and MAdCAM.40 Integrin α6 chain can be assembled with either β1 or β4 chain to form receptors mainly for laminins.23 The α4β7 integrin is expressed in progenitors and may contribute to their homing into BM.40 In contrast, the expression and function of the α6β4 integrin during hematopoietic cell development is so far unclear. Integrin β1 chain is ubiquitously expressed in mouse LSKThy-1lo primitive hematopoietic cells.21 The induced deletion of β1 integrin in LSK cells was shown to lead to loss of surface expression of integrin α4 and severely reduced expression of α6 integrin, suggesting that integrin β1 chain is the major β chain partner for α4 and α6 chains on these cells.7 Gene deletion of β1 chain leads to embryonic lethality before the emergence of hematopoiesis.50 However, studies in which mice chimeric for integrin β1 gene deletion or mice given transplants of β1–/– BM cells have been used6,7 have shown that homing of β1–/– cells to hematopoietic tissues, fetal liver, and adult BM and spleen is completely abolished, emphasizing the critical role of β1 integrins as hematopoietic cell homing receptors.
The β1 integrin chain assembles with 12 different α chain partners (α1-11, αv).8 Consequently, ablation of β1 chain can lead to the loss of 12 different integrin receptors, each with distinct ligand-binding specificities. The extreme homing and migration deficits resulting from deletion of the β1 chain suggest that they are caused by concurrent absence of several receptors. Of β1 integrins, integrin α2 and α5 chains, in addition to α4 and α6 chains, are expressed at high levels in mouse HSCs.21 Integrin α2β1, a receptor for collagen and laminins, has not been found to be functional during HSC homing, as shown by antibody perturbation assay.21 Integrin α5β1, a receptor for fibronectin, has been found to be functional in stem and progenitor cell homing and engraftment in some but not all studies, and its role in these processes is so far unclear.44,46,51,52 The present findings, together with previous results, suggest that integrin α6β1 receptor, in addition to α4 integrins, is an important homing receptor in HSCs and HPCs, contributing to their transmigration to BM. Whether inhibition of stem and progenitor cell homing by anti–integrin α6 antibodies is a direct effect on stem and progenitor cells, as shown for integrin α4 receptor on LSK Thy-1lo cells,21 or is, in part, mediated by other cell types having a facilitating role,53 should be addressed in further studies.
The major ligands for α6 integrins are ECM laminins, although other ligands have also been described.8 Laminins have established biologic roles in regulation of tissue organization and cell-specific functions, including cell adhesion, differentiation, proliferation, and migration.23 All laminins are heterotrimeric proteins composed of genetically distinct variants of α, β, and γ chains. The binding sites for several receptors, including integrin α6β1, are localized in the carboxyterminal LG-domains of the α chains, and consequently the cell-binding activities of different laminins are exclusively or largely determined by the α chains. Laminin-8, containing α4β1γ1 chains and laminin-10, containing α5β1γ1 chains, recently renamed as laminin-411 and -511, respectively,54 are present in subendothelial basement membranes of sinusoids in BM.25,26 Both laminin α4 and α5 chain LG-domains contain binding sites for α6 integrins,55,56 and therefore might influence HSC and HPC phenotype and functions.
Gene deletion of laminin α4 chain in mice is compensated by early postnatal synthesis of laminin α5 chain in vascular basement membranes and leads to a viable phenotype with mild muscular dystrophy and bleeding in neonatal stage.8,57-59 In contrast, absence of laminin α5 chain results in a severe, embryonic lethal phenotype with multiple defects.60 Therefore, the tissue- or cell-specific functions and interactions of α4- and α5-laminins have been studied by using purified laminin molecules or recombinant proteins. We have here used recombinantly produced laminin-827 and laminin-10 molecules28 and placental laminin 10/11 (α5β1γ1/α5β2γ1). Our present findings suggest that the interaction of integrin α6 receptor on HSCs and HPCs with BM laminins is involved in homing of both clonogenic progenitors and long-term HSCs to BM. This is supported by previous in vitro studies showing that both α4 and α5 laminins promote migration of hematopoietic cells, including human CD34+ progenitors, neutrophils, lymphocytes, and monocytes.20,61-63 Accordingly, in vivo analysis of laminin α4 chain gene-deleted mice has shown impaired neutrophil transmigration from blood vessels in response to inflammatory stimuli.63 Both α3 and α6 integrins have been shown to mediate migration of hematopoietic tumor-derived64 and other cell lines on laminins in vitro.27,28,65,66 However, integrin α3 chain was not expressed in human HPCs, whereas the integrin α6 receptor mediated human HPC migration on both α4 and α5 laminins in vitro.20
During steady-state hematopoiesis, a small number of primitive HSCs are circulating.67 This circulating pool of HSCs can be increased by enforced mobilization with growth factors, cytokines, cytostatic drugs, and by perturbation of adhesion receptor function.2 Comparisons on the antigenic and functional phenotype of mobilized progenitors and steady-state BM cells have revealed decreased expression or reduced functional state of integrin α4 receptor.68 Accordingly, the role of integrin α4 receptor in HSC mobilization and in synergistic enhancement of G-CSF–stimulated mobilization is well established.9,14,48 In contrast, intravenous injection of anti–integrin α6 antibodies into mice did not increase the number of circulating progenitors and did not enhance the mobilizing effect of G-CSF. These divergent effects on mobilization by antibodies against α6 and α4 integrin receptors suggest that the functional effects of α6 and α4 integrins on HSC and HPC homing and mobilization are mediated by distinct signal transduction pathways and not by a collaborative signaling effect.
In conclusion, the present study on mouse hematopoietic cells corroborates our previous findings on human BM, showing that laminin isoforms present in BM and blood vessel walls are adhesive substrates for primitive hematopoietic cells, and that their adhesion to laminins is mediated by the α6β1 integrin. Embryonic deletion of integrin α6 chain leads to severe skin blistering, cerebral malformations, and neonatal death, and specific aspects of hematopoiesis in integrin α6-deficient mice have not so far been studied.69 We have here for the first time showed by in vivo transplantation assays that α6 integrin receptor functions in vivo as a homing receptor for progenitors during their transmigration into BM. Furthermore, we show that both integrin α4 and α6 receptors are crucial for homing of long-term multilineage reconstituting HSCs and that these receptors act in synergy during homing of short-term HSCs into BM. The possible collaboration of other receptors and ligands with α6 integrins in HSC homing should be addressed in further studies.
Prepublished online as Blood First Edition Paper, January 26, 2006; DOI 10.1182/blood-2005-10-3932.
Supported by grants from ALF (Government Public Health Grant), Crafoord Foundation, Kungliga Fysiografiska Sällskapet in Lund, Swedish Cancer Society, University Hospital of Lund Foundation, Georg Danielssons Foundation, and Tobias Foundation.
K.T. has declared a financial interest in a company (BioStratum) whose products (recombinant laminin-8 and laminin-10) were studied in the present work.
H.Q. and M.E. designed and performed research, analyzed data, and wrote the paper; K.T. contributed vital new reagents (recombinant laminins); and S.E.J. designed research and analyzed data.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
We thank Lilian Wittman, Zhi Ma, and Anna Fossum for expert technical assistance.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal