Abstract
Pregnancy is associated with hemostatic challenges that may lead to thrombosis. Heparin cofactor II (HCII) is a glycosaminoglycan-dependent thrombin inhibitor present in both maternal and fetal plasma. HCII activity increases during pregnancy, and HCII levels are significantly decreased in women with severe pre-eclampsia. Dermatan sulfate (DS) specifically activates HCII and is abundant in the placenta, but the locations of DS and HCII in the placenta have not been determined. We present evidence that DS is the major anticoagulant glycosaminoglycan in the human placenta at term. DS isolated from human placenta contains disaccharides implicated in activation of HCII and has anticoagulant activity similar to that of mucosal DS. Immunohistochemical studies revealed that DS is associated with fetal blood vessels and stromal regions of placental villi but is notably absent from the syncytiotrophoblast cells in contact with the maternal circulation. HCII colocalizes with DS in the walls of fetal blood vessels and is also present in syncytiotrophoblast cells. Our data suggest that DS is in a position to activate HCII in the fetal blood vessels or in the stroma of placental villi after injury to the syncytiotrophoblast layer and thereby inhibit fibrin generation in the placenta.
Introduction
Thrombotic lesions in the placenta are commonly associated with major complications of pregnancy, such as pre-eclampsia, still-birth, fetal growth retardation, preterm labor, and preterm rupture of membranes.1-7 Exchange of blood gases and nutrients occurs at the level of placental villi, which consist of branched fetal blood vessels covered by a layer of syncytiotrophoblasts. Maternal blood circulates in the spaces between the villi. Although a low level of fibrin deposition localized to areas of denudation of the syncytiotrophoblast layer can be detected in normal placentas at term,8 larger fibrin deposits may occur in the intervillous space or in the fetal blood vessels and compromise placental function.9 Thrombin converts fibrinogen to fibrin and must be tightly regulated to prevent placental thrombosis.
Antithrombin and heparin cofactor II (HCII) are glycosaminoglycan (GAG)–dependent thrombin inhibitors found in both maternal and fetal plasma.10 Antithrombin inhibits thrombin rapidly when bound to heparin or heparan sulfate (HS), while HCII is activated by dermatan sulfate (DS) in addition to heparin and HS.11 Antithrombin deficiency is a well-established risk factor for venous thromboembolism and may increase the incidence of fetal loss approximately 1.7-fold.12 The physiologic role of HCII remains unclear, although above-average levels of HCII are reported to be associated with decreased prevalence of carotid artery atherosclerosis13 and in-stent re-stenosis.14,15
Indirect evidence suggests that HCII activity increases during pregnancy. During the third trimester, plasma HCII levels are approximately 140% of normal,16-18 and thrombin-HCII complexes are elevated up to 4-fold.19 At the same time, both maternal and fetal plasma contain trace amounts of a DS proteoglycan that stimulates thrombin inhibition by HCII.20 The placenta is rich in DS21 and may be the source of this proteoglycan. In one study, a DS proteoglycan (decorin) was purified from human placenta and was shown to have HCII-dependent anticoagulant activity.22 Interestingly, HCII levels are only approximately 50% of normal in women with severe pre-eclampsia,16 suggesting an association between decreased HCII activity and placental dysfunction.
In the present work, we isolated DS from human term placenta and characterized its sulfation pattern. We also performed immunohistochemical analyses to determine the location of DS and HCII in the normal term placenta. Our findings suggest that HCII and DS are positioned to interact with one another and inhibit thrombin at sites of villous injury.
Materials and methods
Reagents
Crude papain was purchased from Sigma, St Louis, MO; human α-thrombin from Haematologic Technologies, Essex Junction, VT; and chondroitin AC II–lyase (catalog no. 100335-1A), chondroitin ABC–lyase (catalog no. 100332-1A), chondroitin B–lyase (catalog no. 100337-1A), and Flavobacterium heparitinase I (catalog no. 100704-1) from Seikagaku, Tokyo, Japan. Porcine intestinal mucosal DS and shark cartilage chondroitin sulfate (CS) C were from Sigma, bovine lung heparin from Upjohn, Kalamazoo, MI; and bovine kidney HS from Seikagaku. Disaccharide standards (C-Kit) were obtained from Seikagaku. Tosyl-Gly-Pro-Arg-P-nitroanilide (Chromozym TH) was from Roche Molecular Biochemicals, Indianapolis, IN; and 3,3′-diaminobenzidine (DAB500Pack) from Biocare Medical, Walnut Creek, CA. Mouse monoclonal anti–chondroitin sulfate (CS) ΔDi-4S and anti–human Δ-HS antibodies were purchased from Seikagaku, and affinity-purified goat anti–HCII IgG from Affinity Biologicals, Hamilton, Ontario, Canada. Biotinylated goat anti–mouse IgG, biotinylated rabbit anti–goat IgG, avidin:biotinylated peroxidase complex, and mounting medium were obtained from Vector Laboratories, Burlingame, CA. Tissue-Tek Optimal cutting temperature (OCT) compound was from Sakura, Tokyo, Japan.
Isolation of placental GAGs
Approval for this study was obtained from the Human Studies Committee of Washington University Medical School. Informed consent was provided in accordance with the Declaration of Helsinki. Placentas were obtained from women with uncomplicated pregnancies after vaginal delivery and were kept on ice until being processed (< 1 hour). Maternal decidua, fetal membranes, and visible blood vessels were removed. The remaining tissue was cut into fine pieces and rinsed several times with ice-cold phosphate-buffered saline (PBS). The minced tissue was then homogenized in a blender after adding a small volume of PBS. Approximately 250 g (40 g dry weight determined by lyophilization) of homogenized material was suspended in 0.5 L of 0.1 M sodium acetate buffer (pH 5.5) containing 25 g of crude papain, 5 mM EDTA, and 5 mM cysteine, and was incubated at 60°C for 24 hours. The incubation was then centrifuged at 2000g for 10 minutes at 4°C. The supernatant was collected and saved at 4°C, while the precipitate was resuspended in buffer and digested for another 24 hours with fresh enzyme. The clear supernatants from the 2 digests were combined, and the polysaccharides were precipitated with 250 mL of 50 mg/mL cetylpyridinium chloride for 1 hour at 37°C. The mixture was then centrifuged at 12 000g for 20 minutes at room temperature. The precipitate was washed twice with 4 volumes of 95% ethanol containing 0.5 M sodium acetate and lyophilized. The lyophilized powder was dissolved in 20 mM Tris-HCl, pH 7.4, and applied to a Mono Q HR 16/16 column (Amersham Biosciences, Piscataway, NJ) equilibrated with 50 mM Tris-HCl, pH 7.4. The column was eluted in 3 steps with 50 mM Tris-HCl, pH 7.4, containing 0.25 M NaCl, 0.8 M NaCl, and 2.0 M NaCl. Fractions were analyzed for uronic acid using the carbazole assay23 standardized with known amounts of porcine mucosal DS. The peaks were pooled, dialyzed against water using a Spectra/Por 1000 molecular weight cutoff membrane (Spectrum Laboratories, Los Angeles, CA), lyophilized, and stored at 4°C.
Characterization of placental GAGs
Pooled GAG fractions were treated with nitrous acid as described by Teien et al.24 Degradation of GAGs (2-16 mg/mL) with chondroitin AC II–lyase was carried out using 0.5 unit of enzyme per milligram of GAG in 0.04 M Tris-HCl buffer (pH 6) containing 0.04 M sodium acetate for 12 hours at 37°C; the incubations were continued for another 12 hours after addition of fresh enzyme. Degradation with chondroitin ABC–lyase was performed under the same conditions except that the buffer was 0.1 M Tris-HCl (pH 8) containing 0.03 M sodium acetate. After each step, the samples were dialyzed against water using a Spectra/Por 3500 molecular weight cutoff membrane and lyophilized. A portion of each sample was analyzed by agarose gel electrophoresis as previously described.25
The products of chondroitin ABC–lyase digestion were applied to a Superdex Peptide HR 10/30 column (Amersham Biosciences) equilibrated with 20% acetonitrile, 0.1% trifluoroacetic acid in water to separate disaccharides from partially cleaved or intact glycosaminoglycans. Void volume fractions containing disaccharides were detected by absorbance at 232 nm, pooled, and lyophilized. The disaccharides were then applied to a 250 × 4.6 mm Supelco Spherisorb SAX 5 μm HPLC column (Waters, Milford, MA) equilibrated with water (pH 3.5 adjusted with HCl) at a flow rate of 0.5 mL/minute. The bound material was eluted with a 22.5-mL linear gradient of 0 to 1.0 M NaCl in water (pH 3.5). Disaccharides were identified by comparison with the elution positions of known disaccharide standards.26 Porcine mucosal DS also was digested with chondroitin ABC–lyase as described in the preceding paragraph, and its disaccharide composition was determined.
Anticoagulant activity assays
Human HCII and antithrombin were purified as previously described.27 Placental GAGs at various steps of purification were tested for the ability to stimulate thrombin inhibition by HCII as follows: 10 μL of HCII (50 μg/mL), 10 μL of the GAG, and 10 nM human α-thrombin were mixed in a 1.5-mL semi-microcuvette in a total volume of 100 μL of buffer containing 50 mM Tris-HCl, 150 mM NaCl, and 1 mg/mL polyethylene glycol 8000, pH 7.4 (TS/PEG buffer). Thrombin was added last to initiate the reaction. After a 60-second incubation at room temperature, 500 μL of 100 mM tosyl-Gly-Pro-Arg-P-nitroanilide in TS/PEG buffer was added, and the absorbance at 405 nm was determined continuously for 100 seconds. The rate of change of absorbance was proportional to the concentration of active thrombin that remained in the incubation. The assay was calibrated with known amounts (0-2 μg) of porcine mucosal DS. To determine the ability of GAGs to stimulate thrombin inhibition by antithrombin, 10 μL of antithrombin (28 μg/mL) was substituted for HCII, and the assay was calibrated with bovine lung heparin.
Preparation of placental tissue sections
Small blocks of placental tissue were rinsed several times with ice-cold PBS. For the preparation of frozen sections, the tissue was embedded in Tissue-Tek OCT compound, frozen on dry ice, and stored at –70°C. For paraffin blocks, the tissues were fixed for 48 hours in 10% buffered neutral formalin, pH 6.8. The tissue blocks were washed, dehydrated in graded ethanol, and embedded in paraffin.
Antibody staining of GAGs
To determine the location of GAG chains in placental sections, we used monoclonal antibodies that detect the “stubs” remaining after digestion with various lyases.28,29 Unfixed frozen sections were incubated for 2 hours at 37°C with one of the following enzymes: 0.5 unit/mL of chondroitin ABC–lyase in 0.05 M Tris-HCl, pH 8.0; 0.5 unit/mL of chondroitin B–lyase in 0.05 M Tris-HCl, pH 7.4; 0.5 unit/mL of chondroitin AC II–lyase in 0.05 M Tris-HCl, pH 6.0; or 0.5 unit/mL of Flavobacterium heparitinase in 0.1 M sodium acetate, pH 7.0. Incubations with the respective buffers alone served as controls. The sections were treated with 0.3% (vol/vol) H2O2 in PBS for 10 minutes to eliminate endogenous peroxidase activity and then blocked for 1 hour with a solution containing 20 mg/mL bovine serum albumin and goat serum (1:100 dilution) in PBS, pH 7.4 (blocking buffer). Sections treated with chondroitin ABC-lyase, B-lyase, or AC II-lyase were incubated overnight at 4°C with 10 μg/mL ΔDi-4S antibody in blocking buffer. Sections treated with heparitinase were incubated with Δ-HS antibody under the same conditions. The sections were then incubated for 30 minutes at room temperature with 10 μg/mL biotinylated goat anti–mouse IgG diluted in blocking buffer followed by 30 minutes at room temperature with avidin:biotinylated peroxidase complex prepared according to the manufacturer's instructions. The peroxidase was visualized by incubation with 3,3′-diaminobenzidine, which generates brown color, and the sections were counterstained with Mayer hematoxylin for 1 minute. Between each step, the sections were washed with PBS. The sections were mounted in mounting medium and examined with a Leica DMLS microscope (Leica Microsystems, Bannockburn, IL) equipped with 10 × ocular and 40 ×/0.65 NA objective lenses. Images were acquired with a QImaging MicroPublisher digital camera and QCapture version 1.1.4 software (QImaging, Burnaby, BC, Canada). The original tagged-image file–formatted (TIFF) files were cropped, but not otherwise modified.
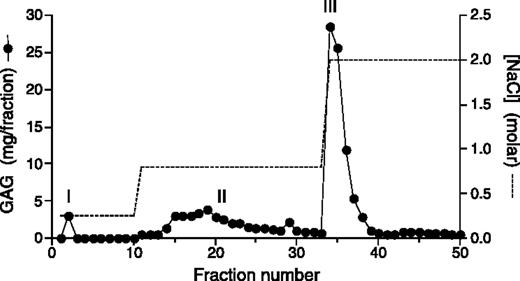
Anion-exchange chromatography of placental extract. Papain-digested and cetylpyridinium chloride/ethanol–precipitated placental extract was applied to a Mono Q column and eluted with NaCl in Tris buffer, pH 7.4, as shown. The amount of GAG in each fraction was determined by the carbazole assay for uronic acid. Fractions were pooled as follows: peak I, fraction 2; peak II, fractions 14-32; peak III, fractions 34-39.
Anion-exchange chromatography of placental extract. Papain-digested and cetylpyridinium chloride/ethanol–precipitated placental extract was applied to a Mono Q column and eluted with NaCl in Tris buffer, pH 7.4, as shown. The amount of GAG in each fraction was determined by the carbazole assay for uronic acid. Fractions were pooled as follows: peak I, fraction 2; peak II, fractions 14-32; peak III, fractions 34-39.
Anti-HCII antibody staining
Formalin-fixed sections cut to a thickness of 6 μm were deparaffinized in xylene, hydrated in graded ethanol, and treated with 3% (vol/vol) H2O2 in PBS for 10 minutes to eliminate endogenous peroxidase activity. After a brief wash with deionized water, the slides were immersed in citrate buffer (1 × Antigen Decloaker, Biocare Medical) and cooked under pressure for 20 minutes in a Decloaking Chamber (Biocare Medical). The slides were cooled for 10 minutes, immersed in PBS for 5 minutes, and blocked for 1 hour with 20 mg/mL bovine serum albumin and rabbit serum (1:100 dilution) in PBS, pH 7.4 (HCII-Ab blocking buffer). The sections were then incubated overnight at 4°C with goat anti–HCII IgG diluted to 4 μg/mL in HCII-Ab blocking buffer or with control nonimmune goat IgG. The sections were then washed in PBS for 5 minutes and incubated with biotinylated rabbit anti–goat IgG for 30 minutes at room temperature. The sections were developed using avidin:biotinylated peroxidase complex and 3,3′-diaminobenzidine as described above and were examined by light microscopy as described in “Antibody staining of GAGs.”
Results
Isolation of placental GAGs
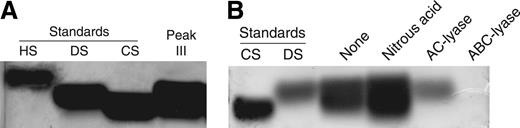
Extraction of 40 g (dry weight) of human placental tissue with papain yielded 171 mg of total GAGs (Table 1). The GAGs were then applied to a Mono Q anion-exchange column and step-eluted with 0.25 M, 0.8 M, and 2.0 M NaCl, yielding 3 peaks (I, II, and III) as shown in Figure 1. The material in peak I did not stimulate thrombin inhibition by HCII and was not characterized further. Peaks II and III were pooled separately and then treated sequentially with nitrous acid to degrade HS, with chondroitin AC II–lyase to degrade chondroitin 4–sulfate (C4S) and chondroitin 6–sulfate (C6S), and finally with chondroitin ABC–lyase to degrade DS (substrate specificities summarized in Table 2). The total amount of GAG recovered after each treatment is indicated in Table 1. Based on these data, peak II contained 35% HS, 16% C4S and/or C6S, 17% DS, and 32% other uronic acid–containing material (eg, hyaluronic acid). Peak III contained 14% HS, 59% C4S and/or C6S, 26% DS, and 1% other material. The composition of peak III was confirmed by agarose gel electrophoresis. Most of the material in this peak comigrated with DS and CS standards (Figure 2A). Little or no material was detectable in the vicinity of an HS standard, but this standard stained relatively poorly with toluidine blue. Treatment of peak III with nitrous acid did not alter the appearance of the stained gel, whereas treatment with chondroitin AC II–lyase left a single band that comigrated with DS (Figure 2B). Treatment with chondroitin ABC–lyase, which cleaves both CS and DS, degraded virtually all of the material in peak III.
Isolation of placental GAGs
. | Total GAG, mg . | Relative HCII activity . | HCII activity yield, % . |
|---|---|---|---|
| Papain extract | 171 | 0.196 | 100 |
| Mono Q peak I | 2.8 | < 0.004 | < 0.03 |
| Mono Q peak II | 29.4 | 0.004 | 0.33 |
| After nitrous acid | 19.1 | 0.022 | 1.24 |
| After AC II-lyase | 14.4 | 0.033 | 1.43 |
| After ABC-lyase | 9.27 | 0.002 | 0.05 |
| Mono Q peak III | 70.5 | 0.444 | 93.4 |
| After nitrous acid | 60.6 | 0.452 | 81.7 |
| After AC II-lyase | 19.1 | 1.300 | 74.2 |
| After ABC-lyase | 0.65 | < 0.06 | < 0.12 |
. | Total GAG, mg . | Relative HCII activity . | HCII activity yield, % . |
|---|---|---|---|
| Papain extract | 171 | 0.196 | 100 |
| Mono Q peak I | 2.8 | < 0.004 | < 0.03 |
| Mono Q peak II | 29.4 | 0.004 | 0.33 |
| After nitrous acid | 19.1 | 0.022 | 1.24 |
| After AC II-lyase | 14.4 | 0.033 | 1.43 |
| After ABC-lyase | 9.27 | 0.002 | 0.05 |
| Mono Q peak III | 70.5 | 0.444 | 93.4 |
| After nitrous acid | 60.6 | 0.452 | 81.7 |
| After AC II-lyase | 19.1 | 1.300 | 74.2 |
| After ABC-lyase | 0.65 | < 0.06 | < 0.12 |
The papain extract was subjected to chromatography on a Mono Q column as shown in Figure 1. Peaks II and III were each treated sequentially with nitrous acid, chondroitin AC II-lyase, and chondroitin ABC—lyase. The material was dialyzed after each step, and the amount of GAG was determined by carbazole assay for uronic acid. Relative HCII activity is defined as the ability of each fraction to stimulate the thrombin-HCII reaction relative to that of porcine mucosal DS (eg, the papain extract had 19.6% of the activity of an equal amount of porcine mucosal DS by weight).
Substrate specificities of lyases
Lyase . | Glycosidic bond(s) cleaved . | Glycosaminoglycan(s) cleaved . | Reference no. . |
|---|---|---|---|
| Chondroitin ABC-lyase | GalNAc4SO3 and/or 6SO3→UA; GalNAc4SO3→UA2SO3 | C4S, C6S, DS | 40 |
| Chondroitin AC II-lyase | GalNAc4SO3 or 6SO3→GlcA | C4S, C6S, DS* | 41 |
| Chondroitin B-lyase | GalNAc4SO3→IdoA | DS | 42 |
| Flavobacterium heparitinase I | GlcNAc6SO3→UA; GlcNSO3→UA; GlcNSO36SO3→UA | HS | 43 |
Lyase . | Glycosidic bond(s) cleaved . | Glycosaminoglycan(s) cleaved . | Reference no. . |
|---|---|---|---|
| Chondroitin ABC-lyase | GalNAc4SO3 and/or 6SO3→UA; GalNAc4SO3→UA2SO3 | C4S, C6S, DS | 40 |
| Chondroitin AC II-lyase | GalNAc4SO3 or 6SO3→GlcA | C4S, C6S, DS* | 41 |
| Chondroitin B-lyase | GalNAc4SO3→IdoA | DS | 42 |
| Flavobacterium heparitinase I | GlcNAc6SO3→UA; GlcNSO3→UA; GlcNSO36SO3→UA | HS | 43 |
GalNAc indicates N-acetylgalactosamine; UA, hexuronic acid; GlcA, glucuronic acid; IdoA, iduronic acid; GlcNAc, N-acetylglucosamine; GlcNSO3, N-sulfoglucosamine; C4S, chondroitin 4-sulfate; C6S, chondroitin 6-sulfate; DS, dermatan sulfate; and HS, heparan sulfate.
Chondroitin AC II-lyase may cleave some linkages in DS that contain GlcA residues.
Anticoagulant activity of GAG fractions
The papain extract stimulated thrombin inhibition by HCII with a specific activity of 0.196 relative to that of porcine mucosal DS. Thus, the total activity of the papain extract was equivalent to that of 33.5 mg of porcine mucosal DS (Table 1). About 93% of the activity eluted from the Mono Q column with 2 M NaCl (peak III), and most of the activity in peak III was resistant to treatment with nitrous acid and chondroitin AC II–lyase but was sensitive to treatment with chondroitin ABC–lyase. Thus, HCII is stimulated by a highly charged DS fraction of the placental extract. The specific activity of the final product was 1.30 relative to that of porcine mucosal DS, and the yield was 74%. Only about 1% of the HCII-stimulating activity was present in peak II. None of the 3 Mono Q fractions stimulated thrombin inhibition by antithrombin to a significant degree.
Agarose gel analysis of Mono Q peak III. (A) Samples of GAG standards (15 μg/well) or desalted Mono Q peak III (50 μg/well) were electrophoresed on a 0.5% agarose gel for 1 hour. Subsequently, the gel was stained with 0.1% toluidine blue. (B) Material from peak III was treated sequentially with nitrous acid, chondroitin AC II–lyase, and chondroitin ABC–lyase. A sample from each step was subjected to electrophoresis. HS indicates heparan sulfate; DS, dermatan sulfate; and CS, chondroitin 6-sulfate.
Agarose gel analysis of Mono Q peak III. (A) Samples of GAG standards (15 μg/well) or desalted Mono Q peak III (50 μg/well) were electrophoresed on a 0.5% agarose gel for 1 hour. Subsequently, the gel was stained with 0.1% toluidine blue. (B) Material from peak III was treated sequentially with nitrous acid, chondroitin AC II–lyase, and chondroitin ABC–lyase. A sample from each step was subjected to electrophoresis. HS indicates heparan sulfate; DS, dermatan sulfate; and CS, chondroitin 6-sulfate.
Disaccharide analysis of placental DS
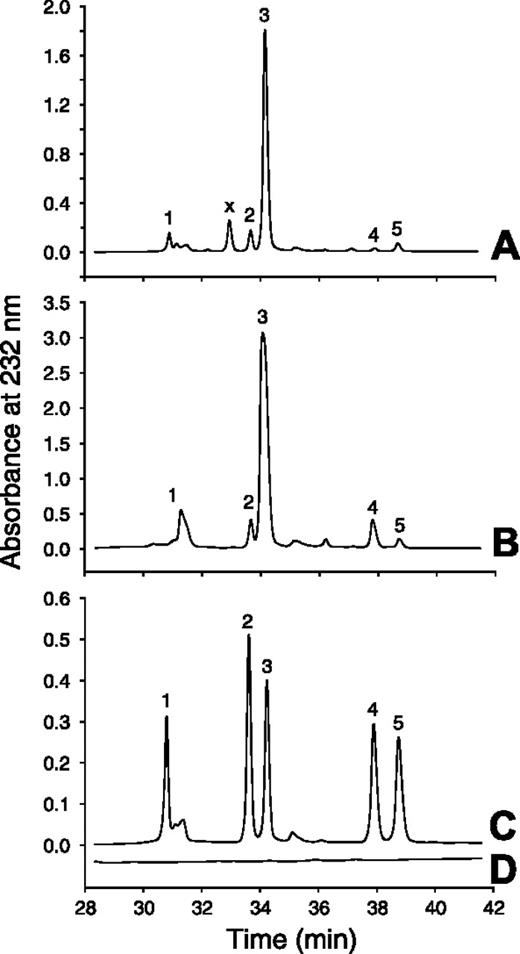
The DS from peak III that remained after treatment with nitrous acid and chondroitin AC II–lyase was dialyzed and then digested with chondroitin ABC–lyase. The resulting disaccharides were analyzed by strong anion-exchange chromatography (Figure 3 and Table 3). The disulfated disaccharides, Δ4,5-hexuronic acid→N-acetylgalactosamine 4,6-sulfate and Δ4,5-hexuronic acid 2-sulfate→N-acetylgalactosamine 4-sulfate, comprised 4.1% of the total disaccharides in placental DS.
Strong anion-exchange HPLC analysis of DS disaccharides
Disaccharide . | Porcine skin DS, %* . | Porcine mucosal DS, %† . | Placental DS,%† . |
|---|---|---|---|
| ΔUA→GalNAc | 0.9 | 1.2 | 2.6 |
| ΔUA→GalNAc6SO3 | 1.8 | 5.3 | 7.2 |
| ΔUA→GalNAc4SO3 | 92.3 | 82.0 | 86.0 |
| ΔUA→GalNAc4,6SO3 | 0.0 | 8.6 | 0.8 |
| ΔUA2SO3→GalNAc4SO3 | 5.0 | 2.8 | 3.3 |
Disaccharide . | Porcine skin DS, %* . | Porcine mucosal DS, %† . | Placental DS,%† . |
|---|---|---|---|
| ΔUA→GalNAc | 0.9 | 1.2 | 2.6 |
| ΔUA→GalNAc6SO3 | 1.8 | 5.3 | 7.2 |
| ΔUA→GalNAc4SO3 | 92.3 | 82.0 | 86.0 |
| ΔUA→GalNAc4,6SO3 | 0.0 | 8.6 | 0.8 |
| ΔUA2SO3→GalNAc4SO3 | 5.0 | 2.8 | 3.3 |
Peak III from the Mono Q column was treated with nitrous acid and chondroitin AC II-lyase. The remaining material was exhaustively degraded with chondroitin ABC-lyase, and the resulting DS disaccharides were analyzed as shown in Figure 3. Areas under peaks corresponding to disaccharide standards were determined from the absorbance at 232 nm.
ΔUA indicates unsaturated Δ4,5-hexuronic acid; GalNAc, N-acetylgalactosamine.
Data from Maimone and Tollefsen, J Biol Chem. 1990;265:18263-18271.
This study.
Immunohistochemical localization of DS in the placenta
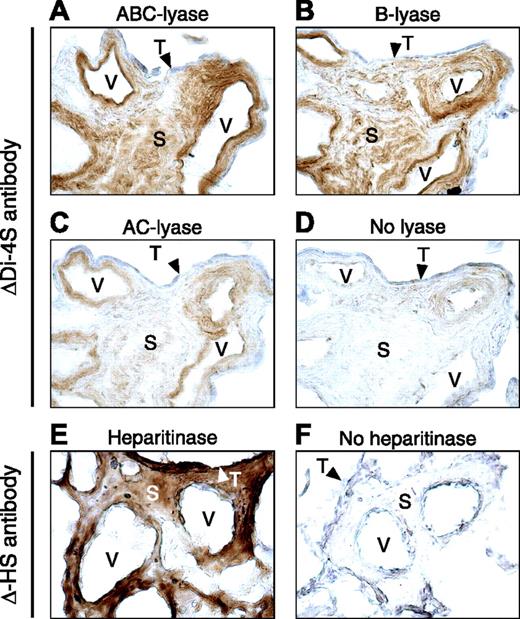
Unfixed frozen sections of placenta were treated with chondroitin B–lyase, AC II–lyase, or ABC–lyase, which cleave the glycosidic linkages between hexosamine and hexuronic acid, leaving behind oligosaccharide “stubs” with Δ4,5-hexuronic acid (unsaturated) residues at the nonreducing termini. The sections were then stained with an antibody (ΔDi-4S) that recognizes the Δ4,5-hexuronic acid→N-acetylgalactosamine 4-sulfate disaccharide. Blood vessels and stroma of the placental villi were strongly stained after treatment with chondroitin ABC–lyase, which digests DS, C4S, and C6S, or with chondroitin B–lyase, which specifically digests DS (Figure 4A,B). Weaker staining of the same structures was observed after treatment with chondroitin AC II–lyase, which digests C4S and C6S, but not DS (Figure 4C). Syncytiotrophoblast cells did not stain after treatment with any of these 3 lyases. Little or no staining with ΔDi-4S antibody was observed in sections that had not been treated with enzyme (Figure 4D). Sections also were treated with Flavobacterium heparitinase, which cleaves HS chains to yield Δ4,5-hexuronic acid at the nonreducing termini. These sections were stained with an antibody (Δ-HS) that recognizes the unsaturated hexuronic acid. In contrast to the results obtained with chondroitin B–lyase, syncytiotrophoblasts were strongly stained after heparitinase treatment; staining of villous stromal regions and blood vessels also was observed (Figure 4E). Minimal background staining with Δ-HS antibody was observed in sections that had not been treated with heparitinase (Figure 4F).
Disaccharide analysis of Mono Q peak III. Mono Q peak III was treated with nitrous acid and chondroitin AC II–lyase to degrade HS, C4S, and C6S as shown in Figure 2. The remaining DS was digested with chondroitin ABC–lyase, and the resulting products were chromatographed on a Spherisorb-SAX column (A). The other chromatograms represent porcine mucosal DS digested with chondroitin ABC–lyase (B), a mixture of Δ4,5-unsaturated disaccharide standards (C), and a blank run (D). The numbered peaks indicate the elution positions of the following disaccharide standards: 1, Δ4,5UA→GalNAc; 2, Δ4,5UA→GalNAc6SO3; 3, Δ4,5UA→GalNAc4SO3; 4, Δ4,5UA2SO3→GalNAc4SO3; and x, unidentified peak.
Disaccharide analysis of Mono Q peak III. Mono Q peak III was treated with nitrous acid and chondroitin AC II–lyase to degrade HS, C4S, and C6S as shown in Figure 2. The remaining DS was digested with chondroitin ABC–lyase, and the resulting products were chromatographed on a Spherisorb-SAX column (A). The other chromatograms represent porcine mucosal DS digested with chondroitin ABC–lyase (B), a mixture of Δ4,5-unsaturated disaccharide standards (C), and a blank run (D). The numbered peaks indicate the elution positions of the following disaccharide standards: 1, Δ4,5UA→GalNAc; 2, Δ4,5UA→GalNAc6SO3; 3, Δ4,5UA→GalNAc4SO3; 4, Δ4,5UA2SO3→GalNAc4SO3; and x, unidentified peak.
Immunohistochemical localization of placental GAGs. Frozen sections were incubated with chondroitin ABC–lyase (A), B-lyase (B), AC II–lyase (C), heparitinase (E), or buffer alone (D,F) and then stained with ΔDi-4S antibody (A-D) or Δ-HS antibody (E,F). V indicates vessel; S, stroma; and T (arrowheads), syncytiotrophoblast layer. Original magnification, 400 ×.
Immunohistochemical localization of placental GAGs. Frozen sections were incubated with chondroitin ABC–lyase (A), B-lyase (B), AC II–lyase (C), heparitinase (E), or buffer alone (D,F) and then stained with ΔDi-4S antibody (A-D) or Δ-HS antibody (E,F). V indicates vessel; S, stroma; and T (arrowheads), syncytiotrophoblast layer. Original magnification, 400 ×.
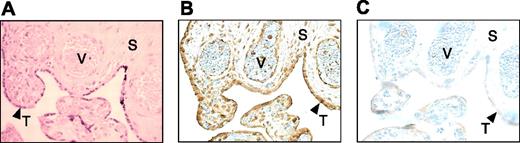
Immunohistochemical localization of endogenous HCII in the placenta
Formalin-fixed human placental sections were stained with goat anti–HCII IgG or nonimmune IgG as a control. HCII was detected in the walls of the villous blood vessels and in the syncytiotrophoblast-cell layer (Figure 5). Relatively little HCII was detectable in the villous stroma.
Discussion
HCII is unique among thrombin inhibitors with regard to its ability to be stimulated by DS.11 DS is a linear polysaccharide found on cellular membranes and in the extracellular matrix of virtually all mammalian tissues.30 It is a repeating polymer of d-glucuronic or l-iduronic acid and N-acetyl-d-galactosamine. O-sulfation of iduronic acid residues at the C2 position and of galactosamine residues at the C4 and/or C6 position occurs to a variable extent, yielding heterogeneous structural domains within the polymer. Porcine skin DS contains a high-affinity binding site for HCII, which consists of a tandem repeat of 3 iduronic acid 2-sulfate→N-acetylgalactosamine 4-sulfate disaccharide subunits.31 Additional experiments suggest that 4-O-sulfation of galactosamine is critical for activity with HCII.32 HCII-binding sites in DS from tissues other than porcine skin also may include unsulfated iduronic acid→N-acetylgalactosamine 4,6-disulfate subunits.33,34
Fractionation of placental GAGs yielded a highly charged DS fraction capable of stimulating thrombin inhibition by HCII. The final product (Mono Q peak III treated with nitrous acid and chondroitin AC II–lyase) comigrated with standard DS on gel electrophoresis and retained 74% of the total HCII stimulating activity of the initial papain extract. Chondroitin AC II–lyase, however, may cleave some linkages in DS that contain glucuronic acid residues. Therefore, the DS polymers obtained after treatment with this enzyme may have an average molecular weight lower than that of the native polysaccharide. Since the molecular weight affects the anticoagulant activity of DS,35 the value of HCII activity reported in Table 1 for Mono Q peak III after AC II–lyase treatmentis a minimum estimate. The activity was entirely abolished by treatment with chondroitin ABC–lyase, which degrades DS. Although Mono Q peaks II and III both contained significant amounts of HS, peak II had almost no activity with HCII, and peak III retained activity after treatment with nitrous acid. Therefore, placental HS does not appear to be capable of activating HCII. Since none of the material in Mono Q peaks I, II, or III stimulated thrombin inhibition by antithrombin in vitro, we conclude that DS is the predominant anticoagulant GAG in the placenta.
Immunohistochemical localization of endogenous placental HCII. Formalin-fixed sections were stained with hematoxylin and eosin (A) or immunostained with anti-HCII antibody (B) or nonimmune purified IgG (C). V indicates vessel; S, stroma; and T (arrowheads), syncytiotrophoblast layer. Original magnification, 400 ×.
Immunohistochemical localization of endogenous placental HCII. Formalin-fixed sections were stained with hematoxylin and eosin (A) or immunostained with anti-HCII antibody (B) or nonimmune purified IgG (C). V indicates vessel; S, stroma; and T (arrowheads), syncytiotrophoblast layer. Original magnification, 400 ×.
The disaccharide composition of peak III DS was comparable to that of DS derived from porcine skin or intestinal mucosa, which contain 5% to 11% of the disulfated disaccharides previously implicated in binding to HCII.31-34
Immunohistochemical experiments indicated that DS is associated with the walls of fetal blood vessels and the villous stroma but is notably absent in the syncytiotrophoblast layer. In agreement with this finding, a previous study found that the core protein of the DS proteoglycan decorin is present in the villous stroma but is absent in trophoblasts.36 These observations suggest that HCII in maternal blood would come in contact with DS only after injury to syncytiotrophoblasts covering the surface of placental villi. Since fibrin deposits have been shown to occur in regions of syncytiotrophoblast loss,8 HCII might serve to limit coagulation at such sites of injury. The pathologic features of pre-eclampsia include syncytiotrophoblast degeneration and increased intervillous thrombosis, as well as damage to the endothelium and thrombosis of villous blood vessels.37,38 Reduction in the levels of HCII in women with severe preeclampsia may contribute to development of these thrombotic lesions.16 The situation may be analogous to that of mice with HCII deficiency, in which thrombosis following injury to the carotid arterial endothelium occurs more rapidly than in wild-type animals.39
Endogenous HCII antigen colocalized with DS in the walls of fetal blood vessels in the placental villi and potentially could be bound there in an activated state. HCII also was present in the syncytiotrophoblast layer, where it appeared to colocalize with HS. The significance of this latter observation is unclear, since we could find no evidence of activation of HCII by placental HS. One possibility is that HS concentrates HCII at the syncytiotrophoblast surface, where it would be readily available to interact with DS in the stroma and inhibit thrombin following trophoblast injury. Relatively little HCII was detectable in the villous stroma, suggesting that the inhibitor is not present in this location in the normal term placenta.
In summary, our data suggest that DS is the major anticoagulant GAG in the human placenta at term. In addition, placental DS contains disaccharide subunits known to interact with HCII and stimulates thrombin inhibition by HCII in vitro. Immunohistochemical studies suggest that DS is positioned to activate HCII in the fetal blood vessels or in the stroma of placental villi after injury to the syncytiotrophoblast layer. Thus, HCII may serve to inhibit fibrin generation in the placenta.
Prepublished online as Blood First Edition Paper, December 8, 2005; DOI 10.1182/blood-2005-09-3755.
Supported by grants from the National Institutes of Health (R01 HL-55520), the Edward Mallinckrodt Jr Foundation, and the American Heart Association, and by a fellowship awarded to T.K.G. by the American Heart Association.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal