Abstract
Feasibility, safety, and efficacy of a 4-course high-dose chemotherapy (HDT) protocol including autologous stem cell transplantation (SCT) after courses 2, 3, and 4 was investigated in 110 patients, aged 18 to 60 years, with primary diagnosis of aggressive NHL (aNHL), and lactic dehydrogenase (LDH) levels above normal. At dose level 1 (DL1), course 1 consisted of cyclophosphamide 1500 mg/m2, doxorubicin (Adriamycin) 70 mg/m2, vincristine 2 mg, etoposide 450 mg/m2, and prednisone 500 mg. With courses 2 and 3 cyclophosphamide and etoposide were escalated to 4500 mg/m2 and 600 mg/m2, respectively. With course 4 cyclophosphamide and etoposide were given at 6000 mg/m2 and 1000 mg/m2, respectively. At DL2 etoposide was further increased to 600, 960, 960, and 1480 mg/m2 with courses 1 to 4, respectively. Therapy as per protocol was completed by 81.8% of patients. Overall survival at 5 years was 67.2%, freedom from treatment failure (FFTF) was 62.1%, and treatment-related mortality was 4.5%. There was a trend to better FFTF at DL2 compared to DL1 (66.9% versus 54.2%). Repetitive HDT with escalated CHOP plus etoposide is feasible and effective treatment of patients with aNHL. DL2 of this therapy is being used in an ongoing phase 3 study.
Introduction
The role of high-dose therapy (HDT) followed by autologous stem cell transplantation (SCT) for primary treatment of aggressive non-Hodgkin lymphoma (aNHL) is still uncertain. Whereas some studies demonstrated superiority of HDT over conventional treatment,1-3 others failed to show significant differences4-6 or reported inferior results.7 Besides differences in patient characteristics the type of HDT and its timing may have important implications for outcome. We performed a phase 1/2 study to test a HDT protocol to investigate the following concepts: maximum dose escalation and dose density of cytotoxic agents with known activity in NHL, intensification of treatment as early as possible after diagnosis, and repeated collection and transplantation of autologous stem cells to exploit the in vivo purging effect exerted by HDT. Four courses of dose-escalated cyclophosphamide, doxorubicin (Adriamycin), vincristine, etoposide, and prednisone were administered, 3 of them followed by autologous SCT. We describe the feasibility, safety, and efficacy of this approach in a multicenter setting.
Patients, materials, and methods
Patients with a primary diagnosis of aNHL, aged 18 to 60 years, and a lactic dehydrogenase (LDH) level above the upper normal limit (UNL) were included in the study. Individual written informed consent was mandatory. The protocol was approved by the Ethics Committee of the Medical Faculty, University of Marburg, Germany and Ethics Committee of the Medical Faculty, University of Kiel, Germany; trial conduct followed the rules of the Declaration of Helsinki. Lymphomas of the central nervous system, lymphoblastic or Burkitt-type lymphoma, major organ dysfunction, known positivity for HIV, or active hepatitis were exclusion criteria. Because repeated stem cell mobilization was planned, bone marrow involvement greater than 25% by histology was an additional exclusion criterion to reduce the risk of transplantation of lymphoma cells. Enrollment started in January 1997 and ended in August 1999. A total of 124 patients were enrolled in the study at 31 institutions (participating institutions are listed in the “Appendix”). Fourteen patients had to be excluded due to correction of histologic diagnosis (n = 8), withdrawal of informed consent (n = 2), bone marrow involvement greater than 25% (n = 2), HIV positivity (n = 1), or LDH level below the UNL (n = 1). The remaining 110 patients were included in the study and received MegaCHOEP treatment (see “Treatment”). Histologic review by an expert hematopathologist was available in 108 of these 110 patients. Distribution of histologic subgroups is given in Table 1. Three fourths of the patients had International Prognostic Index (IPI) of high-intermediate or high-risk features.8 Neither the distribution of histologic subtypes nor age-adjusted IPI risk factors showed significant differences between patients treated at dose level (DL) 1 or 2, respectively (Table 2).
Distribution of histologic subtypes
Histologic subtype . | DL1, no. (%) . | DL2, no. (%) . | Total, no. (%) . |
|---|---|---|---|
| B-cell lymphoma | 31 (84) | 61 (88) | 92 (87) |
| Follicular lymphoma, grade III WHO | 1 (3) | 3 (4) | 4 (4) |
| High-grade, Burkitt-like | 0 (0) | 1 (1) | 1 (1) |
| Mantle cell lymphoma, blastic | 1 (3) | 1 (1) | 2 (2) |
| Diffuse large-cell lymphoma | 25 (68) | 52 (75) | 77 (73) |
| Primary sclerosis, mediastinal type | 4 (11) | 11 (16) | 15 (14) |
| Centroblastic | 15 (41) | 27 (39) | 42 (40) |
| Immunoblastic | 1 (3) | 4 (6) | 5 (5) |
| T-cell—rich B-cell lymphoma | 1 (3) | 4 (6) | 5 (5) |
| Anaplastic large cell lymphoma | 2 (5) | 1 (1) | 3 (3) |
| Not otherwise specified | 2 (5) | 5 (7) | 7 (7) |
| Aggressive B-cell lymphoma, not otherwise specified | 1 (3) | 1 (1) | 2 (2) |
| Aggressive B-cell lymphoma, subtyping not possible | 3 (8) | 3 (4) | 6 (6) |
| T-cell lymphoma | 6 (16) | 8 (12) | 14 (13) |
| Anaplastic large cell lymphoma | 3 (8) | 7 (10) | 10 (9) |
| Peripheral T-cell lymphoma | 3 (8) | 1 (1) | 4 (4) |
Histologic subtype . | DL1, no. (%) . | DL2, no. (%) . | Total, no. (%) . |
|---|---|---|---|
| B-cell lymphoma | 31 (84) | 61 (88) | 92 (87) |
| Follicular lymphoma, grade III WHO | 1 (3) | 3 (4) | 4 (4) |
| High-grade, Burkitt-like | 0 (0) | 1 (1) | 1 (1) |
| Mantle cell lymphoma, blastic | 1 (3) | 1 (1) | 2 (2) |
| Diffuse large-cell lymphoma | 25 (68) | 52 (75) | 77 (73) |
| Primary sclerosis, mediastinal type | 4 (11) | 11 (16) | 15 (14) |
| Centroblastic | 15 (41) | 27 (39) | 42 (40) |
| Immunoblastic | 1 (3) | 4 (6) | 5 (5) |
| T-cell—rich B-cell lymphoma | 1 (3) | 4 (6) | 5 (5) |
| Anaplastic large cell lymphoma | 2 (5) | 1 (1) | 3 (3) |
| Not otherwise specified | 2 (5) | 5 (7) | 7 (7) |
| Aggressive B-cell lymphoma, not otherwise specified | 1 (3) | 1 (1) | 2 (2) |
| Aggressive B-cell lymphoma, subtyping not possible | 3 (8) | 3 (4) | 6 (6) |
| T-cell lymphoma | 6 (16) | 8 (12) | 14 (13) |
| Anaplastic large cell lymphoma | 3 (8) | 7 (10) | 10 (9) |
| Peripheral T-cell lymphoma | 3 (8) | 1 (1) | 4 (4) |
Histologic subtype of NHL as reported by reference pathology. For DL1, n = 39; for DL2, n = 71 (total, n = 110). For 2 patients further histologic subtyping was not available (aggressive lymphoma, not otherwise specified, n = 2). In 2 additional patients reference pathology review was not possible.
Patient characteristics
Characteristic . | DL1 . | DL2 . | Total . |
|---|---|---|---|
| Sex, male/female | 31/8 | 39/32 | 70/40 |
| Age, y (median; range) | 45 (24;60) | 43 (18;60) | 44 (18;60) |
| LDH level above UNL, no. (%) | 39 (100) | 71 (100) | 110 (100) |
| LDH level above 2 times UNL, no. (%) | 9 (23) | 18 (25) | 27 (25) |
| Stage III/IV, no. (%) | 23 (59) | 44 (62) | 67 (61) |
| Performance status (ECOG) greater than 1, no. (%) | 10 (26) | 21 (30) | 31 (28) |
| Age-adjusted IPI, no. (%) | |||
| 1 | 13 (33) | 21 (30) | 34 (31) |
| 2 | 19 (49) | 35 (49) | 54 (49) |
| 3 | 7 (18) | 15 (21) | 22 (20) |
| B symptoms, no. (%) | 27 (69) | 44 (62) | 71 (65) |
| Extranodal disease*, no. (%) | 28 (72) | 49 (69) | 77 (70) |
| Extranodal disease at more than one site*, no. (%) | 7 (18)† | 29 (41) | 36 (33) |
| Bulky disease (> 7.5 cm), no. (%) | 25 (66)† | 45 (63) | 70 (64) |
Characteristic . | DL1 . | DL2 . | Total . |
|---|---|---|---|
| Sex, male/female | 31/8 | 39/32 | 70/40 |
| Age, y (median; range) | 45 (24;60) | 43 (18;60) | 44 (18;60) |
| LDH level above UNL, no. (%) | 39 (100) | 71 (100) | 110 (100) |
| LDH level above 2 times UNL, no. (%) | 9 (23) | 18 (25) | 27 (25) |
| Stage III/IV, no. (%) | 23 (59) | 44 (62) | 67 (61) |
| Performance status (ECOG) greater than 1, no. (%) | 10 (26) | 21 (30) | 31 (28) |
| Age-adjusted IPI, no. (%) | |||
| 1 | 13 (33) | 21 (30) | 34 (31) |
| 2 | 19 (49) | 35 (49) | 54 (49) |
| 3 | 7 (18) | 15 (21) | 22 (20) |
| B symptoms, no. (%) | 27 (69) | 44 (62) | 71 (65) |
| Extranodal disease*, no. (%) | 28 (72) | 49 (69) | 77 (70) |
| Extranodal disease at more than one site*, no. (%) | 7 (18)† | 29 (41) | 36 (33) |
| Bulky disease (> 7.5 cm), no. (%) | 25 (66)† | 45 (63) | 70 (64) |
Characteristics of all patients included in analysis. For DL1, n = 39; for DL2, n = 71 (total, n = 110). Significant differences between dose levels were seen in sex (P = .010) and extranodal disease at more than one site (P = .018).
Bone marrow involvement is defined as extranodal disease.
One missing value.
Treatment
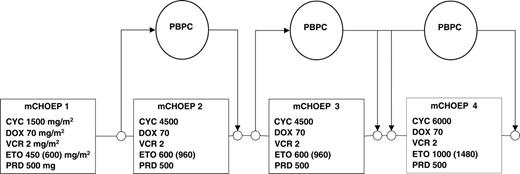
Patients with high tumor load or poor performance status (or both) were to receive a single dose of 2 mg vincristine and 100 mg/d prednisone for 7 days. The MegaCHOEP protocol (Figure 1) consisted of 4 courses of cyclophosphamide (C), doxorubicin (H), vincristine (O), etoposide (E), and prednisolone (P). With course 1, C was given at a dose of 750 mg/m2 on days 1 and 2, H at 35 mg/m2 on days 1 and 2, O at 2 mg on day 1, E at 150 mg/m2 in DL1 and 200 mg/m2 in DL2 on days 1 through 3, and P at 100 mg on days 1 through 5. With courses 2 and 3 the doses of C and E were increased: C 2250 mg/m,2 days 1 and 2, E 100 mg/m2 twice daily on days 1 through 3. With course 4, C was further escalated to 3000 mg/m2 on days 1 and 2, E (125 mg/m2) was given twice daily on days 1 through 4. At DL2 the dose of etoposide was further increased; during courses 2 and 3 E was given at 160 mg/m2 twice daily (days 1-3), and in course 4, E was set at 185 mg/m2 twice daily (days 1-4). To allow for the shortest possible time between chemotherapy courses, time intervals between courses were not fixed. The next course of chemotherapy was to begin as soon as recovery of hematopoiesis was accomplished and nonhematologic toxicities of the preceding therapy had resolved. A platelet count higher than 80 × 109/L (80/nL) was defined as trigger value for hematopoietic recovery.
SCT and supportive therapy
Filgrastim at a dose of 480 μg was started on day 4 after cycle 1 of chemotherapy until 2 × 106 CD34+ progenitor cells/kg body weight (BW) were harvested for transplantation after course 2. Using the same mobilization regimen, peripheral blood progenitor cells were harvested again after the second course of chemotherapy and split into 2 collection products each of which needed to contain 2 × 106 CD34+ cells/kg BW. These harvests were reinfused into the patient after courses 3 and 4 of the protocol. If the yield of the second stem cell collection was not sufficient for transplantation after courses 3 and 4 (< 4 × 106 CD34+ cells/kg BW), a third collection after course 3 was mandatory. Infectious prophylaxis against Pneumocystis carinii with co-trimoxazole was mandatory after courses 2, 3, and 4 of MegaCHOEP. In patients with positive herpes simplex serology, prophylaxis with acyclovir was recommended. Antifungal and antibacterial prophylaxis was administered according to local protocols of the participating centers.
Statistics
The primary end point of this study was feasibility of MegaCHOEP defined as the proportion of patients receiving therapy according to protocol. Safety was evaluated using the Bearman toxicity scale9 ; infectious complications were graded according to World Health Organization (WHO) criteria. To describe the recovery we used box plots with the upper and lower limits describing the 25th and 75th percentiles, respectively. Efficacy was measured by calculating the complete remission (CR) rate, freedom from treatment failure (FFTF), and overall survival (OS). Criteria of remission were used as published.10 Remission status was evaluated by clinical examination and repetition of all diagnostic measures at least 3 months after the last course of therapy. Remission with any type of residual mass stable at least 2 months after final restaging was defined as complete remission, unconfirmed (CRu). Death due to any cause or progression of disease or relapse was defined as treatment failure. FFTF and OS were measured from start of therapy to the respective event. FFTF and OS were estimated according to the method of Kaplan and Meier. The estimators at 2 or 5 years are given with the 95% confidence limits (CLs). Differences in patient characteristics and nonhematologic toxicities between DLs were tested for significance by the χ2 test and, if required, by the Fisher exact test. Differences in the hematologic recovery for leukocytes, in the numbers of CD34+ cells collected or infused between DL11 and DL2, were tested with the Mann-Whitney U test. Univariate analysis for the factors age, sex, extranodal disease, bulky disease, performance status, B symptoms, stage, age-adjusted IPI, and DL for FFTF was done by the log-rank test. Multivariate analysis was done using the Cox regression model. All tests for significance were at the 5% significance level and were not adjusted for multiple comparisons.
MegaCHOP treatment protocol. Cumulative doses per course were given. Dose of etoposide at DL2 is set in brackets; all other drugs were given at identical doses at DL1 and DL2. PBPC indicates peripheral blood progenitor cell; CYC, cyclophosphamide; DOX, doxorubicin; VCR, vincristine; ETO, etoposide; PRD, prednisone.
MegaCHOP treatment protocol. Cumulative doses per course were given. Dose of etoposide at DL2 is set in brackets; all other drugs were given at identical doses at DL1 and DL2. PBPC indicates peripheral blood progenitor cell; CYC, cyclophosphamide; DOX, doxorubicin; VCR, vincristine; ETO, etoposide; PRD, prednisone.
The protocol specified the decision to terminate DL1 for futility or for opening DL2 according to a group sequential Fleming design. This was calculated for 40 patients with inspections after every 10 evaluable patients (α= 0.052, power = 0.91, p0 = 0.08, p1 = 0.25). The criterion for opening DL2 was met after 30 evaluable patients; because further patients were still under treatment at this time 39 patients finally were treated at DL1. For obtaining sufficiently precise estimators it was considered necessary to include at least 60 patients on DL2.
Results
A total of 110 patients were treated at DL1 (39 patients) or DL2 (71 patients). The major characteristics of the patients are shown in Table 2. No significant differences between patients treated at either DL were noted except for a strong male preponderance in the cohort treated at DL1 and a higher proportion of patients with more than one extranodal manifestation of lymphoma at DL2. Ninety patients (81.8%) completed all therapy as per protocol. Twenty patients (18.2%) stopped treatment after course 1 (8 patients), course 2 (7 patients), or course 3 (5 patients) due to toxicity (15 patients), progression of disease (1 patient), protocol violation (2 patients), or insufficient mobilization of stem cells (1 patient); 1 patient terminated therapy after accidental fracture of the humerus (Table 3).
Feasibility
. | Last course of therapy completed, DL1/DL2 . | . | . | . | . | |||
|---|---|---|---|---|---|---|---|---|
| Event . | mCHOEP 1 . | mCHOEP 2 . | mCHOEP 3 . | mCHOEP 4 . | Total, DL1/DL2 . | |||
| Regular therapy | — | — | — | 33/57 | 33/57 | |||
| Progress | 0/0 | 0/1 | 0/0 | 0/0 | 0/1 | |||
| Toxicity | 2/5 | 3/2 | 0/3 | 0/0 | 5/10 | |||
| Protocol violation | 0/0 | 1/0 | 0/1 | 0/0 | 1/1 | |||
| Mobilization failure | 0/0 | 0/0 | 0/1 | 0/0 | 0/1 | |||
| Other | 0/1 | 0/0 | 0/0 | 0/0 | 0/1 | |||
| Total | 2/6 | 4/3 | 0/5 | 33/57 | 39/71 | |||
. | Last course of therapy completed, DL1/DL2 . | . | . | . | . | |||
|---|---|---|---|---|---|---|---|---|
| Event . | mCHOEP 1 . | mCHOEP 2 . | mCHOEP 3 . | mCHOEP 4 . | Total, DL1/DL2 . | |||
| Regular therapy | — | — | — | 33/57 | 33/57 | |||
| Progress | 0/0 | 0/1 | 0/0 | 0/0 | 0/1 | |||
| Toxicity | 2/5 | 3/2 | 0/3 | 0/0 | 5/10 | |||
| Protocol violation | 0/0 | 1/0 | 0/1 | 0/0 | 1/1 | |||
| Mobilization failure | 0/0 | 0/0 | 0/1 | 0/0 | 0/1 | |||
| Other | 0/1 | 0/0 | 0/0 | 0/0 | 0/1 | |||
| Total | 2/6 | 4/3 | 0/5 | 33/57 | 39/71 | |||
Reasons for early termination of study treatment shown as number of patients terminating therapy after courses 1 to 3 (DL1/DL2). Feasibility of SCT: 102 of 110 (93%) patients received at least 1 stem cell transplant, 90 of 110 completed all therapy and received 3 stem cell transplants.
— indicates not applicable.
The median dose of cyclophosphamide actually administered was 98% of the planned dose; 96% of the planned dose of etoposide was actually given without significant differences between DLs. The median duration of therapy from start of first course to recovery of platelets after course 4 was 81 days at DL1 and 86 days at DL2. Compared to 6 cycles of standard CHOEP given at 21-day intervals, the dose intensity of cyclophosphamide was 5.5-fold higher at DL1 and 5.3-fold higher at DL2. The dose intensity of etoposide was increased 2.2-fold (DL1) or 3.1-fold (DL2), respectively.
Hematologic toxicity and time between treatment courses
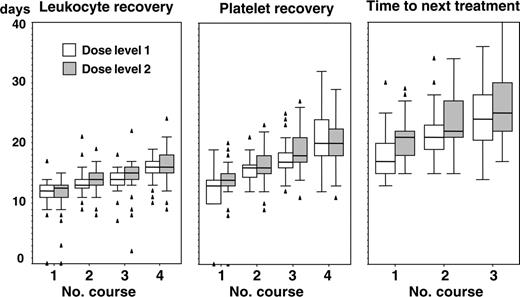
Hematologic recovery after course 1 (white blood cell [WBC] count > 1 × 109/L [1/nL], platelet count > 80 × 109/L [80/nL]) occurred at a median of 12 days (range, 0-17 days) and 14 days (range, 0-20 days), respectively. The median time interval to the next course of therapy was 19 days (range, 13-30 days). The difference between time to hematologic recovery and time to next treatment was even more pronounced after courses 2 and 3 when transplantation of hematopoietic stem cells accelerated recovery. At DL1 recovery of WBCs and platelets needed a median of 13 days (DL2, 14 days) and 16 days (DL2, 16 days) after course 2, and 14 days (DL2, 15 days) and 17 days (DL2, 18 days) after course 3, respectively. Time to next treatment was 21 days (DL2, 22 days) after course 2 and 24 days (DL2, 25 days) after course 3. Hematologic recovery after course 4 occurred at day 16 for leukocytes and day 20 for platelets with no significant differences between dose levels (Figure 2). All patients receiving autologous stem cells ultimately recovered their peripheral blood counts.
Recovery of white blood cells and platelets and time intervals between courses.
Recovery of white blood cells and platelets and time intervals between courses.
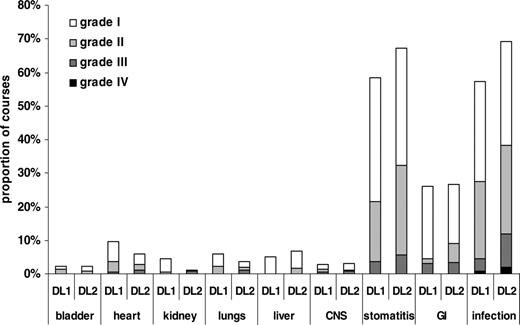
Nonhematologic toxicity. Toxicity graded according to Bearman criteria with exception of infections, which were graded according to WHO. The total number of courses evaluable was 397, with 142 courses at DL1 and 255 at DL2. GI indicates gastrointestinal; CNS, central nervous system.
Nonhematologic toxicity. Toxicity graded according to Bearman criteria with exception of infections, which were graded according to WHO. The total number of courses evaluable was 397, with 142 courses at DL1 and 255 at DL2. GI indicates gastrointestinal; CNS, central nervous system.
Nonhematologic toxicity
Mucositis and infection were the most important nonhematologic toxicities encountered by patients treated with MegaCHOEP. In 26% of all cycles gastrointestinal toxicity (diarrhea or abdominal pain) was reported. All other toxicities were infrequent and usually mild (Figure 3). Life-threatening toxicity was rare and mostly caused by infection. Five of 110 patients (4.5%) died due to therapy-related toxicity. One patient at DL1 experienced cardiac arrest, and 4 patients at DL2 died as a consequence of infectious complications. There was a small but not significant increase in the number of treatment courses with stomatitis and infection when the dose of etoposide was escalated from 2650 mg/m2 (DL1) to 4000 mg/m2 (DL2).
Mobilization of hematopoietic stem cells
Mobilization failure after the third course of MegaCHOEP occurred in a single patient. In all other patients, sufficient numbers of CD34+ cells for all planned transplantation procedures were collected. After the first and second mobilization more than 2 × 106 CD34+ cells/kg BW were harvested from 102 of 102 (100%) and 89 of 91 (97.8%) evaluable patients.
The median yield of CD34+ progenitor cells was 15.1 and 9.4 × 106/kg BW after courses 1 and 2, respectively. A third harvest was performed in 24 patients (25.3%). In patients who needed a third harvest 4.3 × 106 CD34+ cells/kg could be collected. At DL1 the difference of stem cell yield from mobilization 2 to mobilization 1 was –1.4 × 106 CD34+ cells/kg BW (P = .833), whereas it was significantly different at DL2 with a difference of –8.6 × 106 CD34+ cells/kg BW (P = .008). Less than 6% of patients had more than 2 leukaphereses per mobilization. After course 2, the median number of progenitor cells harvested was lower at DL2 (8.4 × 106 CD34+ cells/kg BW) than at DL1 (12 × 106 CD34+ cells/kg BW). However, the number of progenitor cells actually administered after courses 2, 3, and 4 were 7.3, 4.7, and 4.4 × 106 CD34+ cells/kg BW without significant differences between DL1 and DL2.
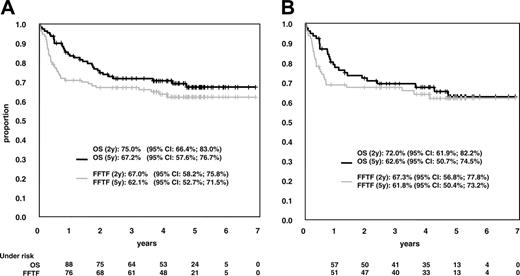
OS and FFTF. (A) OS and FFTF in 110 patients receiving MegaCHOEP at DLs 1 and 2. (B) Results in 77 patients who had a diagnosis of diffuse large-cell lymphoma according to WHO classification.
OS and FFTF. (A) OS and FFTF in 110 patients receiving MegaCHOEP at DLs 1 and 2. (B) Results in 77 patients who had a diagnosis of diffuse large-cell lymphoma according to WHO classification.
Remission rate, time to treatment failure, and survival
Six patients could not be evaluated for response to MegaCHOEP because they terminated therapy early (2 patients after cycle 1, 3 patients after cycle 2, and 1 patient after cycle 3) without a report on the disease status at that time. All of them achieved CR/CRu after additional treatment, 4 patients are in continuous CR. Six additional patients died of disease early prior to the first reevaluation of disease. In 5 of these cases, death was assumed to be related to treatment; in one case death was due to progressive lymphoma. Seventy-seven of 110 patients (70.0%; 95% CI, 61.4%-78.6%) achieved a CR or a CRu. Twenty-one patients had refractory disease at the end of therapy, 18 patients after completion of all 4 courses of MegaCHOEP therapy. There were 3 cases of progressive disease under therapy; all other patients progressed within 2 months after the final restaging of MegaCHOEP. Of the 77 patients achieving CR/CRu, 11 patients had a relapse between 2 and 46 months after the end of therapy. Nine of these 11 patients had completed all therapy. Only 4 relapses occurred later than 2 years from start of treatment and none of the patients had a relapse after the median observation time of 55 months. FFTF was 67.0% at 2 years and 62.1% at 5 years. Treatment of relapse or progressive disease on MegaCHOEP induced a second remission in a small proportion of patients. Salvage therapy was not defined by protocol and patients received various therapy regimens. Four of 14 patients with progressive disease achieved CR/CRu. Four of 9 patients who had a relapse after completed MegaCHOEP achieved CR/CRu after salvage therapy. Overall, survival function followed FFTF function with a small time lag. OS was 75.0% and 67.2% after 2 and 5 years, respectively (Figure 4A). The subgroup of 77 patients with the diagnosis of diffuse large B-cell lymphoma according to the WHO definition had results similar to the whole group (Figure 4B) The rate of CR did not differ between both DLs with 69.2% (95% CL, 52.4%, 83.0%) at DL1 and 70.4% (95% CL, 58.4%, 80.7%) at DL2. There is a trend to better long-term outcome for patients receiving MegaCHOEP at DL2 compared to DL1 with FFTF at 5 years 66.9% versus 54.2% and OS 70.2% versus 61.3%. Patients receiving treatment at DL2 of MegaCHOEP had a 0.63 relative risk of treatment failure compared to patients receiving DL1 MegaCHOEP in a Cox regression model adjusting for the factors of the age-adjusted IPI and those being imbalanced between DLs (Table 4). This, however, was not statistically significant.
Multivariate analysis of risk factors
Risk factor . | Relative risk (95% CL) . | P . |
|---|---|---|
| DL2 | 0.63 (0.31,1.25) | .184 |
| LDH greater than 2 times UNL | 2.04 (1.01,4.08) | .045 |
| Stage III and IV | 1.76 (0.81,3.82) | .154 |
| Performance status (ECOG) greater than 1 | 1.52 (0.77,3.03) | .230 |
| Female sex | 1.20 (0.56,2.57) | .631 |
| Extranodal disease at more than 1 site | 1.11 (0.53,2.33) | .774 |
Risk factor . | Relative risk (95% CL) . | P . |
|---|---|---|
| DL2 | 0.63 (0.31,1.25) | .184 |
| LDH greater than 2 times UNL | 2.04 (1.01,4.08) | .045 |
| Stage III and IV | 1.76 (0.81,3.82) | .154 |
| Performance status (ECOG) greater than 1 | 1.52 (0.77,3.03) | .230 |
| Female sex | 1.20 (0.56,2.57) | .631 |
| Extranodal disease at more than 1 site | 1.11 (0.53,2.33) | .774 |
Multivariate analysis of risk factors with respect to FFTF (number of events, 40). DL, factors of age-adjusted IPI (performance status, stage, LDH level at diagnosis) and those being imbalanced between DLs (extranodal disease, sex) were taken into account for Cox regression analysis.
Prognostic factors
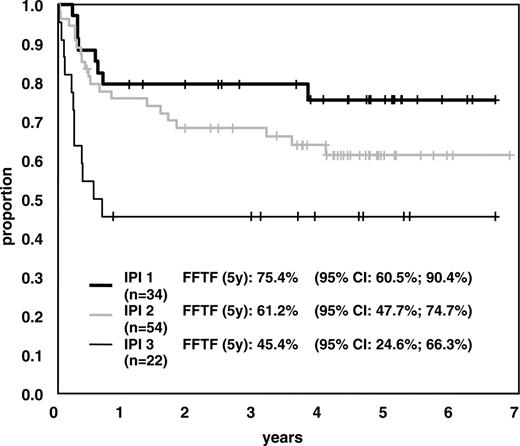
Aside from IPI (Figure 5), 3 single factors showed prognostic significance for long-term results (FFTF) in univariate analysis: LDH level higher than twice the UNL, Ann Arbor stage III/IV, and Eastern Cooperative Oncology Group (ECOG) performance status greater than 1.
It was interesting to note that patients with an LDH level higher than twice the UNL have an increased risk of failure compared to patients with LDH level more than 1 to less than 2 UNL. This finding was confirmed in a multivariate Cox model for FFTF (Table 4).
Late effects: myelodysplasia and secondary neoplasia
One patient presented with a myelodysplastic syndrome (MDS) classified as MDS-refractory anemia with excess of blasts in transformation (RAEBt) 36 months after treatment. Hematopoietic function was still sufficient at that time and no specific treatment was necessary. The patient had a relapse with lymphoma 42 months after start of treatment and died due to progressive disease. No other cases of MDS or secondary neoplasia were reported so far.
Discussion
Here we report the results of a phase 1/2 study in younger patients with primary diagnosis of aggressive lymphoma and high-risk features. We chose LDH level to define high risk because it is the strongest risk factor for treatment failure8 and all studies of the DSHNHL used age and LDH level as discriminating factors at the time our study began.
MegaCHOEP is a complex treatment protocol that requires the availability of adequate resources and prolonged periods of hospital stay. We feel this is absolutely justified given the otherwise poor prognosis of young high-risk patients with aggressive lymphoma. The protocol is characterized by early and repeated administration of high to myeloablative doses of cyclophosphamide, doxorubicin, and etoposide—drugs that all have proven efficacy in aggressive lymphoma. In an attempt to escalate CHOP without stem cell support, Shipp et al11 found 4 g/m2 cyclophosphamide and 70 mg/m2 doxorubicin to be the maximum tolerated dose with thrombocytopenia being dose limiting. With additional administration of high doses of etoposide, transplantation of hematopoietic stem cells is necessary to avoid prolonged cytopenias. Furthermore, high-dose intensity, which is critical in fast-growing NHL, could only be maintained with the help of stem cell support. Finally, a dose intensity that is 3-fold (etoposide) to 5-fold (cyclophosphamide) higher than that of conventional CHOEP-21 could be achieved. We wanted to demonstrate that such an approach is feasible, safe, and suitable to overcome the resistance of tumor cells to more conventional chemotherapy.
Etoposide was given at escalating doses (DL1, 2650 mg/m2; DL2, 4000 mg/m2), whereas all other drugs were given in identical doses. We did not observe clinically relevant differences concerning hematopoietic recovery or nonhematologic toxicity between DLs. However, there is a remarkable trend to better results in terms of FFTF and survival with DL2 compared to DL1 (relative risk FFTF, 0.63) although with the small number of patients treated a statistical difference could not be proven. We chose to use DL2 of MegaCHOEP for further comparative studies.
With regard to the safety of this procedure, treatment-related mortality (4.5%) did not really differ from that reported for a comparable population undergoing conventional chemotherapy. Life-threatening complications were rare and mostly due to mucositis and infections. Treatment had to be stopped due to toxicity in 15 patients (14%), frequently after the first or second course of therapy when poor performance status, high tumor load, and side effects of chemotherapy caused morbidity and sometimes mortality. Hematologic toxicity was not really a problem. For 109 of 110 patients sufficient numbers of stem cells could be obtained to perform all 3 planned transplantations. We noticed mild cumulative hematotoxicity because hematologic recovery tended to occur more slowly and stem cell yields to become lower as patients passed through repeated courses of stem cell collection, HDT, and transplantation. Planned courses of chemotherapy could be given to the vast majority of patients at full dose with approximately 3-week time intervals. If only hematologic recovery was considered, it should have been possible to start the next treatment course already after 2 weeks; this, however, was impossible because of persisting nonhematologic toxicity and because many treating physicians felt that patients were not fit enough to receive further aggressive therapy.
It is noteworthy that despite the high doses of cyclophosphamide and etoposide given with MegaCHOEP the risk of MDS or secondary acute leukemia was low with only one patient having secondary acute myelogenous leukemia (sAML) after a median observation time of 55 months and a maximum of 7 years of follow-up. This is in contrast to reports of significantly higher rates of MDS/sAML observed after dose-escalated CHOP without11 and HDT with autologous transplantation.12-14 We can only note that stress exerted on hematopoietic stem and progenitor cells by MegaCHOEP differs from that of other forms of therapy because stem cell support starts with the second cycle of therapy. However, there is no proof that this design is responsible for the low incidence of sAML seen so far.
FFTF according to age-adjusted IPI. FFTF in patients with age-adjusted IPI 1 (intermediate-low risk), IPI 2 (intermediate-high risk), or IPI 3 (high risk).
FFTF according to age-adjusted IPI. FFTF in patients with age-adjusted IPI 1 (intermediate-low risk), IPI 2 (intermediate-high risk), or IPI 3 (high risk).
Although comparison of the results of MegaCHOEP with conventional chemotherapy is difficult it should be mentioned that the International Non-Hodgkin Lymphoma Prognostic Factors Project reported CR rates of 78%, 57%, and 46% and OS rates of 69%, 46%, and 32% at 5 years for younger patients with age-adjusted IPI 1, 2, or 3. Given the distribution of patients with IPI 1 to 3 in the study (30.9%, 49.1%, and 20.0%) our results compare favorably with these data.8 The National High Priority Lymphoma study reported OS of 50% to 54% for patients treated with CHOP or one of the third-generation protocols used (methotrexate, bleomycin, cyclophosphamide, vincristine, and dexamethasone [m-BACOD]; cyclophosphamide, doxorubicin, etoposide, prednisone, cytarabine, bleomycin, vincristine, methotrexate, and leucovorin [ProMACE-CytaBOM]; or methotrexate with leucovorin, doxorubicin, cyclophosphamide, vincristine, prednisone, and bleomycin [MACOP-B]).15 The conventional arm of the German NHL-A study treated a cohort of patients comparable to our patients with 5 courses of CHOEP-21. The 3-year survival rate was 63%; the event-free survival rate was only 49%, however.6 Thus, results obtained with MegaCHOEP seem to be superior to standard CHOP or CHOP-like regimens.
Another issue is whether moderate dose escalation of active drugs may suffice to improve treatment results, thus helping to avoid the necessity to collect and transplant stem cells. A number of studies have addressed this question by escalating the dose of one or more of the drugs used in the CHOP regimen.11,16-19 In general, this strategy has been met with limited success because early toxicity was substantial and time intervals between therapies had to be extended, a high incidence of early secondary MDS/AML was reported, and superior outcome could not be demonstrated.20 This latter finding is also supported by the results of a recent study of the DSHNHL that compared 6 courses of standard CHOEP-21 with a dose-escalated version of this regimen (cumulative doses of cyclophosphamide and etoposide were 750 versus 1600 and 300 versus 600 mg/m2 per cycle, respectively). This trial had to be stopped after the first interim analysis because toxicity in the treatment arm using higher doses of cyclophosphamide and etoposide was significantly increased; survival curves, however, were superimposable (data on file). Overall, moderate dose escalation of cytotoxic agents without SCT did not result in convincing improvements of efficacy. The results of HDT as part of first-line treatment in young high-risk patients with aggressive lymphoma are heterogenous and not easy to interpret. The randomized studies showing results in favor of HDT restricted this therapy to patients who had already achieved a CR with conventional chemotherapy21 or excluded patients with high-risk disease according to IPI1 ;inthe latter study, HDT was compared to 8 courses of CHOP administered every 3 weeks, a treatment we would no longer consider optimal for this age group.22 The third study that reported positive results for HDT used an approach consisting of early administration of high doses of single-agent etoposide, methotrexate, and melphalan with or without total body irradiation.3 This was a single-center study that has never been updated; results of multicenter trials comparing this treatment protocol to conventional therapy have not yet been published. All other studies either failed to demonstrate a significant advantage of HDT4-6,23 or showed inferior event-free and overall survival after HDT and transplantation.7 Although other interpretations are possible we believe that the major reason for the negative results with HDT was that a high proportion of patients scheduled to receive HDT actually never received it. In the German trial6 one third of patients randomized to HDT did not receive it; similar observations were reported from the Italian and the Dutch trials4,5 where 29% and 39% of patients scheduled for HDT never made it to transplantation. Disease progression was the leading cause of failure in all these trials.
The French trial published recently also highlighted this problem and drew attention to the fact that it is absolutely mandatory to use high doses of active drugs from the very beginning of treatment. The authors concluded that inadequate dose intensity in the experimental arm (HDT) during the first 8 weeks may have caused lack of improvement.7 We agree with this interpretation. Therefore, we wanted to intensify cytotoxic therapy as soon as possible after diagnosis, and keep doses high and time intervals short throughout treatment to avoid early failures and drop outs. We largely succeeded in achieving these goals because progression under treatment was very rare (2 of 110 patients) and more than 80% of all patients were able to complete all planned therapy. Unfortunately, among patients with IPI high-risk features 40% could not receive the total treatment program and we speculate that this was an important cause of limited success of MegaCHOEP in these patients. Special efforts will be necessary to increase the percentage of patients completing all therapy also in this risk group to further improve results. Because mucosal damage and infection are major toxicities of MegaCHOEP, protection of mucosa by keratinocyte growth factor (palifermin)24 and intensified antibiotic prophylaxis may be valuable approaches.
In prior studies, the prognostic impact of the IPI in a primary transplantation setting could not be verified.25 This seems to be in contrast to our findings. However, one should keep in mind that in some of the transplantation studies, HDT was used for consolidation in patients who had achieved CR/PR to conventional therapy, whereas our study included not only chemosensitive but all patients starting treatment.
The “purging” effect of repeated courses of HDT prior to the last stem cell harvest and the use of the “gold standard” combination chemotherapy CHOP supplemented with etoposide also may have contributed to the success of this treatment program. The results of a small pilot study using 3 courses of dose-escalated CHOP followed by 3 courses of CHOP plus etoposide and cisplatin and SCT may support this notion; however, only 13 patients with diffuse large-cell lymphomas were included.26
In conclusion, we were able to demonstrate the feasibility and safety of MegaCHOEP, which represents a new treatment program for high-risk patients with aggressive lymphoma characterized by early and repeated administration of a combination of drugs with known activity in this disease.
FFTF and OS are encouraging. The DSHNHL is currently running a prospective, randomized trial comparing MegaCHOEP at DL2 with conventional chemotherapy (8 courses of CHOEP given every 2 weeks) supplemented with rituximab in both treatment arms. This study will elucidate if high-dose chemotherapy followed by autologous SCT is necessary in the era of rituximab.
Appendix
The membership of the DSHNHL is composed of all of the individuals who participated in the study. The following is a list of study participants. Pathologic review committee: A. C. Feller, M. L. Hansmann, H.-K. Müller-Hermelink, P. Moeller, R. Parwaresch, H. Stein. Coordinating physicians: B. Glass, N. Schmitz. Reference radiotherapists: K. Schnabel, C. Rübe. Biometry: M. Loeffler, D. Hasenclever, M. Kloess. Data management team: B. Mann, U. Schönwiese, A. Schöler, L. Martin Montanez, M. Roskothen, C. Keil. Database: M. Kunert, B. Wicklein. Institutions recruiting patients: Heidelberg: Ruprecht-Karls-Universität Heidelberg, Med. Universitätsklinik und Poliklinik, A. Ho; Homburg: Universitätsklinikum des Saarlandes, Innere Medizin I, R. Schmits, F. Hartmann; Münster: Universitätsklinikum Münster, Innere Medizin A, W. E. Berdel, R. Mesters, P. Koch; Augsburg: Zentralklinikum Augsburg, II. Medizinische Klinik, G. Schlimok; Magdeburg: Medizinische Akademie Magdeburg, Klinik für Innere Medizin/Hämatologie, A. Franke; Cologne: Universitätsklinik Köln, Klinik I für Innere Medizin, A. Engert, M. Reiser; Rostock: Universität Rostock, Innere Medizin/Hämatologie, M. Freund; Kiel: Städtisches Krankenhaus Kiel, II. Medizinischeund Poliklinik, M. Kneba; Essen: Universitätsklinikum Essen, Hämatologische Abteilung, U. Dürsen; Göttingen: Georg-August-Universität Göttingen, Abteilung Hämatologie, G. Brittinger; Lübeck: Universitätsklinikum Schleswig-Holstein, Med. Klinik I-Hämatologie/Onkologie, T. Wagner; Nuremberg: Klinikum Nürnberg, Medizinische Klinik 5, J. Birkmann; Stuttgart: Diakonissenkrankenhaus Stuttgart, Medizinische Klinik II, G. Heidemann; Würzburg: Universität Würzburg, Medizinische Poliklinik, T. Wässa, K. Wilms; Heidelberg: Thoraxklinik Heidelberg, H. Bischoff, P. Drings; Wiesbaden: Dr-Horst-Schmidt-Kliniken, Wiesbaden, N. Frickhofen; Mönchengladbach: Krankenhaus Maria Hilf, Medizinische Klinik, D. Kohl, G. Trenn; Cottbus: Carl-Thiem-Klinikum Cottbus, Ch. Rudolph, H. Steinhauer; Oldenburg: Städtische Kliniken, Hämatologie und Onkologie, B. Metzner; Bremen: Evangelisches Diakonie-Krankenhaus, Medizinische Klinik/Hämatologie und Onkologie, K. H. Pflüger; Hamburg: Universitätskrankenhaus Eppendorf, K. Hossfeld; Kaiserslautern: Westpfalz-Klinikum, H. Link; Bonn: Medizinische Klinik und Poliklinik, A. Glasmacher; Stuttgart: Bürgerhospital Stuttgart, Medizinische Klinik I, H.-G. Mergenthaler; Berlin: Robert-Rössle-Klinikum, B. Dörken; Hamburg: AK Altona, D. Braumann; Potsdam: Klinikum Ernst von Bergmann, R. Pasold; Marburg: Universitätsklinikum Marburg, U. Kaiser; Regensburg: Klinikum der Universität Regensburg, A. Holler; Halle: Klinikum der Universität, H. J. Schmoll.
Prepublished online as Blood First Edition Paper, December 29, 2005; DOI 10.1182/blood-2005-04-1570.
A complete list of the members of the German High Grade Non-Hodgkin Lymphoma Study Group (DSHNHL) appears in the “Appendix.”
Supported by an unrestricted grant from Amgen, Munich, Germany.
B.G., M.B., W.E.B., L.T., M.L., M.P., and N.S. have declared a financial interest in Amgen, whose product (Neupogen) was studied in the present work.
Presented in abstract form at the 46th Annual Meeting of the American Society of Hematology, San Diego, CA, December 5, 2004.27
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal