Abstract
We previously identified the carbohydrate determinant sialyl 6-sulfo Lewis X (Lex) as the major L-selectin ligand on high endothelial venules of peripheral lymph nodes. In this study, we examined the distribution of the sialyl 6-sulfo Lex determinant among peripheral lymphocytes. The determinant was expressed on a subset of helper memory T and NK cells. The helper memory T cells expressing sialyl 6-sulfo Lex were CD45RObright+PSGL-1high+CCR4+L-selectin+CCR7+ but did not express α4β7 integrin or CCR9, indicating that they were the skin-homing population of central memory T cells. The T-cell subset significantly expressed mRNA for 6-sulfotransferase HEC-GlcNAc6ST and fucosyltransferase Fuc-T VII, responsible for the synthesis of sialyl 6-sulfo Lex. Characteristics of the T-cell population were similar to those previously described for cutaneous lymphocyte-associated antigen (CLA)–positive T cells defined by the HECA-452 or 2F3 antibody. The binding of the T-cell subset with the specific anti–sialyl 6-sulfo Lex antibody G152 was almost completely abrogated by HECA-452 or 2F3. Binding of recombinant E-, P-, and L-selectins to the T-cell subset was significantly inhibited by G152 and by HECA-452 antibodies. We propose that CLA, which is expressed without any activation stimuli on peripheral skin-homing helper memory T cells in healthy persons, is at least partly the sialyl 6-sulfo Lex determinant.
Introduction
The cutaneous lymphocyte-associated antigen (CLA) has been effectively applied in clinical studies of inflammatory skin diseases and cutaneous T-cell malignancies1,2 (reviewed in Santamaria-Babi3 and Kim et al4 ). CLA defines a subset of human peripheral helper memory T cells and has long been used as a specific carbohydrate determinant in characterizing skin-homing helper memory T-cell populations.5 The HECA-452 antibody defining CLA was shown to recognize the sialyl Lewis X (Lex) determinant, which is known as a typical carbohydrate ligand for selectins, the cell adhesion molecules that are involved in leukocyte-endothelial and leukocyte-leukocyte interactions.6-10 An antibody, 2F3, one of the antibodies raised against chemically synthesized sialyl Lex determinant, was also shown to detect a similar helper memory T-cell population as HECA-452 and has been used to characterize skin infiltration of adult T-cell leukemia cells.11-14 The puzzling phenomenon, however, has been that some anti–sialyl Lex antibodies, such as CSLEX-1, fail to effectively detect the skin-homing helper memory T-cell subset, whereas only a few anti–sialyl Lex antibodies, such as HECA-452 and 2F3, define the subset. The antigen defined by the 2F3 antibody was designated as variant sialyl Lex.11,12 These findings suggested that CLA on skin homing T cells defined by HECA-452, or the variant sialyl Lex antigen defined by 2F3, is closely related, but not identical, to the conventional sialyl Lex determinant.
Previously, we have shown that sialyl 6-sulfo Lex, but not conventional sialyl Lex, serves as the major ligand for L-selectin on high endothelial venules (HEVs) of human peripheral lymph nodes by generating a monoclonal antibody, G152, specific to this determinant.15,16 The sialyl 6-sulfo Lex determinant on HEV is synthesized through a cooperative action of a fucosyltransferase (Fuc-T VII)17 and 2 GlcNAcβ/6-O-sulfotransferases, GlcNAc6ST-1, and HEC-GlcNAc6ST,18-21 and we have succeeded in reconstituting a functional L-selectin ligand activity on the cell surface by transfecting cDNAs for both enzymes.22 In this study, we investigated the distribution of sialyl 6-sulfo Lex and related determinants on human lymphocyte subpopulations and tested the possibility that sialyl 6-sulfo Lex contributes as a CLA determinant on skin-homing helper memory T cells.
Materials and methods
Monoclonal antibodies directed to sialyl 6-sulfo Lex–related carbohydrate determinants
The antibodies G152 and G72, directed to sialyl 6-sulfo Lex, and AG107, recognizing 6-sulfo Lex, were prepared as described previously.15,18 G152 and G72 are specific to sialyl 6-sulfo Lex and does not react to nonsulfated sialyl Lex. AG107 is reactive to 6-sulfo Lex but not to non-sulfated Lex. An anti–sialyl Lex antibody, CSLEX-1, was obtained from the American Type Culture Collection (Manassas, VA). The anti–sialyl Lex antibody 2F3, which was established against a synthetic sialyl Lex-active glycolipid, was prepared as previously described.11 Anti-CCR4 antibody KM2160 (murine IgG1) was also prepared as described previously.23,24 The anti-CCR7 antibody 150503 and the anti-CCR9 antibody 112509 (both murine IgG2a) were obtained from R&D Systems (Minneapolis, MN). The anti–PSGL-1 antibody KPL1 (IgG1)25 and the anti-α4β7 antibody FIB504 were obtained from PharMingen (San Diego, CA). Among the antibodies used for the cell surface marker analysis, Leu 4 (CD3) for T cells, Leu 12 (CD19) for B cells, Leu 11 (CD16) for NK cells, Leu 8 (CD62L) for L-selectin, and the anti-CD45RO antibody UCHL1 (IgG2a), were obtained from Becton Dickinson Immunocytometry Systems (San Jose, CA). The anti-CD45RA antibody HI100 (IgG2b) was obtained from PharMingen.
Flow cytometric analysis of sialyl 6-sulfo Lex and related carbohydrate determinants
Peripheral blood samples were obtained from healthy donors at Kyoto University Hospital. Approval for these blood samples was obtained from the Kyoto University Hospital institutional review board. Informed consent was provided according to the Declaration of Helsinki. Before the analyses, the samples were checked by flow cytometry for the expression of activation markers on T cells using antibodies directed to HLA-DR, CD25, and CD69, and samples containing activated T cells were excluded from this study. Because expression of the sialyl 6-sulfo Lex determinant defined by the G152 antibody was unstable, whole blood samples were immediately mixed and incubated with the anticarbohydrate monoclonal antibody (purified antibody at 1 μg/mL or culture supernatant at a dilution of 1:10) at 4°C for 30 minutes. Cells were then washed 3 times with PBS containing 0.5% BSA and were stained with 1:200 dilution of fluorescein isothiocyanate (FITC)–conjugated goat anti–mouse IgG (heavy- and light-chain specific; Silenus Laboratories, Hawthorn, Australia) at 4°C for 30 minutes. After lysing red blood cells and fixing on the Q-Prep apparatus with ImmunoPrep Leukocyte Preparation System reagents (Coulter Immunology, Hialeah, FL), the stained cells were analyzed on a FACSCalibur (Becton Dickinson Immunocytometry Systems). For sialidase treatment, whole blood samples were washed 3 times with PBS containing 0.5% BSA and were incubated with 0.05 U/mL neuraminidase from Arthrobacter ureafaciens (Nakarai Chemicals, Kyoto, Japan) before antibodies were added. Subinhibitory concentrations of HECA-452, 2F3, and G152 (0.5 μg/mL) were used for double staining with these antibodies. In inhibition studies, the inhibitory antibodies were used at 20 μg/mL for HECA-452, 2F3, and CSLEX-1 and at 35 μg/mL for G152. Stimulation of peripheral lymphocytes was performed with 10 ng/mL TPA (Sigma Chemical, St Louis, MO) and 1 μg/mL ionomycin (Calbiochem, San Diego, CA) or with 5 μg/mL ConA (Pharmacia, Piscataway, NJ) for 4 days.
Immunohistochemical staining of the skin was performed using formalin-fixed sections of 10-μm thickness prepared from the skin with no apparent abnormalities at autopsy in Kyoto University Hospital. HECA-452 (rat IgM) and G72 (murine IgM) were used as the first antibody for detection of the CLA and sialyl 6-sulfo Lex determinants. The avidin-biotin complex technique for the immunohistochemical study was performed as described in the instructions for the respective kits for murine and rat IgM (Vectastain; Vector, Burlingame, CA). Micrographs were visualized using the Microphot-FXA microscope system equipped with a Plan Apo 10 ×/0.45 numeric aperture objective and a DS-5M-L1 digital camera (Nikon, Tokyo, Japan), and images were processed using Adobe Photoshop software version 5.0J (Adobe Systems, San Jose, CA).
Binding analysis of recombinant E-, P-, and L-selectins
Flow cytometric analyses of recombinant E-, P-, and L-selectins were performed as described.26 Recombinant human E-, P-, and L-selectin IgG chimera were kindly provided by Drs H. Kondo and Y. Inoue (Department of Chemistry, R&D Laboratories, Nippon Organon K.K., Osaka, Japan). Control murine and rat IgM reagents were obtained from Becton Dickinson Immunocytometry Systems (San Jose, CA). Recombinant selectins were preincubated with affinity-purified biotinylated rabbit anti–human IgG (Dako, Glostrup, Denmark) followed by incubation with phycoerythrin-streptavidin (Dako) before application to the staining of peripheral lymphocytes. BD FACS lysing solution was used for lysing red blood cells in the flow cytometric analyses with recombinant selectins instead of the ImmunoPrep Leukocyte Preparation System reagent (Coulter Immunology). Cells were preincubated with blocking antibodies (G152, 35 μg/mL; HECA-452, 20 μg/mL) for 30 minutes at 37°C if indicated.
ELISA and preparation of pure carbohydrate determinants
ELISA was performed using synthetic carbohydrate determinants immobilized at the bottom of 96-well culture plates by a standard method previously described.15 Peroxidase-conjugated rabbit anti–rat IgM (μ-chain specific; Zymed Laboratories, South San Francisco, CA) was used for HECA-452, and peroxidase-conjugated goat anti–mouse IgM (μ-chain specific; Cappel, Malvern, PA) was used for other antibodies. The pure synthetic sialyl 6-sulfo Lex and sialyl Lex determinants used in this study were synthesized as previously described.27,28
RNA extraction and RT-PCR analysis
T lymphocytes of each subset, including CD4+CD45RO+, CD4+CD45RA+, CD3–CD56+, and CD3+CD8+ cells, were collected from healthy donors using FACStar. Samples were frozen rapidly and stored at –80°C until extraction of total RNA. Total cellular RNA was isolated from 2 ×106 cells according to the acid guanidinium thiocyanate–chloroform extraction method using an Isogen kit (Nippon-Gene, Tokyo, Japan). First-strand cDNA was prepared with the use of 300 ng total cellular RNA. Synthesis of cDNA was carried out in a 20-μL reaction volume using the Superscript Pre-amplification System (Gibco BRL, Grand Island, NY) according to the manufacturer's protocol with oligo-d(T) as initiation primer. Primers used in reverse transcription–polymerase chain reaction (RT-PCR) analysis for human GlcNAc6ST-1 reported by Uchimura et al29 were 5′-ATGCAATGTTCCTGGAAGGC-3′ and 5′-TTGATGTAGTTCTCCAGGAAG-3′, which give a 419–base pair product; those for HEC-GlcNAc6ST reported by Bistrup et al19 were 5′-GCAGCATGAGCAGAAACTCAAG-3′ and 5′-TCCAGGTAGACAGAAGATCCAG-3′, which give a 469–base pair product; and those for I-GlcNAc6ST30 were 5′-CAAGACAGTGACAGTGCTCC-3′ and 5′-TACGTCCTGCTTGCTGATGG-3′, which give a 424–base pair product. Primers for GlcNAc6ST-4 were 5′-CGTCCTTCTTGGGCGAACTC-3′ and 5′-CGGAAAAGCTGCACCACCTT-3′, which were to give a 493–base pair product; and those for C-GlcNAc6ST were 5′-ACCTCTTCCAGTGGGCCGTG-3′ and 5′-CGCCAGAGCCTTGGCTGTCT-3′, which were to give a 302–base pair product. The PCR program consisted of 35 cycles with 1 minute of denaturation at 94°C, 45 seconds of annealing at 60°C (for GlcNAc6ST-1), 57°C (for HEC-GlcNAc6ST), 60°C (for I-GlcNAc6ST), 58°C (for GlcNAc6ST-4) or 62°C (for C-GlcNAc6ST), followed by 1 minute of extension at 72°C. The first denaturation step, at 94°C, lasted 4 minutes, and the final extension step lasted 8 minutes at 72°C. Aliquots of each reaction were fractionated by electrophoresis through a 2% agarose gel including ethidium bromide. To control for genomic DNA contamination, parallel amplifications of all samples with no reverse transcriptase were performed. RT-PCR of human Fuc-T VII was performed with the primers 5′-CTCGGACATCTTTGTGCCCTATG-3′ and 5′-CGCCAGAATTTCTCCGTAATGTAG-3′, which give a 291–base pair product, and the primers for Fuc-T IV were 5′-GGTGCCCGAAATTGGGCTCCTGCACAC-3′ and 5′-CCAGAAGGAGGTGATGTGGACAGCGTA-3′, which give a 319–base pair product.31 RT-PCR of human G3PDH was performed with previously reported primers to verify the quality/quantity of RNA.
Results
Distribution of sialyl 6-sulfo Lex and 6-sulfo Lex on human lymphocytes
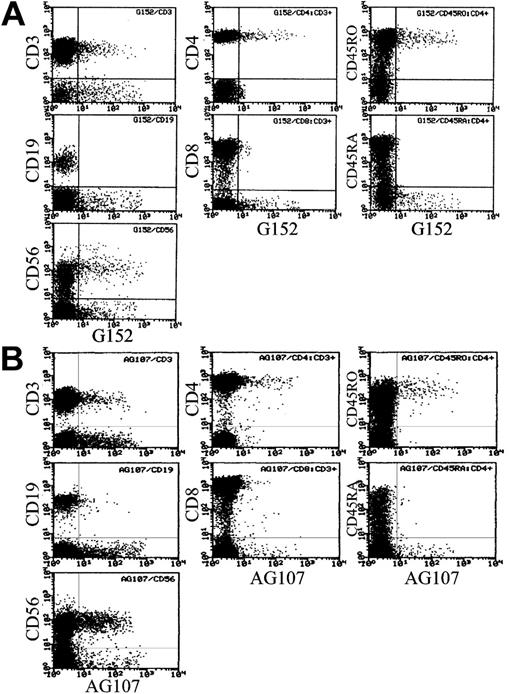
Sialyl 6-sulfo Lex, as defined by the G152 antibody, was expressed on 7.2% ± 4.6% of total lymphocytes in the peripheral blood of healthy persons (n = 24). Strong expression was noted on a part of T cells and NK cells as shown in Figure 1A. Further analyses indicated that T cells expressing sialyl 6-sulfo Lex were CD4+CD45RO+ helper memory T cells (Figure 1A) in all 24 samples tested. Expression of sialyl 6-sulfo Lex among CD4+ cells was confined to CD45RObright+ cells and was not observed on CD45ROdim+ helper T cells.
Distribution of sialyl 6-sulfo Lex and 6-sulfo Lex determinants on human peripheral lymphocyte subsets. (A) Distribution of sialyl 6-sulfo Lex (G152) determinants on human peripheral lymphocyte subsets. (Left column) Two-dimensional distribution of CD3, CD19, or CD56 and sialyl 6-sulfo Lex in total lymphocytes (2-color analyses). (Middle column) Two-dimensional distribution of CD4 or CD8 and sialyl 6-sulfo Lex in gated CD3+ cells (3-color analyses). (Right column) Two-dimensional distribution of CD45RO or CD45RA and sialyl 6-sulfo Lex on gated CD3+CD4+ cells (4-color analyses). (B) Distribution of nonsialylated 6-sulfo Lex (AG107) determinants on sialidase-treated human peripheral lymphocyte subsets in the same settings.
Distribution of sialyl 6-sulfo Lex and 6-sulfo Lex determinants on human peripheral lymphocyte subsets. (A) Distribution of sialyl 6-sulfo Lex (G152) determinants on human peripheral lymphocyte subsets. (Left column) Two-dimensional distribution of CD3, CD19, or CD56 and sialyl 6-sulfo Lex in total lymphocytes (2-color analyses). (Middle column) Two-dimensional distribution of CD4 or CD8 and sialyl 6-sulfo Lex in gated CD3+ cells (3-color analyses). (Right column) Two-dimensional distribution of CD45RO or CD45RA and sialyl 6-sulfo Lex on gated CD3+CD4+ cells (4-color analyses). (B) Distribution of nonsialylated 6-sulfo Lex (AG107) determinants on sialidase-treated human peripheral lymphocyte subsets in the same settings.
To confirm the specific expression of sialyl 6-sulfo Lex on a distinct subset of helper memory T cells and NK cells, we also used antibodies specific to nonsialylated 6-sulfo determinant. The asialo determinant defined by the AG107 antibody, 6-sulfo Lex, was virtually not expressed on lymphocytes (not shown) and appeared only when the lymphocytes were sialidase treated, thus confirming the presence of sialyl 6-sulfo Lex on lymphocytes (Figure 1B). Lymphocytes expressing 6-sulfo Lex after sialidase treatment were mainly helper memory T cells and NK cells, and the percentage of 6-sulfo Lex–positive cells was comparable to the percentage of sialyl 6-sulfo Lex–positive cells before the sialidase treatment in all samples tested, again confirming specific expression of sialyl 6-sulfo Lex on these subpopulations.
Characterization of helper memory T cells and NK cells expressing sialyl 6-sulfo Lex
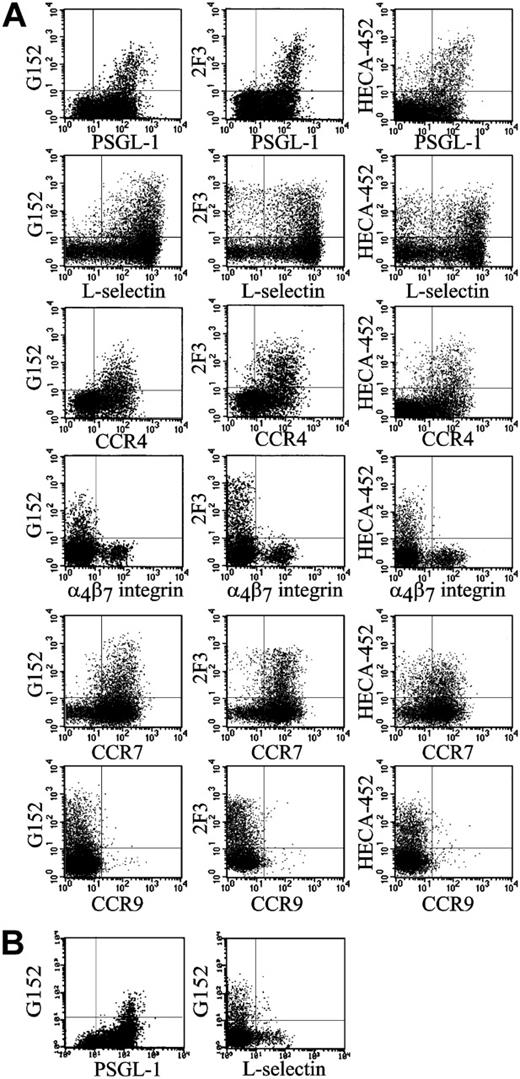
Essentially all the helper memory T cells expressing sialyl 6-sulfo Lex were PSGL-1high+ cells (Figure 2A). Human helper memory T cells are known to be bimodal as to L-selectin expression, and the cells bearing sialyl 6-sulfo Lex belonged to the CD4+CD45RO+ L-selectin+ population (Figure 2A). Virtually all sialyl 6-sulfo Lex–positive helper memory T cells expressed CC chemokine receptor 4 (CCR4), which was proposed to be preferentially expressed on skin homing helper memory T cells.32,33 On the other hand, the sialyl 6-sulfo Lex–positive helper memory T cells did not express α4β7 integrin, which is a marker for the gut homing population.32 These results were consistently observed in analyses of peripheral blood prepared from 12 healthy persons. It has been known that helper memory T cells are poorly reactive to conventional anti–sialyl Lex antibodies but strongly reactive to antibodies having a distinct specificity, such as HECA-452 and 2F3.5,11 Characteristics of the sialyl 6-sulfo Lex–positive subset of helper memory T cells coincided with the population defined by HECA-452 or 2F3 (Figure 2A). These findings suggested that at least part of the determinant previously detected with HECA-452 or 2F3 antibodies on helper memory T cells was sialyl 6-sulfo Lex.
CCR7, the marker for lymph node–homing T cells,34 is proposed to be a marker for central memory T cells.35 Most HECA-452–positive helper memory T cells in peripheral blood are known to express CCR7.51 CCR7 was also strongly expressed on the sialyl 6-sulfo Lex–positive population (Figure 2A). On average, 93.0% ± 6.7% of G152-positive cells (n = 8) expressed CCR7. The percentage was significantly higher than that for HECA-452–positive cells evaluated in the same series of blood samples, which was 82.7% ± 7.7% (P < .05). L-selectin, another marker for central memory T cells, was expressed on 96.4% ± 1.0% of G152-positive cells—again, significantly higher than the positive rate in HECA-452–positive cells, which was 81.8% ± 3.2% (P < .001). These findings indicated that sialyl 6-sulfo Lex–expressing cells are the skin-homing population of central helper memory T cells and do not belong to effector helper memory T cells.
Characterization of the helper memory T cells and NK cells expressing sialyl 6-sulfo Lex by 4-color analyses. Representative results obtained from analysis of 12 persons. (A) Two-dimensional distribution of sialyl 6-sulfo Lex (G152), variant sialyl Lex (2F3) and CLA (HECA-452) with PSGL-1 (PL1), L-selectin (Leu 8), CCR4 (KM2160), α4β7 integrin (FIB504), CCR7 (150 503), or CCR9 (112 509) on the gated CD4+CD45RO+ helper memory T-cell population. (B) Two-dimensional distribution of sialyl 6-sulfo Lex with PSGL-1 or L-selectin on the gated CD56+CD3– NK-cell population.
Characterization of the helper memory T cells and NK cells expressing sialyl 6-sulfo Lex by 4-color analyses. Representative results obtained from analysis of 12 persons. (A) Two-dimensional distribution of sialyl 6-sulfo Lex (G152), variant sialyl Lex (2F3) and CLA (HECA-452) with PSGL-1 (PL1), L-selectin (Leu 8), CCR4 (KM2160), α4β7 integrin (FIB504), CCR7 (150 503), or CCR9 (112 509) on the gated CD4+CD45RO+ helper memory T-cell population. (B) Two-dimensional distribution of sialyl 6-sulfo Lex with PSGL-1 or L-selectin on the gated CD56+CD3– NK-cell population.
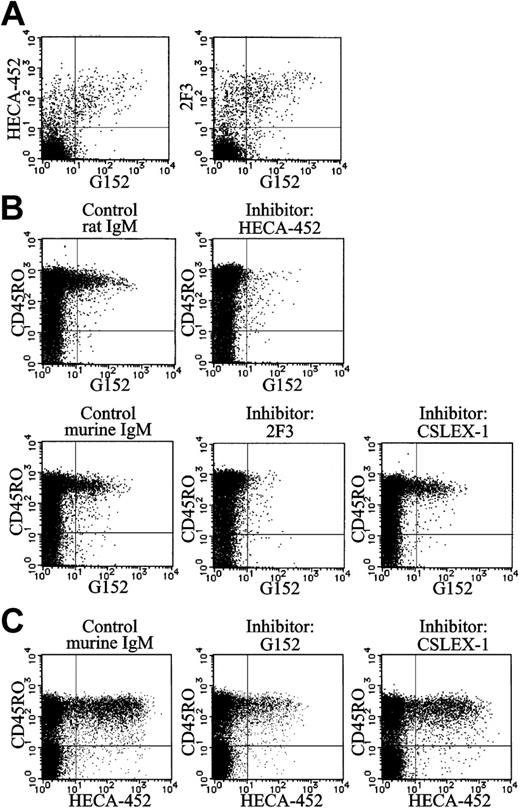
Identification of sialyl 6-sulfo Lex as CLA and variant sialyl Lex. (A) Results of 4-color analyses of normal peripheral blood lymphocytes using HECA-452 or 2F3 with anti-CD4, anti-CD45RO, and G152 antibodies. Two-dimensional distribution of sialyl 6-sulfo Lex (G152) with CLA (HECA-452) or variant sialyl Lex (2F3) on a gated CD4+CD45RO+ cell population was plotted. (B) Inhibition of staining of sialyl 6-sulfo Lex (biotin-G152) by HECA-452 (anti-CLA), 2F3 (anti–variant sialyl Lex), or CSLEX-1 (anti–sialyl Lex) antibody. Results of 4-color analyses using anti-CD3, anti-CD4, anti-CD45RO, and G152 antibodies are shown. Two-dimensional distribution of sialyl 6-sulfo Lex with CD45RO on gated CD3+CD4+ T-cell population was plotted. (C) Inhibition of staining of CLA (HECA-452) by G152 (anti–sialyl 6-sulfo Lex) or CSLEX-1 (anti–sialyl Lex) antibody. Results of 4-color analyses using anti-CD3, anti-CD4, anti-CD45RO, and HECA-452 antibodies. Two-dimensional distribution of CLA with CD45RO on gated CD3+CD4+ T-cell population was plotted.
Identification of sialyl 6-sulfo Lex as CLA and variant sialyl Lex. (A) Results of 4-color analyses of normal peripheral blood lymphocytes using HECA-452 or 2F3 with anti-CD4, anti-CD45RO, and G152 antibodies. Two-dimensional distribution of sialyl 6-sulfo Lex (G152) with CLA (HECA-452) or variant sialyl Lex (2F3) on a gated CD4+CD45RO+ cell population was plotted. (B) Inhibition of staining of sialyl 6-sulfo Lex (biotin-G152) by HECA-452 (anti-CLA), 2F3 (anti–variant sialyl Lex), or CSLEX-1 (anti–sialyl Lex) antibody. Results of 4-color analyses using anti-CD3, anti-CD4, anti-CD45RO, and G152 antibodies are shown. Two-dimensional distribution of sialyl 6-sulfo Lex with CD45RO on gated CD3+CD4+ T-cell population was plotted. (C) Inhibition of staining of CLA (HECA-452) by G152 (anti–sialyl 6-sulfo Lex) or CSLEX-1 (anti–sialyl Lex) antibody. Results of 4-color analyses using anti-CD3, anti-CD4, anti-CD45RO, and HECA-452 antibodies. Two-dimensional distribution of CLA with CD45RO on gated CD3+CD4+ T-cell population was plotted.
NK cells are known to express the conventional sialyl Lex determinant because several anti–sialyl Lex antibodies were reactive to them.11,36 Our observation was that NK cells, preferentially the CD56dim+ population, also expressed sialyl 6-sulfo Lex. Essentially all the NK cells expressing sialyl 6-sulfo Lex were PSGL-1high+ but L-selectin–. This was consistently observed in all samples prepared from 4 persons (a typical sample is shown in Figure 2B).
Identification of sialyl 6-sulfo Lex as CLA and variant sialyl Lex determinants
Results of double staining (Figure 3A) indicated that essentially all cells expressing sialyl 6-sulfo Lex (G152) express CLA (HECA-452) or variant sialyl Lex (2F3). More than half the cells expressing CLA (HECA-452) or variant sialyl Lex were G152+, indicating that the cells expressing sialyl 6-sulfo Lex are a major subset of the HECA-452+ and 2F3+ cells. There were virtually no G152+CLA– or G152+2F3– cells.
In the binding inhibition experiments, the binding of anti–sialyl 6-sulfo Lex antibody (G152) was almost completely inhibited by anti-CLA (HECA-452) or anti–variant sialyl Lex (2F3) antibodies (Figure 3B), indicating that at least a part of CLA or variant sialyl Lex previously detected by these antibodies was sialyl 6-sulfo Lex. This was also confirmed by the partial (approximately 66%) inhibition of the binding of HECA-452 by the addition of G152 (Figure 3C). The antibody directed to conventional nonsulfated Lex showed no appreciable inhibition, confirming that the determinant carried by these cells was sialyl 6-sulfo Lex.
Selectin ligand activity of sialyl 6-sulfo Lex on helper memory T cells
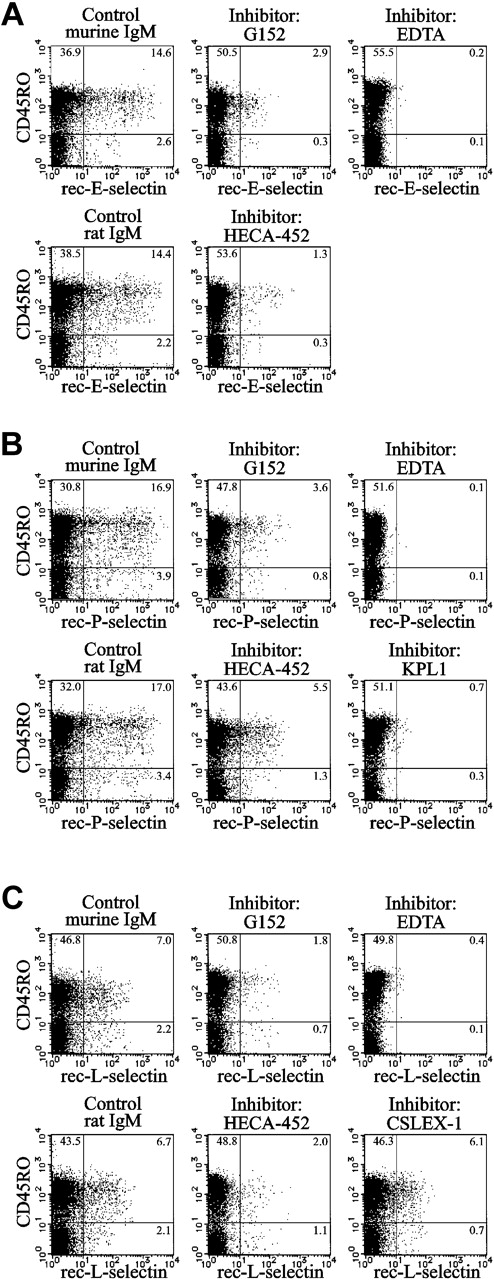
The sialyl 6-sulfo Lex determinant is known to serve as a ligand for E-, P- and L-selectins.26,37,38 The binding of recombinant E-, P- and L-selectins to helper memory T cells was significantly inhibited by the G152 antibody (Figure 4), indicating that the sialyl 6-sulfo Lex determinant on these cells serves as a ligand for all 3 selectins. Specific binding of selectins was ascertained by the inhibition using EDTA. The G152 antibody attained 81% inhibition of specific binding of E-selectin and 79% for binding of P- or L-selectin (Figure 4). The degree of inhibition was comparable with those exerted by the HECA-452 antibody, which were 92% for E-selectin and 68% each for P- and L-selectin (Figure 4). The nature of carbohydrate ligands involved in the remaining binding activity is unclear at present; one possibility could be that carbohydrate moieties internally modified by fucose or sulfate served as ligands. Such internal ligands may not be recognized by the G152 and HECA-452 antibodies because these antibodies are directed to terminal carbohydrate determinants.
Selectin binding activity of sialyl 6-sulfo Lex expressed on helper memory T cells. Inhibition of the binding of recombinant E-selectin (A), P-selectin (B), or L-selectin (C) immunoglobulin to CD45RO+ cells by anti–sialyl 6-sulfo Lex (G152) or anti-CLA (HECA-452) antibody. Specific binding of recombinant selectin immunoglobulin was ascertained by the inhibition with EDTA. KPL1 is an anti–PSGL-1 antibody. Results of 4-color analyses using anti-CD3, anti-CD4, and anti-CD45RO antibodies and recombinant selectin immunoglobulin in the presence or absence of inhibitory antibodies. Two-dimensional distribution of recombinant selectin immunoglobulin staining with CD45RO on gated CD3+CD4+ T-cell population was plotted.
Selectin binding activity of sialyl 6-sulfo Lex expressed on helper memory T cells. Inhibition of the binding of recombinant E-selectin (A), P-selectin (B), or L-selectin (C) immunoglobulin to CD45RO+ cells by anti–sialyl 6-sulfo Lex (G152) or anti-CLA (HECA-452) antibody. Specific binding of recombinant selectin immunoglobulin was ascertained by the inhibition with EDTA. KPL1 is an anti–PSGL-1 antibody. Results of 4-color analyses using anti-CD3, anti-CD4, and anti-CD45RO antibodies and recombinant selectin immunoglobulin in the presence or absence of inhibitory antibodies. Two-dimensional distribution of recombinant selectin immunoglobulin staining with CD45RO on gated CD3+CD4+ T-cell population was plotted.
Carbohydrate specificities of anti-CLA and anti–variant sialyl Lex antibodies
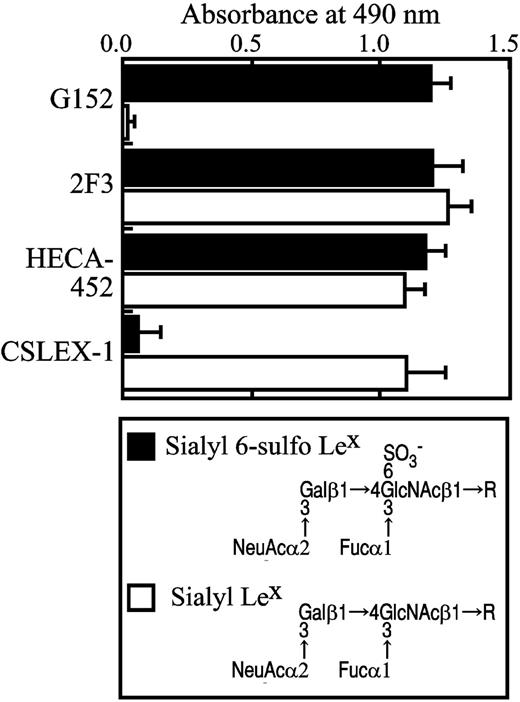
Enzyme-linked immunosorbent assay using pure synthetic sialyl 6-sulfo Lex and nonsulfated conventional sialyl Lex determinants indicated that anti–sialyl 6-sulfo Lex antibody (G152) is reactive only to sialyl 6-sulfo Lex and not to the conventional nonsulfated sialyl Lex determinant (Figure 5). This confirmed that the subset of helper memory T cells expresses sialyl 6-sulfo Lex. Anti-CLA (HECA-452) or anti–variant sialyl Lex (2F3) antibodies react to both determinants, whereas the anti–sialyl Lex antibody CSLEX-1 reacted to only nonsulfated conventional sialyl Lex and not to sialyl 6-sulfo Lex determinant (Figure 5). These findings explained well why HECA-452 and 2F3 react, whereas CSLEX-1 failed to react, to the subset of helper memory T cells.
Enzymes involved in the expression of sialyl 6-sulfo Lex determinant
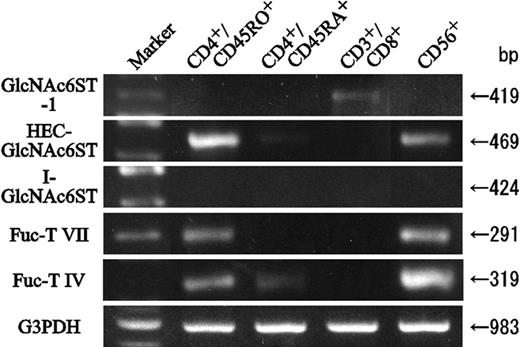
To identify which Fuc-T isoenzyme mediated sialyl 6-sulfo Lex expression, the transcription of genes for fucosyltransferases and 6-sulfotransferases was determined by RT-PCR. As expected, mRNA for the fucosyltransferase Fuc-T VII, the only enzyme known to synthesize sialyl Lex in leukocytes,17,39,40 was detected only in helper memory T cells and NK cells; it was undetectable in helper naive and cytotoxic T cells (Figure 6). The mRNA for another fucosyltransferase, Fuc-T IV, which is known to synthesize Lex determinant in leukocytes but reportedly not to contribute in sialyl Lex or sialyl 6-sulfo Lex synthesis,17,41 was strongly expressed in NK cells and weakly expressed in helper memory T cells. A much weaker band was detected also in helper naive T cells (Figure 6). Among the major isoenzymes of 6-sulfotransferases, strong expression of mRNA for HEC-GlcNAc6ST was detected in helper memory T cells. Moderate expression of HEC-GlcNAc6ST was noted in NK cells. HEC-GlcNAc6ST was not detectable in helper naive or cytotoxic T cells (Figure 6). Weak expression of GlcNAc6ST-1 was noted in cytotoxic T cells, and mRNA for I-GlcNAc6ST, GlcNAc6ST-4, or C-GlcNAc6ST was not detectable in either cell population. These findings suggested that HEC-GlcNAc6ST play a principal role in the synthesis of sialyl 6-sulfo Lex in the skin-homing helper memory T cells.
Carbohydrate structure of sialyl 6-sulfo Lex and nonsulfated sialyl Lex and specificity of antibodies as ascertained by ELISA. The specificity of antibodies directed to sialyl 6-sulfo Lex (G152), CLA (HECA-452), variant sialyl Lex (2F3), and conventional sialyl Lex (CSLEX-1) was ascertained by ELISA using pure synthetic carbohydrate determinants.
Carbohydrate structure of sialyl 6-sulfo Lex and nonsulfated sialyl Lex and specificity of antibodies as ascertained by ELISA. The specificity of antibodies directed to sialyl 6-sulfo Lex (G152), CLA (HECA-452), variant sialyl Lex (2F3), and conventional sialyl Lex (CSLEX-1) was ascertained by ELISA using pure synthetic carbohydrate determinants.
RT-PCR analyses of 6-sulfo- and fucosyl transferase mRNA in human peripheral lymphocyte subsets. Helper memory T (CD4+CD45RO+), helper naive T (CD4+CD45RA+), cytotoxic T (CD3+CD8+), and NK (CD3–CD56+) cells were sorted from peripheral blood and analyzed by RT-PCR using primers specific to 6-sulfo- and fucosyl transferases.
RT-PCR analyses of 6-sulfo- and fucosyl transferase mRNA in human peripheral lymphocyte subsets. Helper memory T (CD4+CD45RO+), helper naive T (CD4+CD45RA+), cytotoxic T (CD3+CD8+), and NK (CD3–CD56+) cells were sorted from peripheral blood and analyzed by RT-PCR using primers specific to 6-sulfo- and fucosyl transferases.
Decreased sialyl 6-sulfo Lex expression by activation of human peripheral lymphocyte
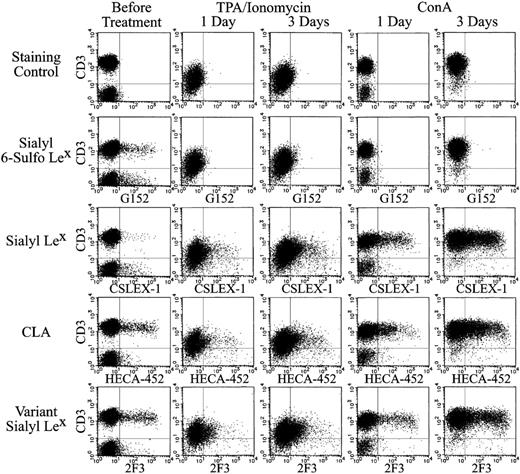
It is well known that the activation of lymphocytes induces strong expression of sialyl Lex.39 In contrast, expression of the sialyl 6-sulfo Lex-positive CD3+ lymphocytes was rapidly decreased after activation of lymphocytes with TPA or ConA. Its decrease was evident on day 1 after stimulation, as shown in the second panel of Figure 7.
The disappearance of the sulfated determinant was in clear contrast to the prominent induction of conventional nonsulfated sialyl Lex expression on the activated T cells. Nonsulfated sialyl Lex was expressed only on some NK cells and virtually not expressed on unstimulated T lymphocytes. TPA and ConA stimulation induced a gradual and strong expression of sialyl Lex, as shown in the third panel of Figure 7.
Given that HECA-452 and 2F3 antibodies are reactive to nonsulfated sialyl Lex and sialyl 6-sulfo Lex, it was predicted that these antibodies would stain the sum of G152-positive and CSLEX-1–positive populations. As expected, these antibodies stained some T lymphocytes under resting conditions as G152 before TPA or ConA stimulation (Figure 7, fourth and fifth rows). CLA defined by HECA-452 and variant sialyl Lex defined by 2F3 showed significantly increased expression in activated lymphocytes, in spite of the diminished expression of sialyl 6-sulfo Lex. The staining patterns obtained with HECA-452 and 2F3 in activated T lymphocytes were essentially the same as those of nonsulfated sialyl Lex, as defined by CSLEX-1 antibody (Figure 7, fourth and fifth rows). These results are compatible with the finding that HECA-452 and 2F3 cross-react with nonsulfated sialyl Lex and with sialyl 6-sulfo Lex.
Expression of sialyl 6-sulfo Lex and related determinants on ConA- or TPA-treated human peripheral lymphocytes. Human peripheral lymphocytes were stimulated with 10 ng/mL TPA and 1 μg/mL ionomycin (middle panel) or with 5 μg/mL ConA (right panel) for 1 or 3 days. Each panel indicates 2-dimensional distribution of sialyl 6-sulfo Lex (G152), conventional sialyl Lex (CXLEX-1), CLA (HECA-452), and variant sialyl Lex (2F3) with CD3.
Expression of sialyl 6-sulfo Lex and related determinants on ConA- or TPA-treated human peripheral lymphocytes. Human peripheral lymphocytes were stimulated with 10 ng/mL TPA and 1 μg/mL ionomycin (middle panel) or with 5 μg/mL ConA (right panel) for 1 or 3 days. Each panel indicates 2-dimensional distribution of sialyl 6-sulfo Lex (G152), conventional sialyl Lex (CXLEX-1), CLA (HECA-452), and variant sialyl Lex (2F3) with CD3.
The increased expression of conventional sialyl Lex in activated T cells was accompanied by the increased mRNA for fucosyltransferase VII.39,40 The decreased expression of sialyl 6-sulfo Lex, however, was not accompanied by a significant decrease of 6-sulfotransferase mRNA (not shown). It is suggested that some posttranslational mechanism was involved in the decrease of sialyl 6-sulfo Lex on the activation of lymphocytes.
Distribution of sialyl 6-sulfo Lex and determinants defined by HECA-452 and 2F3 in human peripheral leukocytes and in normal skin
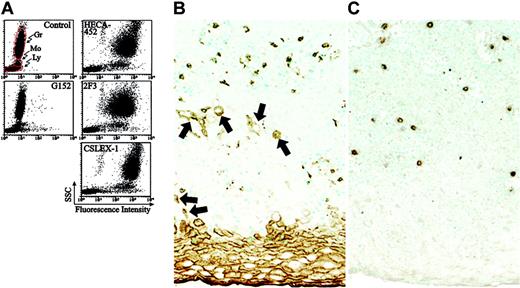
Conventional nonsulfated sialyl Lex is known to be expressed on granulocytes and monocytes. This prompted us to study and compare the distribution of sialyl 6-sulfo Lex with nonsulfated sialyl Lex or with the determinants defined by HECA-452 and 2F3 in peripheral leukocyte populations. As shown in Figure 8A, sialyl 6-sulfo Lex defined by G152 antibody was expressed significantly in a small subset of lymphocytes, only weakly in monocytes, and only in a small and limited population in granulocytes. This was in clear contrast to the distribution of conventional nonsulfated sialyl Lex, defined by CSLEX-1 in Figure 8A, which was strongly expressed on granulocytes and monocytes. As expected from the finding that HECA-452 and 2F3 cross-react with conventional sialyl Lex, these antibodies were also strongly reactive to granulocytes and monocytes. It is concluded that these antibodies detect conventional sialyl Lex on these cells, which do not perform skin homing. Immunohistochemical examination of the normal skin indicated sporadic distribution of lymphoid cells bearing CLA and sialyl 6-sulfo Lex in the dermis and proximal layers of the epidermis, as shown in Figure 8B-C.
Expression of sialyl 6-sulfo Lex and related determinants on human peripheral leukocytes and in the skin. (A) Freshly isolated human peripheral leukocytes from a healthy donor were stained for sialyl 6-sulfo Lex (G152), CLA (HECA-452) variant sialyl Lex (2F3), and conventional sialyl Lex (CXLEX-1). Gr indicates granulocytes; Mo, monocytes; Ly, lymphocytes. (B-C) Distribution of CLA- and sialyl 6-sulfo Lex–positive lymphoid cells in the normal skin, as ascertained by immunohistochemistry. Note that HECA-452 (B) is reactive to keratinizing and parakeratinizing squamous epithelial cells (arrows) in addition to lymphoid cells, whereas sialyl 6-sulfo Lex is expressed only in lymphoid cells (C).
Expression of sialyl 6-sulfo Lex and related determinants on human peripheral leukocytes and in the skin. (A) Freshly isolated human peripheral leukocytes from a healthy donor were stained for sialyl 6-sulfo Lex (G152), CLA (HECA-452) variant sialyl Lex (2F3), and conventional sialyl Lex (CXLEX-1). Gr indicates granulocytes; Mo, monocytes; Ly, lymphocytes. (B-C) Distribution of CLA- and sialyl 6-sulfo Lex–positive lymphoid cells in the normal skin, as ascertained by immunohistochemistry. Note that HECA-452 (B) is reactive to keratinizing and parakeratinizing squamous epithelial cells (arrows) in addition to lymphoid cells, whereas sialyl 6-sulfo Lex is expressed only in lymphoid cells (C).
Discussion
The present study indicated that sialyl 6-sulfo Lex is expressed on distinct subsets of human lymphocytes. The sialyl 6-sulfo Lex determinant was significantly expressed on helper memory T and NK cells among human peripheral lymphocytes. These nonactivated helper memory T cells were known to adhere to E-selectin,6 but the selectin ligand on this T-cell subset had been poorly detected by anti–sialyl Lex antibodies such as CSLEX-1. Only distinct anti–sialyl Lex antibodies, such as HECA-452 and 2F3, effectively detected the ligand.11,42 Hence, the selectin ligand on this cell population has been considered closely related, but not identical, to the conventional sialyl Lex determinant and has been termed cutaneous lymphocyte-associated antigen (CLA)42 or variant sialyl Lex.”11,12
We suggest that at least some of this distinct ligand on helper memory T cells must be sialyl 6-sulfo Lex because the determinant is significantly expressed on the same subpopulation of lymphocytes, as previously characterized by the HECA-452 or 2F3 antibody, which is a CD45RObright+PSGL-1high+ L-selectin+ subset of helper memory T cells. The strong expression of CCR4, the receptor for the TARC chemokine,43 and the absence of α4β7 integrin on the sialyl 6-sulfo Lex–positive helper memory T-cell subset, also support this notion. It was recently proposed that CCR4 is a good marker for skin-homing lymphocytes32 and α4β7 integrin is a good marker for gut-homing lymphocytes.44 The presence of CCR7 on the sialyl 6-sulfo Lex–positive helper memory T-cell subset suggests that these cells are mostly central helper memory T cells and do not belong to so-called effector helper memory T cells because CCR7 was proposed to be a good marker for central memory T cells.35 Sialyl 6-sulfo Lex was initially shown to be an L-selectin ligand, but recently we showed that it also binds to E- and P-selectins,26,38 and the sialyl 6-sulfo Lex–positive helper memory T-cell subset can participate as a ligand for these selectins in the process of skin homing.
The major population of skin-homing helper memory T cells is characterized as expressing sialyl 6-sulfo Lex but no detectable conventional nonsulfated sialyl Lex. The antibodies HECA-452 and 2F3 are reactive to sialyl 6-sulfo Lex, whereas anti–sialyl Lex antibody such as CSLEX-1 is not. This must be why only the former group of anti–sialyl Lex antibodies effectively detects the skin-homing helper memory T cells. NK cells express conventional nonsulfated sialyl Lex11,36 in addition to sialyl 6-sulfo Lex. There are some discrepancies as to the distribution of sialyl 6-sulfo Lex and to HECA-452–defined CLA with regard to lymphocyte subsets other than helper memory T cells. Roughly similar subsets of CD4+ and CD8+ T cells are reportedly HECA-452+,45 whereas sialyl 6-sulfo Lex was more preferentially expressed on CD4+ than on CD8+ T cells. The nature of CLA on the CD8+ T-cell subset is unclear. Sialyl 6-sulfo Lex and CLA are not detectable or are only weakly expressed on B cells and CD45RO– T cells.
Among the major isoenzymes of human 6-sulfotransferases,18,19,30 HEC-GlcNAc6ST was found to be expressed exclusively in helper memory T cells and NK cells. Sialyl 6-sulfo Lex on HEV was shown to be synthesized through a pair of 6-sulfotransferases, HEC-GlcNAc6ST and GlcNAc6ST-1.20-22,46 HEC-GlcNAc6ST seems to play a major role in peripheral helper memory T cells and NK cells because HEC-GlcNAc6ST mRNA was significantly detected and mRNA for the other 6-sulfotransferases was virtually not detected in these cells. Initially, HEC-GlcNAc6ST was thought to be exclusively localized in HEVs, but our results indicated that it is also expressed on some epithelial cells47,48 and on human peripheral leukocytes (this study). The strong expression of sialyl 6-sulfo Lex on the subset of helper memory T cells is in line with the stronger expression of HEC-GlcNAC6ST mRNA compared with that in NK cells.
Peripheral helper memory T lymphocytes significantly expressed mRNAs for sulfotransferase HEC-GlcNAc6ST and fucosyltransferase Fuc-T VII, which cooperate in synthesizing the sialyl 6-sulfo Lex determinant on these cells. It has recently been reported that Fuc-T VII participates in the synthesis of CLA in the murine system.49 Our results suggest that this enzyme is involved in the synthesis of sialyl 6-sulfo Lex in human skin-homing cells. Helper memory T cells and NK cells exhibit constitutive activation of HEC-GlcNAc6ST and Fuc-T VII compared with other human peripheral lymphocyte subpopulations.
Conventional nonsulfated sialyl Lex was virtually not expressed on resting peripheral blood T lymphocytes, whereas its expression was strongly induced on the activation of T lymphocytes, as reported previously.11,36 In contrast, expression of the sialyl 6-sulfo Lex determinant was not increased, but rather was decreased, on activation. Sialyl 6-sulfo Lex expression on the resting helper memory T cells in healthy persons with no signs of inflammatory diseases suggests that the determinant serves as a ligand for selectins in routine trafficking of lymphocytes in healthy persons. Conventional sialyl Lex, in contrast, would play a leading role in inflammation given that its expression is strongly induced on inflammatory stimulation. These findings imply that sialyl 6-sulfo Lex is engaged mainly in the routine trafficking of skin-homing lymphocytes, whereas conventional nonsulfated sialyl Lex is involved in the extensive and irreversible mobilization of leukocytes in inflammation.
Skin-homing helper memory T cells in the peripheral blood of healthy persons is characterized by their activation-independent expression of the selectin ligand CLA.6,7 The HECA-452 antibody is reactive to sialyl 6-sulfo Lex and nonsulfated conventional sialyl Lex, but the determinant detected by this antibody on the skin-homing helper memory T cells in normal peripheral blood must be mainly sialyl 6-sulfo Lex because nonsulfated conventional sialyl Lex is hardly detectable on these cells. The advantage of HECA-452 in studies of various skin diseases to date is that it has long been an unrivaled reagent in detecting sialyl 6-sulfo Lex specifically expressed on skin-homing helper memory T cells. It should be noted in future clinical applications of HECA-452 for defining CLA, however, that this antibody cross-reacts with conventional, nonsulfated sialyl Lex, which is induced on activated lymphocytes, making it appear that CLA is carried by virtually all activated lymphocytes in certain in vitro settings.50 Moreover, the antibody essentially reacts to all granulocytes and behaves merely as an anti–sialyl Lex antibody. We propose that the major and essential part of CLA on skin-homing helper memory T cells in normal peripheral blood is sialyl 6-sulfo Lex, which can be defined as HECA-452+CSLEX-1– or simply as G152+.
Prepublished online as Blood First Edition Paper, December 27, 2005; DOI 10.1182/blood-2005-05-2185.
Supported in part by grants-in-aid from the Ministry of Education, Science, Sports and Culture, Japan (17590258 and on priority areas 17015051); grantsin-aid for the Third Term Comprehensive 10-year Strategy for Cancer Control from the Ministry of Health and Welfare, Japan; and a grant from the CREST program of the Japan Science and Technology Agency.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
We thank Dr Sen-itiroh Hakomori (Pacific Northwest Research Foundation, Seattle, WA) for the gifts of monoclonal antibodies and Drs Yoshimasa Inoue and Hirosato Kondo (Department of Chemistry, Nippon Organon K.K., R&D Laboratories, Osaka, Japan) for the gifts of recombinant selectins.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal