Abstract
2B4 belongs to the CD2 subset of the IgG family of receptors. Members in this family have been shown to function as coreceptors via homophilic or heterophilic interactions. Both 2B4 and CD2 bind to CD48, another member of this family. Because all 3 molecules are expressed on natural killer (NK) cells, it raises a possibility that the binding of 2B4 and CD2 to CD48 among NK cells may have functional consequences. Using specific monoclonal antibodies and gene-deficient NK cells, we found that 2B4/CD48, but not CD2/CD48, interaction is essential for IL-2–driven expansion and activation of murine NK cells. In the absence of 2B4/CD48 interaction, NK cytotoxicity and IFN-γ secretion on tumor target exposure is severely impaired. Impaired activation of NK cells in 2B4-deficient mice was also demonstrated by poor NK-mediated clearance of syngeneic tumor cells in these mice. Functional impairment of NK cells in the absence of 2B4/CD48 interactions was accompanied by defective calcium signaling, suggesting that the early signaling pathway of NK receptors is inhibited. Finally, homotypic interactions among NK cells through 2B4/CD48 was visualized by specific localization of GFP-tagged 2B4 onto NK-NK conjugation sites. Thus, these data identify a novel mechanism whereby NK effector function is regulated via homotypic 2B4/CD48 interactions.
Introduction
The CD2 family of receptors is part of the immunoglobulin (Ig) superfamily, which includes CD2, 2B4 (CD244), CD48, CD58, SLAM (CD150), CS1, CD84, Ly-9, NTBA (SF2000, Ly108), SF2001 (CD2-F10), and BLAME (B-lymphocyte activator macrophage expressed).1,2 The CD2 family members are expressed predominantly on hematopoietic cells and have been shown to interact with other molecules of the same subfamily or with themselves. SLAM, CD84, CS1, and NTBA are found to be self-ligands and to mediate homophilic interaction. For example, SLAM expressed on activated T cells binds to SLAM on B cells and promotes their activation.3-5 CD84 on T cells binds to CD84-Ig fusion protein and enhances IFN-γ secretion on anti-CD3 mAb–mediated T-cell crosslinking.6 CS1 and NTBA on natural killer (NK) cells augment NK cytotoxicity by homophilic interactions.7,8 Unlike these receptors, there is no evidence for 2B4- or CD2-mediated homophilic interaction. Instead, CD48, expressed widely on hematopoietic cells including T and NK cells, has been identified as a ligand for 2B4 and CD2. Although both 2B4 and CD2 bind to CD48, the affinity of 2B4 to CD48 is 5- to 10-fold higher than that of CD2.9 Thus, 2B4/CD48 interaction is likely to dominate over CD2/CD48 interaction in cells coexpressing 2B4, CD2, and CD48, such as NK cells. However, in naive T cells, 2B4 is not expressed; thus, the primary receptor for CD48 in such cells appears to be CD2.10
2B4 was initially identified as an activating receptor.11-15 However, more recent studies with human NK cells suggest that 2B4 may not itself be a triggering receptor but instead function as a coreceptor for other NK-associated activating receptors such as NKp46.16 Similarly, ectopic expression of 2B4 in activated mouse CD8 T cells resulted in T-cell receptor (TCR)–dependent augmentation of cytolysis against antigenic targets.17 These data suggest that the primary role of 2B4 in both T cells and NK cells may be to regulate other receptor/ligand interactions. There is, however, evidence that 2B4 can act as an inhibitory receptor in both humans18 and mice.19,20 Our recent studies show that in murine NK cells 2B4 functions as an inhibitory receptor rather than a costimulatory receptor when engaged by CD48-expressing tumor targets.19 The mechanism by which 2B4 mediates such opposing functions in mice still remains to be determined. Nevertheless, these data strongly suggest that the major function of 2B4 is to regulate other activating or inhibiting receptor/ligand interactions.
Because 2B4, CD2, and CD48 are all expressed in NK cells, the question arises whether 2B4 and/or CD2 binding to CD48 among NK cells (homotypic interaction) can have functional consequences. Indeed, a recent study by Assarson et al21 shows that 2B4/CD48 interaction among NK cells and NK-T cells exists and regulates cell proliferation. We confirm that 2B4 interaction with CD48 among NK cells is necessary for optimal expansion and reveal that such an interaction is critical for optimal cytolytic activation of NK cells, IFN-γ secretion, and elimination of tumor cells in vivo. Our data, therefore, reveal a previously unknown mechanism of augmented NK effector functions by homotypic 2B4/CD48 interaction.
Materials and methods
Mice
C57BL/6 mice between 6 and 12 weeks of age were purchased from Frederick Cancer Research and Developmental Center (National Cancer Institute, Frederick, MD). CD48-KO22 and 2B4-KO mice19 were generated as previously described. All mice were maintained at the University of Chicago and Brigham and Women's Hospital animal housing facilities in a specific pathogen-free environment.
Antibodies, surface and intracellular staining, and flow cytometry
Fluorescently labeled anti-CD48 mAb (clone HM48-1), anti-2B4 mAb (clone 2B4), anti-CD2 mAb (clone RM2-5), anti-IFNγ mAb (clone XMG1.2), and their isotype controls as well as purified anti-2B4 mAb, anti-CD2 mAb, and anti-CD16/32 mAb (clone 2.4G2) were purchased from BD Pharmingen (San Diego, CA). Cell-surface and intracellular staining was performed as previously described.19
Preparation of LAK cultures and 51Cr release assay
NK cells were enriched from spleen-cell suspensions via negative depletion of B cells by passing through a nylon wool column followed by magnetic depletion of CD3+ T cells using miniMACS (magnetic cell sorting) according to the manufacturer's protocol (Milteny Biotech, Auburn, CA).19 NK-cell percentage following negative depletion varied from 13% to 16%. Alternatively, DX5+CD3– NK cells were positively selected using MoFlo high-speed cell sorter (Becton Dickinson, Franklin Lakes, NJ), which resulted in NK purity greater than 99%. Enriched NK cells (2 × 106) were resuspended in 1 mL DMEM or RPMI supplemented with 10% FCS containing 5000 U/mL h-rIL-2 (Proleukin; NIH, Bethesda, MD) and plated in a single well of 24-well plate. Cells were incubated at 37°C in 10% CO2 atmosphere for 6 to 7 days prior to use. Cytotoxicity assays against 51Cr-labled RMA/S(CD48–), Yac-1(CD48+), or P815(CD48–) target cells (1 × 106) was performed as described previously.19 All the cytotoxicity assays were done at least 5 times.
Ex vivo assays of NK-cell function
Mice were given an intraperitoneal injection of 500 μg poly-inosiniccytidylic acid (poly:IC; Sigma-Aldrich, St Louis, MO); where indicated, 200 μg blocking mAbs (anti-CD48 or anti-2B4) was administered. At 48 hours after injection, spleens were removed, and NK cells were partially enriched using negative depletion. The percentage of NK cells in each preparation was determined by fluorescence-activated cell sorting (FACS), and an equal number of CD3–NK1.1+ NK cells were used in 51Cr release assay.
In vivo tumor clearance assay
Tumor clearance assay was performed as previously described.19,23 Briefly, RMA/S cells (H-2b low thymoma cells) suspended in PBS were labeled with 5 μM CFSE (Molecular Probes, Eugene, OR) at 37°C for 10 minutes, and the reaction was quenched with an equal volume of FBS. Cells were washed in PBS, and 10 × 106 RMA/S cells was injected intraperitoneally in 500 μL PBS. After 3 days, mice were killed, and peritoneal cells were recovered. CFSE+ tumor cells were identified by forward versus side scatter.
Measurement of intracellular calcium
Lymphokine-activated killer (LAK) cells were loaded with 5 μM fura 2-AM (Molecular Probes) in HBSS supplemented with 1 mg/mL bovine serum albumin and 0.025% pluronic F-127 and unloaded in HBSS for another 30 minutes. Cells were then plated onto poly-L-lysine–coated 25-mm coverslips and incubated for 20 minutes. Coverslips were mounted at the bottom of a chamber that was placed on the stage of a Nikon Diaphot inverted epifluorescence microscope (Nikon, Garden City, NJ). Cells in a chamber were placed in HBSS, and changes of intracellular calcium concentration were measured.
Generation of 2B4-GFP fusion protein and video fluorescent microscopy
The green fluorescent protein (from the vector pEGFPN1; BD Clontech, Palo Alto, CA) was fused to the C-terminus of murine 2B4 (2B4-long because it is the major form present in LAK cells; data not shown) with a 17-amino acid linker. The fusion protein was expressed in WT LAK cells using a Moloney murine leukemia virus–based retroviral system as used previously.24 FACS analysis of transduced and nontransduced LAK cells demonstrated that the total 2B4 expression level was doubled by transduction. Comparison between live cell images of 2B4-GFP–transduced LAK cells and the 2B4 staining of nontransduced LAK cells showed that introduction of 2B4-GFP did not substantially influence accumulation patterns of 2B4 at the NK/NK interface (data not shown). Video fluorescence microscopy systems and procedures, including the identification of productive cell couples, have been described previously.25 Animal care and experiments were performed in accordance with institutional and National Institutes of Health guidelines, and were approved by the animal use committee at the University of Chicago, IL.
Results
Blocking 2B4/CD48 interaction among NK cells results in decreased NK cytotoxicity
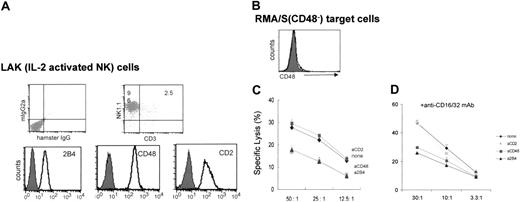
To examine whether 2B4 interaction with CD48 expressed on neighboring NK cells affects NK cytotoxicity, we designed a killing assay using class I low (TAP–/–) RMA/S syngeneic tumor cells. Because the number of NK cells in spleens of normal mice is low and the spontaneous NK activity of resting NK cells is limited, purified splenic NK cells were expanded in IL-2 for 6 to 7 days to generate lymphokine-activated killer (LAK) cells. LAK cultures prepared in this manner contained approximately 96% of NK cells (CD3–NK1.1+), and all expressed 2B4, CD48, and CD2 on their cell surface (Figure 1A). A subline of RMA/S cells that does not express surface CD48 was selected as a target (Figure 1B) to ensure that the interruption of 2B4/CD48 interaction could be localized to NK/NK sites. Initially, we examined the effect of blocking 2B4/CD48 interaction by adding either anti-2B4 or anti-CD48 mAbs during the cytotoxicity assay. As shown in Figure 1C, LAK cells killed CD48–RMA/S targets, but the addition of anti-2B4 or anti-CD48 mAbs, not their isotype control mAbs (data not shown), during the cytotoxicity assay inhibited their lysis. In contrast, addition of anti-CD2 mAbs did not affect the killing of RMA/S cells by NK cells despite that CD2 is expressed on all NK cells and can bind CD48 (Figure 1C). Because RMA/S cells do not express CD48, the inhibition of NK cytotoxicity by anti-2B4 or anti-CD48 mAbs suggests that 2B4 was most likely engaged by CD48 expressed on neighboring NK cells and that this interaction activated NK cytotoxicity. To eliminate nonspecific Fc receptor–mediated Ab crosslinking of NK cells by added mAbs, a cytotoxicity assay was carried out in the presence of anti-CD16/32 (2.4G2) mAbs to block Fc receptors. As seen in Figure 1D, anti-2B4 or anti-CD48 mAbs reduced the lysis of RMA/S target even in the presence of FcR blocking, whereas anti-CD2 mAbs did not have any effect. Together, these data demonstrate that 2B4 and CD48 expressed on NK cells interact with each other to stimulate NK cytotoxicity against CD48– tumor targets.
Blocking 2B4/CD48 interaction using anti-2B4 or anti-CD48 mAbs reduces NK killing against CD48–RMA/S tumor targets. (A) Phenotype of LAK cells as determined by FACS; LAK cultures, produced by either negative depletion or positive selection, contain greater than 96% CD3–NK1.1+ cells (top). These NK cells are all 2B4+, CD48+, and CD2+ (bottom). (B) RMA/S tumor cells do not express any measurable level of CD48 on their surface. Solid line represents staining from anti-CD48 mAbs, whereas filled line represents its isotype control (hamster IgG). (C-D) RMA/S targets (2000) were mixed with LAK cells at the E/T ratio indicated in each figure and subjected to 4-hour 51Cr release assay. Where indicated, 5 μg/mL anti-CD48, anti-2B4, or anti-CD2 mAbs was added in the presence or absence of 5 μg/mL anti-CD16/32 mAbs (2.4G2) to the effector cells prior to addition of target cells. Data are shown as mean ± SD and are representative of data from 5 independent experiments.
Blocking 2B4/CD48 interaction using anti-2B4 or anti-CD48 mAbs reduces NK killing against CD48–RMA/S tumor targets. (A) Phenotype of LAK cells as determined by FACS; LAK cultures, produced by either negative depletion or positive selection, contain greater than 96% CD3–NK1.1+ cells (top). These NK cells are all 2B4+, CD48+, and CD2+ (bottom). (B) RMA/S tumor cells do not express any measurable level of CD48 on their surface. Solid line represents staining from anti-CD48 mAbs, whereas filled line represents its isotype control (hamster IgG). (C-D) RMA/S targets (2000) were mixed with LAK cells at the E/T ratio indicated in each figure and subjected to 4-hour 51Cr release assay. Where indicated, 5 μg/mL anti-CD48, anti-2B4, or anti-CD2 mAbs was added in the presence or absence of 5 μg/mL anti-CD16/32 mAbs (2.4G2) to the effector cells prior to addition of target cells. Data are shown as mean ± SD and are representative of data from 5 independent experiments.
Anti-2B4 or anti-CD48 mAbs, but not anti-CD2 mAbs, inhibit the generation of LAK cells and exhibit impaired cytotoxicity
Because purified NK cells used to generate LAK cells express 2B4 and CD48 even prior to culture in IL-2, we hypothesized that 2B4 could have interacted with CD48 during IL-2–induced NK-cell activation and expansion. To test this possibility, we blocked 2B4/CD48 interaction by culturing NK cells with anti-2B4 or anti-CD48 mAbs at the onset of IL-2 stimulation and examined the ability of these mAb-treated LAK cells to lyse the RMA/S targets. In the presence of anti-2B4, anti-CD48, or anti-CD2 mAbs, NK cells were able to undergo blastogenesis (Figure 2A), but anti-2B4 or anti-CD48 mAb treatment caused a 40% to 50% reduction in the number of LAK cells generated as compared with the cells not treated or cultured with anti-CD2 mAb (Figure 2B). Poor expansion of NK cells in the absence of 2B4/CD48 interactions was also shown by Assarson et al.21 To further investigate the basis for the reduced numbers of LAK cells generated under these conditions, 3H-thymidine incorporation was measured. As shown in Figure 2C, both anti-2B4 and anti-CD48 mAb-treated NK cells show reduced DNA synthesis compared with the no mAb-, isotype control mAb- (data not shown), or anti-CD2 mAb-treated cells. These data suggest that the engagement of 2B4 with CD48 during NK-cell activation is critical for proper expansion of NK cells through regulation of NK-cell proliferation.
Next, we determined whether these mAb-treated LAK cells developed lytic activity. Interestingly, anti-2B4 or anti-CD48 but not anti-CD2 mAb-treated LAK cells showed a profound reduction in the lysis of RMA/S targets (Figure 2D), indicating that 2B4/CD48, but not CD2/CD48, interaction among NK cells is essential for generating lytic NK cells. To confirm that 2B4/CD48 interactions occurred among NK cells, but not between NK cells and the few contaminating splenocytes expressing CD48, NK cells were further purified to greater than 99% purity (DX5+CD3–) by high-speed MoFlow sorter prior to culture in IL-2. Addition of anti-2B4 or anti-CD48 mAbs during IL-2–induced activation of these NK cells resulted in similar inhibition of NK expansion and cytotoxicity (data not shown). Impaired cytotoxicity of anti-2B4 or anti-CD48 mAb-treated cells was not due to poor viability or the absence of lytic machinery in these cells because NK1.1 mAb-induced redirected lysis of FcR+P815 targets was completely normal as shown in Figure 2E. These data demonstrate that blocking of 2B4/CD48 interaction during NK-cell activation results in significant impairment of NK cytotoxicity by regulating a lytic pathway involved in natural cytotoxicity but not by destroying the cells' lytic machinery.
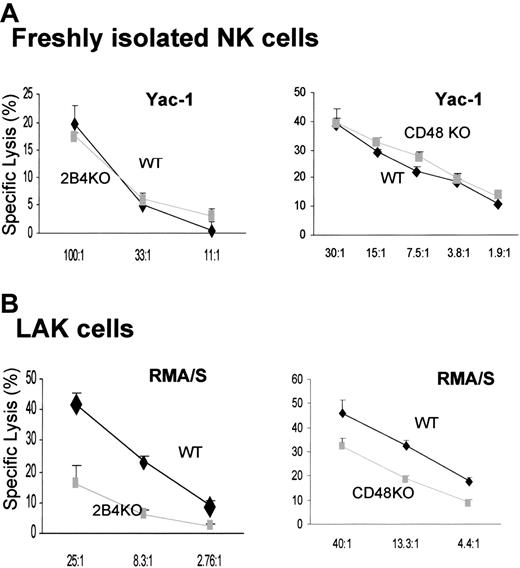
LAK cells, but not freshly isolated NK cells, generated from 2B4-KO and CD48-KO mice showed defective cytotoxicity
To determine the role of 2B4/CD48 signaling in the generation of NK effector function, we decided to study NK cells from 2B4-deficient19 and CD48-deficient mice.22 Despite the inhibition of NK-cell expansion by blocking 2B4/CD48 interaction in vitro (Figure 2B), the number of mature NK cells in 2B4- or CD48-KO mice is equivalent to that of the WT mice,26 indicating that the absence of 2B4 or CD48 does not affect the development of NK cells. Furthermore, freshly isolated splenic cells had normal lytic activity against the NK-sensitive target, Yac-1 [Figure 3A; Yac-1 cells were used because RMA/S targets were not lysed by resting NK cells (Figure 4)]. These data suggest that resting splenic NK cells, developed in the absence of 2B4 or CD48, are competent in mediating cytotoxicity. In contrast, LAK cells derived from 2B4-KO or CD48-KO mice showed impaired killing of RMA/S targets (Figure 3B). This observation confirms the results obtained by anti-2B4 or anti-CD48 mAb treatment of LAK cells and is not subject to any artifacts resulting from antibody treatment in vitro. Thus, it appears that 2B4/CD48 interaction is required during the effector phase of NK-cell activation, rather than during the development of NK cells.
Generation of LAK cells in the presence of anti-2B4 or anti-CD48, but not anti-CD2, mAbs inhibits expansion and cytotoxicity of NK cells. NK cells from B6 mice were enriched using negative depletion and cultured in 5000 U/mL/h-rIL-2 in the presence of 5 μg/mL anti-2B4, anti-CD48, or anti-CD2 mAbs for 7 days. (A) Incubation of NK cells with anti-CD48 or anti-2B4 mAbs did not inhibit the formation of IL-2–induced NK blasts. LAK cells generated in the presence of anti-CD48 or anti-2B4 mAbs showed slightly larger size in both FSC and SSC as compared with those not treated with mAbs or treated with anti-CD2 mAbs. (B) Reduced LAK-cell expansion in the presence of anti-2B4 or anti-CD48 mAbs, but not with anti-CD2 mAbs. LAK cells excluding trypan blue were counted using hemocytometer. This reduction in NK-cell expansion was not likely due to antibody-mediated toxicity, because there was no significant difference in the frequency of nonviable cells between the control and antibody-treated cells up to 3 days in culture (data not shown). Furthermore, neither isotype controls nor anti-CD2 mAbs prevented IL-2–induced NK-cell expansion. (C) 3H-thymidine incorporation into anti-2B4 and anti-CD48 mAb-treated cells was inhibited as compared with untreated or anti-CD2 mAb-treated LAK cells. Negatively enriched NK cells (15 000) were plated on the 96-well plates and cultured as described above. 3H-thymidine (1 μCi [0.037MBq]) was added at the last 16 hours of incubation. (D) LAK cells were subjected to conventional 51Cr release assay at the effector ratio indicated in each figure. Because the number of NK cells derived from anti-2B4 or anti-CD48 mAb-treated groups was reduced by approximately 50%, the cell numbers in each assay were adjusted such that equivalent numbers of viable LAK cells (CD3–NK1.1+) were contained. Target cells used were CD48–RMA/S. (E) Redirected lysis of NK cells was induced by adding 10 μg/mL anti-NK1.1 mAbs, which activates NK1.1(NKRP1-C) through Fc crosslinking via FcR present on P815 (CD48–) cells. In the absence of FcR crosslinking, LAK cells were not able to lyse P815 targets. Targets (2000) were used in each assay. Data are shown as mean ± SD and are representative of data from 5 independent experiments.
Generation of LAK cells in the presence of anti-2B4 or anti-CD48, but not anti-CD2, mAbs inhibits expansion and cytotoxicity of NK cells. NK cells from B6 mice were enriched using negative depletion and cultured in 5000 U/mL/h-rIL-2 in the presence of 5 μg/mL anti-2B4, anti-CD48, or anti-CD2 mAbs for 7 days. (A) Incubation of NK cells with anti-CD48 or anti-2B4 mAbs did not inhibit the formation of IL-2–induced NK blasts. LAK cells generated in the presence of anti-CD48 or anti-2B4 mAbs showed slightly larger size in both FSC and SSC as compared with those not treated with mAbs or treated with anti-CD2 mAbs. (B) Reduced LAK-cell expansion in the presence of anti-2B4 or anti-CD48 mAbs, but not with anti-CD2 mAbs. LAK cells excluding trypan blue were counted using hemocytometer. This reduction in NK-cell expansion was not likely due to antibody-mediated toxicity, because there was no significant difference in the frequency of nonviable cells between the control and antibody-treated cells up to 3 days in culture (data not shown). Furthermore, neither isotype controls nor anti-CD2 mAbs prevented IL-2–induced NK-cell expansion. (C) 3H-thymidine incorporation into anti-2B4 and anti-CD48 mAb-treated cells was inhibited as compared with untreated or anti-CD2 mAb-treated LAK cells. Negatively enriched NK cells (15 000) were plated on the 96-well plates and cultured as described above. 3H-thymidine (1 μCi [0.037MBq]) was added at the last 16 hours of incubation. (D) LAK cells were subjected to conventional 51Cr release assay at the effector ratio indicated in each figure. Because the number of NK cells derived from anti-2B4 or anti-CD48 mAb-treated groups was reduced by approximately 50%, the cell numbers in each assay were adjusted such that equivalent numbers of viable LAK cells (CD3–NK1.1+) were contained. Target cells used were CD48–RMA/S. (E) Redirected lysis of NK cells was induced by adding 10 μg/mL anti-NK1.1 mAbs, which activates NK1.1(NKRP1-C) through Fc crosslinking via FcR present on P815 (CD48–) cells. In the absence of FcR crosslinking, LAK cells were not able to lyse P815 targets. Targets (2000) were used in each assay. Data are shown as mean ± SD and are representative of data from 5 independent experiments.
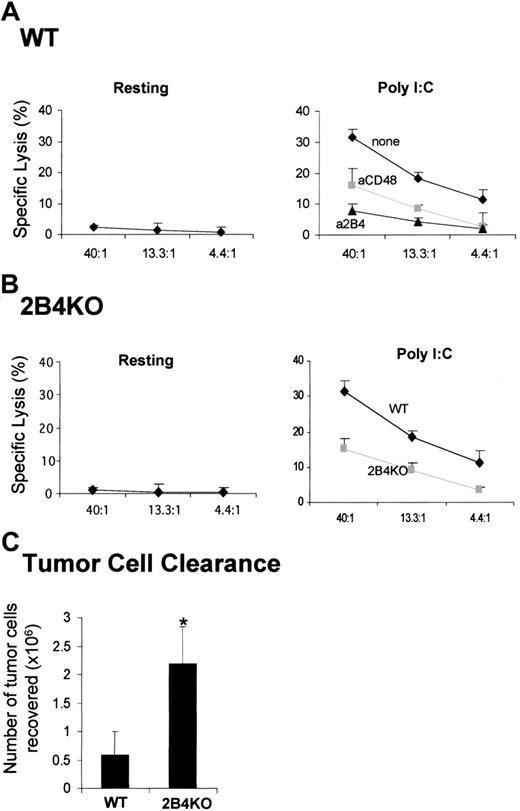
Activation of NK cells in vivo is impaired in the absence of 2B4/CD48 interaction
The hypothesis emerging from the in vitro studies presented in Figures 1 and 2 is that activation of resting NK cells by IL-2 can be regulated by 2B4/CD48 interactions. To further determine the relevance of such interactions, we tested whether 2B4/CD48 interaction can regulate NK-cell activation in vivo. If the interaction of 2B4 and CD48 is critical in activating NK effector functions, blocking 2B4/CD48 interaction by administration of anti-2B4 or anti-CD48 mAbs in WT mice prior to NK-cell stimulation should result in inhibition of NK activity. Polyinosine-polycytidylic acid (poly I:C) is a synthetic analog of double-stranded RNA (dsRNA), which induces production of interferons (IFN-α/β) and other cytokines and stimulates NK cytotoxicity.27 Resting NK cells isolated from WT mice did not show any lytic activity against RMA/S targets as shown in Figure 4A (left). On injection of poly I:C, these NK cells were able to lyse RMA/S targets (Figure 4A, right). When anti-2B4 or anti-CD48 mAbs were injected at the time of poly I:C administration, NK cytotoxicity was reduced significantly (Figure 4A, right). Because there is partial depletion of NK cells on injection with anti-2B4 but not anti-CD48 mAbs, the number of NK cells isolated from each mouse was adjusted to contain equal number of CD3–NK1.1+ cells in each assay. Thus, on a cell-per-cell basis, the lysis of RMA/S targets was greatly reduced by anti-2B4 or anti-CD48 mAb injection. To rule out any artifacts resulting from antibody-mediated depletion of NK cells, 2B4-KO mice were used to test whether their NK cells could be activated by poly I:C treatment (Figure 4B). Similar to WT mice, resting NK cells from 2B4-KO mice were not able to lyse RMA/S cells (Figure 4B, left). However, consistent with the results in Figure 4A, NK cells from 2B4-KO mice show reduced cytotoxicity on poly I:C administration, indicating that 2B4/CD48 interaction is essential for the optimal activation of NK cells in vivo.
To further investigate the significance of the 2B4/CD48 interaction in vivo, we examined whether NK-cell–mediated clearance of tumor cells was altered in the absence of 2B4. Thus, CFSE-labeled CD48–RMA/S tumor cells were injected into the peritoneum of both WT and 2B4-KO mice, and the number of tumor cells remaining in the peritoneum after 3 days was counted. Previously we19 and others23 have found that the peritoneal clearance of RMA/S cells under these conditions is NK-cell mediated. As shown in Figure 4C, the number of RMA/S cells remaining in 2B4-KO mice was approximately 4-fold higher than that recovered in WT mice, suggesting that NK cells in 2B4-KO mice were defective in controlling RMA/S tumor-cell growth. These data are consistent with our in vitro findings and demonstrate that 2B4 plays an important role in regulating NK-cell functions in mice challenged with tumors.
LAK cells generated from 2B4- or CD48-KO mice showed reduced cytotoxicity, but their spontaneous NK cytotoxicity is intact when analyzed ex vivo. (A) Ex vivo analysis of NK cytotoxicity from WT, 2B4-KO, or CD48-KO mice; enriched NK cells using negative depletion (left, WT and 2B4-KO) or positive selection of DX5+CD3– (right, WT and CD48-KO) were used for 4-hour 51Cr release assay using Yac-1 targets. Yac-1 targets were used for ex vivo analysis because resting NK cells did not lyse RMA/S cells. (B) NK cells were enriched from WT, 2B4-KO, or CD48-KO mice using negative depletion and cultured in 5000 U/mL h-rIL-2 for 6 days prior to the 4-hour 51Cr release assay using RMA/S targets. Data are shown as mean ± SD and are representative of data from 4 independent experiments.
LAK cells generated from 2B4- or CD48-KO mice showed reduced cytotoxicity, but their spontaneous NK cytotoxicity is intact when analyzed ex vivo. (A) Ex vivo analysis of NK cytotoxicity from WT, 2B4-KO, or CD48-KO mice; enriched NK cells using negative depletion (left, WT and 2B4-KO) or positive selection of DX5+CD3– (right, WT and CD48-KO) were used for 4-hour 51Cr release assay using Yac-1 targets. Yac-1 targets were used for ex vivo analysis because resting NK cells did not lyse RMA/S cells. (B) NK cells were enriched from WT, 2B4-KO, or CD48-KO mice using negative depletion and cultured in 5000 U/mL h-rIL-2 for 6 days prior to the 4-hour 51Cr release assay using RMA/S targets. Data are shown as mean ± SD and are representative of data from 4 independent experiments.
Ex vivo and in vivo analysis of NK cytotoxicity from WT or 2B4-KO mice. (A-B) Each mouse was injected with 500 μg polyI:C in the presence or absence of 200 μg anti-2B4 or anti-CD48 mAbs as indicated in the figures. At 48 hours after injection, spleens were removed, and NK cells were partially enriched using negative depletion. The resulting splenocytes were subjected to 4-hour 51Cr release assay using RMA/S cells as a target. In panel A, injection of anti-2B4 mAb partially depleted (∼50%) NK cells from the spleen of WT (wild type) mice (right); thus, the number of NK cells from anti-2B4 mAb-treated mice was adjusted to that of untreated controls. Data are shown as mean ± SD and are representative of data from 6 independent experiments. (C) WT and 2B4-KO mice were injected intraperitoneally with 10 × 106 CFSE-labeled tumor cells. The number of tumor cells recovered from the peritoneum after 3 days was calculated on the basis of absolute number of peritoneal exudate cells multiplied by the percentage of CFSE-labeled tumor cells as determined by flow cytometry. Data are shown as mean ± SE pooled from 3 independent experiments (n = 15 for both WT and 2B4-KO). *P < .05 as compared with WT versus 2B4KO.
Ex vivo and in vivo analysis of NK cytotoxicity from WT or 2B4-KO mice. (A-B) Each mouse was injected with 500 μg polyI:C in the presence or absence of 200 μg anti-2B4 or anti-CD48 mAbs as indicated in the figures. At 48 hours after injection, spleens were removed, and NK cells were partially enriched using negative depletion. The resulting splenocytes were subjected to 4-hour 51Cr release assay using RMA/S cells as a target. In panel A, injection of anti-2B4 mAb partially depleted (∼50%) NK cells from the spleen of WT (wild type) mice (right); thus, the number of NK cells from anti-2B4 mAb-treated mice was adjusted to that of untreated controls. Data are shown as mean ± SD and are representative of data from 6 independent experiments. (C) WT and 2B4-KO mice were injected intraperitoneally with 10 × 106 CFSE-labeled tumor cells. The number of tumor cells recovered from the peritoneum after 3 days was calculated on the basis of absolute number of peritoneal exudate cells multiplied by the percentage of CFSE-labeled tumor cells as determined by flow cytometry. Data are shown as mean ± SE pooled from 3 independent experiments (n = 15 for both WT and 2B4-KO). *P < .05 as compared with WT versus 2B4KO.
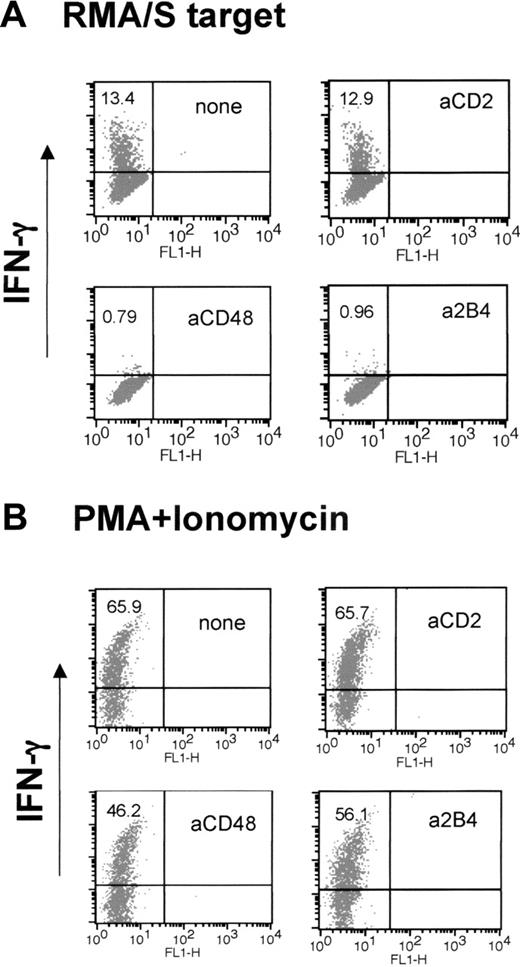
Production of IFN-γ is severely impaired in anti-2B4 and anti-CD48 mAb-treated LAK cells
IFN-γ production is another major function of NK cells critical for stimulating innate and adaptive immune response. Thus, we asked whether anti-2B4 or anti-CD48 mAb treatment during IL-2–induced NK-cell activation affected the ability of LAK cells to produce IFN-γ when exposed to target cells. On mixing with RMA/S cells, a subset of LAK cells rapidly accumulated intracellular IFN-γ (Figure 5A). However, neither anti-2B4 nor anti-CD48 mAb-treated cells were able to up-regulate IFN-γ (Figure 5A; 13.4% versus 0.79% and 0.96%). Impaired production of IFN-γ by anti-2B4 or anti-CD48 mAb-treated LAK cells was directly correlated with the impaired cytotoxicity seen in Figure 2D. Similarly, when 2B4-KO LAK cells were mixed with RMA/S targets, IFN-γ production was greatly reduced (data not shown). The abrogation of IFN-γ production was not due to the loss of the cells' ability to produce IFN-γ, because treatment with PMA and ionomycin, which bypasses proximal activating receptor signaling, was able to produce IFN-γ in anti-2B4 or anti-CD48 mAb-treated cells (Figure 5B). Thus, our data show that 2B4/CD48 signaling regulates cytotoxicity and production of IFN-γ in NK cells by affecting a proximal step of IL-2 signaling necessary for NK-cell activation.
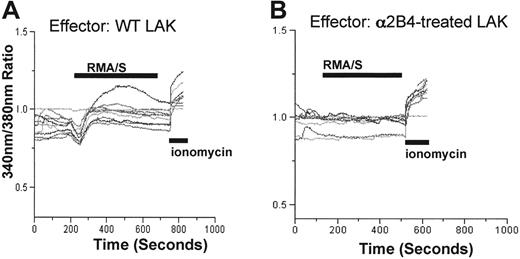
Intracellular calcium release induced on contact with RMA/S tumor cells is defective in a2B4 mAb-treated NK cells
Defective cytotoxicity and IFN-γ production in a2B4 mAb– or aCD48 mAb–treated cells suggests that the signaling pathway initiated from NK-activating receptors on tumor target exposure might not have operated properly. To examine whether the functional defect seen in antibody-treated cells occurred at the membrane proximal or distal level, intracellular calcium influx was measured. Previously, it has been demonstrated that crosslinking of NKRP1A using its specific antibodies resulted in redirected lysis by rat RNK-16 cells,28,29 which was accompanied by a rise in intracellular calcium because of the activation of PLC-γ.30 Similarly, we observed that murine NK cells show increased intracellular calcium concentration on encounter of RMA/S tumor cells (Figure 6A). Within each preparation of NK cells, the percentage of NK cells showing elevation of intracellular calcium varied anywhere from 50% to 70%. In contrast, LAK cells generated either in the presence of a2B4 or aCD48 (data not shown) mAbs were unable to release intracellular calcium (Figure 6B). Impaired calcium release by a2B4 or aCD48 mAb-treated cells was not due to poor viability because these cells show normal intracellular calcium concentration that can be released by the addition of calcium ionophore, ionomycin (Figure 6B). These data suggest that the signaling defect in the absence of homotypic 2B4/CD48 interaction might have been generated at the proximal step of PLC-γ activation.
LAK cells generated in the presence of anti-2B4 or anti-CD48 mAb, but not anti-CD2 mAb, do not make IFN-γ when mixed with RMA/S targets. WT LAK cells were generated in the presence or absence of 5 μg/mL mAbs indicated in the figure. (A) LAK cells (1 × 105) were mixed with RMA/S cells at 1:1 ratio, and IFN-γ production was measured by FACS using anti–IFN-γ-PE, as described in “Materials and methods.” (B) LAK cells were treated with 50 ng/mL phorbol myristate acetate (PMA) and 500 ng/mL ionomycin and analyzed for IFN-γ production by FACS. The numbers in the top left quadrant indicate the percentage of NK cells expressing IFN-γ. The results of this particular experiment represent those from 5 other independent experiments.
LAK cells generated in the presence of anti-2B4 or anti-CD48 mAb, but not anti-CD2 mAb, do not make IFN-γ when mixed with RMA/S targets. WT LAK cells were generated in the presence or absence of 5 μg/mL mAbs indicated in the figure. (A) LAK cells (1 × 105) were mixed with RMA/S cells at 1:1 ratio, and IFN-γ production was measured by FACS using anti–IFN-γ-PE, as described in “Materials and methods.” (B) LAK cells were treated with 50 ng/mL phorbol myristate acetate (PMA) and 500 ng/mL ionomycin and analyzed for IFN-γ production by FACS. The numbers in the top left quadrant indicate the percentage of NK cells expressing IFN-γ. The results of this particular experiment represent those from 5 other independent experiments.
Intracellular calcium mobilization was defective in the absence of homotypic 2B4/CD48 interaction. WT LAK cells generated in the absence or presence of a2B4 mAbs were loaded with Fura-2, and intracellular calcium concentration was monitored as described in “Materials and methods.” The calcium concentration was depicted as the emission ratio of 380:340 nm. RMA/S cells (2 × 106) were added at the time indicated, and 4 μM ionomycin was added at the end of the experiments to ensure that the cells are viable. (A) Thirteen of 20 cells in a field induced elevation of intracellular calcium and are presented in the figure. (B) Thirteen of 20 cells were depicted in the figure. None of the cells in a2B4-treated group showed elevation of intracellular calcium.
Intracellular calcium mobilization was defective in the absence of homotypic 2B4/CD48 interaction. WT LAK cells generated in the absence or presence of a2B4 mAbs were loaded with Fura-2, and intracellular calcium concentration was monitored as described in “Materials and methods.” The calcium concentration was depicted as the emission ratio of 380:340 nm. RMA/S cells (2 × 106) were added at the time indicated, and 4 μM ionomycin was added at the end of the experiments to ensure that the cells are viable. (A) Thirteen of 20 cells in a field induced elevation of intracellular calcium and are presented in the figure. (B) Thirteen of 20 cells were depicted in the figure. None of the cells in a2B4-treated group showed elevation of intracellular calcium.
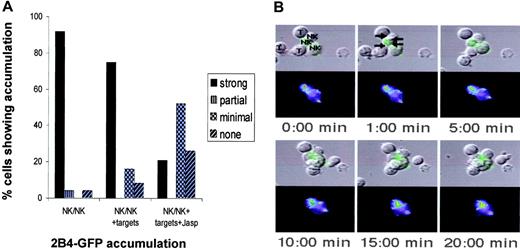
2B4 localizes toward NK/NK interaction sites even in the presence of CD48-expressing targets. 2B4-GFP fusion protein is expressed in WT LAK cells, and the accumulation at the NK/NK interface was analyzed as described in “Materials and methods.” Briefly, for detection of NK-cell couples, cells in PBS with 10% FCS, 1 mM CaCl, and 0.5 mM MgCl were placed on an inverted epifluorescence microscope equipped with a Nikon Plan Apo 20 ×/0.75 air objective lens (Nikon, Melville, NY) and analyzed using videomicroscopy (Princeton Scientific MicroMax, Trenton, NJ). The Metamorph 6.6/6.0 imaging system was also used for analysis (Universal Imaging, Downingtown, PA). (A) The percentage of NK-cell/NK-cell couples showing 2B4-GFP interface accumulation is given. Briefly, 2B4-GFP accumulation, designated as strong, partial, minimal, or no accumulation in the figure, was classified as follows. Average 2B4-GFP fluorescence intensity of the area of 2B4 accumulation at a cellular interface was measured with Metamorph Software (Universal Imaging, Downingtown, PA). Strong accumulation at interface is greater than 40% of anywhere else in the cell. Partial accumulation is greater than 40% of the background but greater than anywhere else in the cell. Minimal is an interface accumulation of greater than 40% above background but less than another area of accumulation in the cell. Addition of all 4 patterns of accumulation equals 100%. NK/NK + targets indicates 2B4 accumulation at the NK-cell/NK-cell interface in a cell cluster where a particular NK cell also had a NK-cell/target-cell interface; Jasp, the presence of Jasplakinolide, which disrupts actin cytoskeleton. Twenty (on average 25) cell couples from at least 5 independent experiments were analyzed per condition. Statistical significance as determined with a Mann-Whitney U test was P < .005 between NK/NK or NK/NK+ targets and NK/NK + targets + Jasp. (B) A time-lapse video image of the 2B4-GFP distribution in a cell cluster of 3 NK cells (NK) and 3 target cells (T) as labeled in the first panel; productive NK-cell/NK-cell and NK-cell/target-cell interfaces are labeled in the top middle panel with arrows pointing at the center of the interfaces. The leftmost target cell only binds to the bottom of the cluster at 10:00 minutes. 2B4-GFP fluorescence intensity is shown as a differential interference contrast (DIC) image on top as well as the top-down projection of three-dimensional data in a false color scale (increasing from purple to red). As the punctuate 2B4-GFP distribution makes alignment of fluorescence and DIC images difficult, the 2B4-GFP fluorescence is also directly laid over the DIC image in a green intensity scale. At the bottom right of the bottom NK cell, 2B4-GFP is enclosed in a bright vesicle that is not continuous with the rest of the cell membrane. In the first 4 panels, the translocation of a 2B4-GFP cluster on the same NK cell (blue-green to yellow in the GFP intensity image) toward an NK-cell/NK-cell interface can be seen on the top of the cell. A minimal 2B4-GFP accumulation at the interface of the same NK cell with the bottom target cell can also be seen in the last 2 panels.
2B4 localizes toward NK/NK interaction sites even in the presence of CD48-expressing targets. 2B4-GFP fusion protein is expressed in WT LAK cells, and the accumulation at the NK/NK interface was analyzed as described in “Materials and methods.” Briefly, for detection of NK-cell couples, cells in PBS with 10% FCS, 1 mM CaCl, and 0.5 mM MgCl were placed on an inverted epifluorescence microscope equipped with a Nikon Plan Apo 20 ×/0.75 air objective lens (Nikon, Melville, NY) and analyzed using videomicroscopy (Princeton Scientific MicroMax, Trenton, NJ). The Metamorph 6.6/6.0 imaging system was also used for analysis (Universal Imaging, Downingtown, PA). (A) The percentage of NK-cell/NK-cell couples showing 2B4-GFP interface accumulation is given. Briefly, 2B4-GFP accumulation, designated as strong, partial, minimal, or no accumulation in the figure, was classified as follows. Average 2B4-GFP fluorescence intensity of the area of 2B4 accumulation at a cellular interface was measured with Metamorph Software (Universal Imaging, Downingtown, PA). Strong accumulation at interface is greater than 40% of anywhere else in the cell. Partial accumulation is greater than 40% of the background but greater than anywhere else in the cell. Minimal is an interface accumulation of greater than 40% above background but less than another area of accumulation in the cell. Addition of all 4 patterns of accumulation equals 100%. NK/NK + targets indicates 2B4 accumulation at the NK-cell/NK-cell interface in a cell cluster where a particular NK cell also had a NK-cell/target-cell interface; Jasp, the presence of Jasplakinolide, which disrupts actin cytoskeleton. Twenty (on average 25) cell couples from at least 5 independent experiments were analyzed per condition. Statistical significance as determined with a Mann-Whitney U test was P < .005 between NK/NK or NK/NK+ targets and NK/NK + targets + Jasp. (B) A time-lapse video image of the 2B4-GFP distribution in a cell cluster of 3 NK cells (NK) and 3 target cells (T) as labeled in the first panel; productive NK-cell/NK-cell and NK-cell/target-cell interfaces are labeled in the top middle panel with arrows pointing at the center of the interfaces. The leftmost target cell only binds to the bottom of the cluster at 10:00 minutes. 2B4-GFP fluorescence intensity is shown as a differential interference contrast (DIC) image on top as well as the top-down projection of three-dimensional data in a false color scale (increasing from purple to red). As the punctuate 2B4-GFP distribution makes alignment of fluorescence and DIC images difficult, the 2B4-GFP fluorescence is also directly laid over the DIC image in a green intensity scale. At the bottom right of the bottom NK cell, 2B4-GFP is enclosed in a bright vesicle that is not continuous with the rest of the cell membrane. In the first 4 panels, the translocation of a 2B4-GFP cluster on the same NK cell (blue-green to yellow in the GFP intensity image) toward an NK-cell/NK-cell interface can be seen on the top of the cell. A minimal 2B4-GFP accumulation at the interface of the same NK cell with the bottom target cell can also be seen in the last 2 panels.
2B4 preferentially accumulates at the NK-cell/NK-cell interface
To visually demonstrate the homotypic interaction among NK cells expressing 2B4 and CD48, we monitored the localization of 2B4 in NK cells. To this end, we fused GFP to the C-terminus of murine 2B4 and transduced the fusion protein into WT LAK cells. The interaction of 2B4/GFP-transduced LAK cells with either CD48– (data not shown) or CD48+ P815 targets (Figure 7) was monitored using video fluorescence microscopy.25 Interestingly, despite the presence of CD48 on admixed target cells, 2B4 accumulated primarily at the NK/NK interface, not at the NK/target interface (Figure 7A-B, arrows). Productive cell couples were identified by the formation of a tight cellular interface. Using the live cell imaging approach, we found 25 NK-cell/NK-cell couples, 41 NK-cell/target-cell couples, and 9 clusters of cells with both NK-cell/NK-cell and NK-cell/target-cell interfaces in which 2B4 localization could be analyzed. Using a scoring system described,25 92% of NK cells within NK/NK conjugates showed strong 2B4 interface accumulation (Figure 7A, first panel, NK/NK). However, even after addition of CD48+ target cells, greater than 75% of cells exhibited strong accumulation of 2B4 at the NK/NK interface, not the NK/target interface (Figure 7A, middle panel, NK/NK + targets). To examine whether this 2B4 accumulation at the NK/NK interface was the result of an active cellular translocation or of passive diffusion, we slowed NK-cell actin dynamics with a small concentration of Jasplakinolide, the inhibitor of actin depolymerization.24,25 As shown in Figure 7A (last panel, NK/NK + Jasp), 2B4 accumulation at NK-cell/NK-cell interphase was significantly (P < .005) inhibited with the reduction of strong accumulation from 92% down to 22%, indicating that 2B4 accumulation at the NK/NK interface was actin dependent. Together, these data provide visual evidence for homotypic interaction among NK cells expressing 2B4 and CD48.
Discussion
In this study, we demonstrate that optimal expansion and activation of NK cells is dependent on the engagement of 2B4 with CD48 expressed on neighboring NK cells. Clustering of 2B4 at NK/NK-cell contact sites provides a morphologic correlate for such interaction. Because both 2B4-KO and CD48-KO mice developed normal numbers of lytically active NK cells, our data indicate that 2B4 or CD48 are not needed for NK-cell development from their progenitors. Instead, it appears that 2B4/CD48 interactions control NK-cell activation in response to signals received from cytokines (IL-2 and IFN-α/β) or tumor target exposure (Figures 2, 3, 4, 5). Both cytolytic and secretory effector functions are influenced by such homotypic NK/NK interactions. Our conclusions are reinforced by the fact that results obtained by the use of receptor/ligand blocking antibodies are fully supported by the use of receptor or ligand knockout mice. Thus, these studies provide compelling evidence for an important role of 2B4 in regulating NK-cell functions.
Evidence has previously been presented to support the role of 2B4 as a triggering receptor in NK cells. However, because the ligand of 2B4, CD48, is widely expressed in most of hematopoietic cells, this model raises a question as to how NK-cell autoreactivity against normal surrounding cells is regulated. It is possible that 2B4/CD48-mediated lysis of autologous cells may be dominantly inhibited by MHC class I–binding inhibitory receptors. Alternatively, 2B4 may function to regulate other triggering receptors, rather than to directly trigger lysis. Our data presented here support this hypothesis, in that 2B4 functions as a costimulatory receptor for cytokine (IL-2 or IFN-α) receptors. In addition, we have recently reported that the effect of 2B4/CD48 ligation can be inhibitory when 2B4-bearing NK cells interacted with tumor cells expressing CD48.19 Thus, it appears that 2B4/CD48 interactions can have 2 opposing effects in NK cells. CD48 expressed on non-NK cells inhibits NK cytotoxicity by engaging 2B4 in NK cells. In contrast, 2B4/CD48 interactions among NK cells costimulate cytotoxicity and IFN-γ secretion induced by IL-2 or IFN-α. Because CD48 expressed on target cells inhibits NK killing, this implies that such inhibition is dominant over the costimulating effect of NK/NK interaction, despite that a substantial number of NK cells make conjugates among NK cells through 2B4/CD48 (Figure 7). Such dominance of inhibition provides a mechanism of tolerance against autologous CD48-bearing hematopoietic cells.
The conclusion that 2B4/CD48 interaction can be inhibitory or activating is evident not only from in vitro experiments but also in vivo by utilization of CD48+ or CD48– tumor cells in 2B4-KO or WT mice. How 2B4 can perform such opposing roles in a given cell is not clear at present. Because murine 2B4 in NK cells exists as 2 isoforms, 2B4-long and 2B4-short, the opposing functions of 2B4 could be due to a distinct role of each isoform. Indeed, our previous studies show the inhibitory role of 2B4-long, but not 2B4-short31 ; thus, 2B4-short may function as a costimulatory molecule in mediating homotypic NK activation. Another possibility is that CD48 expressed on NK cells is structurally dissimilar to that expressed on non-NK cells (perhaps because of cell-specific after translational modifications); thus, 2B4 can signal differentially on binding to CD48 on NK cells versus that on non-NK cells. Alternatively, 2B4 binding to CD48 on target cells may produce steric hindrance that prevents other NK triggering receptors from engaging with their own ligands on target cells. Other possibilities such as “heavy versus light crosslinking” of 2B4 because of the differences in the density of CD48 on NK cells and target cells, differences in “on and off” rate of 2B4 binding to CD48, or distinct clustering of 2B4 involving GEM could be the basis for these opposing roles of 2B4. Some of these possibilities are currently under investigation.
There are several possible mechanisms of 2B4/CD48-mediated augmentation of NK cytotoxicity. The first possibility is that 2B4/CD48 interactions among NK cells deliver costimulatory signals that augment those delivered by simultaneous ligation of other triggering receptors such as NKG2D, NKp46, or Ly49H.32-34 Such costimulation could be delivered either through 2B4 or CD48, because both of these molecules have signaling capacity.35-40 However, such costimulation in cytotoxicity, occurring at the time of target-cell recognition by other NK triggering receptors, is not likely to play a major role in natural cytotoxicity of NK cells because interruption of 2B4/CD48 interactions during the cytotoxicity assay has modest effect on NK functions (Figure 1C-D). Much more profound is the reduction in cytotoxicity that is seen after sustained interruption of 2B4/CD48 interactions during the culture in IL-2 (Figure 2D) or stimulation with IFN-α/β in vivo (Figure 4), prior to any engagement of the target cells. It appears, therefore, that 2B4/CD48 interactions are most critical during the generation of activated NK cells. The absence of such signaling among NK cells leads to the formation of lytically compromised NK cells. Such “compromise” in NK function could occur at any step in the NK lytic pathway from receptor expression to signaling intermediates, to adequacy of lytic moieties such as granzymes and perforin. However, the latter seems unlikely because NK cells that developed in culture containing IL-2 and blocking anti-CD48 mAbs were quite capable of killing P815 targets when NKRP-1C was crosslinked by anti-NK1.1 mAbs (Figure 2E). The alternative possibility is that 2B4/CD48 interactions regulate the expression of other triggering receptors such as NKG2D, Ly49H, and NKp46 during NK-cell activation. Our preliminary microarray analysis data indicate that the mRNA level of these genes was not changed on antibody treatment or in 2B4-KO mice (data not shown). Therefore, at least at the transcriptional level, absence of 2B4/CD48 interaction did not alter the expression of these activating receptors. Moreover, RMA/S target cells do not express the ligand for NKG2D41 or Ly49H, and the reagents to study NKp46 in mice are not yet available. Thus, it still remains unknown which triggering receptor is involved in the killing of RMA/S targets. Nevertheless, our calcium flux data show that the NK signaling defect in the absence of 2B4/CD48 interactions appeared to have occurred at the proximal level of PLC-γ activation (Figure 6).
During the course of our studies, it was demonstrated that 2B4/CD48 interactions can costimulate both NK and T-cell proliferation.21 It was shown in this study that 2B4 served as a ligand for CD48 and delivered the costimulating signals to T cells. This raises the question whether the expansion and activation of NK cells shown here is mediated by the delivery of signals initiated from 2B4 or CD48. Because both are expressed on NK cells, at least in principle, the signal could be generated in either direction. However, several pieces of our unpublished data (K.-M.L., 2004) argue against the possibility of CD48 as the major signaling receptors for activating NK cells. First, when 2B4-KO NK cells (expressing CD48) were cultured in the presence of syngeneic tumor cell lines (EL-4) expressing 2B4, no significant enhancement of NK proliferation in response to IL-2 was detected. Second, cytotoxicity of 2B4-KO LAK (NK) cells was not affected by the addition of 2B4-expressing EL-4 cells in an in vitro cytotoxicity assay. Together, these data suggest that activating signals on 2B4/CD48 interaction are likely to be transmitted from 2B4 rather than from CD48.
Although our data show that homotypic interactions among NK cells occur during NK-cell activation in vitro, we cannot rule out the possibility that in vivo interaction involving 2B4/CD48 may occur between NK cells and other hematopoietic cells expressing CD48. For example, on MCMV infection, NK cells are recruited to the inflammatory foci in the liver where CD48-expressing dendritic cells and macrophages are also enriched.42 Similarly, on virus infection, NK cells migrate into the splenic marginal zones rich in macrophages and dendritic cells.43 Therefore, these homotypic or heterophilic 2B4/CD48 interactions among NK cells or NK cells and other non-NK hematopoietic cells (eg, dendritic cells) may provide a basis for the potent and prompt NK response on viral infection or tumor formation.
Prepublished online as Blood First Edition Paper, May 19, 2005; DOI 10.1182/blood-2005-01-0185.
Supported by the National Institutes of Health (grant 5RO1AI20451-18), the Korea Research Foundation (grant R08-2004-000-10478-0) and the National Nuclear R&D Program of the Ministry of Science and Technology of Korea (grant BAERI).
K.-M.L., J.P.F., and M.E.McN. contributed equally to this work.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
We thank NIH AIDS Research and Reference Reagent Program, Division of AIDS, NIAID, NIH for the generous gift of h-rIL-2.

![Figure 2. Generation of LAK cells in the presence of anti-2B4 or anti-CD48, but not anti-CD2, mAbs inhibits expansion and cytotoxicity of NK cells. NK cells from B6 mice were enriched using negative depletion and cultured in 5000 U/mL/h-rIL-2 in the presence of 5 μg/mL anti-2B4, anti-CD48, or anti-CD2 mAbs for 7 days. (A) Incubation of NK cells with anti-CD48 or anti-2B4 mAbs did not inhibit the formation of IL-2–induced NK blasts. LAK cells generated in the presence of anti-CD48 or anti-2B4 mAbs showed slightly larger size in both FSC and SSC as compared with those not treated with mAbs or treated with anti-CD2 mAbs. (B) Reduced LAK-cell expansion in the presence of anti-2B4 or anti-CD48 mAbs, but not with anti-CD2 mAbs. LAK cells excluding trypan blue were counted using hemocytometer. This reduction in NK-cell expansion was not likely due to antibody-mediated toxicity, because there was no significant difference in the frequency of nonviable cells between the control and antibody-treated cells up to 3 days in culture (data not shown). Furthermore, neither isotype controls nor anti-CD2 mAbs prevented IL-2–induced NK-cell expansion. (C) 3H-thymidine incorporation into anti-2B4 and anti-CD48 mAb-treated cells was inhibited as compared with untreated or anti-CD2 mAb-treated LAK cells. Negatively enriched NK cells (15 000) were plated on the 96-well plates and cultured as described above. 3H-thymidine (1 μCi [0.037MBq]) was added at the last 16 hours of incubation. (D) LAK cells were subjected to conventional 51Cr release assay at the effector ratio indicated in each figure. Because the number of NK cells derived from anti-2B4 or anti-CD48 mAb-treated groups was reduced by approximately 50%, the cell numbers in each assay were adjusted such that equivalent numbers of viable LAK cells (CD3–NK1.1+) were contained. Target cells used were CD48–RMA/S. (E) Redirected lysis of NK cells was induced by adding 10 μg/mL anti-NK1.1 mAbs, which activates NK1.1(NKRP1-C) through Fc crosslinking via FcR present on P815 (CD48–) cells. In the absence of FcR crosslinking, LAK cells were not able to lyse P815 targets. Targets (2000) were used in each assay. Data are shown as mean ± SD and are representative of data from 5 independent experiments.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/107/8/10.1182_blood-2005-01-0185/4/m_zh80080694040002.jpeg?Expires=1769333101&Signature=i-JaDX0CMZr42PXp-Rab0kAWAF1nR2EGiKFm1vE4pU~FGJzRzbtCmy-TW1P9WX-zgbMGlaavGGYr9qvGVDLKd2I6BL~14tvLrMK8w0ms1vT5ZAfmpogtX20iWVfRJYu1PSoqILhyPHQGd3nOCPmc3Rjk9b69y0YAjCMtxLJVCYEBIJ~ndR4B6hqojC-1sMdLZpzb4uEzjVkfP4ow1moPYOw9~mNvHW-X3Yk5DNWMhg4h-hX6CDPUHK-YMlGmplHAWQCJcPFZjT3GQOP2j09mCg1D58E43zxeoeiJKV6bAoISHKEVO6zNkcRe3fEpGslcqJPVV0E0ICJ-BbiVNZ5pRw__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
This feature is available to Subscribers Only
Sign In or Create an Account Close Modal