Abstract
Recent studies suggest that thrombophilic abnormalities and the use of oral contraceptives (OCs) are the leading causes of cerebral vein thrombosis (CVT). The purpose of this study was to assess the association between CVT and thrombophilic states, OCs, and their interaction. For data sources, we used the MEDLINE, EMBASE, and Cochrane Library databases (January 1994 to March 2005), reference lists of retrieved articles, and contact with content experts. We selected studies comparing the prevalence of OC use and the prevalence of prothrombitic abnormalities in patients with CVT compared with healthy controls. Two reviewers independently selected studies and extracted study characteristics, quality, and outcomes. Odds ratios (ORs) were calculated for each trial and pooled using the Mantel-Haenszel method. Seventeen studies were included. There was an increased risk of CVT in patients using OCs (OR 5.59; 95% confidence interval [CI] 3.95 to 7.91; P < .001), and in patients with factor V Leiden (OR 3.38; 95% CI 2.27 to 5.05; P < .001), with mutation G20 210A of prothrombin (OR 9.27; 95% CI 5.85 to 14.67; P < .001) and with hyperhomocysteinemia (OR 4.07; 95% CI 2.54 to 6.52; P < .001). We concluded that OC users, and patients with factor V Leiden, the prothrombin G20 120A mutation, and hyperhomocysteinemia are at a significantly increased risk of CVT.
Introduction
Cerebral vein thrombosis (CVT) is a relatively uncommon but potentially life-threatening disease. Recent series showed that as many as 8% of patients with CVT die.1 In previous studies, the majority of CVT cases were found to be secondary to local or systemic infections and more than 30% of CVT cases were considered idiopathic.2,3 However, more recent trials reported other risk factors, such as thrombophilia or the use of oral contraceptives (OCs) to be associated with CVT.1,4 In the last decade several inherited or acquired factors causing hypercoagulable state have been studied in patients with deep vein thrombosis (DVT) and pulmonary embolism (PE). Resistance to activated protein C, the most common cause of inherited thrombophilia, was discovered in 1993.5 One year later, factor V Leiden was identified as the most frequent cause of this resistance.6 Finally, in 1996, a mutation in the prothrombin regulatory sequence was found to be a common prothrombotic factor.7 Several large studies and meta-analyses have confirmed that factor V Leiden and G20 210A of mutation of prothrombin are associated with an increased risk of DVT and PE.8-10 Large epidemiologic studies have also confirmed that OC users, particularly users of third-generation OCs, are at increased risk of venous thromboembolism (VTE).11 Furthermore, the absolute risk of venous thrombosis in women who use OCs and have a thrombophilic state is higher than expected from the addition of these risks, suggesting a possible enhancement in their individual procoagulant effect.12
Unfortunately, due to the rarity of this pathology, the prevalence of these factors in patients with CVT has been evaluated only in small studies and the results are often conflicting or not conclusive.
The aims of our systematic review and meta-analysis are therefore to assess the prevalence and the risk of CVT associated with different inherited and acquired thrombophilic states, to evaluate the prevalence and the risk of CVT associated with use of OCs, and to evaluate the possible interaction between these different prothrombotic factors.
In particular, we considered the following factors: use of OCs, factor V Leiden, prothrombin mutation G20 210, hyperhomocysteinemia, anti–thrombin III, protein C, or protein S deficiency and antiphospholipid syndrome, and either anticardiolipin antibodies and/or lupus anticoaugulants.
Methods
Study identification
We tried to identify all published studies that evaluated the presence of inherited or acquired risk factors for CVT using the MEDLINE (1994 to March 2005 Week 5), EMBASE (1994 to 2005 Week 15) and The Cochrane Library (2005, Issue 2) electronic databases. We excluded articles published before 1994 since that was the year that substantial evidence for the importance of activated protein C resistance/factor V Leiden began to become available. The search strategy was developed in collaboration with a professional librarian, had no language restrictions, and used the keywords and subject headings presented in Appendix 1. We supplemented our search by manually reviewing the reference list of all retrieved articles and contacted content experts for additional published or unpublished trials.
Study selection
Study selection was performed independently by 2 reviewers with disagreements resolved through discussion and by opinion of a third reviewer, if necessary. Studies were included if they met the following criteria: (1) diagnosis of CVT was objectively confirmed; (2) patients were 18 years or older; (3) patients were compared with a control group of healthy subjects without a history of thromboembolic disease or genetic relationship with the patients; (4) prevalence of at least one of the following risk factors was measured in patients and in control group: use of OCs, factor V Leiden, prothrombin mutation G20 210, hyperhomocysteinemia, anti–thrombin III, protein C, or protein S deficiency and antiphospholipid syndrome (anticardiolipin antibodies and/or lupus anticoaugluant positive); (5) inherited or acquired factors causing hypercoagulable state were measured in an objectively and commonly accepted manner. We excluded case series of patients and all the studies in which CVT was diagnosed only on the basis of clinical symptoms and not confirmed by an objective imaging. When multiple papers for a single study had been published, we decided to use the latest publication and to supplement it, if necessary, with data from the earlier publications.
To assess the agreement between reviewers for study selection, we used the kappa (k) statistic, which measures agreement beyond chance.13 According to Maclure and Willett, k values higher than 0.6 are considered to represent a substantial agreement and values higher than 0.8 an almost perfect agreement.14
Study validity assessment
Two unmasked investigators independently completed the assessment of study validity (F.D., W.A.). Because the use of quality scoring systems or quality scales in observational studies is controversial,15 the internal validity of each study was evaluated considering 2 potential sources of bias of case-control studies.16 Studies were considered of low quality when subjects were arbitrarily excluded from either the case or control groups, and when baseline characteristics of the control group (age, sex) were not matched with characteristics of the patient group. Otherwise studies were considered of higher quality.
Data extraction
Two reviewers independently completed data extraction. Disagreement was resolved by consensus and by the opinion of a third reviewer, if necessary. The following baseline characteristics for cases and control groups were collected: number of subjects studied, mean age, variation in age, sex, and race. One or more of the following risk factors was collected in each study: (1) number and proportion of patients and controls with factor V Leiden, prothrombin mutation G20 210, hyperhomocisteinemia, anti–thrombin III, protein C, or protein S deficiency and antiphospholipid syndrome, either anticardiolipin antibodies and lupus anticoaugluant positive; (2) number and proportion of women of reproductive age using OCs (pregnant, postpartum, and postmenopausal women were excluded). If the required data could not be located in the published report, we contacted the corresponding author by mail, with a reminder e-mail sent in 15 days.
Statistical analysis
We used Review Manager (RevMan; version 4.2 for Windows; Oxford, England; The Cochrane Collaboration, 2003) to pool data for each risk factor by using the Mantel-Haenszel method and a fixed-effects model.17 Pooled results are reported as odds ratio (OR) and are presented with 95% confidence interval (CI) and with 2-sided P values. A P value of .05 or less was considered statistically significant. Statistical heterogeneity was evaluated using the I2 statistic, which assesses the appropriateness of pooling the individual study results.18 The I2 value provides an estimate of the amount of variance across studies due to heterogeneity rather than chance. When heterogeneity was found, the analysis was repeated using the random-effects model, which includes a measure of variance between studies.19 To further clarify the role of thrombophilic states and OCs as risk factors for CVT, a priori secondary analysis was planned. We decided to assess, whenever possible, the prevalence and the risk of CVT associated with different inherited and acquired thrombophilic states and associated with the use of OCs, in patients without any other risk factor known to predispose to thrombosis. Finally, funnel plots of effect size against standard error were completed, whenever possible, to assess for the presence of publication bias.20
Results
Study identification and selection
We identified 436 studies using our search strategy: 187 from MEDLINE, 346 from EMBASE, and 20 from The Cochrane Library (Figure 1). There were 97 studies identified in duplicate. We could exclude 410 studies after title and abstract screening using the predefined inclusion and exclusion criteria, and 26 studies were retrieved for more detailed evaluation.21-46 The interobserver agreement for the study selection was excellent, with a k of 0.99. Contact with the experts and manual review of references did not reveal any additional studies. Nine of the 26 studies were subsequently excluded for the following reasons: 8 did not meet inclusion criteria27,28,33,34,36,40,42,44 and 1 contained duplicate data.25 Seventeen studies were therefore included in our systematic review. 21-24,26,29-32,35,37-39,41,43,45,46
Study characteristics
Of the 17 studies included, 16 were written in the English language21-24,26,29-31,35,37-39,41,43,45,46 and 1 in the Chinese language.32 All included studies were case-control studies. The number of subjects studied ranged from 48 to 2285. Baseline characteristics of included studies are summarized in Table 1. Eight studies evaluated the frequency of OC use in patients with CVT and in a control population.21-23,29,30,39,41,45 Sixteen studies evaluated the role of different thrombophilic factors in the CVT pathogenesis21-23,25,29-32,35,37-39,43,45,46 ; 13 studies considered Leiden mutation of factor V22-24,26,29-32,34,38,43,45,46 ; 9 mutation G20 210 of factor II22-24,29-31,35,39,46 ; 4 hyperhomocysteinemia21-23,31 ; 2 anti–thrombin III, protein C, and protein S deficiency23,24 ; 1 antiphospholipid syndrome23 ; and 1 anticardiolipin antibodies.37 No studies specifically studied patients with more than 1 hereditary or acquired prothrombotic defect, except as discussed in “OCs and thrombophilic factor interactions.”
Baseline characteristics
Study, year (reference no.) . | Risk factors . | Cases, n . | Mean or median age for patients (range), y . | Cases, description . | Controls, n . | Mean or median age for controls (range), y . | Controls, description . | Excluded patients . |
|---|---|---|---|---|---|---|---|---|
| Gadelha et al, 2005 (29) | FV, FII, OCs | 26 | 28.5 (3-46) | Patients < 50 aa with CVT confirmed by DA or MRA | 217 | 29 (15-62) | Age- and racially matched healthy individuals with no history of thrombosis or genetic relationship brought by patients and volunteering physicians and health care workers | Patients with major systemic diseases known to predispose to thrombosis or with any positive antiphospholipid test |
| Boncoraglio et al, 2004 (31) | FV, FII, Hyper-Hcy | 26 | 43 (21-73) | Consecutive patients with first episode of CVT confirmed by DA or MRA | 100 | 42 (21-72) | Healthy hospital employees | NA |
| Ventura et al, 2004 (22) | FV, FII, Hyper-Hcy, OCs | 30 | 35 (16-49) | Patients with CVT confirmed by CT DA or MRA | 40 | 34 (18-51) | Age- and sex-matched healthy individuals with no history of thrombosis or vascular disease | CVT related to other risk factors |
| Rodrigues et al, 2004 (30) | FV, FII, OCs | 42 | 28 (2-68) | Patients with objectively confirmed CVT | 134 | 34 (-) | Healthy subjects with no history of thrombosis or genetic relationship with patients | NA |
| Cantu et al, 2004 (21) | Hyper-Hcy, OCs | 45 | 28 (14-55) | Patients with CVT confirmed by DA or MRA | 90 | 28 (16-53) | Age- and sex-matched healthy individuals with no history of thrombosis or vascular disease | Patients dead or lost to follow up |
| Martinelli et al, 2003 (23) | FV, FII, OCs, Hyper-Hcy, AT III, PC, PS, APL | 121 | 33 (12-64) | Patients with first episode of CVT confirmed by CT DA or MRA | 242 | 36 (13-62) | Age- and sex-matched healthy individuals with no history of thrombosis | Patients with incomplete thrombophilia screening, uncertainness in diagnosis, or previous thrombotic episode |
| Meng, 2002 (32) | FV | 20 | 31 (20-48) | NA | 50 | 38 (18-58) | NA | NA |
| Bombeli et al, 2002 (24) | FV, FII, AT III, PC, PS | 51 | 36.7 (17-61) | Patients with CVT confirmed by DA or MRA | 120 | 37.4 (19-62) | Healthy individuals with no history of thrombosis or vascular disease and without any medication | Patients with unclear diagnosis of acquired or hereditary protein C or S deficiency |
| Margaglione et al, 2001 (46) | FV, FII | 28 | NA | Patients with CVT confirmed by DA or MRA | 1304 | NA (22-66) | Healthy hospital employees | Patients < 14 aa or with previous thromboembolic episodes |
| Voetsch et al, 2000 (35) | FV, FII | 14 | 24.8 (16-31) | Patients with CVT confirmed by DA or MRA considered idiopathic | 225 | 34.1 (16-50) | Age- and sex-matched volunteer physicians, students, and laboratory staff | Patients with deficiency of natural anticoagulants or antiphospholipid antibodies: underlying venous malformation |
| Reuner et al, 1998 (39) | FII, OC | 45 | 37 (3-69) | Patients with CVT confirmed by CT, DA, or MRA | 354 | 39 (18-65) | Healthy blood donors | NA |
| De Bruijn et al, 1998 (41) | OC | 37 | NA (18-49) | Women with CVT confirmed by DA or MRA | 2248 | NA (18-49) | Age-matched randomly selected women | Women < 18 aa or pregnant |
| Weih et al, 1998 (26) | FV | 12 | 33.8 (21-60) | Consecutive patients with CVT confirmed by DA or MRA | 36 | 34.2 (NA) | Age- and sex-matched subjects with no history of thrombosis | Patients with septic venous thrombosis, neoplasm or non-German descendent |
| Cristopher et al, 1998 (37) | ACL | 31 | 27.6 (NA) | Patients with CVT confirmed by CT or DA | 31 | 26.6 (NA) | Age- and sex-matched asymptomatic subjects | Patients with CVT related to infection or trauma |
| Ludemann et al, 1998 (38) | FV | 55 | 40 (11-83) | Patients with CVT confirmed by DA or MR imaging | 272 | NA (18-55) | Healthy adult subjects | NA |
| Zuber et al, 1998 (43) | FV | 19 | 38 (20-72) | Patients with CVT confirmed by DA or MR imaging | 57 | NA | Age- and sex-matched subjects with no history of cerebrovascular disease | NA |
| Martinelli et al, 1996 (45) | FV, OC | 25 | 32 (21-64) | Patients with CVT confirmed by CT, DA, or MR imaging | 75 | NA | Age- and sex-matched subjects with no history of thrombosis | CVT secondary to a local infection |
Study, year (reference no.) . | Risk factors . | Cases, n . | Mean or median age for patients (range), y . | Cases, description . | Controls, n . | Mean or median age for controls (range), y . | Controls, description . | Excluded patients . |
|---|---|---|---|---|---|---|---|---|
| Gadelha et al, 2005 (29) | FV, FII, OCs | 26 | 28.5 (3-46) | Patients < 50 aa with CVT confirmed by DA or MRA | 217 | 29 (15-62) | Age- and racially matched healthy individuals with no history of thrombosis or genetic relationship brought by patients and volunteering physicians and health care workers | Patients with major systemic diseases known to predispose to thrombosis or with any positive antiphospholipid test |
| Boncoraglio et al, 2004 (31) | FV, FII, Hyper-Hcy | 26 | 43 (21-73) | Consecutive patients with first episode of CVT confirmed by DA or MRA | 100 | 42 (21-72) | Healthy hospital employees | NA |
| Ventura et al, 2004 (22) | FV, FII, Hyper-Hcy, OCs | 30 | 35 (16-49) | Patients with CVT confirmed by CT DA or MRA | 40 | 34 (18-51) | Age- and sex-matched healthy individuals with no history of thrombosis or vascular disease | CVT related to other risk factors |
| Rodrigues et al, 2004 (30) | FV, FII, OCs | 42 | 28 (2-68) | Patients with objectively confirmed CVT | 134 | 34 (-) | Healthy subjects with no history of thrombosis or genetic relationship with patients | NA |
| Cantu et al, 2004 (21) | Hyper-Hcy, OCs | 45 | 28 (14-55) | Patients with CVT confirmed by DA or MRA | 90 | 28 (16-53) | Age- and sex-matched healthy individuals with no history of thrombosis or vascular disease | Patients dead or lost to follow up |
| Martinelli et al, 2003 (23) | FV, FII, OCs, Hyper-Hcy, AT III, PC, PS, APL | 121 | 33 (12-64) | Patients with first episode of CVT confirmed by CT DA or MRA | 242 | 36 (13-62) | Age- and sex-matched healthy individuals with no history of thrombosis | Patients with incomplete thrombophilia screening, uncertainness in diagnosis, or previous thrombotic episode |
| Meng, 2002 (32) | FV | 20 | 31 (20-48) | NA | 50 | 38 (18-58) | NA | NA |
| Bombeli et al, 2002 (24) | FV, FII, AT III, PC, PS | 51 | 36.7 (17-61) | Patients with CVT confirmed by DA or MRA | 120 | 37.4 (19-62) | Healthy individuals with no history of thrombosis or vascular disease and without any medication | Patients with unclear diagnosis of acquired or hereditary protein C or S deficiency |
| Margaglione et al, 2001 (46) | FV, FII | 28 | NA | Patients with CVT confirmed by DA or MRA | 1304 | NA (22-66) | Healthy hospital employees | Patients < 14 aa or with previous thromboembolic episodes |
| Voetsch et al, 2000 (35) | FV, FII | 14 | 24.8 (16-31) | Patients with CVT confirmed by DA or MRA considered idiopathic | 225 | 34.1 (16-50) | Age- and sex-matched volunteer physicians, students, and laboratory staff | Patients with deficiency of natural anticoagulants or antiphospholipid antibodies: underlying venous malformation |
| Reuner et al, 1998 (39) | FII, OC | 45 | 37 (3-69) | Patients with CVT confirmed by CT, DA, or MRA | 354 | 39 (18-65) | Healthy blood donors | NA |
| De Bruijn et al, 1998 (41) | OC | 37 | NA (18-49) | Women with CVT confirmed by DA or MRA | 2248 | NA (18-49) | Age-matched randomly selected women | Women < 18 aa or pregnant |
| Weih et al, 1998 (26) | FV | 12 | 33.8 (21-60) | Consecutive patients with CVT confirmed by DA or MRA | 36 | 34.2 (NA) | Age- and sex-matched subjects with no history of thrombosis | Patients with septic venous thrombosis, neoplasm or non-German descendent |
| Cristopher et al, 1998 (37) | ACL | 31 | 27.6 (NA) | Patients with CVT confirmed by CT or DA | 31 | 26.6 (NA) | Age- and sex-matched asymptomatic subjects | Patients with CVT related to infection or trauma |
| Ludemann et al, 1998 (38) | FV | 55 | 40 (11-83) | Patients with CVT confirmed by DA or MR imaging | 272 | NA (18-55) | Healthy adult subjects | NA |
| Zuber et al, 1998 (43) | FV | 19 | 38 (20-72) | Patients with CVT confirmed by DA or MR imaging | 57 | NA | Age- and sex-matched subjects with no history of cerebrovascular disease | NA |
| Martinelli et al, 1996 (45) | FV, OC | 25 | 32 (21-64) | Patients with CVT confirmed by CT, DA, or MR imaging | 75 | NA | Age- and sex-matched subjects with no history of thrombosis | CVT secondary to a local infection |
ACL indicates anticardiolipin antibodies; ATIII, deficiency of anti-thrombin III; CT, computerized tomography; DA, digital angiography; FII, mutation 20210 factor II; FV, mutation 1691 factor V (Leiden); Hyper-Hcy, hyperhomocysteinemia; MR, magnetic resonance; MRA, magnetic resonance angiography; OCs, oral contraceptives; PC, protein C deficiency; PS, protein S deficiency; and NA, not applicable.
Of the 4 studies that evaluated the presence of hyperhomocysteinemia, 2 measured only fasting levels of homocysteine22,31 and 2 also postmethionine load levels.21,23 Therefore, we decided to pool only fasting levels of homocysteine in our meta-analysis.
Study quality
Oral contraceptives
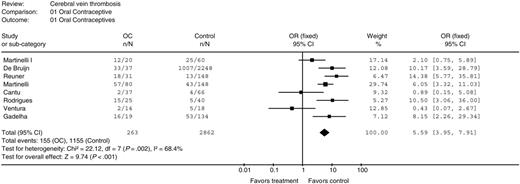
There were 8 studies that evaluated the role of OCs in CVT.21-23,29,30,39,41,45 The pooled analysis included 263 women with CVT and 2862 women without CVT. In women using OCs, the summary OR for developing CVT was 5.59 in comparison to controls (95% CI 3.95 to 7.91; P < .001), Figure 2. Heterogeneity between the studies was significant (I2 = 68.4%; P = .002) and was maintained after the exclusion all the possible outliers from the analysis (data not shown). However, a similar OR was obtained using the random-effects model (OR 4.79; 95% CI 2.40 to 9.58; P < .001). This result probably reflects the difference among the studies in the selection of the population analyzed. Funnel plot of OR versus standard error appeared asymmetric with an absence of studies in the bottom right hand corner, suggesting that smaller, unpublished studies that demonstrate an increased OR of CVT in patients taking OCs were not included in our meta-analysis.
Odds ratio for cerebral vein thrombosis for oral contraceptive users. n indicates the number positive; N, total number.
Odds ratio for cerebral vein thrombosis for oral contraceptive users. n indicates the number positive; N, total number.
Odds ratio for cerebral vein thrombosis in factor V Leiden carriers. n indicates the number positive; N, total number.
Odds ratio for cerebral vein thrombosis in factor V Leiden carriers. n indicates the number positive; N, total number.
Factor V Leiden
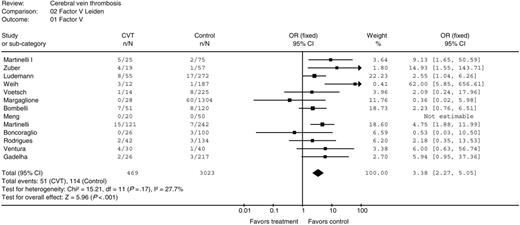
Thirteen studies included 469 case and 3023 control subjects.22-24,26,29-32,35,37,43,45,46 In 12 studies the method used to determine the presence of the factor V Leiden mutation was described22-24,26,29,31,32,35,38,43,45,46 ; in all studies detection of Leiden mutation was carried out by amplification of a fragment of exon 10 of the factor V and posterior digestion with an endonuclease, according to the method used by Bertina et al.6 Compared with controls, the pooled OR for CVT in patients with factor V Leiden mutation was 3.38 (95% CI 2.27 to 5.05; P < .001), Figure 3. Heterogeneity between the studies was extremely low (I2 = 27.7%; P = .18).
Funnel plot of OR versus standard error evaluated 12 of 13 studies included in our meta-analysis since in 1 study there were neither patients nor controls with Leiden mutation of factor V.32 The plot appeared symmetric, suggesting absence of publication bias.
Mutation G20 210A of factor II
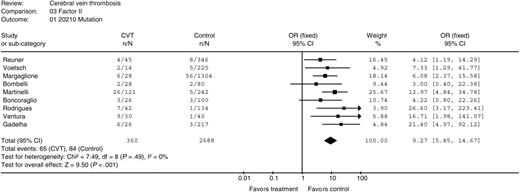
Nine studies evaluated the role of mutation G20 210A in the risk of CVT.22-24,29-31,35,39,46 In the pooled analysis 360 patients and 2688 controls were studied. In 8 of 9 studies, the method to detect the G20 210A mutation of factor II was described.22-24,29,31,35,39,46 All the studies used the method described by Poort et al.7 The pooled OR of developing CVT was 9.27 (95% CI 5.85 to 14.67; P < .001) in patients with mutation G20 210A of factor II compared with controls, Figure 4. There was no significant between-study heterogeneity (I2 = 0%; P = .49). The funnel plot appeared symmetric, suggesting absence of publication bias.
Odds ratio for cerebral vein thrombosis in G20 210A factor II mutation carriers. n indicates the number positive; N, total number.
Odds ratio for cerebral vein thrombosis in G20 210A factor II mutation carriers. n indicates the number positive; N, total number.
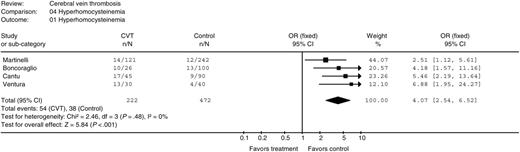
Hyperhomocysteinemia
Four case-control studies evaluated hyperhomocysteinemia in patients with CVT.21-23,31 Fasting hyperhomocysteinemia was defined as above the 95th percentile of normal population value in 2 studies,23,31 as above the 90th percentile of normal population value in 1 study,21 and as higher than 12 μM in 1 study.22 Compared with controls, the pooled OR for CVT in patients with hyperhomocysteinemia was 4.07 (95% CI 2.54 to 6.52; P < .001), Figure 5. There was no heterogeneity between the studies (I2 = 0%; P = .48). In the 2 studies that also evaluated postmethionine load levels of homocysteine, patients with fasting or postload hyperhomocysteinemia had an OR of developing CVT of 4.3 and 4.2, respectively, compared with controls.21,23 Due to the low number of studies, funnel-plot analysis could not be done. Therefore, the presence of publication bias could not be excluded.
Other risk factors
Two studies analyzed the role of the deficiency of anti–thrombin III, protein C, and protein S23,24 as risk factors for CVT. Only 1 study considered antiphospholipid syndrome23 and 1 anticardiolipin antibodies.37 Antithrombin levels were measured using functional assay and then confirmed by an immunologic assay. The combined OR of the 2 studies was 2.69 (95% CI 0.66 to 10.96; P = .19). Protein C and protein S were measured by functional and immunologic assays. The combined OR of the 2 studies was 11.10 (95% CI 1.87 to 66.05; P = .009) for protein C and 12.49 for protein S (95% CI 1.45 to 107.29; P = .03). Due to the low number of eligible patients, the confidence interval is very wide and it is impossible to draw any major conclusion from these results.
Odds ratio for cerebral vein thrombosis in patients with hyperhomocysteinemia. n indicates the number positive; N, total number.
Odds ratio for cerebral vein thrombosis in patients with hyperhomocysteinemia. n indicates the number positive; N, total number.
The only study that considered the antiphospholipid antibodies syndrome23 found a higher incidence of antiphospholipid antibodies in patients with CVT (9/121) compared with controls (0/242).
Finally, Christopher et al37 evaluated the role of anticardiolipin antibodies in CVT. They found a significantly higher incidence of anticardiolipin antibodies in patients (7/31) in comparison to controls (1/31) (OR 8.75; 95% CI 1.01 to 75.64).
OCs and thrombophilic factor interactions
Few studies provided separate analyses of OR of CVT in thrombophilic women taking OCs.23,29 Therefore, it was not possible to pool these data. In their recent study, Martinelli et al23 stratified patients for the presence of hyperhomocysteinemia, factor V Leiden, or prothrombin mutation and the intake of OCs. They found that the presence of both risk factors gave an OR of 19.5 (95% CI 5.7 to 67.3) for hyperhomocysteinemia, 30.0 (95% CI 3.4 to 263.0) for factor V Leiden, and 79.3 (95% CI 10.0 to 629.4) for prothombin mutation in comparison to controls.
The multivariate analysis performed in the study conducted by Gadelha et al29 confirmed the independent association between CVT, prothrombin mutation, and OC use.
Discussion
In the first meta-analysis published on this particular topic, we have reviewed 17 publications that analyzed the association between CVT and the most frequent prothrombotic states. We found a strong association between CVT and use of OCs, the factor V Leiden mutation, the G20 210A mutation of prothrombin, and hyperhomocysteinemia. Our conclusions are strengthened by the uniform nature of our results and the narrow confidence interval about the resulting ORs. Only 1 study failed to detect factor V Leiden mutation in both patient and control groups,32 but this study was carried out in Chinese patients within whom the prevalence of factor V Leiden is far lower than is seen in white patients.47 Data on antiphospholipid antibodies syndrome, deficiencies of anti–thrombin III, protein C, and protein S deficiency were inadequate to allow a reliable statistical analysis. However, a non–statistically significant trend toward an association with the disease for antithrombin, protein C, and protein S was observed. Finally, only 2 studies have evaluated the effect of a concomitant thrombophilia in women of reproductive age using OCs. These data suggest a possible interaction between OCs and some prothrombotic states (hyperhomocystinemia, factor V Leiden, prothrombin G20 210A). In particular, the risk of CVT in women on OCs who are carriers of the mutation G20 210A of prothrombin seemed to be much higher than in controls.
Several well-designed large trials, reviews, and meta-analyses have demonstrated that use of OCs and the presence of 1 or more thrombophilic factors is associated with an increased risk of DVT and PE.8-12,48-50 Testing for most common thrombophilic factors in young patients presenting with unprovoked serious thrombotic events is currently recommended,51 a recommendation supported by our observation of a strong association between thrombophilic states and CVT.
Our meta-analysis has limitations. First, our systematic review was restricted to case-control studies, and the application of formal meta-analytic methods to observational studies is controversial, since bias implicit in the study design may misrepresent the strength of associations within the data.15 To minimize this potential bias, we selected only studies in which the diagnosis of CVT was objectively confirmed and in which prothrombotic factors were measured with objectively and widely accepted methods. Second, studies included in our meta-analysis have different inclusion and exclusion criteria, and to combine results across studies may be inappropriate. However, the heterogeneity between the studies, calculated using the I2 statistic, was generally low. Only when we pooled studies that evaluated OCs in patients with CVT was the heterogeneity between studies remarkable, and it remained high after adjustment for possible outliers. These results suggest that baseline characteristics of patients included in the analysis may be different and that results should be interpreted cautiously. However, after repeating the analysis using a random-effects model, an approach that accounts for some of the variance between studies, we found similar results. Third, despite a careful review of references and contact with content experts, we failed to identify any published or unpublished study not found in our initial literature search. Finally, despite several efforts, it was not possible to have information about the methods used to diagnose CVT in one study in which the authors stated that CVT was objectively confirmed in all patients.30 However, since only 42 patients were included in that study, it is very unlikely that even in the worst-case scenario (the diagnosis of CVT was wrong in all patients) this could influence the results of our meta-analysis. Because it is recognized that publication bias can affect the results of meta-analyses, we attempted to assess this potential bias using a funnel plot. However, funnel plots appeared symmetric, suggesting the absence of publication bias in the 3 analyses that considered factor V Leiden, mutation G20 210A of the prothrombin, and hyperhomocysteinemia. In the funnel plat analysis of the association of OCs with CVT we observed asymmetry, with an absence of studies in the bottom right hand corner of the plot. This suggests that smaller, unpublished studies likely to demonstrate an increased risk of CVT with use of OCs were not included in our meta-analysis. However, inclusion of these studies and elimination of this bias, if it really existed, would increase the observed association between OCs and CVT.52
In conclusion, our meta-analysis shows that CVT is strongly associated with factor V Leiden mutation, with mutation G20 210A of prothrombin, and with hyperhomocysteinemia. The role of the other thrombophilic abnormalities in the pathogenesis of CVT is less clear. The use of OCs significantly increases the risk of CVT in women and preliminary data suggest that such risk is further increased in OC-using women with prothrombotic abnormalities. Therefore, our observations suggest that women with CVT should avoid use of OCs, and that the use of OCs in women with known thrombophilic states should be evaluated on an individual basis assessing the risk of CVT for different thrombophilic abnormalities. Future studies, including careful prospective studies of patients with CVT, are now warranted to better delineate the risk of recurrent thrombosis in patients with and without thrombophilic defects. However, due to the rarity of the disease, it is very unlikely that these studies will be performed.
Appendix: MEDLINE search strategy
(Intracranial embolism and thrombosis).mp. [mp = title, original title, abstract, name of substance word, subject heading word]/(7436)
Exp cerebral veins/(1672)
Exp intracranial thrombosis/ or exp sinus thrombosis, intracranial/ (2032)
1 or 2 or 3/ (10 728)
Exp factor V/ (4134)
Anti–thrombin III/ (5505)
Protein C/ (3904)
Protein S/ (1375)
Homocysteine/ or 5-methyltetrahydrofolate-homocysteine S-methyltransferase/ (6860)
Exp antibodies, antiphospholipid/ (5050)
Exp contraceptives, oral/ (33 169)
Prothrombin/ (6075)
Cerebral vein thrombosis.tw./ (79)
Cerebral venous thrombosis.tw./ (548)
Hyperhomocysteinemia/ (1470)
4 or 13 or 14/ (10 833)
5 or 6 or 7 or 8 or 9 or 10 or 11 or 12 or 15/ (61 761)
16 and 17/ (495)
Limit 18 to year = 1994-2005/ (236)
Limit 19 to “review articles”/ (28)
19 not 20/ (208)
Limit 21 to “all infant (birth to 23 months)”/ (21)
21 not 22/ (187)
Prepublished online as Blood First Edition Paper, January 5, 2006; DOI 10.1182/blood-2005-09-3578.
M.C. is a Career Investigator of the Heart and Stroke Foundation of Canada.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal