Abstract
Signals derived from nonhematopoietic tissues are essential for normal primitive erythropoiesis in vertebrates, but little is known about the nature of these signals. In Xenopus, unidentified factors secreted by ectodermal cells during gastrulation are required to enable the underlying ventral mesoderm to form blood. Steel is expressed in the ectoderm of early Xenopus embryos and is known to regulate definitive erythroid progenitor survival and differentiation in other organisms, making it an excellent candidate regulator of primitive erythropoiesis. In this study, we tested whether steel signaling is required for primitive red blood cell differentiation in mice and frogs. We show that Xsl is expressed in the ectoderm in Xenopus gastrulae and that c-kit homologs are expressed in the underlying mesoderm at the same stages of development. We present loss of function data in whole Xenopus embryos and explants that demonstrate a requirement for ectodermally derived steel to signal through c-kit in the mesoderm to support early steps in the differentiation of primitive erythroid but not myeloid cells. Finally, we show that primitive erythropoiesis is not disrupted in mouse embryos that lack c-kit function. Our data suggest a previously unrecognized and unique function of steel/c-kit during primitive erythropoiesis in Xenopus.
Introduction
The first phase of vertebrate blood development, termed primitive hematopoiesis, gives rise primarily to embryonic erythrocytes and some leukocytes. In Xenopus laevis, primitive hematopoiesis occurs intraembryonically in a ventral mesodermal cell population known as the ventral blood island (VBI), which is the functional equivalent of the mammalian yolk sac.1,2 The VBI has 2 distinct early embryonic origins: the anterior portion of the VBI is derived from dorsal blastomeres, whereas the posterior VBI is derived from ventral blastomeres.3-5 Primitive blood cells begin to differentiate within the VBI in an anterior-to-posterior wave at the tail bud stage of development, and subsequently begin to circulate after stage 33/34 when the heart starts to beat. In Xenopus, definitive hematopoiesis initiates in a separate mesodermal population located near the dorsal lateral plate and contributes adult hematopoietic stem cells.3,6,7 This compartment is equivalent to the murine aorta, gonad mesonephros region, which is the first site of definitive hematopoiesis.8-11
Embryologic experiments have shown that signals derived from nonhematopoietic tissues are essential for normal primitive erythropoiesis in vertebrates.12-14 Numerous lines of evidence suggest that ectodermal cells, which come into contact with underlying ventral mesoderm during gastrulation, serve as an important source of such signals in Xenopus. For instance, when embryos are induced to exogastrulate, which prevents the prospective ectoderm from coming into apposition with the mesoderm, primitive erythrocytes fail to differentiate although other mesodermal cell types develop normally.15 Tissue recombination studies also demonstrate that signals provided by the ectoderm are required for differentiation of primitive erythrocytes in Xenopus. Specifically, early gastrula stage ventral mesoderm, which normally gives rise to the posterior VBI, fails to produce primitive red blood cells (RBCs) when explanted from embryos before physical contact with the ectoderm is established through gastrulation movements. When the same mesoderm is explanted but then recombined with ectodermal fragments, differentiation of primitive erythrocytes is restored.16,17 By the early neurula stage (stage 16) explanted ventral mesoderm is competent to differentiate as RBCs in the absence of ectoderm, suggesting that the ectodermal signals required for erythropoiesis are transmitted at some time between stages 10 and 16.16 Misregulation of the bone morphogenetic protein (BMP) or calmodulin-dependent protein kinase IV (CaM KIV) signaling pathways within ectodermal cells of intact embryos also leads to loss of RBC differentiation and survival, further underscoring the influence that the ectoderm has on primitive erythropoiesis.18-20 It remains to be determined precisely which signaling molecules produced by the ectoderm are required for this process.
Steel (also known as stem cell factor or kit ligand) is a reasonable candidate for a cytokine that might signal from the ectoderm to support primitive erythropoiesis in Xenopus. Two Xenopus steel homologues, Xsl-1 and Xsl-2, were recently reported and both are expressed in the prospective ectoderm.21 Moreover, low doses of soluble murine steel protein can induce globin expression when added to the culture media of Xenopus ventral mesoderm explants.22 The consequences of disrupting steel function in Xenopus have not been reported, but in mouse loss of steel or its receptor causes severe macrocytic anemia due to defects in proliferation of definitive erythroid progenitors.23 Little is known, however, about steel function during primitive murine hematopoiesis.
Steel signals through c-kit, a type III receptor tyrosine kinase related to the PDGF receptor.24,25 The c-kit extracellular region contains multiple immunoglobulin-like domains that bind steel and possibly mediate dimerization. Intracellularly, c-kit contains 2 catalytic domains separated by a kinase insert. As for other receptor tyrosine kinases, binding of ligand induces receptor dimerization and autophosphorylation of intracellular tyrosine residues. Activated c-kit interacts with multiple signaling components, including but not limited to JAK/STAT, Src, PI3K, Ras-Raf-MAPK, and PLC. During murine definitive erythropoiesis, c-kit is expressed in erythroid progenitor cells but not in differentiated erythrocytes.26 Two c-kit receptors have been identified in Xenopus, but their functions are not known.27,28 Xenopus kit–related-kinase1 (Xkrk1) shares approximately 60% amino acid identity with the mouse and chick c-kit receptors. The only other reported Xenopus c-kit homolog, Xenopus kit-like1 (Xkl1), and a closely related isoform, Xkl1A, share over 80% identity with Xkrk1 at the nucleotide level. Both Xenopus c-kit homologs are highly related to mammalian c-kit receptors within the intracellular domain, suggesting a high degree of functional conservation.
In the current study, we examined the expression and function of Xsl and its receptors during primitive hematopoiesis in Xenopus. We show that Xsl is expressed in the ectoderm and that c-kit homologs are expressed in the underlying mesoderm from gastrula through early neurula stages. We present loss-of-function data in whole embryos and explants that demonstrate a requirement for ectodermal steel to signal to c-kit in the mesoderm to support early differentiation steps in primitive erythroid cells, but not in primitive myeloid cells. Finally, to determine whether a requirement for steel signaling in primitive RBC development is conserved among vertebrates, primitive erythropoiesis was analyzed in mouse embryos that lack c-kit function. Our data suggest a previously unrecognized and unique function of steel/c-kit during primitive erythropoiesis in Xenopus.
Materials and methods
Embryo injection, culture, and recombination assays
Xenopus eggs were obtained and the embryos were injected with synthetic mRNAs or morpholinos and cultured as previously described.29 Embryonic stages are according to Nieuwkoop and Faber.30 Capped synthetic RNA was generated by in vitro transcription of linearized cDNA templates using a MegaScript kit (Ambion, Austin, TX). The Xsl morpholino 5′-GGTTAGCTTGTCTATTATCCCCTTAG-3′ and a control morpholino were obtained from Gene Tools (Philomath, OR). For embryo recombinant assays, embryo fragments were collected and cultured on a bed of 1.5% agarose in half-strength normal amphibian medium.31 Ventral mesoderm was collected from stage 10+ embryos as described by Maeno et al.17 Ectodermal explants from stage 10+ embryos were placed pigment side down in the dish and ventral mesoderm was placed on top of each explant. After the embryo fragments healed together (10-30 minutes), the recombinants were transferred to a new dish and cultured until sibling embryos reached stage 33. For a given experiment, equal numbers of explants (between 12 and 20) from each experimental group were pooled for analysis of globin expression by Northern blot.
Cloning ofXsl-1,Xsl-2b, andDN-kit
cDNAs containing the open reading frame (ORF) of Xsl-1 and Xsl-2b were amplified from stage 19 cDNA using the following primers: XslORF5′: 5′-ATGAAGAAGACAAAAACTTGG-3′ and XslORF3′: 5′-GAGATTTAGCCTCTGTAAACC-3′. Clones were ligated into pCS2+ for RNA transcription. To generate Xsl-1– and Xsl-2–specific probes for ribonuclease protection assays, cDNA fragments were amplified by polymerase chain reaction (PCR) from full-length Xsl-1 and Xsl-2 using the primers 5′-ATGATGAAATGGACTTTGACT-3′ and 5′-CTACTATTCTTTCGTGTGCGTT-3′ and subcloned into pGEMT easy (Promega, Madison, WI). To enhance translation of both clones, Kozak consensus sequence was added upstream of the ATG. Xsl-2b including 109 bp of 5′ untranslated region (UTR) immediately upstream of the ATG was cloned from stage 31 cDNA using the primers 5′-GGAATTCTGCTGGGGCTCAGAGCAGCT-3′ and XslORF3′. An Xsl-1 clone containing both morpholino target and Kozak consensus sequence upstream of the ATG in pCS2+ was generated by PCR from the Xsl-1 ORF template using the primers 5′-ACGAGATCTAGCACTAAGGGGATAATAGACAAGCTACCATGAAGAAGACAAAAA and XslORF3′. Xkrk was cloned from stage 19 cDNA using the primers 5′-TACGATCACAATGAGCTGGAC-3′ and 5′-CCAGACGAGAACCTTGTTGAC-3′. Xkl1A (IMAGE clone 4030854) was purchased from Open Biosystems (Huntsville, AL) on behalf of the IMAGE consortium.32 cDNAs encoding dominant-negative versions of Xkrk1 and Xkl1-A (consisting of the first 573 amino acids of each receptor) were generated by subcloning a about 1.7 kb EcoRI fragment from each cDNA into pCS2+.
In vitro translation
[35S]Met/Cys-labeled Xsl-1 was synthesized in rabbit reticulocyte lysates (Promega) following the manufacturer's instructions. Products were analyzed by sodium dodecyl sulfate–polyacrylamide gel electrophoresis (SDS-PAGE) followed by autoradiography.
In situ hybridization, ribonuclease protection assays, Northern blot analysis, and RT-PCR
Embryos were processed for in situ hybridization as previously described.33 Embryos were photographed using a Leica (Bannockburn, IL) MZ FLIII microscope equipped with a 1.0 ×/0.078 numeric aperture objective lens and an Optronics (Goleta, CA) CE digital camera in conjunction with ImagePro plus software (Media Cybernetics, Silver Spring, MD) for embryo sections and In-Focus Z-stack 1.31 software (Meyer Instruments, Houston, TX) for whole embryos. Images were further processed using Adobe Photoshop 7.0 software (Adobe Systems, San Jose, CA). Total RNA and DNA were isolated from individual mouse embryos using TRIzol (Invitrogen, Carlsbad, CA), following the manufacturer's protocol. Total RNA was isolated from Xenopus embryos and ribonuclease protection assays were performed as previously described34 using 40 to 50 μg total RNA per sample. RNAs were hybridized with Xsl-1–specific (240-bp protected fragment) or Xsl-2b–specific (240-bp protected fragment) riboprobes together with an Fgfr-specific probe (200-bp protected fragment) as a loading control. Northern blots were hybridized with Xenopus alpha T3 globin and ornithine decarboxylase (odc) antisense riboprobes, or with a mouse beta h1-globin riboprobe, as described previously.35 Reverse transcriptase (RT)–PCR was performed as previously described,34 using Gata1 primers 5′-CCTAGGAGGAGGAGGGCAGG-3′ and 5′-AGTGTACCTTTATGCTAAC-3′ and odc primers 5′-AATGGATTTCAGAGACCA-3′ and 5′-CCAAGGCTAAAGTTGCAG-3′.
Collection and analysis of peripheral blood from Xenopus and mouse embryos
Tails were severed from Xenopus embryos; peripheral blood cells were collected into amphibian PBS containing 0.5% BSA and 10 IU/mL heparin and concentrated onto slides using a cytocentrifuge as previously described.20 For each experiment, a minimum of 24 embryos were bled per age-matched experimental group and, for each embryo, the number of cells in 4 random fields was counted using a Fischer Scientific (Pittsburgh, PA) MicroMaster microscope equipped with a 40 × 0.65 objective lens. Results from 3 separate experiments were pooled for presentation. Because stage 40 embryos were analyzed in one experiment, and stage 43 embryos in 2 later experiments, and because the number of blood cells increases dramatically between these 2 stages, results are presented as percent of control rather than absolute cell number. KitW/+ mice were obtained from the Jackson Laboratory (Bar Harbor, ME). Mouse embryos from intercrosses were harvested at embryonic day (E) 11.5 or E12.5 and individual embryos with their yolk sacs were exsanguinated in 35-mm dishes of PB1 supplemented with heparin as described.36 Media containing peripheral blood was passed though a cell strainer to remove debris. Blood samples were counted using a hemoctyometer, and cytospins were prepared as described.36 Cytospins were photographed using a Nikon (Melville, NY) Optiphoto2-UD microscope equipped with a 20 ×/0.50 objective lens and used in conjunction with a Nikon DXM 1200F digital camera and Nikon Act-1 version 2.62 imaging software. Images were further processed with Adobe Photoshop 7.0 software. Each exsanguinated mouse embryo was digested with tissue lysis buffer (0.1 M Tris, pH 8.0, 5 mM EDTA, 0.2 M NaCl, 0.2% SDS, 100 μg/mL proteinase K) to extract DNA. Embryo genotypes were ascertained from isopropanol-precipitated DNA using primers, PCR conditions, and HphI restriction digest as described.37
Results
Xenopussteel is a candidate ectodermal regulator ofXenopusprimitive erythropoiesis
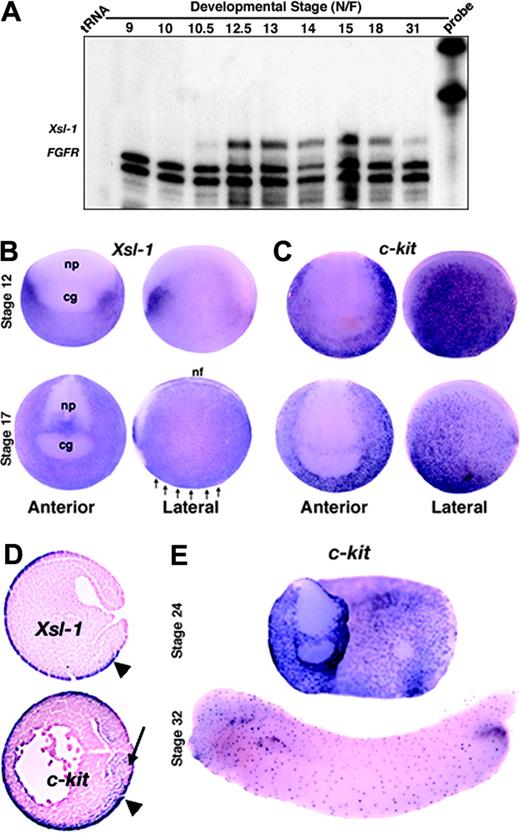
We cloned 2 Xenopus steel (Xsl) cDNAs that share approximately 76% identity in their coding regions. One of these cDNAs is identical to the recently published steel homologue, Xsl-1, and the other shares 92% identity with a second steel homologue, Xsl-2,21 and will thus be referred to as Xsl-2b. Expression of Xsl-1 and Xsl-2b was barely detectable by Northern blot analysis, although numerous hybridizing bands were observed (data not shown), possibly representing different splice isoforms as has been reported for mammalian steel.38 We therefore used ribonuclease protection assays to examine the temporal pattern of expression of Xsl. Expression of Xsl-1 was first detected at the onset of gastrulation (stage 10-10.5) and was maintained through to tail bud stages (Figure 1A). An identical pattern of expression was observed for Xsl-2b but transcripts were less abundant (data not shown). Maternal transcripts were not detected for either isoform. Analysis of the spatial pattern of expression of Xsl-1 by whole-mount and section in situ hybridization showed that transcripts are restricted to nonneural ectoderm from gastrula through early neurula stages (Figure 1B,D). By stage 24, Xsl transcripts are no longer detected in the ectoderm overlying the hematopoietic mesoderm but are instead restricted to the neural tube, pronephric duct, otic placode, and proctodeum (data not shown; Martin and Harland21 ). This pattern of expression persists until at least the tail bud stage.
We also examined expression of the 2 known Xenopus c-kit homologues, Xkrk28 and Xkl,27 using a probe that recognizes both genes. In addition to detecting c-kit expression in previously reported locations, including the nonneural ectoderm (Figure 1C-D), we also detected expression in the mesoderm at late gastrula through neural stages (Figure 1D arrow and data not shown), which is when Xsl-1 and Xsl-2b mRNAs are present in the overlying ectoderm. At stage 24 and later, c-kit is expressed in a punctate pattern throughout the surface ectoderm (Figure 1E). Together, these data are consistent with the possibility that ectodermally derived Xsl signals to the prospective hematopoietic mesoderm via activation of the c-kit receptor during late gastrula through neurula stages. After this time, neither the receptor nor the ligand is expressed in an appropriate pattern to directly influence primitive hematopoiesis.
Xenopus steel and c-kit are expressed at the right time and place to support primitive blood development. (A) Ribonuclease protection analysis of the temporal pattern of expression of Xsl-1. FGFR is included as a loading control and protects a doublet. (B-C) Expression of Xsl-1 (B) and c-kit (C) analyzed by whole-mount in situ hybridization at stages 12 and 17; cg indicates cement gland; nf, neural fold; np, neural plate. Arrows point to prospective VBI. (D) In situ hybridization of Xsl-1 and c-kit riboprobes to sagittal sections of stage 12.5 embryos. Both are expressed in the ectoderm (arrowhead), whereas c-kit is also expressed in prospective ventral mesoderm (arrow). (E) Expression of c-kit analyzed by whole-mount in situ hybridization at stages 24 and 32.
Xenopus steel and c-kit are expressed at the right time and place to support primitive blood development. (A) Ribonuclease protection analysis of the temporal pattern of expression of Xsl-1. FGFR is included as a loading control and protects a doublet. (B-C) Expression of Xsl-1 (B) and c-kit (C) analyzed by whole-mount in situ hybridization at stages 12 and 17; cg indicates cement gland; nf, neural fold; np, neural plate. Arrows point to prospective VBI. (D) In situ hybridization of Xsl-1 and c-kit riboprobes to sagittal sections of stage 12.5 embryos. Both are expressed in the ectoderm (arrowhead), whereas c-kit is also expressed in prospective ventral mesoderm (arrow). (E) Expression of c-kit analyzed by whole-mount in situ hybridization at stages 24 and 32.
Steel function is essential for primitive erythropoiesis inXenopus
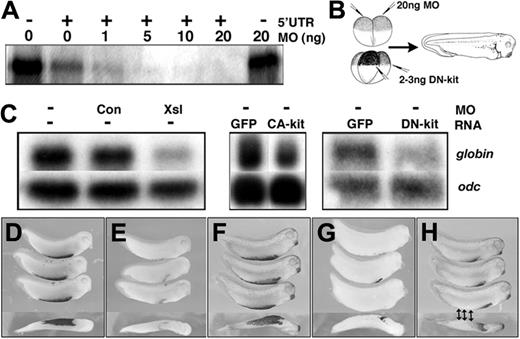
We used antisense morpholino oligonucleotides (MOs) to knock down expression of Xsl to determine whether it is necessary for primitive erythropoiesis in Xenopus. We designed an MO that shares identity with both Xsl-1 and Xsl-2 within their 5′ UTRs and confirmed that it can block translation of Xsl-1 using an in vitro translation assay. As shown in Figure 2A, addition of 1 to 20 ng Xsl MO to rabbit reticulocyte lysates programmed with Xsl-1 mRNA containing the target 5′ UTR sequence blocked Xsl-1 translation in a dose-dependent fashion. In contrast, addition of 20 ng Xsl MO to lysates programmed with Xsl-1 mRNA lacking the target sequence did not affect Xsl-1 translation. We could not perform a similar in vitro assay with Xsl-2b because this mRNA is not readily translated in vitro, even when the endogenous sequence upstream of the start site is replaced by Kozak consensus sequence and all of the 5′ UTR is removed (data not shown).
Injection of a total of 20 ng of the Xsl MO into the animal pole of 2-cell embryos, as illustrated in Figure 2B, leads to loss of expression of alpha T3 globin (globin), a marker of differentiating primitive RBCs, as detected by Northern blot in pooled stage 33 embryos in a minimum of 5 independent experiments (Figure 2C). Examination of globin expression by whole-mount in situ hybridization revealed that globin expression was decreased throughout the VBI (n = 42 of 47; Figure 2E). In contrast, injection of 20 ng control MO into the animal pole of 2-cell embryos had no effect on globin expression levels by Northern (Figure 2C) or in situ hybridization analysis (n = 40 of 50; Figure 2D). We attempted to rescue blood development in Xsl MO-injected embryos by coinjection of plasmids or mRNAs encoding versions of either, or both, Xsl-1 and Xsl-2b optimized for translation (see “Materials and methods”), but we were not able to consistently rescue globin expression in this manner (data not shown). This may be due to poor translation of Xsl-1 and Xsl-2 in vivo. In an attempt to circumvent this complication, we overexpressed a constitutively active form of human c-kit (CA-kit), in which the valine at position 816 is substituted with aspartic acid (for reviews, see Ronnstrand25 and Lennartsson et al39 ). Injection of various doses (50-500 pg) of CA-kit mRNA near the ventral marginal zone of 4-cell embryos not only failed to rescue globin expression in Xsl MO-injected embryos but was itself sufficient to suppress globin expression in uninjected controls (Figure 2C middle panel and data not shown). This finding is consistent with published data showing that overexpression of high doses of mouse steel suppresses primitive erythroid differentiation in Xenopus,22 and suggests that strict regulation of Xsl dosage is critical for normal primitive hematopoiesis.
Xsl and c-kit function are required for primitive erythropoiesis in Xenopus. (A) Xsl-1 RNA containing (+) or lacking (–) the 5′ UTR sequence targeted by the antisense MO was translated in vitro in the presence of increasing doses of MO. Radiolabeled translation products were analyzed by SDS-PAGE and autoradiography. (B) Schematic representation of experiments shown in panels C-H. MOs were targeted to prospective ectoderm by injection into the animal pole and DN-kit mRNAs were targeted to the prospective VBI by injection near the marginal zone. Embryos were allowed to develop to stage 33/34 for analysis of globin. (C) Northern blot analysis of expression of globin and odc (loading control) in embryos injected with MOs or synthetic RNAs as indicated above each lane. (D-H). Whole-mount in situ hybridization analysis of expression of globin in embryos injected with control MO (D), Xsl MO (E), GFP RNA (F) DN-kit RNA targeted to the entire VBI (G), or DN-kit RNA targeted to the posterior VBI only (denoted by arrows; H). Anterior is to the right. The bottom embryo in each panel is a ventral view; all other embryos are shown in a lateral view.
Xsl and c-kit function are required for primitive erythropoiesis in Xenopus. (A) Xsl-1 RNA containing (+) or lacking (–) the 5′ UTR sequence targeted by the antisense MO was translated in vitro in the presence of increasing doses of MO. Radiolabeled translation products were analyzed by SDS-PAGE and autoradiography. (B) Schematic representation of experiments shown in panels C-H. MOs were targeted to prospective ectoderm by injection into the animal pole and DN-kit mRNAs were targeted to the prospective VBI by injection near the marginal zone. Embryos were allowed to develop to stage 33/34 for analysis of globin. (C) Northern blot analysis of expression of globin and odc (loading control) in embryos injected with MOs or synthetic RNAs as indicated above each lane. (D-H). Whole-mount in situ hybridization analysis of expression of globin in embryos injected with control MO (D), Xsl MO (E), GFP RNA (F) DN-kit RNA targeted to the entire VBI (G), or DN-kit RNA targeted to the posterior VBI only (denoted by arrows; H). Anterior is to the right. The bottom embryo in each panel is a ventral view; all other embryos are shown in a lateral view.
To further test whether steel function is required for primitive hematopoiesis, we overexpressed dominant-negative versions of c-kit (DN-kit) in the ventral mesoderm of Xenopus embryos to block activation of the endogenous, wild-type receptor by steel. Dominant-negative versions of both isoforms of Xenopus c-kit (Xkrk and Xkl) were made by deleting sequence encoding the intracellular portion of the receptor, downstream of the juxtamembrane domain. These deletion mutants are capable of dimerizing with wild-type receptors, but lack the tyrosine kinase domain and thus cannot transphosphorylate and activate the endogenous receptor. A similar strategy has been used to generate dominant mutant forms of other transmembrane tyrosine kinase receptors.40 Furthermore, naturally occurring deletions and point mutations in murine c-kit that abrogate tyrosine kinase activity have been shown to function in a dominant mutant fashion.41 RNA encoding one or the other of the DN-kit receptor isoforms was injected near the marginal zone of 4-cell embryos as illustrated in Figure 2B, either into all 4 blastomeres (0.5-0.75 ng/blastomere) to target the entire prospective blood island, or into the 2 ventral blastomeres (1-1.5 ng/blastomere) to selectively target the posterior VBI. Ventral injection of RNA encoding DN-kit led to a loss of globin expression, as analyzed by Northern blot hybridization, in a minimum of 5 experiments (Figure 2C). Disruption of c-kit signaling in the entire marginal zone blocked expression of globin throughout the VBI in most embryos at stage 33 (n = 20 of 22; Figure 2G), whereas disruption of c-kit signaling in the ventral marginal zone blocked expression of globin only in the posterior VBI (n = 68 of 114; Figure 2H). Injection of 200 ng GFP mRNA into the ventral marginal zone had no effect on expression of globin as assessed by Northern blot (Figure 2C) or in situ hybridization analysis (n = 34 of 36; Figure 2C,F). Taken together, these results demonstrate that steel function is required for primitive erythropoiesis and further suggest that c-kit functions cell autonomously within the VBI.
Expression of Xsl in the ectoderm and activation of c-kit in the mesoderm are required for primitive erythropoiesis
Although these results are consistent with a requirement for ectodermal steel to activate c-kit in prospective hematopoietic mesoderm, targeted injections do not restrict Xsl MO or DN-kit exclusively to the ectoderm and ventral mesoderm, respectively. We therefore used a tissue recombination assay to definitively test which cells produce kit ligand and which cells require activated c-kit for primitive RBC differentiation. Previous studies have shown that when gastrula stage ventral mesoderm is explanted and cultured to the tail bud stage, erythrocytes will not differentiate from this tissue unless it is cocultured with ectodermal cells.6,16 To ascertain whether ectodermally derived Xsl is required to enable explanted mesoderm to differentiate as blood, we compared expression of globin in recombinants composed of ectoderm and mesoderm isolated from control embryos to those in which expression of Xsl had been suppressed in the ectodermal component, by injection of Xsl MOs, or in which reception of the Xsl signal had been suppressed in the mesodermal component, by injection of DN-kit RNA (illustrated in Figure 3A). Expression of globin was not detected in ventral mesodermal explants cultured in the absence of ectoderm (Figure 3B). By contrast, robust expression of globin was observed in pooled control recombinants but not in pooled recombinants lacking Xsl in the ectoderm (4 of 5 experiments) or expressing DN-kit in the mesoderm (2 of 3 experiments; Figure 3B). These results demonstrate that kit ligand produced in the ectoderm signals to its receptor in the ventral mesoderm to support RBC differentiation.
Steel is required for an early step in erythropoiesis
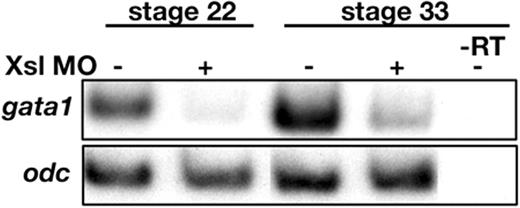
The loss of expression of globin observed in Xsl-deficient embryos could be due to an early defect in the hematopoietic program, such as failure of mesodermal cells to commit to an erythropoietic fate, or it could be due to a later failure of committed progenitors to survive or differentiate as erythrocytes. To begin to distinguish between these possibilities, we analyzed expression of gata1, a transcription factor whose expression is restricted to hematopoietic mesoderm prior to RBC differentiation, and whose expression is maintained in erythroblasts as they progress through early stages of differentiation.42-45 Expression of gata1 was severely repressed in Xsl MO-injected embryos at stages 18 (not shown), 22, and 33 in 2 independent experiments (Figure 4). An identical result was obtained when RNA encoding DN-kit was injected in an additional experiment (data not shown). The observation that expression of gata1 is diminished in Xsl-deficient embryos as early as stage 18, which is prior to the onset of RBC differentiation, suggests that steel function is required for an early step in primitive erythroid development. These data do not rule out the possibility that activation of the steel signaling cascade during gastrulation leads to secondary signals that function to promote the survival, proliferation, or differentiation of RBCs at later stages of development.
Expression of Xsl in the ectoderm and activation of c-kit in the mesoderm are required for primitive erythropoiesis. (A) Schematic showing experimental design. Recombinants were cultured until stage 33/34, pooled, and analyzed for expression of globin. (B) Northern blot analysis of globin and odc expression in mesoderm (VM) or ectoderm/mesoderm (Ecto/VM) recombinants generated from control embryos or from embryos that had been injected with Xsl MOs or DN-kit RNA as indicated above each lane.
Expression of Xsl in the ectoderm and activation of c-kit in the mesoderm are required for primitive erythropoiesis. (A) Schematic showing experimental design. Recombinants were cultured until stage 33/34, pooled, and analyzed for expression of globin. (B) Northern blot analysis of globin and odc expression in mesoderm (VM) or ectoderm/mesoderm (Ecto/VM) recombinants generated from control embryos or from embryos that had been injected with Xsl MOs or DN-kit RNA as indicated above each lane.
Loss of gata1 expression in Xsl MO-injected embryos. Expression of gata1 in control and Xsl MO-injected embryos was assayed by semiquantitative RT-PCR at stages 22 and 33. Reverse transcriptase was omitted from one set of samples (–RT) as a control for genomic contamination.
Loss of gata1 expression in Xsl MO-injected embryos. Expression of gata1 in control and Xsl MO-injected embryos was assayed by semiquantitative RT-PCR at stages 22 and 33. Reverse transcriptase was omitted from one set of samples (–RT) as a control for genomic contamination.
Steel is required for formation of primitive erythrocytes but not leukocytes
We examined peripheral blood samples from Xsl MO and DN-kit RNA-injected embryos to determine whether loss of steel function affected the number or morphology of circulating primitive erythrocytes or leukocytes. Injected embryos and control siblings were allowed to develop to stage 40-43, at which time peripheral blood from a minimum of 24 embryos in each experimental group was collected onto slides by cytocentrifugation, stained with a Wright-Giemsa differential stain, and blood cell counts determined (see “Materials and methods”). Injection of either the Xsl MO or DN-kit mRNA drastically reduced the number of erythrocytes but did not have a significant impact on the number of circulating leukocytes in 3 independent experiments (Figure 5A). As shown in Figure 5B, primitive hematopoiesis generates primarily erythrocytes and a smaller number of leukocytes, most of which are of the monocyte/macrophage lineage. No distinguishable differences in primitive RBC or leukocyte morphology were observed among experimental groups (Figure 5B-D). Thus, disruption of steel signaling early in development prevents the production of primitive RBCs but not leukocytes.
Steel is not required for primitive erythropoiesis in mice
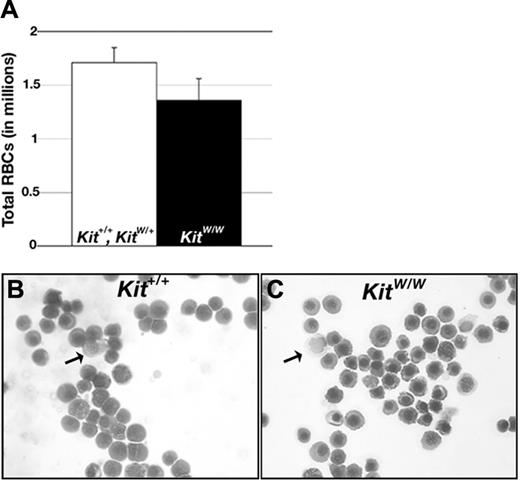
We analyzed primitive erythropoiesis in c-kit–deficient mouse embryos to determine whether the requirement for steel function in early blood development is conserved in mouse. KitW/W mutants contain a point mutation in a splice donor site that causes skipping of the exon encoding the extracellular domain of c-kit.46 These mutants are thus unable to transduce steel signals. KitW/+ mutants were intercrossed and the number and morphology of circulating erythrocytes was determined in individual littermates at E11.5, at which time circulating erythrocytes are exclusively primitive in nature and derived from the yolk sac.36 We found no significant difference in the number (Figure 6A) or morphology (Figure 6B-C) of circulating RBCs in KitW/W embryos relative to wild-type and heterozygous littermates. We also assayed levels of beta h1-globin on Northern blots of RNA extracted from whole embryos (including yolk sacs) at E12.5. At this stage of development, more than 90% of circulating RBCs are primitive erythrocytes.36 Although we detected some variability in signal intensity among individuals, we did not detect a decrease in beta h1-globin signal in KitW/W embryos (not shown). Thus, our data suggest that although steel signaling is necessary for primitive erythropoiesis in Xenopus, it is dispensable for primitive erythroid development in the mouse.
Xsl function is required for differentiation of primitive erythrocytes but not leukocytes. (A) Number of erythrocytes and leukocytes in tadpoles that had been injected with Xsl MOs (▪) or with DN-Kit RNA (▦) expressed as a percentage of cell number in control embryos (□). Error bars show the SEM. Pooled results from 3 independent experiments are presented. (B-D) Wright-Giemsa stained cytospins of representative samples from control (B), Xsl MO- (C), and DN-kit– (D) injected embryos; e indicates erythrocyte; l, leukocyte.
Xsl function is required for differentiation of primitive erythrocytes but not leukocytes. (A) Number of erythrocytes and leukocytes in tadpoles that had been injected with Xsl MOs (▪) or with DN-Kit RNA (▦) expressed as a percentage of cell number in control embryos (□). Error bars show the SEM. Pooled results from 3 independent experiments are presented. (B-D) Wright-Giemsa stained cytospins of representative samples from control (B), Xsl MO- (C), and DN-kit– (D) injected embryos; e indicates erythrocyte; l, leukocyte.
c-kit signaling is not required for primitive erythropoiesis in mice. (A) Average number of primitive RBCs in individual E11.5 littermates generated from KitW/+ intercrosses (Kit+/+, n = 3, KitW/+, n = 7; KitW/W, n = 5). Error bars show the SEM. (C-D) Wright-Giemsa–stained blood collected from Kit+/+ (B) and KitW/W (C) embryos. Arrows point to leukocytes, remaining cells are erythrocytes.
c-kit signaling is not required for primitive erythropoiesis in mice. (A) Average number of primitive RBCs in individual E11.5 littermates generated from KitW/+ intercrosses (Kit+/+, n = 3, KitW/+, n = 7; KitW/W, n = 5). Error bars show the SEM. (C-D) Wright-Giemsa–stained blood collected from Kit+/+ (B) and KitW/W (C) embryos. Arrows point to leukocytes, remaining cells are erythrocytes.
Discussion
Tissue microenvironments regulate many aspects of primitive and definitive hematopoiesis. In Xenopus, results from numerous studies implicate the prospective ectoderm as a source of signals that are required for the mesoderm to form primitive erythrocytes. Similar non–cell autonomous signals that are critical for primitive erythropoiesis in other vertebrates have also been described (reviewed by Baron12 ). In the mouse and chick, for example, signals transmitted from the primitive endoderm are essential for RBC differentiation in the mesoderm. Prospective hematopoietic mesoderm is in direct contact with the primitive endoderm (known as visceral endoderm in the mouse) at pregastrula stages and remains in contact with this tissue during gastrulation, when it migrates into the extraembryonic region. Removal of the visceral endoderm from explanted pregastrula and early gastrula stage mouse epiblasts results in the subsequent failure of RBCs to differentiate, whereas erythropoiesis is restored when epiblasts are cocultured with visceral endoderm.12,47,48 A similar requirement for a diffusible primitive endodermal signal for erythropoiesis in chicks has also been demonstrated, yet the identity of this signal is not known.49 In zebrafish, both the paraxial mesoderm14 and endothelial cells50 have been shown to provide essential signals to differentiating primitive RBCs.
Our data demonstrate that steel is an ectodermally expressed factor that is critical for primitive erythropoiesis in Xenopus. The timing of steel expression is consistent with the onset of ectodermal competence to support RBC differentiation as determined by heterochronic recombination assays. Specifically, whereas ectoderm isolated from early gastrula stage (stage 10) Xenopus embryos supports robust production of primitive RBCs from ventral mesodermal explants, ectoderm isolated at stage 7 does not.17 Ectoderm isolated at stage 7 becomes competent to promote erythropoiesis, however, when it is derived from embryos injected with polyadenylated mRNAs extracted from ectodermal cells of stage 10 gastrulae. This implies that a signaling molecule required to support erythropoiesis is expressed de novo in ectodermal cells by stage 10, which is when expression of steel initiates. Thus, transcription of steel is likely a key process in the ectoderm's acquisition of competence to promote erythropoiesis in adjacent ventral mesoderm. Ventral mesoderm becomes competent to differentiate as RBCs in the absence of ectoderm by stage 16, and shortly after this time expression of c-kit and steel is no longer detected in hematopoietic mesoderm or in adjacent tissues. This suggests that the developmental window during which ectodermally derived steel signals to underlying mesoderm to promote erythropoiesis extends from the gastrula through the early neurula stages.
We have previously shown that BMPs and CaM KIV function in a common pathway within ectodermal cells to regulate primitive erythropoeisis.19 Specifically, activation of the BMP pathway is required within ectodermal cells to generate a secondary signal that enables mesoderm to form RBCs. CaM KIV, on the other hand, is required to negatively regulate BMP-mediated transcriptional responses during hematopoiesis Our current results raise the possibility that Xsl-1 or Xsl-2 or both are transcriptional targets of the BMP pathway and act downstream of it in ectodermal cells. Several lines of evidence suggest that this is not the case. First, although BMPs and Xsl are both required for primitive hematopoiesis, they appear to act at different stages of blood development. Whereas Xsl is required for early expression of gata1 during neurula stages, ectodermal BMP function is dispensable for this process but is required instead to prevent apoptosis of committed erythroid progenitors later in development. Second, in preliminary experiments, we did not detect gross changes in expression of Xsl-1 or Xsl-2 at stages 12-13 in embryos in which BMP or CaM KIV signaling was misregulated. It remains possible, however, that BMPs regulate transcription of Xsl-1 or Xsl-2 at a different developmental stage or regulate expression at a posttranscriptional level. A more likely possibility is that BMPs and Xsl act in parallel pathways, with both signaling cascades being required, but not sufficient, for primitive erythropoiesis.
Our data showing that Xsl is required for expression of gata1 as early as stage 18 suggests that it regulates an early step in erythropoiesis. Recent studies have shown that knock down of gata1 in zebrafish results in excessive production of leukocytes at the expense of erythrocytes, presumably as a consequence of altering the fate of a common myeloid precursor.51,52 By contrast, the early loss of expression of gata1 we observed in Xenopus embryos in which steel function is blocked is followed by a loss of erythrocytes but no change in the number of circulating leukocytes. In addition, no obvious changes in expression of the myeloid cell marker X-pox253 are observed in steel-deficient Xenopus embryos at stages 19-20 (data not shown). Thus, loss of steel signaling in Xenopus likely influences the proliferation, survival, or differentiation of committed erythroid progenitors, similar to its known role in murine definitive erythropoiesis,23 rather than functioning at the level of a common myeloid precursor.
In the mouse, steel is a critical component of the definitive erythroid microenvironment. Steel and c-kit mutants die shortly before or after birth as a result of severe anemia caused by decreased proliferation of definitive erythroid progenitors (for a review, see Broudy23 ). Primitive hematopoiesis has not been examined closely in these mutants although one study reported that KitW/W embryos show a 50% decrease in the number of circulating erythrocytes at E12,54 when nearly all RBCs are derived from primitive hematopoiesis.36 In this study, however, the genotype of individual embryos was inferred from the number of circulating RBCs, raising the possibility that the decrease in RBC number reflected random variability rather than genotype. Consistent with this possibility, we detect some variability in RBC number, and in the level of beta h1-globin transcripts between individual littermates, but do not find any defect in primitive hematopoiesis in KitW/W embryos. Thus, mouse embryos, like zebrafish embryos,55,56 do not require c-kit function for normal primitive erythropoiesis. Hematopoietic cells expressing c-kit are present in the embryonic yolk sac by E857 but we (data not shown) and others58,59 have been unable to detect steel transcripts prior to E9, suggesting that this signaling pathway may not even be active in mouse embryos until well after the first primitive erythrocytes have begun to differentiate. Although c-kit expression domains in Xenopus, zebrafish, and chick largely overlap, there are numerous examples of differences in c-kit expression and function among these species.55,56,60 Given that other receptor tyrosine kinases and their cognate ligands (eg, erythropoietin) that activate the same downstream signaling events as c-kit are expressed during primitive hematopoiesis,61,62 these might function in place of c-kit in mouse and zebrafish.
Prepublished online as Blood First Edition Paper, December 15, 2005; DOI 10.1182/blood-2005-09-3930.
Supported by an award from the American Heart Association (D.C.G.), by a Veterans Affairs Merit Review Grant (M.C.H.), and by grants from the National Institutes of Health (RO3 HD050242; J.L.C.) and the American Heart Association (0150321N0; J.L.C.).
D.C.G. designed and performed research, analyzed data, and wrote the paper; L.K.B. performed research; M.C.H. contributed vital new reagents; and J.L.C. designed and analyzed research and wrote the paper.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
We thank Dr Frank Costantini for permission to use the beta h1-globin probe and Nathan Donley and Riffat Ahmed for excellent technical assistance.



This feature is available to Subscribers Only
Sign In or Create an Account Close Modal