Abstract
The homozygous deletion of the inducible costimulator (ICOS), an activation-induced member of the CD28 family on T cells, causes an antibody deficiency syndrome in affected humans. The identification of a total of 9 ICOS-deficient patients revealed that this monogenic disease comprises the full clinical phenotype described for common variable immunodeficiency (CVID), including recurrent bacterial infections, adult as well as childhood onset, splenomegaly, autoimmune phenomena (autoimmune neutropenia), intestinal lymphoid hyperplasia, and malignancy (carcinoma of the vulva). All patients exhibited a profound hypogammaglobulinemia and a disturbed B-cell homeostasis. The severe reduction of class-switched memory B cells resulted from poor germinal center formation in the absence of ICOS. The additional decrease of naive B cells was associated with a partial inhibition of the early B-cell development at the pre–B-I stage. T-cell homeostasis seemed not to be affected, but low IL-10 production by ICOS-deficient T cells may contribute to the disturbed germinal center reaction. Human ICOS deficiency is indistinguishable from CVID and thus serves as a monogenic model for this complex syndrome.
Introduction
The coevolution of the human immune system in competition with pathogens has led to highly specialized defense mechanisms in which single gene mutations may have deleterious effects, as first described for X-linked agammaglobulinemia.1 Since then more than 100 genetic defects have been defined, resulting in inherited (primary) immunodeficiencies (reviewed by Fischer2 ). Defects of the humoral immune response may originate from mutations in genes affecting antigen-independent early B-lymphocyte development in the bone marrow3 or the peripheral, antigen-dependent maturation in secondary lymphoid organs. Although most defects of the early B-cell differentiation are intrinsic B-cell defects (eg, of btk, μ chain, Igα, λ5, or BLNK),3 disturbed peripheral maturation may be due to intrinsic B-cell defects of enzymes critical in class-switch recombination (AID, UNG) or to a disturbed T to B cooperation in germinal centers.4 The latter develop during immune responses to T-cell–dependent antigens and create a microenvironment which promotes the selection and expansion of antigen-specific B cells. Costimulatory molecules are crucial in this process.5,6
In 1999, the discovery of the inducible costimulator (ICOS)7 extended the list of costimulatory molecules. ICOS belongs to the family of Ig-like molecules, including CD28, CTLA-4 (CD152), and programmed cell death-1 (PD-1). Whereas CD28 is expressed constitutively, CTLA-4, ICOS, and PD-1 are induced after stimulation by the T-cell receptor (TCR).8 CD28 and CTLA-4 share CD80 (B7.1) and CD86 (B7.2) as ligands. ICOS as well PD-1 have unique ligands designated ICOS-L (hGL50, B7h, or B7RP-1) and PD-L1/PD-L2, respectively. Although CTLA-4 and PD-1 provide negative regulatory signals, CD28 and ICOS boost the cognate T- to B-cell interaction (reviewed in Sharpe and Freeman8 ), and thereby are essential for an optimal antibody response.
Human antibody deficiencies lacking a genetic or other causal explanation have been termed common variable immunodeficiency (CVID).9 This syndrome describes a heterogeneous group of patients presenting with a history of recurrent bacterial infections of the respiratory and gastrointestinal tract.10 CVID commonly manifests in adulthood with a subset presenting already in childhood.
We have reported 4 patients with the diagnosis of CVID lacking ICOS expression on activated T cells because of a homozygous genomic deletion of exons 2 and 3.11 This single gene defect had manifested itself as CVID during early adulthood. Immunologically, all 4 patients presented with a marked decrease of total B cells (< 3.5% of peripheral blood mononuclear cells [PBMCs]) and especially of switched memory B cells. Since the first report we identified 5 more patients with ICOS deficiency, including 3 children.12 The purpose of this study was to comprehend the clinical phenotype of ICOS deficiency and to examine the underlying aberrations of the immune system. Here, we describe for the first time that in the absence of ICOS expression patients can develop the full clinical spectrum of CVID. In contrast to our first report11 in which we considered ICOS deficiency an adult-onset disease without splenomegaly or autoimmune phenomena, we learned from the 5 additional cases that the identical monogenetic defect may have an early onset (in 2 patients) and can be associated with splenomegaly, sarcoidlike granulomata, autoimmune phenomena, and malignancy. In addition, human ICOS-deficient T cells exhibit a greatly disturbed IL-10 and IL-17 production. This failure of IL-10 production may underlie the critical role of ICOS in the formation of germinal centers and B-cell memory in humans.
Patients, materials, and methods
Patients and immunizations
Nine patients with the molecular diagnosis of ICOS deficiency were enrolled into ethics board–approved protocols at the University of Freiburg (no. 239/99 to B.G. and EU-IMPAD-QLG1-CT-2001-01536). The molecular cause of the patients' defects have been reported earlier.11,12 Informed written consent was obtained from each patient prior to participation. Diagnosis of CVID was established according to the criteria of the European Society for Immunodeficiencies (ESID) (www.esid.org).9
Vaccination records of 2 ICOS-deficient children (patients no. 8 and no. 9) were reviewed and vaccination responses were examined for specific antibody titers against pertussis, tetanus, diphtheria, measles, mumps, German measles, and hepatitis B. Patients no. 1 and no. 2 had participated in a previous vaccination trial. Patients no. 3 and no. 4 were included in an ongoing CVID vaccination protocol with T-dependent (tetanus, hepatitis A and B), and T-independent antigens (pneumococcal polysaccharides).
Flow cytometric analysis of peripheral blood lymphocytes and bone marrow precursor B-cell compartment
PBMCs were isolated by Ficoll density gradient centrifugation and stained with quadruple combinations of monoclonal antibodies as described before.11 Heparinized bone marrow aspirates (50 μL; 15 × 106 nucleated cells/mL) were incubated for 10 minutes at room temperature with quadruple combinations of monoclonal antibodies as described before.13 Labeled cells were analyzed by flow cytometry (FACSCalibur; Becton Dickinson, San Jose, CA).
Analysis of somatic hypermutation (SHM)
Frequency and characteristics of SHMs in the variable region of the IgM heavy chain (VH region) were studied in purified CD19+CD27+ B cells as previously described.14 PBMCs were labeled with anti-CD19 and anti-CD27 mAbs (Immunotech, Marseille, France) and then purified by FACS sorting, with a purity of sorted CD19+CD27+ B cells greater than 99%. Total RNA was isolated with the Trizol reagent (Invitrogen, Cergy Pontoise Cedex, France), and cDNA was obtained by reverse transcription (Superscript II; Invitrogen) with an oligo dT primer (Invitrogen). SHMs were identified in the VH3 to VH23 region (GenBank accession no. AB019439) after polymerase chain reaction (PCR) amplification using the Pfu polymerase (PfuTurbo; Stratagene, Amsterdam, The Netherlands) and VH3 to VH23 and Cμ primers. Cloning and sequencing were performed as previously described.14
Analysis of T-cell cytokine production
EDTA blood (50-80 mL) was collected from 5 ICOS-deficient patients and 5 control subjects. CD4+ T cells were isolated and purified to greater than 96% by positive selection as previously described.11 Purified CD4+ T cells (106 cells/mL) were stimulated for 18 hours with 200 ng/mL anti-CD3 mAb (clone Hit3a; BD Pharmingen, Heidelberg, Germany), 200 ng/mL anti-CD28 mAb (clone CLB-CD28/1; Sanquin Reagents, Amsterdam, The Netherlands), or 4 μg/mL anti-ICOS mAb (clone F44; a gift of R. Kroczek, Robert Koch Institute, Berlin, Germany7 ). Supernatants were analyzed by enzyme-linked immunosorbent assay (ELISA) for the cytokines IL-10, IL-13, IL-17, and IFN-γ as described previously.11 The ELISA for IL-17 was performed using murine anti–human IL-17 mAb and biotinylated rat anti–human IL-17 (both RD Systems, Wiesbaden, Germany).
Histology
Immunohistochemistry was performed according to standard protocols. All antigens were detected in paraffin sections using the Vecta Stain ABC Kit (Vector Laboratories, Burlingame, CA). Antigens were unmasked by exposure to high temperature (about 96°C for 15 minutes pH8 [CD4], 30 minutes pH6 [CD8]) or proteinase K digestion (10 minutes at room temperature [CD3]). The following primary antibodies were used at optimal working dilution: anti-CD3 (clone F7.2.38, 1:25), anti-CD8 (clone C8/144B, 1:200), anti-CD20 (clone L26, 1:200), and anti-Ki67 (clone MIB-1, 1:2000) (all DAKO Cytomation, Hamburg, Germany) and anti-CD4 (clone 4B12, 1:500) (Novocastra, Edinburgh, United Kingdom).
Sections were analyzed and documented on a Leitz DM RB Axioplan microscope (Leica Microsystems, Wetzlar, Germany) with an L Plan 10 × 25 ocular and the following objectives: 5 ×/0.12 numeric aperture (NA) PL Fluotar (Figures 2A, 5 HE staining) and 20 ×/0.60 PL Apo (Figures 2B-C, 5 CD4 and CD8 staining). Kodak Ektachrome 64 film (Eastman Kodak, Rochester, NY) and a Leica wildMPS52 camera were used to capture the images. Images were processed using Adobe Photoshop (Adobe Systems, San Jose, CA).
Results
Case reports
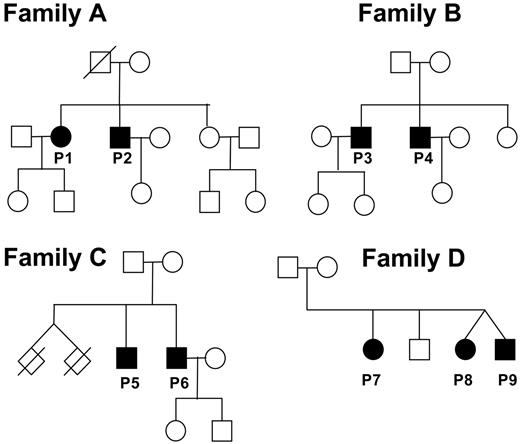
The evaluation of 11 families with autosomal recessive CVID for the integrity of the ICOS gene revealed the identical homozygous loss of a genomic sequence of 1815 bp (base pair), including exons 2 and 3 in 4 families (Figure 1).11,12
Pedigrees of patients with ICOS deficiency. Circles represent females; squares, males, and slashed rhombuses, deceased. Filled symbols represent affected family members; open symbols, unaffected family members. The twins of family C died as a result of complications at premature birth.
Pedigrees of patients with ICOS deficiency. Circles represent females; squares, males, and slashed rhombuses, deceased. Filled symbols represent affected family members; open symbols, unaffected family members. The twins of family C died as a result of complications at premature birth.
ICOS deficiency family A
Patient no. 1 was diagnosed with CVID in 1990 at the age of 28 years after 1 year of recurrent upper and lower respiratory infections, including pneumonia. In addition, she had impetigo caused by Staphylococcus aureus. Under monthly intravenous infusions of 20 g immunoglobulin G (IgG) since 1990 she maintained serum IgG levels of approximately 5 g/L and improved in frequency and intensity of her infections. At age 34 years, she was diagnosed with plurifocal verrucous squamous epidermal carcinoma of the vulva associated with human papilloma virus infection.
Patient no. 2 is the younger brother of patient no. 1. He had recurrent upper and lower respiratory tract infections for 2 years, when in 1991, at age 21 years, the diagnosis of CVID was established. During the further course of disease he developed lambliasis in 1993, salmonellosis in 1997, and Campylobacter enteritis with nodular lymphoid hyperplasia of the intestinal mucosa in 2001. He also had recurrent herpes keratitis. For control of his considerable susceptibility to infections, patient no. 2 receives intravenous replacement therapy with 20 g IgG every month.
Their mother and sister are healthy and have normal immunoglobulin serum levels; the father died at age 81 years of a heart attack.
ICOS deficiency family B
Patient no. 3 had a normal frequency of infections in childhood and adolescence, except for pneumonia at 6 years of age. In 1998, at age 31 years, he was diagnosed with CVID after recurrent upper respiratory infections for approximately 3 years. He was put on intravenous Ig and improved substantially. According to the patient's wish, he receives 20 g intravenous Ig only every 3 to 4 months, which seems to control his susceptibility to infections, although the serum IgG trough levels remain below 3 g/L.
Patient no. 4 is the younger brother of patient no. 3. He had his first pneumonia at age 12 years, but diagnosis of CVID was not established until 1999 at age 22 years, after 3 years of recurrent respiratory infections, including pneumonia and recurrent Salmonella enteritis. Patient no. 4 was put on intravenous Ig replacement therapy in 1999; like his brother, so far 20 g intravenous Ig every 4 to 5 months seem to sufficiently control his susceptibility to infection, although the serum IgG trough levels remain below 3 g/L. The parents and the sister of the patients in family B are healthy and have normal Ig levels.
ICOS deficiency family C
After the loss of twin siblings as a result of complications at birth, the healthy parents gave birth to 2 boys. In his adolescence the older brother (patient no. 5) had recurrent bronchitis and sinusitis. After 3 episodes of pneumonia at 27, 31, and 38 years of age the diagnosis of CVID was established, but intravenous Ig replacement therapy was irregular and discontinued after a severe infusion reaction. The recurrent upper respiratory infections were frequently accompanied by localized herpes simplex virus eruptions. On physical examination a mild splenomegaly and lymphadenopathy were detectable. When he recently presented with neurologic symptoms (vertigo, ataxia, headache) associated with sterile lymphocytic meningoencephalitis, we diagnosed a borreliosis of the central nervous system by means of Borrelia burgdorferi–specific IgM antibodies in the cerebrospinal fluid, initiated antibiotic treatment with ceftriaxone, and reinstituted intravenous Ig replacement therapy without complications.
The younger brother, patient no. 6, developed his first pneumonia at 12 years of age. Like his brother, he had recurrent upper respiratory tract infections and localized herpes simplex virus infections during adolescence.
When referred to our department at the age of 41 years, the patient presented with hepatosplenomegaly (spleen size, 190 × 70 mm) without evidence for viral infection. Laboratory work-up showed a severe autoimmune neutropenia (80 neutrophils/μL) as a result of IgG antineutrophil antibodies. The histologic examination of the bone marrow demonstrated a hyper-regenerative granulopoiesis, nodular T-cell infiltrates, and a depletion of plasma cells similar to most patients with CVID. After the initiation of an immunosuppressive therapy with oral steroids, the neutrophil counts recovered to a lower normal range.
ICOS deficiency family D
In contrast to the first 3 families with ICOS deficiency, 2 patients of the fourth family were diagnosed during childhood. The index patient of this family (patient no. 7) was healthy up to 8 years of age, when she presented with a scarring pneumonia in the lower left lobe of the lung. She was shown to be hypogammaglobulinemic, put on prophylactic antibiotic treatment but not on intravenous Ig replacement therapy. Several flare-ups of pneumonia recurred and destroyed the lower left lobe, necessitating lobectomy. Still without adequate therapy, the pulmonary infections progressed, and bronchiectases formed. At age 15 years she was diagnosed with CVID and put on intravenous Ig. Since then, she has had no more bouts of pneumonia.
Patient no. 9 developed watery and bloody diarrhea as early as 18 months of age, but no pathogen was identified. Under the suspicion of inflammatory bowl disease, a colonoscopy was performed. Histology of the lesions in the colon identified a nodular lymphoid hyperplasia as reported in approximately 20% to 30% of patients with CVID. Subsequently, the diagnosis of CVID was established, and an intravenous Ig replacement therapy was initiated. The diarrhea responded promptly to this treatment.
The dizygotic twin of this patient (patient no. 8) carries the same homozygous mutation as her affected twin brother, causing a severe hypogammaglobulinemia (IgG < 3 g/L; see Table 1), but she is asymptomatic as of today (age 5 years). The heterozygous parents of this family and one son, whose ICOS status is not known, were asymptomatic.
Serum immunoglobulin and IgG subclass levels in ICOS deficiency
. | Age at testing, y . | IgG, g/L . | IgG1, mg/dL . | IgG2, mg/dL . | IgG3, mg/dL . | IgG4, mg/dL . | IgA, g/L . | IgM, g/L . | IgE, IU/mL . |
|---|---|---|---|---|---|---|---|---|---|
| Family A | |||||||||
| Patient 1 | 29 | < 1.9* | ND | ND | ND | ND | < 0.3* | < 0.2* | < 28 |
| Patient 2 | 21 | 4.4* | ND | ND | ND | ND | 0.4* | < 0.2* | < 17.5 |
| Family B | |||||||||
| Patient 3 | 35 | 2.49* | 118* | 71.7* | 21.2* | 7* | 0.46* | 0.26* | < 17.5 |
| Patient 4 | 25 | 1.05* | 78.2* | 10.4* | 38.7 | < 6* | < 0.06* | 0.31* | < 17.5 |
| Family C | |||||||||
| Patient 5 | 46 | 1.01* | < 2.8* | 66.9* | 44.9 | < 0.72* | < 0.06* | 0.38* | < 17.5 |
| Patient 6 | 42 | 0.57* | ND | ND | ND | ND | < 0.06* | 1.8 | < 19.1 |
| Family D | |||||||||
| Patient 7 | 15 | 2.55* | 181* | 36* | 38 | 0* | < 0.25* | 0.26* | < 30 |
| Patient 8 | 4 | 2.13* | 118* | 10* | 11* | 0* | 0.58* | 0.57 | ND |
| Patient 9 | 3 | 0.54* | 50* | 20* | 4* | 0* | 0.2* | 0.43 | < 17.5 |
| Normal range | — | 7.0-16 | 360-940 | 170-550 | 30-120 | 6-120 | 0.7-4 | 0.4-2.3 | < 100 |
. | Age at testing, y . | IgG, g/L . | IgG1, mg/dL . | IgG2, mg/dL . | IgG3, mg/dL . | IgG4, mg/dL . | IgA, g/L . | IgM, g/L . | IgE, IU/mL . |
|---|---|---|---|---|---|---|---|---|---|
| Family A | |||||||||
| Patient 1 | 29 | < 1.9* | ND | ND | ND | ND | < 0.3* | < 0.2* | < 28 |
| Patient 2 | 21 | 4.4* | ND | ND | ND | ND | 0.4* | < 0.2* | < 17.5 |
| Family B | |||||||||
| Patient 3 | 35 | 2.49* | 118* | 71.7* | 21.2* | 7* | 0.46* | 0.26* | < 17.5 |
| Patient 4 | 25 | 1.05* | 78.2* | 10.4* | 38.7 | < 6* | < 0.06* | 0.31* | < 17.5 |
| Family C | |||||||||
| Patient 5 | 46 | 1.01* | < 2.8* | 66.9* | 44.9 | < 0.72* | < 0.06* | 0.38* | < 17.5 |
| Patient 6 | 42 | 0.57* | ND | ND | ND | ND | < 0.06* | 1.8 | < 19.1 |
| Family D | |||||||||
| Patient 7 | 15 | 2.55* | 181* | 36* | 38 | 0* | < 0.25* | 0.26* | < 30 |
| Patient 8 | 4 | 2.13* | 118* | 10* | 11* | 0* | 0.58* | 0.57 | ND |
| Patient 9 | 3 | 0.54* | 50* | 20* | 4* | 0* | 0.2* | 0.43 | < 17.5 |
| Normal range | — | 7.0-16 | 360-940 | 170-550 | 30-120 | 6-120 | 0.7-4 | 0.4-2.3 | < 100 |
ND indicates not determined; —, not applicable.
Pathologic value.
Immunoglobulin levels in ICOS deficiency
ICOS deficiency caused a severe reduction of total IgG and IgA in all patients (Table 1). Serum IgM ranged from reduced levels in 6 patients to low normal values in 3 patients. During his last bronchopneumonia, patient no. 6 developed an IgM level of 3.1 g/L (normal range, 0.4-2.3 g/L). Interestingly, patient no. 6 produced detectable antineutrophil IgG antibodies in an autoimmune context. Among the IgG subclasses, IgG3 was less affected than IgG1, IgG2, and IgG4. IgE was not detectable in 8 tested patients with ICOS deficiency.
Response to vaccination
Vaccination records and sera before the initiation of intravenous Ig therapy were available for patients no. 8 and no. 9. None of the tested sera contained detectable amounts of specific IgG antibodies (except for a low titer of antidiphtheria antibodies in patient no 8). Patients no. 1 and no. 2 had participated in a vaccination trial with the neoantigen phage ϕ17415 before the detection of the genetic defect. Neither patient produced detectable amounts of specific IgG, and only patient no. 1 produced low levels of phage-specific IgM (Andres Rump, Hans Ochs, and H.H.P., unpublished data, June 1996). Patients no. 3 and no. 4 are participating in an ongoing trial examining vaccination responses in patients with CVID (S. Goldacker, unpublished data, March 2005). After 4 weeks no specific IgG response to hepatitis A virus (HAV), tetanus, and diphtheria toxoid were detected. Patient no. 5 produced detectable amounts of anti–B burgdorferi–specific IgM in the cerebrospinal fluid during neuroborreliosis. Overall, ICOS deficiency seems to permit transient IgM responses but no protective IgG responses.
Lymphocyte subpopulations in the peripheral blood in ICOS deficiency
The number of circulating B cells was severely decreased in 5 of 6 adult ICOS-deficient patients, whereas the children presented with normal numbers (Table 2). Absolute numbers of naive B cells were severely reduced in these patients and were normal in the children. Switched memory B cells were virtually absent from the peripheral blood of all ICOS-deficient patients (0.6% ± 0.6%; normal range, 6.5%-33%). IgM memory B cells (5.7% ± 3.0%; normal range, 7.9%-36%) were reduced in most patients (7 of 9). The distribution of T-cell subpopulations appeared normal, except in 3 patients (no. 2, no. 6, and no. 7) who had an inverted CD4+/CD8+ ratio (0.8-0.9; normal range, 1.0-3.6) (Table S1; see the supplemental table link at the top of the online article, at the Blood website). This is found in about 35% of patients with CVID and may indicate an underlying chronic infection.
Phenotypic characterization of the lymphocyte compartment and somatic hypermutations in ICOS deficiency
. | Age at testing, y . | Lymphocyte count, × 109/L . | CD3, %Ly . | CD4, %Ly . | CD8, %Ly . | CD19, %Ly . | CD19/μL . | CD27-IgD+, %Bc . | CD27+IgD+, %Bc . | CD27+IgD-, %Bc . | SHM, %bp . |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Family A | |||||||||||
| Patient 1 | 41 | 1.266 | 95.6* | 59.2* | 31.9 | 0.6* | 7* | 89.6* | 5.9* | 0.8* | ND |
| Patient 2 | 32 | 1.047 | 88.5* | 39.9 | 47.2* | 0.6* | 9* | 88.5* | 7.8* | 1.6* | 3.8 |
| Family B | |||||||||||
| Patient 3 | 35 | 1.171 | 77.6 | 50.5 | 22.8 | 2.8* | 34* | 94.9* | 4.1* | 0.2* | —† |
| Patient 4 | 25 | 0.353* | 91.9* | 44.8 | 33.6 | 3.2* | 5* | 92.9* | 5.1* | 0.3* | ND |
| Family C | |||||||||||
| Patient 5 | 46 | 0.871* | 72.2 | 55.1 | 16.6 | 14.6 | 127 | 90.4* | 9 | 0.2* | 2.2* |
| Patient 6 | 42 | 1.722 | 91.9* | 23.1 | 64.0* | 2.01* | 33* | 90.2* | 11.3 | 1.3* | ND |
| Family D | |||||||||||
| Patient 7 | 18 | 3.031 | 88.5* | 40.8 | 47.5* | 6.1 | 184 | 96.8* | 2.7* | 0* | —† |
| Patient 8 | 5 | 3.584 | 76.1 | 39.0 | 34.5 | 19.1 | 686 | 95.2* | 3.2* | 1.3* | 2.3* |
| Patient 9 | 5 | 4.153 | 70.4 | 32.8 | 34.8 | 21.2 | 882 | 97.7* | 2* | 0* | —† |
| Mean ± SD | — | 1.910 ± 1.262 | 83.6 ± 9.0 | 42.8 ± 10.5 | 37.0 ± 13.4 | 7.8 ± 7.7 | 219 ± 311 | 92.9 ± 3.2 | 5.7 ± 3.0 | 0.6 ± 0.6 | 2.8 ± 0.7 |
| Normal range | — | 1.0-2.8 | 55-83 | 28-57 | 10-39 | 6-19 | 100-500 | 38-80 | 7.9-36 | 6.5-33 | 2.5-6.3 |
. | Age at testing, y . | Lymphocyte count, × 109/L . | CD3, %Ly . | CD4, %Ly . | CD8, %Ly . | CD19, %Ly . | CD19/μL . | CD27-IgD+, %Bc . | CD27+IgD+, %Bc . | CD27+IgD-, %Bc . | SHM, %bp . |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Family A | |||||||||||
| Patient 1 | 41 | 1.266 | 95.6* | 59.2* | 31.9 | 0.6* | 7* | 89.6* | 5.9* | 0.8* | ND |
| Patient 2 | 32 | 1.047 | 88.5* | 39.9 | 47.2* | 0.6* | 9* | 88.5* | 7.8* | 1.6* | 3.8 |
| Family B | |||||||||||
| Patient 3 | 35 | 1.171 | 77.6 | 50.5 | 22.8 | 2.8* | 34* | 94.9* | 4.1* | 0.2* | —† |
| Patient 4 | 25 | 0.353* | 91.9* | 44.8 | 33.6 | 3.2* | 5* | 92.9* | 5.1* | 0.3* | ND |
| Family C | |||||||||||
| Patient 5 | 46 | 0.871* | 72.2 | 55.1 | 16.6 | 14.6 | 127 | 90.4* | 9 | 0.2* | 2.2* |
| Patient 6 | 42 | 1.722 | 91.9* | 23.1 | 64.0* | 2.01* | 33* | 90.2* | 11.3 | 1.3* | ND |
| Family D | |||||||||||
| Patient 7 | 18 | 3.031 | 88.5* | 40.8 | 47.5* | 6.1 | 184 | 96.8* | 2.7* | 0* | —† |
| Patient 8 | 5 | 3.584 | 76.1 | 39.0 | 34.5 | 19.1 | 686 | 95.2* | 3.2* | 1.3* | 2.3* |
| Patient 9 | 5 | 4.153 | 70.4 | 32.8 | 34.8 | 21.2 | 882 | 97.7* | 2* | 0* | —† |
| Mean ± SD | — | 1.910 ± 1.262 | 83.6 ± 9.0 | 42.8 ± 10.5 | 37.0 ± 13.4 | 7.8 ± 7.7 | 219 ± 311 | 92.9 ± 3.2 | 5.7 ± 3.0 | 0.6 ± 0.6 | 2.8 ± 0.7 |
| Normal range | — | 1.0-2.8 | 55-83 | 28-57 | 10-39 | 6-19 | 100-500 | 38-80 | 7.9-36 | 6.5-33 | 2.5-6.3 |
Lymphocyte subpopulations were analyzed by flow cytometry with the following markers: CD3 for total T cells and CD4 and CD8 for the respective subpopulations, CD19 for B cells with naive (CD27-IgD+), IgM memory (CD27+IgD+), and switched memory (CD27+IgD-) subpopulations. Normal ranges are determined for adult population.
%Ly indicates percentage of lymphocytes; %Bc, percentage of B cells; SHM, somatic hypermutation; %bp, percentage of mutated base pairs; ND, not determined; —, not applicable.
Pathologic values.
The percentage of CD19+CD27+ cells was too low for analysis.
Secondary lymphoid tissue in ICOS deficiency
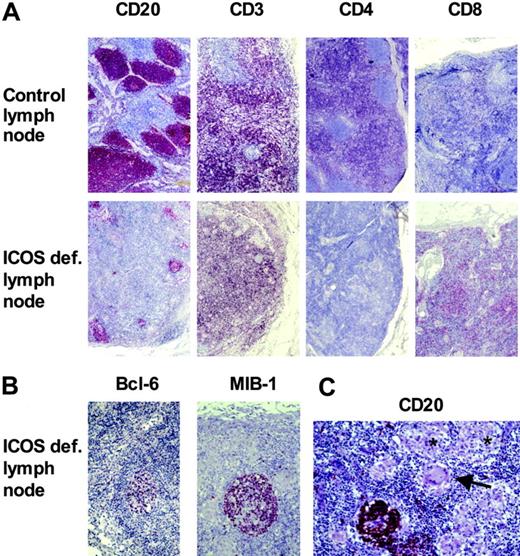
The strong decrease of memory B cells and the poor IgG response in vivo suggested a disturbed germinal center reaction as had been previously shown for murine ICOS deficiency.16-18 We examined secondary lymphoid tissue of patient no. 1. She had undergone lymphadenectomy in 1997 because of the occurrence of a carcinoma of the vulva. All lymph nodes were shown to be free of tumor. Compared with the draining lymph node of an ICOS-positive control patient with vulva carcinoma, the lymph node of patient no. 1 appeared cytopenic with a disturbed secondary follicular structure. B-cell follicles in the ICOS-deficient lymph node were few and small (Figure 2A). In contrast to the control lymph node, the few B-cell follicles were surrounded mostly by ICOS-deficient CD4–, CD8+ T cells. Some of the rudimentary follicles contained BCL-6–positive, proliferating B cells (Figure 2B). One of 2 examined ICOS-deficient lymph nodes presented with multiple small noncaseating granulomas (Figure 2C).
Disturbed secondary structure of lymph nodes in ICOS deficiency. Formalin-embedded inguinal lymph nodes of patient no. 1 and a control patient with carcinoma of the vulva were stained for B cells (CD20), T cells (CD3), and the CD4+ and CD8+ subsets, respectively (A). The ICOS-deficient lymph node contained fewer and smaller B-cell follicles, which were surrounded mainly by CD8+ T cells. The few germinal centers (MIB-1+, bcl-6+) appeared rudimentary (B). In one lymph node of the ICOS-deficient patient, several granulomata (*) with giant cells (arrow) were present, disrupting the follicular structure (C).
Disturbed secondary structure of lymph nodes in ICOS deficiency. Formalin-embedded inguinal lymph nodes of patient no. 1 and a control patient with carcinoma of the vulva were stained for B cells (CD20), T cells (CD3), and the CD4+ and CD8+ subsets, respectively (A). The ICOS-deficient lymph node contained fewer and smaller B-cell follicles, which were surrounded mainly by CD8+ T cells. The few germinal centers (MIB-1+, bcl-6+) appeared rudimentary (B). In one lymph node of the ICOS-deficient patient, several granulomata (*) with giant cells (arrow) were present, disrupting the follicular structure (C).
Somatic hypermutation (SHM) in ICOS deficiency
The generation of SHM represents a major event of B-cell maturation during the antigen-driven germinal center reaction. Because all patients exhibited a profound decrease in CD19+CD27+ memory B cells, SHM in the V region of the IgM gene could be analyzed in only 3 patients. For these patients SHM was found normal or slightly diminished (Table 2) with no abnormality in the pattern of nucleotide substitution.
T-cell compartment in ICOS deficiency
Although ICOS is expressed exclusively on activated T cells,7 the phenotypic and functional analysis of peripheral blood T cells did not reveal obvious abnormalities in ICOS deficiency.11 Because several reports have suggested an influence of ICOS costimulation on CD4+ T-cell cytokine production,19 we tested the secretion of IL-10, IL-13, IL-17, and INF-γ by purified CD4+ T cells after costimulation of the TCR-CD3 complex via ICOS or CD28.
As previously reported, ICOS costimulation superinduced IL-10 production by CD4+ T cells of healthy individuals.19 Correspondingly, IL-10 production was markedly impaired in all ICOS-deficient patients tested. Even after CD28 costimulation, IL-10 production increased only partially in the absence of ICOS and did not reach normal levels (Figure 3A).
Because ICOS-deficient T cells had been reported to produce less IL-17 in a murine model for arthritis,20 IL-17 production was assessed. Like IL-10, the secretion of IL-17 by ICOS-deficient CD4+ T cells was severely reduced after ICOS costimulation and only partially rescued by CD28 costimulation (Figure 3B).
Finally, to study TH1 versus TH2 differentiation, we analyzed INF-γ and IL-13 secretion, respectively. Both cytokines were not noticeably affected by the absence of ICOS costimulation. Unlike IL-10 and IL-17, IFN-γ and IL-13 were fully induced by costimulation through CD28 (Figure 3C-D).
Bone marrow precursor B-cell compartment in ICOS deficiency
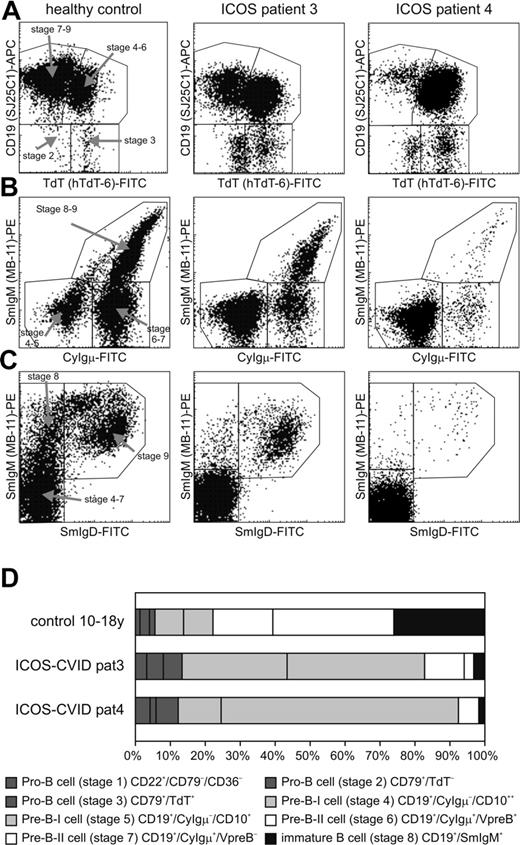
In addition to the severe reduction of memory B cells, 5 of 6 adult patients presented with a profound decrease of circulating naive CD27– B cells. The primary B-cell development was analyzed in bone marrow biopsies of patients no. 3 and no. 4 by flow cytometry. A relative expansion of the cytoplasmic Igμ-negative pre–B-I cells was observed, possibly resulting from a partial block of differentiation into the cytoplasmic Igμ-positive pre–B-II cell stage (Figure 4). This disturbed B-cell differentiation may contribute to the severe B lymphocytopenia in the peripheral blood. Similar though more drastic arrests of B-cell differentiation can be found in patients with X-linked agammaglobulinemia resulting from mutations in the BTK gene.13
Carcinoma of the vulva in ICOS deficiency
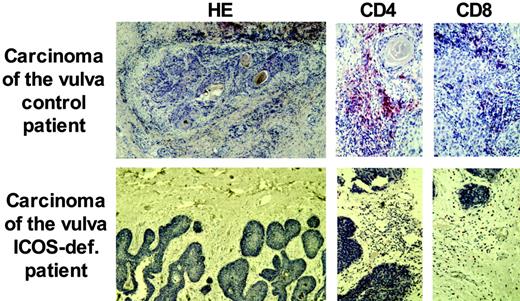
Patients with CVID have an increased incidence of malignancies, mostly lymphomas.21 Here, we report the development of a malignant tumor in ICOS deficiency. Patient no. 1 was diagnosed at age 34 years with a carcinoma of the vulva associated with a papilloma virus infection. Histology showed an epidermal tumor invading the surrounding stroma, with a reduced perilesional lymphocytic infiltrate (Figure 5). The absence of CD4+ T cells at this site was striking when compared with the great abundance of CD4+ T cells in the ICOS+ control patient with papilloma virus–induced carcinoma (Figure 5). Patient no. 1 had not received previous radiotherapy, chemotherapy, or other treatments known to induce lymphopenia. During the further course of the disease, her tumor relapsed 3 times despite extensive surgical removal and radiotherapy.
Reduced IL-10 and IL-17 production by ICOS-deficient T cells after in vitro stimulation. Bar graphs show in vitro cytokine production of isolated CD4+ T cells after stimulation with the indicated stimuli for 5 ICOS-deficient patients (ICOS) and 5 healthy control subjects (HD). Bars display mean and standard deviation for each cohort. Statistical significant differences are indicated (*P < .05; **P < .001).
Reduced IL-10 and IL-17 production by ICOS-deficient T cells after in vitro stimulation. Bar graphs show in vitro cytokine production of isolated CD4+ T cells after stimulation with the indicated stimuli for 5 ICOS-deficient patients (ICOS) and 5 healthy control subjects (HD). Bars display mean and standard deviation for each cohort. Statistical significant differences are indicated (*P < .05; **P < .001).
Relative increase of pre–B-1 B cells in the bone marrow of ICOS-deficient patients. Eight different precursor B-cell stages are defined and 1 mature IgM+/IgD+ B-cell stage. The pro-B and pre–B-I cell subsets (stages 1-5) of ICOS-deficient patients 3 and 4 show a relative increase as compared with the healthy control subject. (A) Analysis of expression of TdT and CD19 within a purified cytoplasmic (cy) CD79a+ lymphogate discriminates 4 different B-cell populations: a TdT–/CD19– (stage 2) and a TdT+/CD19– (stage 3) pro–B-cell subset, a TdT+/CD19+ B-cell population (pre–B-I/pre–B-II stage 6), and a TdT–/CD19+ (pre–B-II stage 7, immature and mature B cells). (B) Analysis of CyIgμ and surface membrane (Sm) IgM within a purified CD19+ lymphogate discriminates pre–B-I (CyIgμ–/SmIgM–), pre–B-II (CyIgμ+/SmIgM–), and immature/mature (CyIgμ+/SmIgM+) B-cell subsets. (C) Analysis of SmIgM and SmIgD within a purified CD19+ lymphogate discriminates the pre–B-I and pre–B-II cell subsets together (SmIgM–/SmIgD–), the immature B cells (SmIgM+/SmIgD–), and the mature B cells (SmIgM+/SmIgD+). (D) Schematic representation of the precursor B-cell compartment in a healthy donor and ICOS-deficient patients 3 and 4. The size of the 8 precursor B-cell stages is set to 100%.
Relative increase of pre–B-1 B cells in the bone marrow of ICOS-deficient patients. Eight different precursor B-cell stages are defined and 1 mature IgM+/IgD+ B-cell stage. The pro-B and pre–B-I cell subsets (stages 1-5) of ICOS-deficient patients 3 and 4 show a relative increase as compared with the healthy control subject. (A) Analysis of expression of TdT and CD19 within a purified cytoplasmic (cy) CD79a+ lymphogate discriminates 4 different B-cell populations: a TdT–/CD19– (stage 2) and a TdT+/CD19– (stage 3) pro–B-cell subset, a TdT+/CD19+ B-cell population (pre–B-I/pre–B-II stage 6), and a TdT–/CD19+ (pre–B-II stage 7, immature and mature B cells). (B) Analysis of CyIgμ and surface membrane (Sm) IgM within a purified CD19+ lymphogate discriminates pre–B-I (CyIgμ–/SmIgM–), pre–B-II (CyIgμ+/SmIgM–), and immature/mature (CyIgμ+/SmIgM+) B-cell subsets. (C) Analysis of SmIgM and SmIgD within a purified CD19+ lymphogate discriminates the pre–B-I and pre–B-II cell subsets together (SmIgM–/SmIgD–), the immature B cells (SmIgM+/SmIgD–), and the mature B cells (SmIgM+/SmIgD+). (D) Schematic representation of the precursor B-cell compartment in a healthy donor and ICOS-deficient patients 3 and 4. The size of the 8 precursor B-cell stages is set to 100%.
Discussion
Worldwide, ICOS deficiency has been identified so far only in these 9 patients,12,22,23 rendering the incidence of ICOS deficiency among patients with CVID rather low. The SNP analysis of the shared haplotype (Christoph Benoist, unpublished data, April 2004) revealed an identical costimulatory locus among all affected members suggestive of a founder effect for all 4 families. This is supported by the notion that 3 of the 4 families derive from the same village in the Black Forest. The fourth family, however, comes from the Austrian Burgenland. These regions are linked historically through the house of Habsburg, because the Black Forest region with Freiburg as capital was the most Western protectorate of the Habsburg empire from the 15th to the early 19th century.
Clinical presentation of ICOS deficiency
The clinical phenotype of ICOS deficiency is indistinguishable from the general clinical presentation of CVID.10 ICOS deficiency manifested with recurrent bacterial infections during the second to third decade of life in 6 patients, with 3 patients presenting in childhood. The adult onset of this inherited disease suggests a sufficient protection against common pathogens by the innate immune system and T-independent primary IgM responses in most ICOS-deficient children. Patients typically presented with frequent upper and/or lower respiratory infections. Some had additional gastrointestinal involvement, including giardiasis, inflammatory bowel disease, salmonellosis, and nodular lymphoid hyperplasia. Splenomegaly, autoimmune phenomena, and a malignant carcinoma were also observed. Especially surprising was the development of IgG-mediated autoimmune neutropenia in patient no. 6, representing an ICOS-independent class switch in an autoimmune setting. This wide range of clinical manifestations of a single gene defect was unexpected and emphasizes the role of so far poorly defined modifying factors. Conversely, because of the multifaceted clinical presentation, the follow-up of ICOS-deficient patients requires awareness of all possible complications found in CVID.
Of note is the development of a carcinoma of the vulva in patient no. 1. The early onset and the viral induction of this carcinoma suggest decreased tumor surveillance in the absence of ICOS. Several reports demonstrated a role of murine ICOS in tumor rejection.24-26 The inadequate lymphocytic infiltration at the site of tumor invasion supports this notion. However, the absence of tumor-infiltrating CD4+ T cells and the scarceness of CD4+ T cells in the draining lymph node are difficult to attribute solely to the lack of ICOS expression.
Immunologic presentation of ICOS deficiency
All ICOS-deficient patients are severely hypogammaglobulinemic, particularly concerning the T-dependent isotypes, suggesting a crucial role of human ICOS in the development of secondary antibody responses. Murine models had demonstrated an insufficient generation of germinal centers in the absence of ICOS.16-18 In humans an indicator of functional germinal centers is the presence of CD27+ switched memory B cells in peripheral blood.27 All ICOS-deficient patients exhibited a severely reduced number of CD27+ switched memory B cells (Freiburg classification type I28 ). The analysis of 2 lymph nodes of patient no. 1 underlined an impaired formation of germinal centers in ICOS-deficient humans. Surprisingly, CD4+ T cells were almost completely absent from the analyzed lymph nodes. This severe reduction of CD4+ T cells remains unexplained because the patient had not received radiotherapy, chemotherapy, or corticosteroids before the removal of the lymph nodes and had at no point of the analysis low numbers of circulating CD4+ T cells.
Reduced perilesional lymphocytic infiltration of an invasive carcinoma of the vulva in ICOS deficiency. Paraffin sections of the carcinoma of the vulva of patient 1 demonstrate a reduced infiltration of lymphocytes at the site of invasion. CD4+ T cells were especially diminished compared with an immunocompetent control patient. The number of CD8+ T cells was comparable.
Reduced perilesional lymphocytic infiltration of an invasive carcinoma of the vulva in ICOS deficiency. Paraffin sections of the carcinoma of the vulva of patient 1 demonstrate a reduced infiltration of lymphocytes at the site of invasion. CD4+ T cells were especially diminished compared with an immunocompetent control patient. The number of CD8+ T cells was comparable.
It is not understood why ICOS-deficient mice and humans do not develop normal germinal centers. The main defect reported here for human ICOS-deficient T cells affects the production of IL-10 and IL-17. IL-10 has been shown to support antibody production and the survival of germinal center B cells.29 Therefore, its impaired secretion may contribute to the poor germinal center and B-cell memory formation in ICOS deficiency. This secondary IL-10 deficiency may not only be a direct effect of the absent costimulation via ICOS, but additionally an indirect effect as a result of the participation of ICOS in the differentiation of an IL-10–producing T-cell subpopulation in vivo. In this context, an effector CD4+ T-cell population has been described as the main source of IL-10 after ICOS costimulation by dendritic cells.30
For IL-17, the other differentially regulated cytokine in ICOS deficiency, no role in germinal center formation has been reported. Interestingly, however, the impaired production of IL-17 by ICOS-deficient T cells may contribute to an increased respiratory infection rate because IL-17–deficient mice succumb to bacterial pneumonia as a result of impaired neutrophil recruitment.31
The absence of fully developed germinal centers and the severe decrease of class-switched memory B cells with retained SHM in the few CD19+CD27+ B cells is reminiscent of what has been observed in CD40L and CD40 deficiencies32,33 and supports the notion that SHM can occur outside germinal centers. It has been proposed that somatically hypermutated IgM+CD27+ B cells are derived from the splenic marginal zone and are involved in T-independent antipolysaccharide responses.34 Because of the effect on germinal center formation, the phenotype of an hyper IgM syndrome was expected,23 but despite transient IgM responses most ICOS-deficient patients presented with low IgM levels, suggesting additional effects of ICOS on the humoral immune response. In this context the severe reduction of circulating B cells is remarkable. This reduction seems to be increasing with age, pointing at an exhaustion of the highly regenerative primary B-cell response35 necessary in patients lacking memory B-cell responses. The examination of B-cell development in the bone marrow of 2 ICOS-deficient patients demonstrated a relative block at the pre-B1 stage, compatible with the idea of exhaustion of the central B-cell development at the time of high demand. Alternatively, ICOS signaling may be required as a survival signal in early, bone marrow–dependent B-cell development. Currently, however, there is no evidence from ICOS–/– or ICOS-L–/– mice to support this assumption.16-18,36,37 Moreover, preliminary bone marrow analysis of ICOS-positive patients with CVID suggests that a partial block between the pre–B-I and -II stage may not be ICOS specific (M.S., H.H.P., unpublished data, November 2004).
Unlike the severe effect on B-cell homeostasis, the distribution of naive, memory, and effector T-cell populations evaluated by the expression of CD4 versus CD8, CD45RA versus CD45RO, including the differentiation of T helper 1 (Th1) versus Th2 memory T cells seemed to be normal in human ICOS deficiency. The murine data as well as the incidence of the carcinoma of the vulva may however hint at more subtle defects.
In summary, ICOS deficiency may be suspected in patients with the clinical phenotype of CVID and an autosomal recessive familial trait. Patients may present as children as well as adults. Most of the adult patients present with a B lymphopenia, and all patients had a severe reduction of switched memory B cells (Freiburg classification, CVID type I28 ). This finding, however, is not specific for ICOS deficiency because about 75% of patients with CVID show an absence of class-switched memory B cells.28 The diagnosis may be confirmed by the absence of ICOS surface expression on activated T cells and by genetic analysis. The immunologic analysis of ICOS-deficient patients has provided new insights into the role of ICOS in the adaptive immune response and has defined a series of new CVID candidate genes. Thus, upstream genes involved in the regulation of ICOS, downstream genes involved in the signaling pathway of ICOS (c-Maf, Akt), ICOSligand, cytokines released after ICOS ligation (IL-10, IL-17), and their respective receptors are potential candidate genes for patients with CVID type I. Therefore, ICOS deficiency serves as an excellent monogenic model for CVID.
Prepublished online as Blood First Edition Paper, December 29, 2005; DOI 10.1182/blood-2005-07-2955.
L.B. and U.S. contributed equally to this work.
Supported by the German Research Foundation (DFG) grant SFB620, project C1 (K.W. and H.H.P.) and project C2, grant GR1617/3 (B.G.); by the European Union EU–Improved healthcare for patients with primary antibody deficiencies through new strategies elucidating their pathophysiology (IMPAD) grant QLG1-CT-2001-01536 (H.H.P.); and by grants from INSERM (A.D.), l'Association la Recherche Contre le Cancer (ARC) (A.D.), la Ligue Contre le Cancer (A.D.), and the French Rare Disease Program (A.D.).
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
We thank Drs W. Mezger, Ekkehard Röther, Sigune Goldacker, Andres Rump, Hans Ochs, Jens Thiel, Peter Vaith, and Elisabeth Förster-Waldl for their involvement in the care of the patients; Dr U. Sachs (detection of antineutrophil antibodies); Margarete Ditter for her technical excellence (immunohistochemistry); and Richard Kroczeck for donation of the F44 antibody.


This feature is available to Subscribers Only
Sign In or Create an Account Close Modal