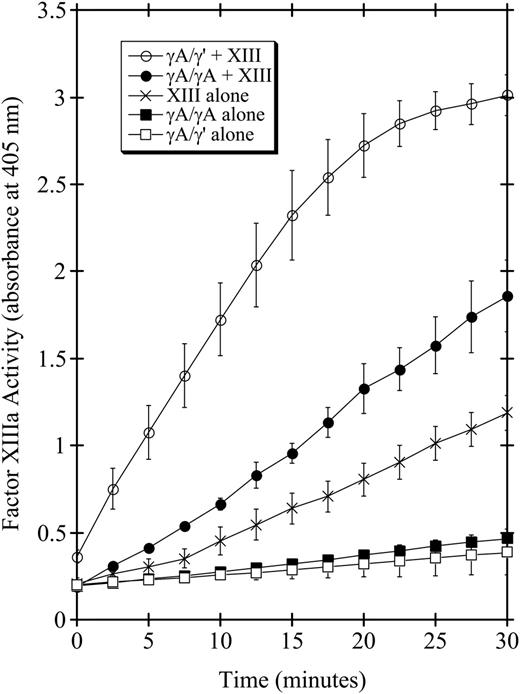
A recently published paper by Siebenlist et al1 presents data that apparently contradicts results published previously by our laboratory2 and others.3 Siebenlist et al's data show that factor XIII activation is more rapid in the presence of γA/γA fibrinogen than in the presence of γA/γ′ fibrinogen.1 These findings are in contrast to our previous results showing that factor XIII activation is more rapid in the presence of γA/γ′ fibrinogen. Siebenlist et al1 reconciled this discrepancy by proposing that our fibrinogen 2 (γA/γ′ fibrinogen) preparations “contained unaccounted for factor XIII.” 1(p2734) Curiously, they did not present any analysis of contaminating factor XIII in their own fibrinogen preparations. But the fibrinogen preparations in our paper were assayed for contaminating factor XIII activity that can copurify with γA/γ′ fibrinogen.4 Although these data were not included in the final version of our paper,2 they are presented here in Figure 1.
Factor VIII activation in the presence or absence of fibrinogen. One NIH unit/mL α-thrombin in 100 μL buffer containing 1 mM CaCl2, and 1 mM GPRP peptide to prevent fibrin polymerization,5 was incubated at room temperature with 43 nM factor XIII (×), 86 nM γA/γA fibrinogen (▪), 86 nM γA/γ′ fibrinogen (□), 86 nM γA/γA fibrinogen + 43 nM factor XIII (•), or 86 nM γA/γ′ fibrinogen + 43 nM factor XIII (○). Thrombin was inactivated at the indicated times with 0.5 mM PPACK. Factor XIIIa activity was measured by the incorporation of 0.5 mM 5-(biotinamido)pentylamine into immobilized N, N′-dimethylcasein, and detected at 405 nm by P-nitrophenyl phosphate hydrolysis following incubation with streptavidin-conjugated alkaline phosphatase.6
Factor VIII activation in the presence or absence of fibrinogen. One NIH unit/mL α-thrombin in 100 μL buffer containing 1 mM CaCl2, and 1 mM GPRP peptide to prevent fibrin polymerization,5 was incubated at room temperature with 43 nM factor XIII (×), 86 nM γA/γA fibrinogen (▪), 86 nM γA/γ′ fibrinogen (□), 86 nM γA/γA fibrinogen + 43 nM factor XIII (•), or 86 nM γA/γ′ fibrinogen + 43 nM factor XIII (○). Thrombin was inactivated at the indicated times with 0.5 mM PPACK. Factor XIIIa activity was measured by the incorporation of 0.5 mM 5-(biotinamido)pentylamine into immobilized N, N′-dimethylcasein, and detected at 405 nm by P-nitrophenyl phosphate hydrolysis following incubation with streptavidin-conjugated alkaline phosphatase.6
These data clearly demonstrate that our fibrinogen preparations did not contain sufficient contaminating factor XIII to account for the increased rate of factor XIII activation that we observed when γA/γ′ fibrinogen was added to factor XIII. Therefore, the reason for the discrepancies between our results and those of Siebenlist et al1 is still unclear. One possibility is that Siebenlist et al1 measured the rate of factor XIIIa-subunit activation peptide cleavage, whereas our laboratory measured the appearance of factor XIIIa activity. These two assays do not, in fact, measure the same thing. While factor XIII activation peptide cleavage is necessary for factor XIII activation under physiologic conditions, it is not sufficient. A second step that must occur prior to activation is the dissociation of the b-subunits of factor XIII.7 The paper by Siebenlist et al1 measured the first step in factor XIII activation, activation peptide cleavage, whereas our paper measured the last step in factor XIII activation, the expression of catalytic activity. Our hypothesis is that γA/γ′ fibrin(ogen), by virtue of its binding to factor XIIIb-subunits through the γ′ chain,4 increases the rate of factor XIII activation by increasing the rate of factor XIIIb-subunit dissociation from the catalytic a′-subunits.
Another possibility for the discrepancy in results is that subtle differences in fibrinogen purification methods among different laboratories may result in different amounts of contaminants or different amounts of fibrinogen degradation that may influence these assays. Such differences could potentially account for the discrepancies between the paper by Siebenlist et al1 and our paper, and possibly the discrepancies between their paper and a previous paper by Cooper et al.3 The data of Cooper et al3 indicate that fibrinopeptide A release from γA/γA and γA/γ′ fibrinogen is identical but that fibrinopeptide B release is delayed from γA/γ′ fibrinogen. In contrast, the data presented by Siebenlist et al shows that fibrinopeptide A release from γA/γ′ fibrinogen is delayed compared to γA/γA fibrinogen, as well as fibrinopeptide B release. The reason for these discrepancies is still unclear.
Fibrinogen containing γ′ chains; is the assay measuring what Farrell's group expects?
Dr Farrell has objected to certain findings in our paper1 that do not agree with his published work2 : for example, in our hands, factor XIII (fXIII) activation in the presence of fibrinogen 2 (γA-γ′) was slower than with fibrinogen 1 (γA-γA). To explain the differences, we suggested that the fibrinogen 2 used in his experiments was likely to have contained fXIII that had coeluted with the fibrinogen 2.As far as we can determine, Farrell's group did not process the fibrinogen 2 in a manner that would have removed the fXIII present in chromatographically isolated fibrinogen 2.3 Contrary to Dr Farrell's assertion, we very carefully redescribed our method for rendering fibrinogen 2 fXIII free and how the product was analyzed and shown to be fXIII free.1,3
Farrell asserted that his “activity” assay for fXIII activation was more appropriate than activation peptide cleavage from fXIII. Activation peptide release is an accepted and often-used surrogate for measuring fXIII activation. Furthermore, activation peptide release is the rate limiting step of fXIII activation, not the Ca++-mediated dissociation of A* from the B subunits.
To support the contention that the fibrinogen 2 Farrell's group used was depleted of fXIII, he included some unpublished experiments in his letter. The methodological design of these experiments raises more questions than they answer. First, since chromatographic separation of fibrinogen 1 from fibrinogen 2 results in fXIII-free fibrinogen 1, why was transglutaminase activity observed with fibrinogen 1 alone? Second, we previously demonstrated that “unactivated” fXIII, such as is present in chromatographically purified fibrinogen 2 and in the activation mixtures that Farrell measured in his assay, cross-links fibrin almost as rapidly as thrombin-activated factor XIIIa. Factor XIII also cross-links fibrinogen and artificial substrates, but at slower rates.4 Since only one (ie, biotinylated pentylamine incorporation into dimethylcasein) of several reactions (eg, fibrin(ogen) cross-linking, dimethylamine incorporation into fibrin(ogen), biotinylated pentylamine incorporation into fibrin(ogen)) was measured in his assay, we wonder what the effect of competing fXIII substrate(s), namely fibrin(ogen), was on the pentylamine incorporation into dimethylcasein?
Dr Farrell also posited that the discrepancies in FPA release between our paper and one by Cooper et al5 might be explained by contaminants or variable degrees of fibrinogen degradation in our preparations. Our explanation is simpler. Differences between our results and those of Cooper et al5 were due to the lower levels of thrombin we employed, and those lower concentrations enabled us to measure differences in FPA release between fibrinogen 2 and fibrinogen 1. Other experiments in our paper, showing down-regulation of thrombin catalytic activity (kcat) when bound to fibrin 2 γ′ chains, provide more than adequate support for our explanation and also for the slower release of activation peptide from fXIII in the presence of fibrinogen 2.
Correspondence: Department of Biomedical Sciences, College of Health Sciences, Marquette University, PO Box 1881, Milwaukee, WI 53233-1881; e-mail: kevin.siebenlist@marquette.edu.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal