Abstract
Prostaglandin E2 (PGE2) blocks mast-cell (MC)-dependent allergic responses in humans but activates MCs in vitro. We assessed the functions of the EP receptors for PGE2 on cultured human MCs (hMCs). hMCs expressed the EP3, EP2, and EP4 receptors. PGE2 stimulated the accumulation of cyclic adenosine monophosphate (cAMP), and suppressed both FcϵRI-mediated eicosanoid production and tumor necrosis factor-α (TNF-α) generation. PGE2 also caused phosphorylation of extracellular signal-regulated kinase (ERK), exocytosis, and production of prostaglandin D2 (PGD2), as well as leukotriene C4 (LTC4) when protein kinase A (PKA) was inhibited. An EP3 receptor-selective agonist, AE-248, mimicked PGE2-mediated ERK phosphorylation, exocytosis, and eicosanoid formation. Selective agonists of both EP2 and EP4 receptors (AE1-259-01 and AE-329, respectively) stimulated cAMP accumulation. No selective agonist, alone or in combination, was as effective as PGE2. AE-248, AE1-259-01, and AE-329 all inhibited FcϵRI-mediated TNF-α generation, while AE1-259-01 blocked eicosanoid production. PGE2 caused the expression of inducible cAMP early repressor (ICER) by a pathway involving PKA and ERK. Thus, while PGE2 activates MCs through EP3 receptors, it also counteracts FcϵRI-mediated eicosanoid production through EP2 receptors and PKA, and blocks cytokine transcription. These functions explain the potency of PGE2 as a suppressor of early- and late-phase allergic responses.
Introduction
Mast cells (MCs) initiate inflammatory responses to infectious organisms and allergens. In allergic diseases, MCs are activated by cross-linkage of their high-affinity Fc receptors for immunoglobulin (Ig) E (FcϵRI), releasing preformed proteases and biogenic amines, and generating leukotriene C4 (LTC4), the parent molecule of the cysteinyl leukotrienes (cysLTs), and prostaglandin D2 (PGD21-3 ). Aspirin-exacerbated respiratory disease (AERD) is characterized by nasal polyposis, asthma, and MC activation in response to challenge with nonselective inhibitors of cyclooxygenase (COX).4,5 MC activation follows allergen challenge in the lungs or nose of susceptible individuals, inducing tissue swelling, bronchconstriction, and vascular leakage (early-phase response [EPR]).6 In vitro, FcϵRI-induced MC activation also initiates transcription through the actions of nuclear factor κB (NF-κB), nuclear factor of activated T cells (NF-AT) and activator protein-1 (AP-1) transcription factors, resulting in sustained cytokine and chemokine generation.7 MC activation recruits leukocytes to allergen-challenged tissues, resulting in sustained swelling and inflammation (late-phase response [LPR]) in a significant proportion of susceptible individuals.8 Mediator generation by MCs stimulated through toll-like receptors (TLRs) also plays an important role in protective innate immunity.9-11 Thus, whether activated through stimulation of FcϵRI, idiosyncratic mechanisms, or pattern recognition receptors, MCs provide eicosanoids and inducible cytokines for inflammatory responses in vivo, benefiting the host in protective immunity, but also potentially inducing exacerbations of allergic diseases.
PGE2, a functionally versatile eicosanoid, acts at 4 divergent G protein-coupled receptors (GPCRs), called the EP1, EP2, EP3, and EP4 receptors (reviewed in Kobayashi and Narumiya12 ). PGE2 can amplify inflammatory gene expression and promote tissue pathology in colon cancer13 and in mouse models of arthritis.14 In contrast, PGE2 strongly suppresses allergic respiratory mucosal inflammation. Inhalation of PGE2 before allergen challenge prevents both EPR and LPR in subjects with asthma,15 and decreases the levels of PGD2 that are detected in the bronchoalveolar lavage fluid after allergen challenge.16 In AERD, PGE2 inhalation prevents bronchconstriction induced by challenge with nonselective COX inhibitors and abrogates the characteristic rise in urinary levels of LTE4, the end product of the cysLTs.5,17,18 Collectively, these observations suggest that PGE2 inhibits MC activation in the respiratory tract. Although PGE2 reportedly suppresses mediator release by some MC subtypes in vitro by raising intracellular levels of cyclic adenosine monophosphate (cAMP),19,20 it also enhances mediator release from mouse MCs.21-23 The mechanisms and EP receptor subtypes responsible for PGE2-mediated inhibition of MC activation are incompletely understood.
We explored the mechanisms and receptors by which PGE2 modulates activation responses of cord blood-derived human MCs (hMCs). hMCs express EP2,EP3, and EP4 receptors, and respond to PGE2 and receptor-selective analogs with anticipated biochemical signatures and signaling events. In contrast to the results of earlier studies of human lung MCs, PGE2 does not suppress FcϵRI-dependent exocytosis by hMCs. Rather, PGE2 causes exocytosis on its own when hMCs are primed with interleukin-4 (IL-4), a function reflecting EP3 receptors. Moreover, EP3 receptor-dependent stimulation of hMCs induces phosphorylaton of extracellular signal-regulated kinases (ERKs) 1 and 2 and PGD2 generation, and also causes the production of LTC4 when protein kinase A (PKA) is inhibited. However, PGE2 interferes with both LTC4 and PGD2 generation by hMCs stimulated by FcϵRI cross-linkage, an effect mediated by EP2 receptors, and strikingly inhibits FcϵRI-mediated production of tumor necrosis factor-α (TNF-α). The latter event reflects complementary signaling through PKA- and ERK-mediated signaling pathways, in turn reflecting combinatorial contributions from EP receptors leading to the expression of the transcriptional repressor protein inducible cAMP early repressor (ICER).24,25 Thus while EP3 receptor-dependent signaling causes or potentiates MC mediator release, EP2 receptors inhibit FcϵRI-initiated mediator generation and may limit tissue pathology in the LPR and other circumstances where MC activation induces inflammation.
Materials and methods
Reagents
PGE2, PGD2, LTD4, the EP2/EP3 dual receptor antagonist AH6809 (EC50 = 50 μM) and the EP2 receptor selective agonist butaprost (Ki ∼10 μM) were purchased from Cayman Chemical (Ann Arbor, MI). EP1, EP2, EP3, and EP4 receptor-selective agonists (DI-004, AE1-259-01, AE-248, and AE-329, respectively) and an EP4 receptor-selective antagonist (AE-208) were obtained from Ono Pharmaceuticals (Osaka, Japan). These agonists are potent (Ki ∼3 nM, 150 nM, 7.5 nM, and 10 nM for AE1-259-01, DI-004, AE-248, and AE-329, respectively) and reportedly show little to no cross-reactivity on cloned EP receptors even at doses 1000 times their Ki values.26,27 In primary cells, agonist doses between 0.1 and 10 μM are reportedly sufficient to elicit maximal functional and signaling responses.28,29 Each selective reagent was dissolved in dimethyl sulfoxide (DMSO; Sigma, St Louis, MO). Pertussis toxin (PTX) was purchased from Sigma. The selective agonist of exchange protein activated by cAMP (Epac), 8-pCPT-2′-O-Me-cAMP, and an inhibitor of glycogen synthase kinase 3 (GSK-3) (GSK-3 inhibitor II) were purchased from Calbiochem (San Diego, CA). A second GSK-3 inhibitor, SB216763, was obtained from Sigma. Inhibitors of PKA (H89; Sigma) and mitogen-activated protein kinase kinase (MEK)/ERK (UO126; Promega, Madison, WI) were used at their recommended concentrations (10 μM and 5 μg/mL, respectively).
Derivation and priming of hMCs
hMCs were derived from cord-blood mononuclear cells cultured in the presence of stem-cell factor (SCF; 100 ng/mL), IL-6 (50 ng/mL), and IL-10 (10 ng/mL) (all from R&D Systems, Minneapolis, MN), as previously described,30 and studied when they reached more than 95% purity based on staining with toluidine blue (6-9 weeks). For FcγRI-dependent exocytosis, cytokine generation, and eicosanoid production, hMCs were transferred to new medium containing SCF and IL-4 (10 ng/mL, R&D Systems) for 5 days before activation.31,32 Because IL-4 did not alter the expression level of any EP receptor and did not change calcium fluxes in response to PGE2, signaling studies were performed with unprimed hMCs. Each donor's cells were used for 1 experiment per assay.
Flow cytometry
Expression of EP receptor proteins was assessed on fixed, permeabilized hMCs as described.33 Polyclonal antibodies (Abs) against each EP receptor (Cayman Chemical) and a monoclonal Ab against Kit (Pharmingen, San Diego, CA) were used at 2 μg/sample. The EP3 receptor Ab was raised against a peptide sequence (amino acids 308-327) common to all isoforms of this receptor.
Reverse transcriptase-polymerase chain reaction (RT-PCR)
Total RNA was extracted from hMCs at 6 to 8 weeks of culture with TRI Reagent (Molecular Research, Cincinnati, OH). RNA samples were primed with oligo(dT) and reverse transcribed using an RT kit (Clontech, Palo Alto, CA). Primer sequences for the amplification of the EP1, EP2, and EP4 receptors34 are as follows, reading from the 5′ to the 3′ direction: EP1, sense ATCTGCTGGAGGCCAATGCTGGTGT, antisense TCGTTGGGCCTCTGGTTGTGCTT; EP2, sense CCTGGCCGTGCTGCCTGTCATCTAT, antisense CCATGGACACCCTTTCCCTCTCCT; and EP4, sense TTTGCAGGCCATCCGAATTGCTTCT, antisense CCTGCCTCCAAGGCCATTTTCACTGG. Because the human EP3 receptor gene gives rise to several splice variants,35,36 we designed primers for the selective amplification of each. Splice variants I to IV were amplified using a common sense strand primer (CTGAACCAGATCTTGGATCC) with the following isoform-specific antisense primers: EP3-I, TCACCATCATAAGCTTATAC; EP3-II, TACGAATGGCAGACTCAACA; and EP3-III and EP3-IV, TCATGGAGCTTCCAGTGATG. Specific primer pairs were used to amplify EP3-V (sense CAGAGGTTTCCCAGAGAGGAAGGCGTGG, antisense TCCTGGACCTGC CTCCATGCATGACAAA) and EP3-VI (sense GAGATGGGGCCTGATGGAAG, antisense TCATGGAGCTTCCAGTGATG). Primers for human glyceraldehyde-3-phosphate dehydrogenase (Clontech) were run as positive controls. PCR was performed in a Perkin-Elmer Thermal Cycler with 0.4 units of Taq polymerase (Perkin-Elmer, Shelton, CT) for 35 cycles (94°C × 1 minute, 94°C × 30 seconds, 56°C × 2 minutes, and 72°C × 4 minutes). Genomic DNA and non-reverse-transcribed RNA were used as positive and negative control templates, respectively. The PCR products were resolved on ethidium bromide-stained 1.5% agarose gels. The analyses for each receptor were repeated 8 times with RNA harvested from the cells of different donors.
Calcium mobilization
Changes in the concentration of cytostolic free Ca++ were assessed by two methods. First, a fluorescence imaging plate reader (FLIPR)-based calcium-imaging assay was used to determine the optimal dosing range for PGE2-mediated calcium flux. hMCs (8 weeks old) were washed into Hanks balanced salt solution (HBSS) containing 1% bovine serum albumin (BSA), 20 mM HEPES, and 2.5 mM probenecid at a density of 1.3 × 106 cells/mL and loaded with 2 μM Fluo-4 (Invitrogen, Carlsbad, CA) according to the manufacturer's protocol. The plates were then placed into a FLIPR (Molecular Devices) to monitor cell fluorescence (ex = 488 nm, EM = 540 nm) before and after the addition of various agonists. Triplicate samples of cells were stimulated with PGE2 (0.01-10 μM) or similar concentrations of PGD2 or LTD4 as negative and positive controls. The susceptibility of PGE2-mediated calcium flux to interference by PTX (4- to 12-hour treatment with 100 ng/mL) and the ability of EP receptor-selective agonists to elicit this response were tested with Fura-2 am (Molecular Probes, Eugene, OR)-loaded hMCs using a fluorescence spectrophotometer (Hitachi F-4500; Hitachi, Greenville, SC) using excitation at 340 and 380 nm to monitor cytosolic free Ca++ as previously described.30
Cell activation for mediator secretion
IL-4-primed hMCs were passively sensitized with human myeloma IgE (2 μg/mL; Chemicon, Tecaluma, CA) overnight on the fourth day of priming. The cells were washed and resuspended in fresh medium at a concentration of 1 × 106 cells/mL, except for the samples used for analysis of exocytosis, which were suspended at a 10 × 106/mL. For studies of exocytosis, samples of 2 × 106 hMCs were challenged with anti-IgE (1 μg/mL; Calbiochem) or medium alone. In some experiments, PGE2, AE-248, AE-329, AE1-259-01, DMSO, or butaprost were added just before activation. The cells were maintained in the presence of SCF (100 ng/mL) throughout to promote optimal viability. Activation was stopped on ice. Supernatants were separated from the pellets by centrifugation at 200g in an Eppendorf microcentrifuge at 4°C. The content of β-hexosaminidase (β-hex) was measured by spectrophotometric analysis as described previously,37 and the percentage of release values were calculated. Data were expressed as mean release from duplicate readings.
For eicosanoid production, triplicate samples of 1 × 105 cells were stimulated with various doses of PGE2 or its mimics (0.01-10 μM) or DMSO (1:1000), with and without anti-IgE (1 μg/mL) in the wells of 96-well flat-bottom plates. Supernatants were collected at 30 minutes and stored at -20° C until further analysis with specific enzyme-linked immunosorbent assays (ELISAs) to detect cysLTs (LTC4, LTD4, and LTE4; Cayman Chemical) and PGD2 (Amersham, Arlington Heights, IL). In some experiments, the selective inhibitor of PKA, H89 (10 μM), was added to the cells 30 minutes before activation. The assay for PGD2 did not detect PGE2 at concentrations as high as 10 μM. For cytokine generation, sensitized, primed hMCs were challenged with Staphylococcus aureus peptidoglycan (PGN) (10 μg/mL; Sigma), anti-IgE (1 μg/mL), or medium alone in the presence or absence of agonists or controls as described for eicosanoids. Supernatants were collected at 6 hours and frozen at -70°C until further analysis with ELISAs for TNF-α and IL-5 (both from eBiosiences, San Diego, CA). The data for each experiment were tabulated as the mean values from triplicate samples.
cAMP measurements
Triplicate samples of 2 × 105 hMCs were stimulated with various agonists or antagonists for 10 minutes as described previously.25 cAMP was measured with a commercial Biotrack cAMP ELISA kit (Amersham). The mean cAMP values for triplicate samples of cells stimulated with each agonist were compared with values from unstimulated cells, and the data expressed as absolute values.
SDS-polyacrylamide gel electrophoresis immunoblotting
Samples of 2 × 105 hMCs were stimulated for various intervals with each agonist. Reactions were stopped on ice and the cells were lysed in a buffer containing 1% sodium dodecyl sulfate (SDS), 1% Triton X-100, 1 mM EDTA, 1 mM EGTA, 0.2 mM PMSF, 5 μg/mL leupeptin, and 1 μg/mL pepstatin in 10 mM Tris (pH 8.0). Western blotting was performed as previously described.25 Dilutions (1:2000) of primary antibodies specific for the active, phosphorylated forms of ERK-1/ERK-2, c-Jun NH2-terminal kinase (JNK), and p38 mitogen-activated protein kinase (MAPK) were used. The same blots were stripped and probed again with rabbit polyclonal antibodies that detect total ERK-1/ERK-2, p38, and JNK at dilutions of 1:2,000. All anti-MAPK antibodies were purchased from Cell Signaling Technologies (Beverly, MA). ICER was detected with a rabbit polyclonal antiserum that recognizes all forms of cAMP response element modulator (CREM), including ICER (provided by Dr Carlos Molina, University of New Jersey Medical School) at a concentration of 1:5000.38
Real-time PCR
Samples of 5 to 10 × 106 primed, sensitized hMCs were stimulated for 2 hours with anti-IgE or medium alone, all in the presence of SCF at a constant concentration of 100 ng/mL, with or without PGE2. The expression of TNF-α mRNA was determined with real-time PCR performed on ABI PRISM 7700 Sequence Detection System (Applied Biosystems, Foster City, CA) as previously described.25 Primers and probes for the amplification of TNF-α and human β2 microglobulin and the corresponding VIC dye were purchased from Applied Biosystems.
Statistics
Data are expressed as means ± SEM from at least 3 experiments except where otherwise indicated. Because quantities of eicosanoids and cytokines generated by hMCs from different donors varied widely, the data for some analyses were converted to percentages of the control (the samples not treated with PGE2 or EP receptor-selective agonists). Differences between treatment groups were determined with the Student t test, with P values less than .05 considered significant. A Q test was used to eliminate outlying experiments.
Results
Expression and function of EP receptors
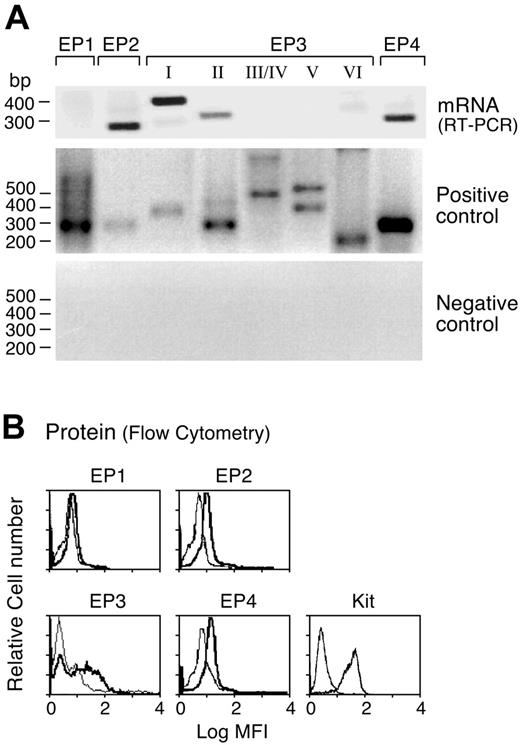
We used RT-PCR to determine the profile of EP receptor mRNAs expressed by hMCs. Bands corresponding to the predicted sizes of the EP2 and EP4 receptors were detected in mRNA samples from all 8 donors tested (as shown for 1 donor, Figure 1A). All 8 donors showed both the EP3-II splice variant and at least one Gi-linked EP3 variant (5 showed EP3-I, 4 showed the EP3-IV, 4 showed EP3-VI, and none showed EP3-V transcript). EP1 receptor transcripts were detected in the RNA from the cells of only 1 donor (not shown). All of the primer sets yielded bands of the expected sizes from the positive control DNA template, and no products were detected from the negative control template (Figure 1). The EP2, EP3, and EP4 receptor proteins were all detected by flow cytometry (n = 3 as shown for 1 experiment; Figure 1B), while the EP1 receptor protein was not detected in the cells from any of these donors. IL-4 priming of the hMCs did not alter the profile or the level of EP receptor mRNA or proteins expressed (not shown). Virtually all of the cells showed high-level cytofluorographic expression of Kit (Figure 1B), confirming the identity of the EP receptor-positive cells as hMCs.
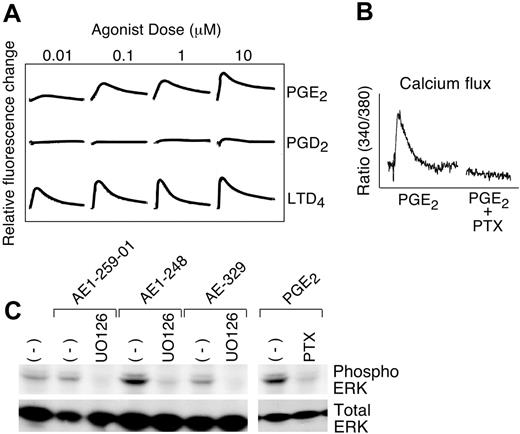
In a FLIPR assay, PGE2 (0.1-10 μM), but not PGD2, induced a calcium flux (an expected signature of both EP1 and EP3 receptors; Figure 2A). Subsequently, fluorescence spectrophotometry was used to measure calcium flux in hMCs following treatment with or without PTX. The cells from 8 donors were tested in this assay, and 3 exhibited calcium flux to PGE2 (1-10 μM); this flux was completely blocked by treatment of the cells with PTX (Figure 2B). LTD4-mediated calcium flux occurred in every donor and was unaffected by PTX. Of the receptor-selective reagents, only the EP3 receptor-selective agonist AE-248 (10 μM) stimulated calcium flux, and was much weaker than PGE2 at the same concentration (n = 2, data not shown). Priming of the cells with IL-4 did not change the strength of PGE2-induced calcium flux.
Profile of EP receptors expressed by hMCs. (A) Total RNA was extracted from 8-week-old hMCs derived in the presence of SCF, IL-6, and IL-10, and samples were subjected to RT-PCR with primers specific for each indicated receptor. Positive control (from genomic DNA) and negative control (from non-reverse-transcribed RNA) are displayed. (B) Eight-week-old hMCs were fixed, permeabilized, and stained with affinity-purified polyclonal antibodies specific for the indicated receptor proteins. IL-4 priming did not change the levels of expression of any EP receptor (not shown). Bold tracings indicate EP receptors; light tracings, control IgG. MFI indicates mean fluorescence intensity. RT-PCR data in panel A and flow cytometry in panel B are from experiments representative of 8 and 3 performed, respectively, each using the cells of a different donor.
Profile of EP receptors expressed by hMCs. (A) Total RNA was extracted from 8-week-old hMCs derived in the presence of SCF, IL-6, and IL-10, and samples were subjected to RT-PCR with primers specific for each indicated receptor. Positive control (from genomic DNA) and negative control (from non-reverse-transcribed RNA) are displayed. (B) Eight-week-old hMCs were fixed, permeabilized, and stained with affinity-purified polyclonal antibodies specific for the indicated receptor proteins. IL-4 priming did not change the levels of expression of any EP receptor (not shown). Bold tracings indicate EP receptors; light tracings, control IgG. MFI indicates mean fluorescence intensity. RT-PCR data in panel A and flow cytometry in panel B are from experiments representative of 8 and 3 performed, respectively, each using the cells of a different donor.
Functional signatures of the EP3 receptor. (A) FLIPR assay revealing dose-dependent calcium fluxes by hMCs stimulated with the indicated concentrations of PGE2, PGD2, or LTD4. Each tracing is representative of triplicate samples, all of which showed virtually identical responses. (B) Effect of overnight treatment with PTX on calcium flux by Fura-2-am-loaded hMCs stimulated with PGE2 (10 μM). Data are from 1 of 3 experiments in which a calcium response was elicited. (C) SDS-PAGE immunoblot showing signals corresponding to phosphorylated and total ERK MAPK in samples of hMCs stimulated for 5 minutes with the indicated agonists (1 μM each) in the presence or absence (-) of the MEK inhibitor, UO126, or PTX. Data are from a single experiment representative of 4 performed, all of which yielded similar results.
Functional signatures of the EP3 receptor. (A) FLIPR assay revealing dose-dependent calcium fluxes by hMCs stimulated with the indicated concentrations of PGE2, PGD2, or LTD4. Each tracing is representative of triplicate samples, all of which showed virtually identical responses. (B) Effect of overnight treatment with PTX on calcium flux by Fura-2-am-loaded hMCs stimulated with PGE2 (10 μM). Data are from 1 of 3 experiments in which a calcium response was elicited. (C) SDS-PAGE immunoblot showing signals corresponding to phosphorylated and total ERK MAPK in samples of hMCs stimulated for 5 minutes with the indicated agonists (1 μM each) in the presence or absence (-) of the MEK inhibitor, UO126, or PTX. Data are from a single experiment representative of 4 performed, all of which yielded similar results.
Stimulation of hMCs with PGE2 (1 μM) rapidly (5 minutes) induced phosphorylation of ERK-2 and ERK-1, with ERK-2 being expressed more strongly (Figure 2C). ERK was also phosphorylated in response to stimulation of the hMCs with the EP3 receptor-selective agonist AE-248 at 1 μM, but not with the EP1, EP2, and EP4 receptor-selective agonists at the same concentration (n = 4 as shown for a single experiment; Figure 2C). ERK phosphorylation in response to stimulation with both PGE2 (Figure 2C) and AE-248 (not shown) was completely blocked by pretreatment of the cells with PTX or with the MEK inhibitor UO126 (5 μg/mL), but was unaffected by treatment with H89 (10 μM, n = 3, not shown). p38 and JNK were constitutively phosphorylated and not affected by PGE2 or its analogs.
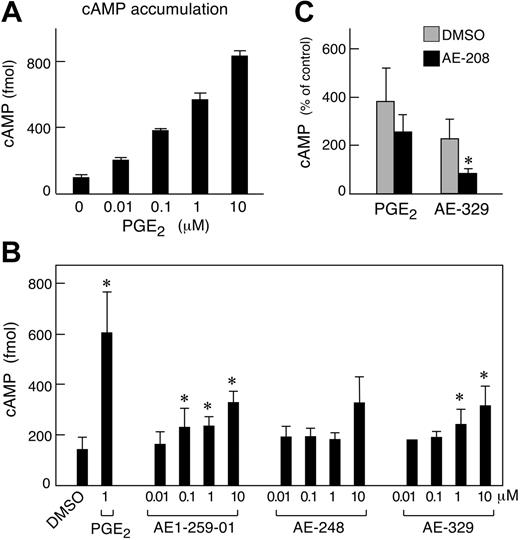
Stimulation of the cells with PGE2 dose-dependently induced the accumulation of cAMP for every donor tested (n = 3 as shown for 1 representative experiment; Figure 3A). The EP2 receptor-selective agonist AE1-259-01 modestly stimulated cAMP accumulation at concentrations of 0.1, 1, and 10 μM (n = 5, Figure 3B). A second EP2 receptor agonist, butaprost, weakly stimulated cAMP accumulation, reaching significance at 10 μM (210 ± 6 vs 158 ± 10 fmol for buffer treated cells; P = .03, n = 3, data not shown). The EP4 receptor-selective agonist AE-329 also induced cAMP accumulation at 1 and 10 μM (Figure 3B). cAMP values in cell samples stimulated with AE-248 at 10 μM tended to be higher than controls, but did not reach significance (P = .08 relative to unstimulated controls, n = 5). The EP1 receptor-selective agonist D1-004 was inactive (n = 3, not shown). Even at the highest doses tested, none of the selective agonists stimulated as much cAMP accumulation as PGE2, and various combinations of the selective agonists did not additively stimulate cAMP over a wide concentration range (0.01-1 μM, n = 3, not shown). The EP4 receptor-selective antagonist AE-208 (10 μM) abrogated cAMP accumulation in response to 10 μM AE-329, but its effect on cAMP accumulation occurring in response to PGE2 (1 μM) was not significant (P = .054, n = 4, Figure 3C). The EP2/EP3 receptor-selective antagonist AH6809 (50 μM) blocked cAMP accumulation in response to 1 and 10 μM PGE2 by 69% ± 6% and 42% ± 16%, respectively, but failed to block cAMP accumulation in response to AE-329 (mean ± ½ range, n = 2, data not shown). PTX pretreatment failed to alter cAMP accumulation in response to any agonist (not shown).
Effects of PGE2 on exocytosis and eicosanoid production
PGE2 at doses as high as 10 μM failed to attenuate FcϵRI-dependent or PGN-dependent exocytosis of β-hex by IL-4-primed hMCs and tended to induce some exocytosis by itself under these conditions (Figure 4A). To determine whether this unanticipated response depended on IL-4, we tested PGE2-mediated exocytosis in both IL-4-primed and unprimed cells. PGE2 induced exocytosis only in the IL-4-primed cells, and this response was blocked by pretreatment of the cells with PTX (Figure 4B). Stimulation of the primed hMCs with the EP3 receptor-selective agonist AE-248 caused a small amount of exocytosis (4.3% ± 1% net release, n = 3, not shown), whereas none of the other agonists induced this response.
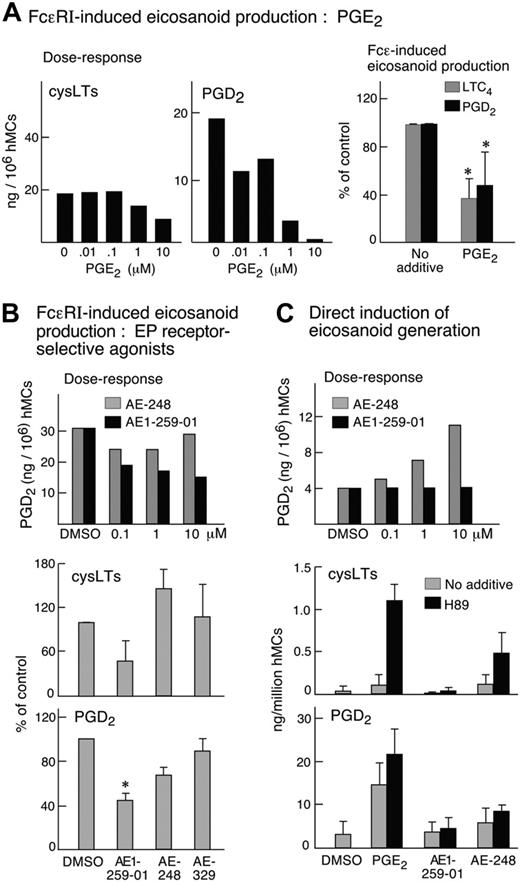
Primed hMCs generated both LTC4 (as determined by the detection of cysLTs) and PGD2 when stimulated by FcϵRI cross-linkage.34 Treatment of the cells with PGE2 inhibited the production of both PGD2 and LTC4 in a concentration-dependent manner (n = 2, as shown for 1 dose-response experiment; Figure 5A). PGE2-induced suppression of both eicosanoids was reversed completely by treatment with AH6809, but not with AE-208 (n = 1, not shown). Of the selective agonists, only AE1-259-01 (Figure 5B) tended to block LTC4 and PGD2 production (n = 5, Figure 5B). The inhibition of LTC4 generation, but not that of PGD2 production, was reversed by the PKA inhibitor H89 (n = 2, data not shown). When used by itself, PGE2 (0.1-10 μM) induced the production of PGD2 (15, 18, and 22 ng/106 hMCs in response to 0.1, 1 and 10 μM PGE2 in a dose-response experiment, data not shown). Both PGE2 and AE-248 (0.1-10 μM each) also induced LTC4 production by the primed hMCs when the cells were pretreated with H89 (as shown for the 10 μM concentration; Figure 5C). PGE2-mediated eicosanoid generation was completely blocked by pretreatment of the cells with PTX (n = 2, not shown). The selective agonists of the EP1,EP2, and EP4 receptors failed to induce eicosanoid generation by themselves, or when used in combination with H89, even at the highest doses tested (10 μM, n = 3, data not shown).
Effect of stimulation of hMCs with PGE2 and receptor-selective analogs on cAMP accumulation. (A) Effect of PGE2. Samples of 2 × 105 hMCs were stimulated for 10 minutes with the indicated concentrations of PGE2. Values are mean ± SEM of triplicate samples from a single experiment. Similar results were obtained with hMCs from 2 additional donors. (B) Effect of selective EP receptor agonists. Cells were stimulated for 10 minutes with the indicated concentrations of selective agonists of the EP1 (D1-004), EP2 (AE1-259-01), EP3 (AE-248), and EP4 (AE-329) receptors, or with DMSO alone. Values are expressed as absolute quantities of cAMP and are expressed as mean ± SEM for 5 experiments. (C) Effect of the EP4 receptor-selective antagonist AE-208 (10 μM) on cAMP accumulation in response to hMC stimulation with PGE2 (1 μM) or AE-329 (10 μM). Values are expressed as percent of control (DMSO alone) and are mean ± SEM from 4 experiments. *Significant.
Effect of stimulation of hMCs with PGE2 and receptor-selective analogs on cAMP accumulation. (A) Effect of PGE2. Samples of 2 × 105 hMCs were stimulated for 10 minutes with the indicated concentrations of PGE2. Values are mean ± SEM of triplicate samples from a single experiment. Similar results were obtained with hMCs from 2 additional donors. (B) Effect of selective EP receptor agonists. Cells were stimulated for 10 minutes with the indicated concentrations of selective agonists of the EP1 (D1-004), EP2 (AE1-259-01), EP3 (AE-248), and EP4 (AE-329) receptors, or with DMSO alone. Values are expressed as absolute quantities of cAMP and are expressed as mean ± SEM for 5 experiments. (C) Effect of the EP4 receptor-selective antagonist AE-208 (10 μM) on cAMP accumulation in response to hMC stimulation with PGE2 (1 μM) or AE-329 (10 μM). Values are expressed as percent of control (DMSO alone) and are mean ± SEM from 4 experiments. *Significant.
Effect of PGE2 on exocytosis of β-hex by hMCs. (A) hMCs were primed for 5 days with IL-4, sensitized with IgE overnight, and then stimulated with medium alone, anti-IgE, or staphylococcal PGN (50 μg/mL) for 30 minutes in the presence or absence of PGE2 (10 μM). Values are expressed as percent of total cellular β-hex release and are the mean ± SEM from 5 experiments. (B) Primed and unprimed hMCs were stimulated for 30 minutes with PGE2 (10 μM) and compared for exocytosis. Some of the primed cells were treated overnight with PTX before activation. Values are mean ± SEM from 3 experiments. *Significant relative to unprimed cells.
Effect of PGE2 on exocytosis of β-hex by hMCs. (A) hMCs were primed for 5 days with IL-4, sensitized with IgE overnight, and then stimulated with medium alone, anti-IgE, or staphylococcal PGN (50 μg/mL) for 30 minutes in the presence or absence of PGE2 (10 μM). Values are expressed as percent of total cellular β-hex release and are the mean ± SEM from 5 experiments. (B) Primed and unprimed hMCs were stimulated for 30 minutes with PGE2 (10 μM) and compared for exocytosis. Some of the primed cells were treated overnight with PTX before activation. Values are mean ± SEM from 3 experiments. *Significant relative to unprimed cells.
Cytokine generation and ICER induction
Primed hMCs generated IL-5 and TNF-α when stimulated with either anti-IgE or PGN. PGE2 interfered with the production of each cytokine in a dose-dependent manner (as shown in a representative experiment; Figure 6A), in response to both anti-IgE and to PGN (Figure 6B). AH6809 (50 μM) and AE-208 (10 μM) reversed the inhibitory effect of PGE2 on TNF-α generation by 24% and 50%, respectively (n = 1, not shown). AE1-259-01 inhibited TNF-α generation by hMCs in a dose-dependent manner, being statistically significant at 10 μM (n = 3; Figure 6C). Butaprost was also active for this function at 10 μM (n = 2, not shown). Both AE-248 and AE-329 also inhibited TNF-α production by hMCs (Figure 6C). H89 failed to reverse the inhibitory effect of any agonist on cytokine generation (n = 3, not shown). PGE2 at 10 μM strongly inhibited the FcϵRI-induced steady-state expression of TNF-α mRNA, as determined by real-time PCR (n = 2); this effect was reproduced by both AE1-259-01 and AE-329 (as shown for 1 experiment; Figure 6D). Pretreatment of the cells with the selective Epac agonist 8-pCPT-2′-O-Me-cAMP (10 μM) modestly inhibited FcϵRI-mediated TNF-α generation (43% inhibition), but had no effect on IL-5 production, and 2 inhibitors of GSK-3 (GSK-3 inhibitor II (20 μM) and SB 216763 (20 μM) had no effect on TNF-α generation (14% and 12% inhibition, respectively; not shown).
At 0.1-10 μM, PGE2 dose-dependently induced ICER expression (Figure 7A), peaking at 2 to 3 hours after stimulation (Figure 7B). This effect was partly mimicked by stimulation of the cells with forskolin at 200 μM (Figure 7D). AE1-259-01 and AE-329 both also induced ICER expression at 1 μM (Figure 7B,D), while the EP3 receptor-selective agonist AE-248 did not strongly induce ICER expression. Pretreatment of the cells with either UO126 or H89 (5 μg/mL and 10 μM) tended to attenuate the PGE2-induced expression of ICER (P = .08 and .06 relative to PGE2 alone, n = 4, as shown for 1 experiment; Figure 7C). Combined treatment with H89 and UO126 totally abrogated ICER induction, and pretreatment with either AH6809 or AE-208 modestly reduced ICER induction (n = 2, Figure 7D; as shown for 1 experiment, Figure 7C). H89, but not UO126, attenuated ICER induction by AE1-259-01 (n = 3, not shown). Treatment of the cells with 8-pCPT-2′-O-Me-cAMP did not induce ICER expression (n = 2, not shown).
Effect of PGE2 and its analogs on FcϵRI-dependent and -independent eicosanoid generation. (A) Dose response (left panel) for the suppressive effect of PGE2 on eicosanoid formation by IL-4-primed, sensitized hMCs stimulated for 30 minutes with anti-IgE. Data in second experiment were similar. Effect of 10 μM PGE2 (right panel) or buffer control. Data are means ± SEM over 5 experiments, expressed as percent of control (quantities generated in the presence of buffer alone). (B) Dose-dependent effects of EP2 receptor-selective antagonist AE1-259-01 and EP3 receptor-selective agonist AE-248 on FcϵRI-mediated PGD2 generation (top panel). Results from a second experiment were similar. Effect of 10 μM AE1-259, AE-248, and the EP4 receptor-selective agonist AE-329 (10 μM each) on FcϵRI-mediated generation of LTC4 (middle panel, as measured by an ELISA for cysLTs) and PGD2 (bottom panel). Values are means ± SEM from 4 experiments, normalized to percentage of control (cells stimulated in the presence of DMSO). (C) Induction of eicosanoid generation directly in response to PGE2 and its analogs. Cells were stimulated for 30 minutes with PGE2 or the indicated receptor-selective analogs, or with buffer control (DMSO), with or without pretreatment with H89 for 30 minutes. Dose-dependent effect of AE-248 and AE1-259-01 on PGD2 generation by primed hMCs without FcϵRI cross-linkage (top panel). Data in a second experiment were similar. The effects of 10 μM PGE2 and selective agonists in the presence or absence of the PKA inhibitor H89 (10 μM) are displayed for cys LTs (middle panel) and PGD2 (bottom panel). Data in the latter 2 panels are means ± SEM from 3 experiments. *Significantly different from control.
Effect of PGE2 and its analogs on FcϵRI-dependent and -independent eicosanoid generation. (A) Dose response (left panel) for the suppressive effect of PGE2 on eicosanoid formation by IL-4-primed, sensitized hMCs stimulated for 30 minutes with anti-IgE. Data in second experiment were similar. Effect of 10 μM PGE2 (right panel) or buffer control. Data are means ± SEM over 5 experiments, expressed as percent of control (quantities generated in the presence of buffer alone). (B) Dose-dependent effects of EP2 receptor-selective antagonist AE1-259-01 and EP3 receptor-selective agonist AE-248 on FcϵRI-mediated PGD2 generation (top panel). Results from a second experiment were similar. Effect of 10 μM AE1-259, AE-248, and the EP4 receptor-selective agonist AE-329 (10 μM each) on FcϵRI-mediated generation of LTC4 (middle panel, as measured by an ELISA for cysLTs) and PGD2 (bottom panel). Values are means ± SEM from 4 experiments, normalized to percentage of control (cells stimulated in the presence of DMSO). (C) Induction of eicosanoid generation directly in response to PGE2 and its analogs. Cells were stimulated for 30 minutes with PGE2 or the indicated receptor-selective analogs, or with buffer control (DMSO), with or without pretreatment with H89 for 30 minutes. Dose-dependent effect of AE-248 and AE1-259-01 on PGD2 generation by primed hMCs without FcϵRI cross-linkage (top panel). Data in a second experiment were similar. The effects of 10 μM PGE2 and selective agonists in the presence or absence of the PKA inhibitor H89 (10 μM) are displayed for cys LTs (middle panel) and PGD2 (bottom panel). Data in the latter 2 panels are means ± SEM from 3 experiments. *Significantly different from control.
Effect of PGE2 and receptor-selective analogs on cytokine generation by hMCs. (A) Effect of various doses of PGE2 on the generation of TNF-α by IL-4-primed, sensitized hMCs stimulated for 6 hours with anti-IgE or PGN (10 μg/mL). Values are the means of triplicate samples from a single experiment. Similar data were obtained with the cells of a second donor. (B) Effect of PGE2 (10 μM) on the production of TNF-α and IL-5 by IL-4-primed, sensitized hMCs stimulated with anti-IgE or PGN for 6 hours. Values are expressed as percentage of control and are the means ± SEM from 3 separate experiments. (C) Effect of receptor-selective agonists on TNF-α generation by hMCs stimulated with anti-IgE. Primed, sensitized hMCs were stimulated for 6 hours in the presence of PGE2 (10 μM) or the indicated concentrations of the EP receptor-selective agonists. Values are expressed as percentage of the DMSO control and are means ± SEM from 3 experiments. (D) Real-time PCR showing effect of PGE2 and receptor-selective agonists on the FcϵRI-induced steady-state expression of TNF-α mRNA. Data are from a single experiment, and are similar to that obtained in a second experiment. *Significantly different from control.
Effect of PGE2 and receptor-selective analogs on cytokine generation by hMCs. (A) Effect of various doses of PGE2 on the generation of TNF-α by IL-4-primed, sensitized hMCs stimulated for 6 hours with anti-IgE or PGN (10 μg/mL). Values are the means of triplicate samples from a single experiment. Similar data were obtained with the cells of a second donor. (B) Effect of PGE2 (10 μM) on the production of TNF-α and IL-5 by IL-4-primed, sensitized hMCs stimulated with anti-IgE or PGN for 6 hours. Values are expressed as percentage of control and are the means ± SEM from 3 separate experiments. (C) Effect of receptor-selective agonists on TNF-α generation by hMCs stimulated with anti-IgE. Primed, sensitized hMCs were stimulated for 6 hours in the presence of PGE2 (10 μM) or the indicated concentrations of the EP receptor-selective agonists. Values are expressed as percentage of the DMSO control and are means ± SEM from 3 experiments. (D) Real-time PCR showing effect of PGE2 and receptor-selective agonists on the FcϵRI-induced steady-state expression of TNF-α mRNA. Data are from a single experiment, and are similar to that obtained in a second experiment. *Significantly different from control.
Discussion
Both COX-1- and COX-2-dependent synthetic pathways mediate PGE2 production in models of experimentally induced allergic pulmonary disease in mice.39,40 The abrogation of functional COX-1 or COX-2 activity through genetic39 or pharmacologic40 approaches amplifies mucosal inflammation, eosinophilia, and airway hyperresponsiveness in such models by enhancing cytokine generation by T-helper cells.41 Because MCs are an apparent target of the protective effects of PGE2 against provocative challenges in humans with asthma15,16 and AERD,17 we used well-characterized nontransformed hMCs derived in vitro to better define the EP receptors contributing to the effects of PGE2 on mediator release, and to determine whether PGE2 might also activate hMCs in certain contexts.
EP receptors induce differential signaling events through specific patters of G protein utilization. Heterologously expressed EP2 and EP4 receptors both use Gs proteins to stimulate adenylyl cyclase and induce accumulation of cAMP,42 with EP2 receptors being more potent. We detected both of these receptors at the protein and mRNA levels (Figure 1), and showed that both were functional based on the cAMP accumulation induced by the selective agonists AE1-259-01 and AE-329 (Figure 3). The complete blockade of AE-329-induced cAMP accumulation by the EP4 receptor antagonist AE-208 (Figure 3C) confirmed the specificity of this agonist at 10 μM, and the functionality of the receptor. However, the failure of AE-208 to significantly suppress PGE2-induced cAMP stimulation suggests a more dominant role for EP2 receptors in this response, also reflected by inhibition by AH6809. Despite reported nanomolar range potency on EP transfectants,26,27 none of the selective agonists, alone or in various combinations, approached the efficacy of PGE2 itself. We speculate that primary cells that express comparatively low levels of individual EP receptor proteins (Figure 1) may require the assembly of ligand-induced, hetero-oligomeric complexes between different EP receptors to amplify PGE2-dependent signaling events, as reported for other coexpressed GPCRs.43 Such complexes may not be efficiently induced by the selective agonists. We also cannot exclude the potential existence of previously unrecognized EP receptors among the orphan GPCRs.
Expression of the transcriptional repressor ICER. (A) hMCs were stimulated with PGE2 at the indicated concentrations for 3 hours. Lysates of the cells were resolved by SDS-PAGE, and immunoblotting was performed with a CREM-specific antiserum that recognizes ICER. (B) Time-dependent induction of ICER expression in response to PGE2 or EP receptor-selective analogs. Lysates were generated from hMCs stimulated with the indicated agonist (1 μM each) for 5 minutes to 3 hours. The samples were subjected to SDS-PAGE and immunoblotting with anti-CREM. The same blot was stripped and probed for β-actin. Data in panels A and B are from single experiments representative of 3 performed for each. (C) Effects of H89 (10 μM), UO126 (5 μg/mL), AH6809 (50 μM), and AE-248 (10 μM) on the induced expression of ICER in response to stimulation with PGE2 (1 μM) for 3 hours. Results in a second experiment were similar. (D) Quantitative densitometry showing induction of ICER following stimulation with the indicated agonists (1 μM) or their respective buffer controls for 3 hours. Results are the means ± SEM from a minimum of 4 separate experiments, except for the combined effect of H89 with UO126 (top), and the effects of the EP2 and EP4 receptor-selective antagonists AH6809 and AE-208, both of which represent mean ± 1/2 range from 2 of these experiments. *P < .05 relative to control (buffer or DMSO alone).
Expression of the transcriptional repressor ICER. (A) hMCs were stimulated with PGE2 at the indicated concentrations for 3 hours. Lysates of the cells were resolved by SDS-PAGE, and immunoblotting was performed with a CREM-specific antiserum that recognizes ICER. (B) Time-dependent induction of ICER expression in response to PGE2 or EP receptor-selective analogs. Lysates were generated from hMCs stimulated with the indicated agonist (1 μM each) for 5 minutes to 3 hours. The samples were subjected to SDS-PAGE and immunoblotting with anti-CREM. The same blot was stripped and probed for β-actin. Data in panels A and B are from single experiments representative of 3 performed for each. (C) Effects of H89 (10 μM), UO126 (5 μg/mL), AH6809 (50 μM), and AE-248 (10 μM) on the induced expression of ICER in response to stimulation with PGE2 (1 μM) for 3 hours. Results in a second experiment were similar. (D) Quantitative densitometry showing induction of ICER following stimulation with the indicated agonists (1 μM) or their respective buffer controls for 3 hours. Results are the means ± SEM from a minimum of 4 separate experiments, except for the combined effect of H89 with UO126 (top), and the effects of the EP2 and EP4 receptor-selective antagonists AH6809 and AE-208, both of which represent mean ± 1/2 range from 2 of these experiments. *P < .05 relative to control (buffer or DMSO alone).
Splice variants of the human EP3 receptor mRNA produce multiple isoforms with identical ligand binding properties but different C-termini that confer the ability to either stimulate PTX-sensitive Gi proteins (all isoforms) or Gs proteins (EP3-II and EP3-IV isoforms),35,36 mediating opposing effects on adenyly cyclase/cAMP.44,45 The consistent expression of the EP3-II message with a combination of Gi-linked variants suggests that the EP3 receptor protein expressed by hMCs (Figure 1B) reflects more than one isoform. The modest increment in cAMP that was induced by AE-248 (Figure 3) suggests the function of the EP3-II receptor, although this effect did not reach statistical significance and only occurred at a dose potentially capable of crossover effects.27 Since cAMP elevation attenuates calcium signaling,46 concomitant Gs-induced signaling from EP3-II or other receptors may explain why EP3 receptor-dependent calcium flux was observed in only 3 of the 8 donors tested (Figure 2). Nonetheless, PTX-sensitive ERK phosphorylation in response to PGE2 and AE-248 was observed in all donors tested (Figure 2), reflecting a robust signal through the Gi-linked EP3 receptor variants responsible for the secretory events observed (Figures 4, 5).
For mediator release, we primed hMCs with IL-4 because this cytokine augments both exocytosis and eicosanoid generation.32,47 While earlier studies reported that PGE2 (by raising cAMP levels) inhibited FcϵRI-dependent exocytosis and eicosanoid release from MCs,20,21 other studies reported that PGE2 potentiates histamine release22 and IL-6 production23 by mBMMCs through effects at the Gi-linked EP3 receptor. Despite raising cAMP, PGE2 did not inhibit FcϵRI-dependent exocytosis, instead inducing some exocytosis by itself (Figure 4A). This response required IL-4, was blocked by PTX pretreatment (Figure 4B), and was partly mimicked only by AE-248. IL-4 may thus alter the coupling of Gi-linked EP3 receptor signaling to exocytosis, presumably distal to phospholipase C, inositol phosphate production, protein kinase C activation, and calcium flux since priming was not required for the latter event (Figure 1). IL-4, which is abundant in allergic inflammation, could promote MC activation responses to PGE2 in tissues where MCs express EP3 receptors.
FcϵRI-dependent MC activation simultaneously induces preformed mediator release (histamine and proteases) and eicosanoid generation (LTC4 and PGD2) de novo. Eicosanoid generation requires ERK-dependent phosphorylation of cytosolic PLA2,48 providing arachidonic acid to both the COX-PGD synthase and 5-lipoxygenase-LTC4 synthase (5-LO/LTC4S) pathways. Because PGE2 blocks the production of both PGD2 and cysLTs in response to provocative challenges in vivo, it was not surprising that pretreatment of the cells with PGE2 (or with the EP2 receptor-selective antagonist AE1-259-01) attenuated FcϵRI-mediated generation of both PGD2 and cysLTs (Figure 5A-B). Unexpectedly, hMCs stimulated with PGE2 also generated PGD2, but not LTC4 (reflected by the cysLT-specific ELISA). cAMP-dependent PKA phosphorylates 5-LO, preventing its translocation to the nuclear envelope to synthesize the LT precursor LTA4.49 This likely explains why cysLT generation occurred in response to PGE2 when PKA was inhibited by H89 (Figure 5C). This response was also elicited, albeit weakly, by AE-248. Combined with the abrogation of PGE2-induced exocytosis and eicosanoid generation by pretreatment of the cells with PTX, these findings firmly implicate EP3 receptors in these effector responses. The clearly opposing cellular and signaling responses to EP2 and EP3 receptor agonists strongly supports the selectivity of these reagents for their intended receptors at the doses used. It appears likely that EP2 and EP3 receptors counterbalance one another's effects on cellular activation responses when coexpressed. EP2 receptor-selective stimulation was recently reported to induce the production of vascular endothelial growth factor by hMCs.50 Thus EP2 receptor signaling in allergic disease may attenuate effector eicosanoid formation while simultaneously promoting tissue remodeling to facilitate the resolution phase of mucosal inflammation.
TNF-α localizes to MCs in airway biopsy specimens from patients with severe asthma who respond therapeutically to TNF-α-selective antagonists51 and promotes granulocyte influx in MC-dependent innate immune responses in mice.11 In our previous studies, adenine nucleotides suppressed TNF-α generation by hMCs,25 initiating a cAMP-dependent, PKA-independent signaling cascade resulting in the expression of ICER, a transcriptional repressor protein that is the product of an alternate promoter in the gene encoding cAMP response element modulator.38 ICER prevents binding of NF-AT and AP-1 transcription factors to the promoter regions of the genes encoding TNF-α and other cytokines.52 The marked suppression of TNF-α protein and mRNA expression by PGE2 (Figure 6) suggested a process involving ICER. Indeed, PGE2 strongly induced ICER expression (Figure 7), as did the adenylyl cyclase activator forskolin. Importantly, this response likely involved input from more than 1 EP receptor, since both AE1-259-01 and AE-329 both induced ICER on their own at 1 μM (Figure 7B,D). Moreover, H89 (10 μM) only partly blocked ICER induction, but completely blocked it when used in combination with UO126 (Figure 7C,D). Thus PKA (reflecting the EP2 and/or EP4 receptors) and ERK (implicating EP3 receptors; Figure 2) cooperate to induce ICER. Since the Epac agonist and GSK-3 antagonists did not block cytokine generation or induce ICER expression, the effect of PGE2 in our study was not a consequence of the recently reported Epac/GSK-3 transcriptional repressor pathway.53 Thus PGE2 suppresses cytokine generation by cell types with different EP receptor expression profiles34,52-55 through multiple pathways. This versatility may ensure that PGE2, when not suppressed by COX inhibitors, tempers cytokine generation and consequent inflammation in the hours after MC activation occurs in allergic disease.
Our data reveal that PGE2 can either induce (via EP3 receptors) or suppress (via EP2 receptors) mediator release from MCs that induce the EPR. EP2 receptor-dependent signaling is an attractive candidate to explain PGE2-dependent suppression of eicosanoid generation in allergen or aspirin challenge. Moreover, the suppressive effect of PGE2 on allergen-induced LPRs15 could in part reflect transcriptional repression through cooperative signaling from multiple EP receptors. Finally, EP3 receptor-dependent MC activation is reported to occur in the skin in a mouse model of cutaneous inflammation,21 and this may reflect both the release of histamine and PGD2. It seems likely that the pro- and anti-MC-activating effects of PGE2 reported for respectively for skin21 and lung15 reflect regional differences between MCs EP receptor expression and function.
Prepublished online as Blood First Edition Paper, December 15, 2005; DOI 10.1182/blood-2005-07-2772.
Supported by National Institutes of Health grants AI-48802, AI-52353, AI-31599, and HL-36110, and by grants from the Charles Dana Foundation and the Vinik Family Fund for Research in Allergic Diseases.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.




This feature is available to Subscribers Only
Sign In or Create an Account Close Modal