Abstract
B-cell posttransplantation lymphoproliferative disorder (B-PTLD) is a rare but severe complication of transplantation, with no consensus on best treatment practice. This prospective trial, the first to test a treatment for PTLD, was designed to evaluate the efficacy and safety of rituximab in patients with B-PTLD after solid organ transplantation (SOT). Fortysix patients were included and 43 patients were analyzed. Patients were eligible if they had untreated B-PTLD that was not responding to tapering of immunosuppression. Treatment consisted of 4 weekly injections of rituximab at 375 mg/m2. At day (d) 80, 37 (86%) patients were alive, and the response rate was 44.2%, including 12 complete response/unconfirmed complete response (CR/CRu). The only factor predictive of a response at d80 was a normal lactate dehydrogenase level (P = .007, odds ratio [OR] = 6.9). At d360, responses were maintained in 68% of patients, and 56% of patients were alive. The overall survival rate at 1 year was 67%. We conclude that rituximab is effective and safe in PTLD, with stable responses at 1 year. The response rate and overall survival might be improved by combining rituximab with other treatments.
Introduction
Patients who have received solid-organ transplants (SOTs) have a 20- to 120-fold higher incidence of non-Hodgkin lymphoma (NHL).1 These posttransplantation lymphoproliferative disorders (PTLDs) characteristically have rapid onset, aggressive behavior, and tropism for extranodal sites. They are mostly of B-cell origin and are often associated with active Epstein-Barr virus (EBV) infection. There is no consensus on the optimal treatment for PTLD, and no prospective trials of PTLD treatments have previously been published. A dose reduction or termination of immunosuppressive therapy is the first step in the management of PTLD, and can lead to partial or complete regression in some cases.2 Surgery, radiotherapy, and pharmacotherapy (with interferon-α or antiviral drugs) have also been used in small series.3,4 Chemotherapy is another alternative, but is associated with frequent morbidity and mortality (up to 50%).5 Infusion of expanded donor-derived EBV-specific cytotoxic T cells (CTLs) has shown some efficacy for preventing and treating PTLD in hematopoietic stem-cell transplantation (HSCT) patients, but this option is restricted to a few centers.6 For several years, monoclonal anti–B-cell antibodies have been used to treat PTLD. Antibodies directed against B-cell CD21 and CD24 surface antigens yielded complete remission in 60% of patients with B-cell PTLD (B-PTLD), with long-term survival rates of 35% in HSCT patients and 55% in SOT patients,7 but these antibodies are no longer produced. Recently, rituximab, a mouse/human chimeric anti-CD20 monoclonal antibody, introduced for the treatment of follicular lymphoma and diffuse large B-cell lymphomas, has been used in PTLD.8,9 The results of retrospective studies are encouraging but inconsistent, with response rates ranging from 20% to 100%, and the effect of anti-CD20 therapy on PTLD is difficult to assess because of the small number and heterogeneity of patients enrolled in published studies.10 To test the efficacy and safety of rituximab monotherapy in this setting, we conducted a prospective, multicenter, phase 2 trial in 46 patients with B-PTLD after SOT.
Patients, materials, and methods
Patients
This study was conducted at 15 French and 4 Belgian centers between May 2000 and December 2001. Patients were eligible for inclusion if they were 1 to 75 years of age, and had a performance status of 0 to 3 according to the criteria of the Eastern Cooperative Oncology Group (ECOG). Patients who had received SOT were included if they had untreated B-PTLD diagnosed according to the World Health Organization (WHO) classification,11 and if their tumor was stable at 1 month, or was stable at 2 weeks but with a clinical impact, or progressed within 1 month after reduction or discontinuation of immunosuppressive drug therapy (all immunosuppressive drugs had to be stopped if possible, or their dosage reduced by at least 50% and/or the number of drugs reduced to no more than 2); changes in immunosuppressive therapy were decided by the local transplantation teams; and at least 1 tumor of more than 2 cm and/or bone marrow involvement was mandatory. All patients in whom PTLD was diagnosed underwent a thorough work-up to detect lymphoproliferative sites by means of computed tomography (CT) of the chest and abdomen, and bone marrow biopsy. Patients were not eligible if they had central nervous system (CNS) involvement or any serious concomitant disease. The study complied with the Declaration of Helsinki and its current amendments, and was conducted in accordance with Good Clinical Practice guidelines.12 All patients or legal representatives gave their written informed consent. The protocol was approved by the local and national institutional review board of each participating center and country. The study was supervised by an independent data and safety monitoring committee.
Treatment
Rituximab treatment consisted of 4 intravenous (IV) infusions, each of 375 mg/m2, on days (d) 1, 8, 15, and 22. Treatment was stopped if the lymphoma progressed, if the patient declined to continue, or if the investigator considered it necessary to do so because of concomitant illness or adverse events. Premedication consisting of paracetamol and dexchlorpheniramine was administered before each rituximab infusion. Immunosuppression was stable throughout the study, except that it could be augmented in case of rejection.
Study endpoints
The primary endpoint was the overall response rate on d80. Tumor responses were assessed by means of computed tomography (and bone marrow biopsy in case of initial marrow involvement), 80, 180, and 360 days after treatment outset (d80, d180, and d360, respectively). Responses were classified as complete (CR), complete unconfirmed (CRu), partial (PR), stable disease (SD), or progressive disease (PD), based on the International Workshop criteria.13 In patients with bone marrow involvement, CR was defined as less than 30% of lymphocytes in marrow, with normal features and phenotype, and by a normal peripheral-blood smear. The overall response rate was defined as the sum of the rates of CR, CRu, and PR. Patients who progressed on treatment could be reevaluated earlier than d80 if necessary. All CT scans were reviewed by an independent expert panel (Alain Delmer, Department of Hematology, Robert Debré Hospital, Reims; Yves Menu, Department of Radiology, Beaujon Hospital, Clichy; and Antoine Scherrer, Department of Radiology, Foch Hospital, Suresnes, France) to confirm that the response criteria had been met. Clinical responses and overall survival were also assessed on d180 and d360. The effect of rituximab on graft rejection was also evaluated.
The safety and tolerability profile of rituximab was assessed by using the National Cancer Institute's Common Toxicity Criteria (CTC) grading system.14
Study assessments
Tissues. When possible, diagnostic tissue samples were collected and processed at a single institution (Nantes). All samples thus collected were reviewed by 4 pathologists (A.M.; Nicole Brousse, Department of Pathology, Necker Hospital, Paris; Françoise Berger, Department of Pathology, Lyon-Sud Hospital Pierre, Benite; and Martine Raphael, Department of Pathology, Bicetre Hospital, Le Kremlin, Bicetre, France) and were classified morphologically according to the WHO classification.11 CD20 and latent membrane protein 1 (LMP1) were detected by using the avidin-biotin-peroxidase complex method and monoclonal antibodies L26 and CS.1-4 (Dakopatts, Trappes, France). Epstein-Barr–encoded RNA (EBER) in situ hybridization was done on LMP1-negative samples (paraffin sections) with a Benchmark automated slide stainer (Ventana Medical Systems SA, Ilkirch, France) with fluorescein isothiocyanate (FITC)–labeled EBER 1 + 2–specific oligonucleotides (Ventana Medical Systems SA) following the manufacturer's instructions. Positive control sections were run with each sample.
EBV load measurement. EBV load was measured in all the patients before treatment. EBV load was measured in peripheral-blood mononuclear cells (PBMCs) and plasma samples collected on d0 and d80 by means of LightCycler (LC) real-time quantitative polymerase chain reaction (PCR) (Roche Molecular Biochemicals, Indianapolis, IN), as previously described.15 An equivalent of 0.5 μg PBMCs and 10 μL plasma were used for LC PCR. The primer pair for EBV DNA amplification was situated in the BXLF1 gene (encoding thymidine kinase) and generated a product of 169 bp. This method was able to detect 5 EBV genome copies in 1 μg total DNA and 50 copies in 1 mL plasma. EBV DNA was quantified by using a serial 10-fold dilution of DNA extracted from Namalwa cells containing 2 integrated copies of the EBV genome per cell. The results were expressed as the number of copies per microgram of DNA for PBMCs and as the number of copies per milliliter of plasma. High EBV DNA load was defined as 600 copies or more per microgram of DNA in PBMCs and 250 copies or more per milliliter of plasma. β-globin real-time PCR was performed on all EBV-DNA–negative samples in order to check for PCR inhibitors.
Statistical methods
All analyses were done on an intention-to-treat basis. The overall response rate (CR+CRu+PR) was calculated, together with the 95% exact binomial confidence interval (CI), overall survival (OS), and event-free survival (EFS) rates were calculated by using the nonparametric Kaplan-Meier method. OS was measured from the first dose of rituximab until death or last contact, and EFS was calculated from the first dose of rituximab to the date of relapse, disease progression, death, or last contact. Factors predictive of the response at d80 were analyzed by using logistic regression, and the Cox proportional hazards model was used to identify factors predictive of survival. Continuous parameters are reported as means ± standard deviation. All statistical tests had a significance cutoff of 5% (2-sided), and were run on SAS software (SAS Institute, Cary, NC).
Results
Patients
A total of 46 SOT recipients were enrolled. All received at least 1 dose of rituximab and were assessable for safety. Immunosuppressive drug therapy was reduced in all patients, in keeping with the inclusion criteria, but was never totally withdrawn. Efficacy was assessable in 43 patients (the per-protocol population); 3 patients were excluded because of major protocol violations (immunosuppressive therapy was not reduced before screening in 1 patient, 1 patient was in complete remission at the time of inclusion, and another patient had mantle-cell lymphoma, a type not described in the WHO classification of PTLD). SOT consisted of kidney grafting in 18 patients, heart grafting in 11 patients, liver grafting in 7 patients, lung grafting in 4 patients, and heart and lung grafting in 3 patients; 41 of these patients had a measurable lesion, and 2 had bone marrow infiltration. The time from transplantation to PTLD diagnosis was 1 year or less in 14 (35%) patients. Median age at enrollment was 50 years (range, 13-73 years), and 2 patients were younger than 18 years old. The patients' baseline characteristics are shown in Table 1.
Baseline characteristics: per-protocol population
Characteristics . | n = 43 . |
|---|---|
| Age, mean y ± SD (range) | 47.9 ± 14.8 (13-73) |
| Less than 18 y, no. patients | 2 |
| 18 y or older, no. patients | 41 |
| Sex, no. patients | |
| Male | 32 |
| Female | 11 |
| Performance status (ECOG), no. patients | |
| 0 | 9 |
| 1 | 20 |
| 2 | 10 |
| 3 | 4 |
| Median time from transplantation to PTLD diagnosis, m (range) | 51.8 (2.9-186) |
| 1 y or less, no. patients | 14 |
| More than 1 y, no. patients | 29 |
| Ann Arbor lymphoma staging, no. patients | |
| I-II | 11 |
| III-IV | 32 |
| No. involved sites, no. patients | |
| 1 | 14 |
| 2-3 | 12 |
| 4-5 | 12 |
| More than 5 | 5 |
| Lactate dehydrogenase levels, no. patients | |
| Normal | 15 |
| Above normal | 28 |
| Cell EBV viral load, no. patients | |
| Less than 600 copies/μg | 21 |
| 600 copies/μg or more | 18 |
| Missing | 4 |
| Histologic findings, no. patients | |
| Polymorphic | 4 |
| Monomorphic | 28 |
| Unclassified | 5 |
| Not reviewed | 6 |
Characteristics . | n = 43 . |
|---|---|
| Age, mean y ± SD (range) | 47.9 ± 14.8 (13-73) |
| Less than 18 y, no. patients | 2 |
| 18 y or older, no. patients | 41 |
| Sex, no. patients | |
| Male | 32 |
| Female | 11 |
| Performance status (ECOG), no. patients | |
| 0 | 9 |
| 1 | 20 |
| 2 | 10 |
| 3 | 4 |
| Median time from transplantation to PTLD diagnosis, m (range) | 51.8 (2.9-186) |
| 1 y or less, no. patients | 14 |
| More than 1 y, no. patients | 29 |
| Ann Arbor lymphoma staging, no. patients | |
| I-II | 11 |
| III-IV | 32 |
| No. involved sites, no. patients | |
| 1 | 14 |
| 2-3 | 12 |
| 4-5 | 12 |
| More than 5 | 5 |
| Lactate dehydrogenase levels, no. patients | |
| Normal | 15 |
| Above normal | 28 |
| Cell EBV viral load, no. patients | |
| Less than 600 copies/μg | 21 |
| 600 copies/μg or more | 18 |
| Missing | 4 |
| Histologic findings, no. patients | |
| Polymorphic | 4 |
| Monomorphic | 28 |
| Unclassified | 5 |
| Not reviewed | 6 |
EBV viral load
Cellular EBV load was determined in 39 patients, and was high (≥ 600 copies/μg DNA) in 18 (46%) patients (median, 4232 copies; range, 625-449 400 copies).
Histopathologic findings
Baseline tissue samples from 37 of 43 SOT patients underwent centralized histologic assessment. Of these, 37 were classified as typical PTLD (4 polymorphic PTLD; 28 monomorphic PTLD or diffuse large B-cell lymphoma; and 5 unclassifiable). All samples were CD20-positive. Baseline tissue samples from 32 PTLD patients were screened for EBV: 21 were positive (16 LMP1-positive, 5 only EBER-positive) and 11 were negative.
Efficacy
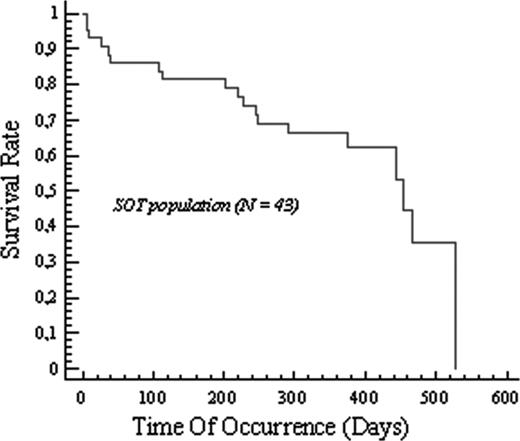
Thirty-two (74%) of the 43 patients completed their rituximab therapy (4 infusions). Among the 43 patients who were assessable on d80, 19 responded to the treatment, giving an overall response rate of 44.2% (95% CI: 29%-60%); there were 9 CRs, 3 CRu's, and 7 PRs. Two patients had stable disease on d80. On d360, the overall response rate was 34.1% (95% CI: 20%-51%), with 12 CRs, 1 CRu, and 1 PR. It is noteworthy that among the 19 responders at d80, the responses were maintained at d360 in 13 (68%) patients; the disease subsequently progressed in 3 patients who entered PR. Only 1 patient relapsed after entering CR, and 2 patients were not assessed. The only factor predictive of the response at d80 was a normal level of lactate dehydrogenase (LDH) (P = .007; odds ratio [OR] = 6.9) (Table 2). The overall survival rate at 1 year, estimated with the Kaplan-Meier method, was 67% (Figure 1), and the event-free survival rate at 1 year was 71.6%. With a median overall follow-up of 12 months, 24 (56%) of 43 patients were alive, giving a median survival time of 454 days. Thirteen of the 19 deaths were due to disease progression, and 1 was due to chemotherapy toxicity after PTLD relapse; 1 patient died of bronchospasm associated with pulmonary edema after the first infusion, but the death was not considered to be treatment-related (Table 3). The only factor predictive of survival was the number of sites (P = .0354; hazard ratio = 3.8, Cox proportional hazards model) (Table 2). Nine patients (1 liver, 1 lung, 4 heart, and 3 kidney recipients) developed graft rejection (acute or chronic). Except for 1 case that occurred on d27, these rejections occurred more than 5 months after the start of treatment. One rejection was fatal, 1 year after treatment completion. Twenty-three patients have been retreated for PTLD progression or relapse, 5 with rituximab alone (3 PDs, 1 SD, and 1 PR), and 18 with chemotherapy (mainly cyclophosphamide/doxorubicin/vincristine/prednisone [CHOP]; 5 CR,s 2 PRs, 3 SDs, and 6 PD). Three stem-cell autografts were done (2 CRs).
Baseline factors predictive of the response at d80 (logistic regression analysis) and of overall survival (Cox proportional hazards model)
Category pair for each baseline variable . | N . | ORP* . | Odds ratio (95% CI) . | OSP* . | Hazard ratio (95% CI) . |
|---|---|---|---|---|---|
| 48 y or younger/older than 48 y | 19/24 | .390 | 0.583 (0.171; 1.993) | .665 | 1.248 (0.459; 3.392) |
| Female/male | 11/32 | .922 | 1.072 (0.270; 4.247) | .838 | 1.123 (0.368; 3.429) |
| Performance status (ECOG) less than 2/ECOG 2 or higher | 29/14 | .158 | 2.679 (0.681; 10.534) | .113 | 2.276 (0.823; 6.290) |
| Time from transplantation to PTLD diagnosis, 1 y or less/more than 1 y | 14/29 | .439 | 0.595 (0.160; 2.214) | .584 | 1.318 (0.490; 3.542) |
| No. sites, 1/more than 1 | 14/29 | .238 | 2.182 (0.596; 7.984) | .035 | 3.821 (1.096; 13.326) |
| Histologic type, monomorphic/polymorphic† | 28/4 | .208 | 0.216 (0.020; 2.347) | .383 | 2.476 (0.323; 19.0) |
| Cell EBV viral load, 600 copies/μg DNA or less/more than 600 copies/μg DNA‡ | 21/18 | .167 | 0.400 (0.109; 1.466) | .666 | 1.238 (0.470; 3.259) |
| Lactate dehydrogenase, normal/abnormal | 15/28 | .007 | 6.875 (1.682; 28.10) | .119 | 2.313 (0.807; 6.631) |
Category pair for each baseline variable . | N . | ORP* . | Odds ratio (95% CI) . | OSP* . | Hazard ratio (95% CI) . |
|---|---|---|---|---|---|
| 48 y or younger/older than 48 y | 19/24 | .390 | 0.583 (0.171; 1.993) | .665 | 1.248 (0.459; 3.392) |
| Female/male | 11/32 | .922 | 1.072 (0.270; 4.247) | .838 | 1.123 (0.368; 3.429) |
| Performance status (ECOG) less than 2/ECOG 2 or higher | 29/14 | .158 | 2.679 (0.681; 10.534) | .113 | 2.276 (0.823; 6.290) |
| Time from transplantation to PTLD diagnosis, 1 y or less/more than 1 y | 14/29 | .439 | 0.595 (0.160; 2.214) | .584 | 1.318 (0.490; 3.542) |
| No. sites, 1/more than 1 | 14/29 | .238 | 2.182 (0.596; 7.984) | .035 | 3.821 (1.096; 13.326) |
| Histologic type, monomorphic/polymorphic† | 28/4 | .208 | 0.216 (0.020; 2.347) | .383 | 2.476 (0.323; 19.0) |
| Cell EBV viral load, 600 copies/μg DNA or less/more than 600 copies/μg DNA‡ | 21/18 | .167 | 0.400 (0.109; 1.466) | .666 | 1.238 (0.470; 3.259) |
| Lactate dehydrogenase, normal/abnormal | 15/28 | .007 | 6.875 (1.682; 28.10) | .119 | 2.313 (0.807; 6.631) |
N = 43.
Difference between 2 specified categories.
N = 32.
N = 39.
Causes of death
. | n = 43 . |
|---|---|
| Death, no. patients (%) | |
| No | 24 (56) |
| Yes | 19 (44) |
| Causes of death, no. patients | |
| Septic shock | 2 |
| Bronchospasm* | 1 |
| Chemotherapy toxicity | 1 |
| Unknown | 1 |
| PTLD | 13 |
| Acute rejection | 1 |
. | n = 43 . |
|---|---|
| Death, no. patients (%) | |
| No | 24 (56) |
| Yes | 19 (44) |
| Causes of death, no. patients | |
| Septic shock | 2 |
| Bronchospasm* | 1 |
| Chemotherapy toxicity | 1 |
| Unknown | 1 |
| PTLD | 13 |
| Acute rejection | 1 |
Bronchospasm followed by pulmonary edema in a patient with pre-existing heart failure.
Tolerability and safety
Safety was analyzed in all 46 patients, all of whom received at least 1 dose of rituximab. Fifty-five adverse events (AEs) of CTC grade 3 or 4 were reported in 26 (57%) patients. Only 2 of these events (hypertension and purpura with myalgia) were considered by the investigator to be related to rituximab. Irrespective of attributability, the most frequently reported AEs were transplant rejection (22%), abdominal pain (20%), and dyspnea (17%). Four AEs led to treatment withdrawals in 2 (4%) patients, all between d1 and d50. Fifty-two serious adverse events (SAEs) were reported in 29 patients; 2 of these events were considered by the investigator to be related to rituximab: they consisted of an intestinal perforation at a site of PTLD involvement, and purpura with myalgia, occurring 2 months after the last dose of rituximab. Episodes of neutropenia were reported in 2 patients, after completion of the study treatment; 1 patient had a tacrolimus overdose.
Discussion
This is the first prospective trial of a treatment for PTLD. Overall, 44.2% of patients responded to rituximab monotherapy. In previous, retrospective, studies, anti-CD20 therapy was often combined with other treatments, including a reduction in immunosuppressive drug therapy and donor lymphocyte infusion,16 making it difficult to determine the specific effect of the antibody in some cases.
The only previously published study of factors predictive of the response to anti–B-cell monoclonal antibodies showed that complete responses were associated with a shorter interval between transplantation and PTLD onset, a smaller number of involved sites, and the absence of CNS involvement.7 In our study, the only factor predicted of a poor response in univariate analysis was an elevated LDH level; CNS involvement was an exclusion criteria in our study, and could not therefore be assessed. PTLD relapsed in only 21% of patients who had responded after treatment completion (PR in 3 cases, and CR in 1 case), indicating that rituximab provides durable disease control. Interestingly, some patients with CRu, PR, or SD on d80 entered CR by d180. The only factor predictive of survival was the number of involved sites, confirming our previous results in 61 PTLD patients who received various treatments.17
Rituximab was well tolerated, although the profound B-cell depletion it induces can further exacerbate these patients' immunosuppression.18,19 Only 2 deaths were due to infections, a rate compatible with that expected in this type of population. Late-onset neutropenia has recently been described in patients treated with rituximab.20 The calculated postmarketing rate of notified late-onset neutropenia is currently less than 1 case per 1000 patients.21 In the present study, only 1 of the 2 cases of neutropenia observed after treatment completion was not associated with alternative causes. It is noteworthy that this patient's neutropenia was asymptomatic and resolved spontaneously, as in another recent report.22
The rituximab response rate in this study (44.2%) is encouraging but still inadequate. A combination of rituximab and chemotherapy (CHOP regimen) has been shown to increase the response and survival rates in immunocompetent NHL patients.23 Chemotherapy is commonly the treatment of choice for aggressive PTLD, yielding CR in nearly 50% of patients, but the results are inconsistent and survival is poor among patients with PTLD treated with chemotherapy because of severe toxic effects and an increase in life-threatening infections.
A combination of rituximab and T-cell therapy could be an alternative.24 Indeed, infusion of expanded donor-derived EBV-specific cytotoxic T lymphocytes (CTLs) has shown some preventive and therapeutic efficacy in HSCT patients.6 Autologous EBV-specific CTLs can be generated in SOT recipients but remains challenging,25 and can only be used in PTLD of recipient origin. Another alternative could be the use of CTLs generated from human leukocyte antigen (HLA)–matched donors, although this approach may have an allogeneic effect on the host, leading to graft-versus-host disease or graft rejection.26 Because of the progressive nature of PTLD, the key to the management of high-risk patients may be early or even preemptive treatment with anti–B-cell antibodies, when EBV load increases in peripheral blood.9
In this carefully designed prospective trial, we showed that treatment of PTLD with rituximab was effective and well tolerated, and associated with a low relapse rate. This trial is a basis for future prospective studies using new therapeutic approaches.
Prepublished online as Blood First Edition Paper, October 27, 2005; DOI 10.1182/blood-2005-01-0377.
Supported by research support from Roche France.
Several of the authors (N.B.-V., L.B.) are employed by a company (Roche) whose product was studied in the present work.
N.M. was the coordinator; V.L. and A.F. were co-coordinators; S.C., W.F, A.F., J.L.G., R.H, O.H., A.J, T.L., P.L., Y.L., F.M., F.O., E.O., M.-N.P., G.S., G.S., A.M.S., P.V., and E.V. were investigators; S.C., N.M., and V.L. wrote the manuscript (the manuscript was reviewed and accepted by all authors); A.M. coordinated the independent expert panel for the centralized anatomopathology review and analyzed the pathologic data; S.F.-K. and P.M. carried out the centralized EBV viral load assay and analyzed the data; N.B.-V. and L.B., representing Roche France, cooperated in preparation of the document by providing access to the data from M39037 trial; and L.B. did the statistical analysis.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
We thank Profs Nicole Brousse, Martine Raphael, and Françoise Berger for their participation in the independent expert panel for the centralized anatomopathology review. We thank Profs AlainDelmer and Yves Menu and Dr Antoine Scherrer for their participation in the independent expert panel for the centralized CT scan review. We thank Prof André Bosly, Dr Eric Deconinck, Prof François Dreyfus, Dr Jan Van Droogenbroeck, Dr Cyrille Feray, Prof George Fillet, Dr Rémi Gressin, Prof Bruno Moulin, and Dr Pierre Zachéed. We thank Nicole Piatysek, clinical study manager (Roche France); David Pau, data manager (Roche France); Carine Keppens (Roche Belgium); and Lut Lemmens (Roche Belgium).

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal