Abstract
Critical signals for erythroblast formation are transduced by activated, tyrosine-phosphorylated erythropoietin receptor (EpoR) complexes. Nonetheless, steady-state erythropoiesis is supported effectively by EpoR alleles that are deficient in cytoplasmic phosphotyrosine sites. To better define core EpoR action mechanisms, signaling capacities of minimal PY-null (EpoR-HM) and PY343-retaining (EpoR-H) alleles were analyzed for the first time in bone marrow–derived erythroblasts. Jak2 activation via each allele was comparable. Stat5 (and several Stat5-response genes) were induced via EpoR-H but not via EpoR-HM. Stat1 and Stat3 activation was nominal for all EpoR forms. For both EpoR-HM and EpoR-H, Akt and p70S6-kinase activation was decreased multifold, and JNK activation was minimal. ERKs, however, were hyperactivated uniquely via EpoR-HM. In vivo, Epo expression in EpoR-HM mice was elevated, while Epo-induced reticulocyte production was diminished. In vitro, EpoR-HM erythroblast maturation also was attenuated (based on DNA content, forward-angle light scatter, and hemoglobinization). These EpoR-HM–specific defects were corrected not only upon PY343 site restoration in EpoR-H, but also upon MEK1,2 inhibition. Core EpoR PY site-independent signals for erythroblast formation therefore appear to be Stat5, Stat1, Stat3, p70S6-kinase, and JNK independent, but ERK dependent. Wild-type signaling capacities, however, depend further upon signals provided via an EpoR/PY343/Stat5 axis.
Introduction
Signals provided by Epo and its single transmembrane receptor (EpoR) are essential for erythroblast formation.1 Physicochemical studies have revealed unique mechanisms for Epo binding2 and conformation-dependent activation of EpoR-Jak2 kinase complexes.3 Epo-activated signaling pathways also are well studied,4 yet gaps in knowledge persist concerning the nature of key signals for EpoR biofunction. Interest in this basic problem also is provoked by apparent EpoR cytoprotection of injured myocardial, neuronal, endothelial, and renal cells,5 and by the association of EpoR action with angiogenesis,5 VHL carcinomas,6 melanoma,7 and myoma8 formation. To better elucidate core signals for erythroid progenitor cells, we presently have performed first-time analyses of the signaling capacities of minimal knocked-in murine EpoR alleles in primary bone marrow–derived erythroblasts.
Epo binding occurs via Epo high-affinity A, B, D helix site-1, and low-affinity A, C helix site-2 interactions with bipartite 7 beta-strand ligand binding sites in appositioned EpoR dimers.2 Via a cytoplasmic juxtamembrane box-1 domain, the EpoR also preassembles with Jak2 kinase.9 Epo-EpoR interactions stimulate Jak2 phosphorylation at Y1007/Y1008 sites,10 and Jak2 (potentially in concert with Src, Btk, STK, and/or Kit tyrosine kinases)11-15 then mediates the phosphorylation of multiple EpoR cytoplasmic tyrosine motifs. In particular, within the EpoR of mouse, human, and zebra fish,16 8 distal phosphotyrosine motifs are conserved that possess established binding specificities for SH2-domain–encoding effectors. These include the following: PY343 binding of Stat517 ; PY401 binding of cytokine-inducible SH2-domain–containing protein Cis-1, SH2 inositol 5-phosphatase, SHIP-1, Gab-2, SOCS-3, and/or Syp/SH2-PTP218-22 ; PY429 and PY431 binding of SOCS-3 and/or SHP-123,24 ; PY460 binding of CrkL25 and regulation of intracellular calcium flux26 ; PY464 and/or PY479 binding of Lyn27 ; and PY479 binding of alpha-p85/PI3 kinase.28 In the human EpoR, an additional juxtamembrane cytoplasmic PY285 site also exists and has been demonstrated in 32D cells to modulate Stat5 and Stat1 activation.29
Based on the evolutionary conservation of these EpoR PY sites and their demonstrated role as an assembling scaffold for downstream effectors,4 EpoR phosphotyrosine motifs are predicted to be important for Epo's actions. The extent to which these EpoR regions (and linked pathways) act in central or perhaps only modulatory capacities, however, is controversial. This point is highlighted by the ability of PY-deficient EpoR alleles to support steady-state erythropoiesis in vivo.30 Specifically, steady-state erythropoiesis in mice expressing a knocked-in PY-null EpoR-HM allele is affected to the extent that hematocrits are decreased approximately 8 points on average, and red blood cell (RBC) counts are decreased approximately 15%.30 Unexpectedly, this suggests that core signals provided by Jak2 (in the absence of EpoR PY signals) efficiently support Epo-dependent erythroblast formation. These findings raise basic questions concerning the nature of core signaling pathways that are used by these minimal EpoR alleles (and the wt-EpoR). Due to the challenges of working with low-abundance progenitor cells, however, analyses of molecular signals relayed by minimal murine EpoR forms in bone marrow–derived erythroblasts are limited to date to single-point electrophoretic mobility shift assay (EMSA) analyses of Stat5 activity.30
To address the above basic problems in Epo signaling, primary culture systems presently have been implemented to investigate Epo-activated signals in erythroblasts derived from adult bone marrow of mice expressing a PY-null EpoR-HM allele, or a related knocked-in EpoR-H allele in which a single PY343 Stat5 binding site is selectively restored.30 These analyses provide several new lines of insight into signals that are relayed via EpoR-HM/Jak2 and EpoR-H/Jak2/Stat5 axes. Overall, core EpoR-plus-Jak2–activated erythropoietic signals appear to be relayed primarily via Stat5–, Stat1–, Stat3–, JNK-, and p70S6K-independent, but ERK 1,2–dependent, routes. EpoR-PY343 signals, by comparison, restore wild-type erythropoietic capacities via suggested actions of key Stat5-target genes. This dissection of core EpoR signal transduction mechanisms in primary marrow-derived erythroblasts also may shed useful light on cytoprotective mechanisms that are stimulated by Epo in injured cardiac, neuronal, endothelial, and renal cells.5
Materials and methods
Mice and primary erythroid progenitor cell culture
Mice expressing knocked-in EpoR-HM and EpoR-H alleles were those described by Zang et al.30 Bone marrow progenitor cells were prepared as follows. Femurs and tibiae were isolated, and marrow cells were gently flushed from cavities using 21-gauge (femur) or 23-gauge (tibia) needles and 10 mL Iscove modified Dulbecco medium (IMDM, no. 12440-053; Invitrogen, Carlsbad, CA) plus 2% fetal bovine serum (FBS). Cells then were passed thrice slowly through a 21-gauge needle and 40-μm strainer. Collected cells were resuspended initially in 1 mL phosphate-buffered saline (PBS. no. 14190-144; Invitrogen) and were exposed for 2 minutes to 9 mL potassium bicarbonate–buffered 0.8% ammonium chloride, 0.1 mM Na2 EDTA (ethylenediaminetetraacetic acid) solution, pH 7.5. Then, 10 × PBS (1.1 mL) was added. Cells were collected through 16 mL of 50% FBS in PBS and washed in IMDM, 2% FBS. In expansions, cells were cultured (at 7.5 × 105 cells/mL) in StemPro-34 medium (Invitrogen) supplemented with 2.5 U/mL Epo (Epoetin-alpha; Amgen, Thousand Oaks, CA), 100 ng/mL mSCF (Peprotech, Rocky Hill, NJ), 1 μM dexamethasone, 1 μM beta-estradiol, 40 ng/mL IGF-1, 75 μg/mL h-transferrin, 0.5% BSA (Stem Cell Technologies, Vancouver, BC), 0.1 mM 2-mercaptoethanol, and 1.5 mM l-glutamine (ie, SP34-EX medium). At 24 hours of culture, 0.5 volumes of medium were added. At 48 hours, cells were replated at 7.5 × 105 cells/mL in 80% new media plus 20% residual conditioned media. For certain experiments, CD71highTer119neg erythroblast populations were prepared (at day 3.5 of culture) via 2 rounds of Ter119pos cell depletion (no. 130-049-901; Miltenyi Biotech, Auburn, CA). In differentiation experiments, expanded erythroblasts (at day 3 of expansion) were cultured (at 7.5 × 105 cells/mL) in 2.5 U/mL Epo, 150 μg/mL transferrin, 10 μg/mL insulin, 0.5% BSA, 0.1 mM 2-mercaptoethanol, 10% FBS (no. SH30070.03; Hyclone, Logan, UT) in IMDM. Differentiation was assayed based on side- and forward-angle light scatter, DRAQ5 (10 μM; ALEXIS, San Diego, CA) staining of DNA content,31 hemoglobinization,32 and Ter119 expression. PP2, PP3, SB202190, SP600125, and U0126 (Calbiochem, San Diego, CA) were prepared upon use in DMSO (at × 1000 concentrations) and were added directly to SP34-EX cultures (with supplementation at 24 and 48 hours). U0126, SB202190, and SP600125 also were included in differentiation medium.
Flow cytometry, fluorescence-activated cell sorter (FACS) analysis, and cytospins
Cells (1 × 106) were incubated in 0.2 mL PBS, 1% BSA with 1 μg rat IgG (15 minutes), and with PE-Ter119 (2 μg), FITC-CD71 (1 μg), and/or APC-Kit (1 μg) (BD Biosciences, San Diego, CA). PE/FITC–annexin-V binding assays (BD Biosciences) were performed in 140 mM NaCl, 2.5 mM CaCl2, 10 mM HEPES (pH 7.4) (20 minutes). Washed cells were analyzed via flow cytometry (BD FACScalibur; BD Immunocytometry, San Jose, CA). In all experiments, equivalent numbers of gated events were analyzed. FACS analysis was performed with a Vantage-SE system. Cytospin analyses (1 × 105 cells) involved slide centrifugation (15 minutes, 100g [300 rpm], Hettich Universal-16A cyto-centrifuge; Mettich Universal, Tuttlingen, Germany) and Dip-Stain reagent staining (Volu-Sol, Salt Lake City, UT).
Reverse transcriptase–polymerase chain reaction (RT-PCR)
RNA was purified using Trizol reagent (Invitrogen). RT was with Superscript III (Invitrogen). Q-PCR (I-Cycler) used Sybr-green reagents (BioRad, Hercules, CA) and the following oligo pairs: Pim1, 5′-TTC-TGG-ACT-GGT-TCG-AGA-GG-3′ and 5′-GCT-CCT-CGT-TCG-GTG-ATA-AA-3′; oncostatinM, 5′-AAC-TGA-GCA-AGC-CTC-ACT-TCC-3′ and 5′-ATG-CCG-AGG-ATA-TTG-TGC-CG-3′; Socs3, 5′-CCG-CTT-CGA-CTG-TGT-ACT-CAA-G-3′ and 5′-TCT-TCT-CGC-CCC-CAG-AAT-AGA-T-3′; Cis1, 5′-CCA-CTG-GCT-TTG-TCA-AGA-AGG-3′ and 5′-AGG-CCA-CAT-AGT-GCT-GCA-CAA-3′; Bclx, 5′-ACT-GTG-CGT-GGA-AAG-CGT-AGA-3′ and 5′-TGC-TGC-ATT-GTT-CCC-GTA-GAG-3′; Epo, 5′-AGA-ATG-GAG-GTG-GAA-GAA-CAG-G-3′ and 5′-CTG-GTG-GCT-GGG-AGG-AAT-TG-3′; and Actin, 5′-CGT-GCG-TGA-CAT-TAA-AGA-GAA-G-3′ and 5′-TGG-ATG-CCA-CAG-GAT-TCC-ATA-3′.
Signal transduction factor analyses
In signal transduction experiments, expanded erythroblasts (1 × 107) were washed, incubated for 6 hours in 0.5% BSA, 10 μg/mL transferrin, 10 ng/mL insulin, 0.1 mM 2-mercaptoethanol in IMDM, and then exposed to Epo. Upon washing in 3 volumes of 2°C PBS, cells were lysed in 0.2 mL 1% Igepal, 150 mM NaCl, 50 mM NaF, 2 mM Na2 EDTA, 0.1 mM NaVO3, 1 mM dithiothreitol, 10 mM sodium pyruvate, 25 mM beta-glycerol phosphate, 10% glycerol, 50 mM HEPES (pH 7.5) plus 0.25 mg/mL phenylmethylsulfonylfluoride, 1 × protease and phosphatase inhibitor cocktails (no. P8340, no. P5726; Sigma-Aldrich, St Louis, MO). Then, 1% Triton-X-100, 0.5% sodium deoxycholate, 0.1% SDS, 112.5 mM NaCl, 37.5 mM Tris-HCL (pH 7.4) were added (0.2 mL), and cleared extracts (25 μg) were denatured, electrophoresed, and transferred to PVDF membranes. Blocked membranes (0.05% Tween-20, 3% fat-free milk, 1% BSA, 0.15 M NaCl, 20 mM Tris, pH 7.4) were incubated with antibodies to Akt (no. sc-1618; SantaCruz Biotechnology, Santa Cruz, CA), p60Src (no. sc-8056; SantaCruz Biotechnology), p38 MAPK (no. 535; SantaCruz Biotechnology), Bax (no. AB2915; Chemicon, Temecula, CA), DAPK-2 (no. 3606; Chemicon), Bcl-x (no. 610211; BD Biosciences), and the following antibodies from Cell Signaling (Beverly, MA): Stat5 (no. 9352), PY-Stat5 (no. 9351), PS-Akt (no. 9271), PY-p60Src (no. 2101), PY/T-p38MAPK (no. 9211), ERK1,2 (no. 9102), PY/T-ERK1,2 (no. 4375), SAPK/JNK (no. 9252), PY/T-SAPK/JNK (no. 9251), PT/S p70S6-kinase (no. 9204), and p70S6-kinase (no. 9202). For Jak2, Ipegal lysates (without SDS, Triton-X-100, or deoxycholate) were incubated with anti-Jak2 antibodies (no. 06-255; Upstate, Waltham, MA), and protein-A magnetic microbeads (Miltenyi Biotech), and immunoprecipitates were isolated. Phospho-Jak2 was detected with 4G10 (Upstate). Chemiluminescence used HRP-conjugated secondary antibodies (Jackson Immunoresearch, Westgrove, PA) and Dura reagent (no. 34076; Pierce, Rockford, IL). Band signal intensities were analyzed with ImageQuant-TL (Amersham Biosciences, Piscataway, NJ). Certain Western blots represent composites of more than one experiment with matched enhanced chemiluminescence (ECL) exposures.
Epo dosing and reticulocyte assays
At 0 and 24 hours, EpoR-HM, EpoR-H, and wt-EpoR mice were injected intraperitoneally with Epo (2.5 U/gram per mouse). On day 5, reticulocyte levels were determined via thiazole orange staining and flow cytometry.
Results
Jak, Stat, and Stat5-target gene activation via minimal EpoR-HM and EpoR-H alleles
To enable quantitative analyses of EpoR allele signaling in primary bone marrow–derived erythroblasts, a system for the efficient in vitro expansion of erythroid progenitor cells was implemented. This involved gentle disaggregation of marrow, limited exposure to NH4Cl, and culture in serum-free SP34 media with optimized supplements. Supplement sources were important, as were subculture details. At day 3.5 of culture, 2 × 107 cells were propagated on average per mouse—and 45% to 50% of these cells reproducibly were highly Epo-responsive CD71highTer119low erythroblasts. The balance of cells included Mac-1pos (12%), B220pos (5%), and CD41pos (5%) cells (data not shown). This was the case for marrow cells from wt-EpoR mice, as well as mice expressing the minimal EpoR alleles EpoR-HM (PY-null form) and EpoR-H (PY343-retaining form) (Figure 1A,Bi).
Jak2 and Stat5 activation via EpoR-HM and EpoR-H alleles in primary bone marrow–derived erythroblasts. (A) Minimal EpoR alleles. Diagrammed are knocked-in PY-null EpoR-HM and PY343-encoding EpoR-H alleles, together with the wt-EpoR. (B) Jak2 activation profiles via minimal EpoR alleles. (Bi) Erythroid progenitor cells from wt-EpoR, EpoR-HM, and EpoR-H mice were expanded to yield (on day 3) 45% to 50% frequencies of CD71high erythroblasts. Washed cells were cultured for 6 hours in the absence of hematopoietic cytokines (10 μg/mL transferrin, 10 ng/mL insulin, 0.5% BSA in IMDM). Cells were then exposed to Epo (2.5 U/mL), and at the indicated intervals lysates were prepared for Western blot analyses. For phospho-Jak2, note the fairly uniform activation profiles supported via EpoR-H, EpoR-HM, and wt-EpoR erythroblasts. In all expansion experiments, CD71 and Ter119 marker expression was assessed, and representative distributions are shown. (Bii) Jak2 activation was analyzed as above, except for erythroblast preparations from which Ter119pos cells were depleted. The numbers in quadrants (clockwise from bottom left) indicate the percentage of CD71negTer119neg, Ter119negCD71pos, CD71posTer119high, and Ter119highCD71neg cells among total live-gated cells. (C) Stat5 activation via EpoR-H, but not EpoR-HM, alleles. In parallel analyses, EpoR allele–mediated activation of Stat5 was assessed both for expansion cultures (Ci) and for Ter119-depleted cultures (CD71posTer119neg populations) (Cii).
Jak2 and Stat5 activation via EpoR-HM and EpoR-H alleles in primary bone marrow–derived erythroblasts. (A) Minimal EpoR alleles. Diagrammed are knocked-in PY-null EpoR-HM and PY343-encoding EpoR-H alleles, together with the wt-EpoR. (B) Jak2 activation profiles via minimal EpoR alleles. (Bi) Erythroid progenitor cells from wt-EpoR, EpoR-HM, and EpoR-H mice were expanded to yield (on day 3) 45% to 50% frequencies of CD71high erythroblasts. Washed cells were cultured for 6 hours in the absence of hematopoietic cytokines (10 μg/mL transferrin, 10 ng/mL insulin, 0.5% BSA in IMDM). Cells were then exposed to Epo (2.5 U/mL), and at the indicated intervals lysates were prepared for Western blot analyses. For phospho-Jak2, note the fairly uniform activation profiles supported via EpoR-H, EpoR-HM, and wt-EpoR erythroblasts. In all expansion experiments, CD71 and Ter119 marker expression was assessed, and representative distributions are shown. (Bii) Jak2 activation was analyzed as above, except for erythroblast preparations from which Ter119pos cells were depleted. The numbers in quadrants (clockwise from bottom left) indicate the percentage of CD71negTer119neg, Ter119negCD71pos, CD71posTer119high, and Ter119highCD71neg cells among total live-gated cells. (C) Stat5 activation via EpoR-H, but not EpoR-HM, alleles. In parallel analyses, EpoR allele–mediated activation of Stat5 was assessed both for expansion cultures (Ci) and for Ter119-depleted cultures (CD71posTer119neg populations) (Cii).
In expanded wt-EpoR, EpoR-HM, and EpoR-H erythroblast preparations, Epo-induced Jak2 activation first was analyzed (Figure 1Bi). Hematopoietic cytokines were withdrawn (for 6 hours), and erythroblasts then were exposed to Epo (2.5 U/mL) for the indicated intervals. Via each EpoR allele, Jak2 activation was rapid (> 50% maximum activation by 3 minutes) and progressed over highly similar time courses. To confirm this result, and to account for possible variable responsiveness among Ter119pos subpopulations, analyses were repeated using Ter119-depleted preparations (and extended time courses) (Figure 1Bii). Jak2 again was activated by the wt-EpoR, EpoR-HM, and EpoR-H at similar levels and rates. Differential Jak2 activation therefore does not appear to underlie differences in EpoR allele biosignaling capacities. No Epo-dependent activation of Jak1, Jak3, or Tyk2 was detected (data not shown).
Stat activation via EpoR alleles next was studied. Stat5 is most frequently linked to Epo signaling17,33,34 and was first analyzed. In time-course experiments, EpoR-H activation of Stat5 paralleled that of the wt-EpoR. Stat5, however, was not detectably activated via EpoR-HM (Figure 1Ci). Next, analyses of Stat5 activation were repeated in independent erythroblast preparations following Ter119pos cell depletion. Essentially equivalent results were obtained (Figure 1Cii). In addition, the abilities of EpoR-HM, EpoR-H, and wt-EpoR alleles to support Epo induction of 5 genes that have been indicated in cell line studies to comprise Epo and Stat5-response genes were studied: Pim1, oncostatinM, Socs3, Cis1, and Bclx. This involved cytokine withdrawal, exposure to Epo (2.5 U/mL, 90 minutes), RNA isolation, and quantitative RT-PCR. Pilot experiments for Cis-1 indicated maximal Epo induction at 90 minutes (Figure S2, available on the Blood website; see the Supplemental Figures link at the top of the online article). Pim1, oncostatinM, Socs3, and Cis1 each were induced via the wt-EpoR and EpoR-H between 5- to 35-fold (Table 1) but not via EpoR-HM. Somewhat unexpectedly, Bcl-x was not significantly induced by any EpoR alleles. Together, these results further discount EpoR PY–independent mechanisms for Stat5 activation via EpoR-HM. In cell line models, Epo activation of Stat1 and Stat3 has been reported.35 In addition, Stat1-deficient mice exhibit decreased erythroid progenitor cell levels,36 and Stat1–– BFU-Es show decreased Epo responsiveness.36 Activation of these Stats via EpoR alleles in primary marrow-derived erythroblasts therefore was analyzed. Stat3 activation was undetectable. Stat1 activation was detected, but only at the limits of optimized ECL sensitivity (Figure S1). This suggests limited contributions of these Stats to Epo bioactivities in this primary erythroblast system.
Induction of candidate STAT5-response genes in WT-EpoR, EpoR-HM, and EpoR-H erythroblasts
. | Pim1 . | OncoM . | Socs3 . | Cis1 . | Bclx . |
|---|---|---|---|---|---|
| wt-EpoR | 6.3 ± 0.8 | 10.6 ± 1.2 | 16 ± 1.1 | 37.3 ± 1.7 | 1.8 ± 0.5 |
| EpoR-HM | 1.9 ± 0.5 | 1.4 ± 0.3 | 1.8 ± 0.5 | 1.0 ± 0.4 | 0.9 ± 0.4 |
| EpoR-H | 8.1 ± 0.4 | 15.6 ± 1.2 | 19.7 ± 0.8 | 31.3 ± 5.0 | 1.1 ± 0.2 |
. | Pim1 . | OncoM . | Socs3 . | Cis1 . | Bclx . |
|---|---|---|---|---|---|
| wt-EpoR | 6.3 ± 0.8 | 10.6 ± 1.2 | 16 ± 1.1 | 37.3 ± 1.7 | 1.8 ± 0.5 |
| EpoR-HM | 1.9 ± 0.5 | 1.4 ± 0.3 | 1.8 ± 0.5 | 1.0 ± 0.4 | 0.9 ± 0.4 |
| EpoR-H | 8.1 ± 0.4 | 15.6 ± 1.2 | 19.7 ± 0.8 | 31.3 ± 5.0 | 1.1 ± 0.2 |
Values are mean levels, ± SE, of transcript induction over baseline at 90 minutes of Epo exposure, 2.5 U/mL.
Epo receptor allele regulation of Akt, p70S6-kinase, p60-Src, and MAPKs
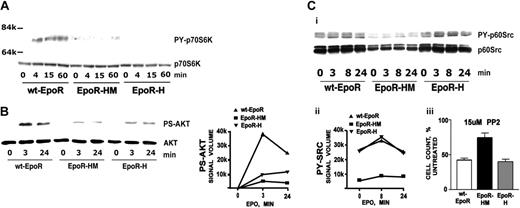
In cell lines and in fetal liver cells, Akt and p70S6K each have been shown to be activated by Epo.28,37,38 These response pathways are linked in that PI3-kinase stimulates Akt, Akt activates mTOR, and mTOR comprises a major p70S6K regulator.39,40 Each response also can affect progenitor cell survival.40 p70S6K and Akt activation therefore was examined in primary EpoR-HM and EpoR-H erythroblasts. p70S6K was activated efficiently via the wt-EpoR, but was not significantly stimulated via EpoR-H or EpoR-HM alleles (Figure 2A). This outcome is consistent with an indicated role for an EpoR PY479 site recruitment of PI3-kinase upstream of p70S6K activation28 (and p70S6K therefore may be nonessential for efficient EpoR function). For Akt, activation via EpoR-H and EpoR-HM alleles was diminished markedly, but each nonetheless activated Akt at residual levels (∼ 20% of wt-EpoR levels) (Figure 2B). The extent to which this limited Akt activation may affect EpoR-H and EpoR-HM bioactivity is unclear.
EpoR-HM, EpoR-H, and wt-EpoR modulation of p70S6-kinase, Akt, and p60-Src. (A) p70S6-kinase activation via the wt-EpoR, but not EpoR-H or EpoR-HM. Erythroblasts from wt-EpoR, EpoR-HM, and EpoR-H mice were expanded, washed, cultured for 6 hours in the absence of cytokines, and then stimulated for the indicated intervals with Epo (2.5 U/mL). Lysates then were prepared and levels of phosphorylated and total p70S6-kinase were assayed by Western blotting. (B) Deficient Akt activation via EpoR-HM and EpoR-H alleles. Erythroblasts were prepared as described for panel A, exposed to Epo, and analyzed for Akt activation. Note the multifold deficit activation of Akt via EpoR-HM and Epo-H alleles. (Ci-ii) Deficient PY-416 p60-Src expression via EpoR-HM. In the wt-EpoR, EpoR-HM, and EpoR-H mouse-derived cells and samples (B), levels of phospho-p60-Src (and p60-Src) were assayed by Western blotting (and digital densitometry imaging). (Ciii) Differential PP2 inhibition of EpoR-HM, EpoR-H, and wt-EpoR erythroblast expansion. During in vitro expansions, wt-EpoR, EpoR-H, and EpoR-HM erythroblasts were exposed to 15 μM PP2. Effects on erythroblast formation were assessed by direct cumulative cell counts at day 3 of expansion and are normalized to numbers for parallel DMSO-exposed control cultures. PP3 also was tested but was without significant effects (data not shown). Plotted values are mean values plus or minus SE; n = 3 wt EpoR, EpoR-HM, and EpoR-H mice per group.
EpoR-HM, EpoR-H, and wt-EpoR modulation of p70S6-kinase, Akt, and p60-Src. (A) p70S6-kinase activation via the wt-EpoR, but not EpoR-H or EpoR-HM. Erythroblasts from wt-EpoR, EpoR-HM, and EpoR-H mice were expanded, washed, cultured for 6 hours in the absence of cytokines, and then stimulated for the indicated intervals with Epo (2.5 U/mL). Lysates then were prepared and levels of phosphorylated and total p70S6-kinase were assayed by Western blotting. (B) Deficient Akt activation via EpoR-HM and EpoR-H alleles. Erythroblasts were prepared as described for panel A, exposed to Epo, and analyzed for Akt activation. Note the multifold deficit activation of Akt via EpoR-HM and Epo-H alleles. (Ci-ii) Deficient PY-416 p60-Src expression via EpoR-HM. In the wt-EpoR, EpoR-HM, and EpoR-H mouse-derived cells and samples (B), levels of phospho-p60-Src (and p60-Src) were assayed by Western blotting (and digital densitometry imaging). (Ciii) Differential PP2 inhibition of EpoR-HM, EpoR-H, and wt-EpoR erythroblast expansion. During in vitro expansions, wt-EpoR, EpoR-H, and EpoR-HM erythroblasts were exposed to 15 μM PP2. Effects on erythroblast formation were assessed by direct cumulative cell counts at day 3 of expansion and are normalized to numbers for parallel DMSO-exposed control cultures. PP3 also was tested but was without significant effects (data not shown). Plotted values are mean values plus or minus SE; n = 3 wt EpoR, EpoR-HM, and EpoR-H mice per group.
p60-Src also has been demonstrated to interact with the EpoR and to affect EpoR phosphorylation.11 Epo-induced activation of p60-Src in EpoR-HM, EpoR-H, and wt-EpoR erythroblasts therefore was analyzed. Via the wt-EpoR, p60-Src was activated several fold, and was maximally activated at 8 minutes of Epo stimulation (Figure 2C). In cytokine-deprived wt-EpoR cells, background levels of activated p60-Src, however, were sustained (as contrasted with Jak2 and Stat5, for example). In EpoR-HM erythroblasts, Epo detectably (but nominally) activated p60-Src. Levels of PY-p60-Src overall were also decreased several fold compared directly with wt-EpoR or EpoR-H cells (and to an extent this involved an apparent decrease in EpoR-HM cells of total p60-Src levels). Restoration of PY343 in EpoR-H cells rescued essentially wild-type levels of Epo-induced (and background) p60-Src activation and expression. These results indicate a previously unappreciated role for EpoR PY343 signals in up-modulating p60-Src, possibly via Stat5. Epo can also activate Lyn kinase. The possibility that the SFK studied might be Lyn, however, was discounted by the parallel blotting of lysates from expanded Lyn–/– erythroblasts (ie, Lyn migrated at a lower apparent Mr, and did not react with anti–p60-Src antibodies) (data not shown). Possible differential effects of PP2 (an SFK inhibitor) on the in vitro expansion of wt-EpoR, EpoR-HM, and EpoR-H erythroblasts also were assessed (Figure 2Ciii). PP2 inhibited the expansion of each, but was significantly less effective against EpoR-HM expansion. PP3 (as an inactive orthologue) exerted only nominal overall effects (data not shown). In pilot experiments, PP2 doses higher than 15 μM incurred toxicity, while lower doses did not efficiently inhibit p60-Src activation. Overall outcomes indicate only limited use of p60-Src by EpoR-HM and are consistent with Src contributions to EpoR-H and wt-EpoR activities.
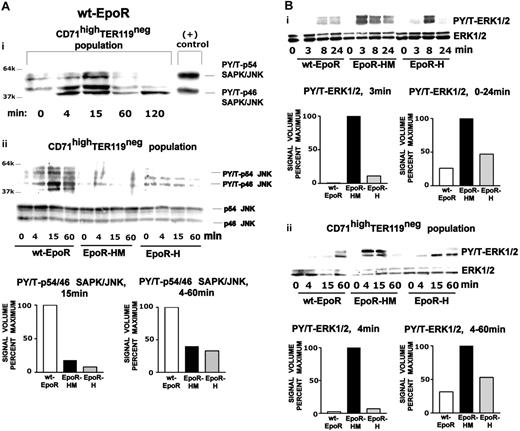
With regard to MAPKs, p38, JNKs, and ERKs have each been demonstrated in cell lines and/or primary erythroid cell preparations to be activated by Epo. Regulation of each in wt-EpoR, EpoR-H, and EpoR-HM in bone marrow–derived erythroblasts therefore was analyzed. For p38, activation was supported by each EpoR allele, but fold induction over background was limited (Figure S3). For JNKs, 2 isoforms were rapidly stimulated by Epo via the wt-EpoR (Figure 3A). Little to no JNK activation was detected, however, via EpoR-HM or EpoR-H alleles. ERKs, in contrast, proved to be induced via each Epo receptor allele, but, of interest, were discovered to be selectively hyperactivated via the PY-null EpoR-HM allele (Figure 3Bi). This latter finding was also examined further and was confirmed in Ter119pos-depleted erythroblast preparations (Figure 3Bii).
Epo receptor allele activation of JNKs and ERKs. (A) Efficient JNK activation via the wt-EpoR, but not via EpoR-H or Epo-HM alleles. Erythroblasts expanded from wt-EpoR, EpoR-HM, and EpoR-H bone marrow preparations were washed, cultured for 6 hours in the absence of cytokines, and stimulated with Epo (2.5 U/mL) for the indicated intervals. Levels of phospho-JNKs (and total JNKs) were assayed by Western blotting and digital densitometry imaging. The top panel (Ai) illustrates results for the wt-EpoR and includes coanalyzed positive controls (no. 9253; Cell Signaling). In the bottom panel (Aii), note the nominal activation of JNKs via EpoR-HM and Epo-H alleles. (B) ERK hyperactivation via EpoR-HM. (Bi) Erythroblasts expanded from wt-EpoR, EpoR-HM, and EpoR-H bone marrow preparations were washed, cultured for 6 hours in the absence of cytokines, and stimulated with Epo (2.5 U/mL) for the indicated intervals. Levels of phospho-ERK1,2 (and total ERK1,2) were assayed by Western blotting and digital densitometry imaging. (Bii) Parallel analyses of EpoR allele activation of ERKs were performed using expanded, Ter119-depleted wt-EpoR, EpoR-HM, and EpoR-H CD71high erythroblasts (and Epo exposure was extended to 60 minutes). For comparison, levels of Epo-stimulated phosphorylated p38-MAPK (PT180 and PY182) (and total p38-MAPK) also were assayed (Figure S3).
Epo receptor allele activation of JNKs and ERKs. (A) Efficient JNK activation via the wt-EpoR, but not via EpoR-H or Epo-HM alleles. Erythroblasts expanded from wt-EpoR, EpoR-HM, and EpoR-H bone marrow preparations were washed, cultured for 6 hours in the absence of cytokines, and stimulated with Epo (2.5 U/mL) for the indicated intervals. Levels of phospho-JNKs (and total JNKs) were assayed by Western blotting and digital densitometry imaging. The top panel (Ai) illustrates results for the wt-EpoR and includes coanalyzed positive controls (no. 9253; Cell Signaling). In the bottom panel (Aii), note the nominal activation of JNKs via EpoR-HM and Epo-H alleles. (B) ERK hyperactivation via EpoR-HM. (Bi) Erythroblasts expanded from wt-EpoR, EpoR-HM, and EpoR-H bone marrow preparations were washed, cultured for 6 hours in the absence of cytokines, and stimulated with Epo (2.5 U/mL) for the indicated intervals. Levels of phospho-ERK1,2 (and total ERK1,2) were assayed by Western blotting and digital densitometry imaging. (Bii) Parallel analyses of EpoR allele activation of ERKs were performed using expanded, Ter119-depleted wt-EpoR, EpoR-HM, and EpoR-H CD71high erythroblasts (and Epo exposure was extended to 60 minutes). For comparison, levels of Epo-stimulated phosphorylated p38-MAPK (PT180 and PY182) (and total p38-MAPK) also were assayed (Figure S3).
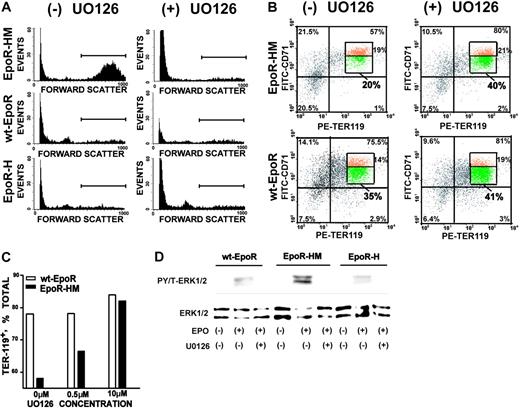
Faltered late-stage development of EpoR-HM erythroblasts and rescue by PY343 signals or MEK1,2 inhibition
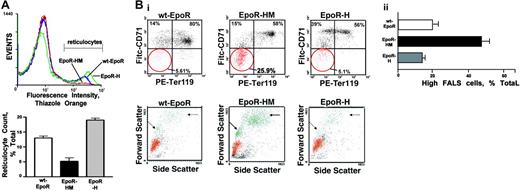
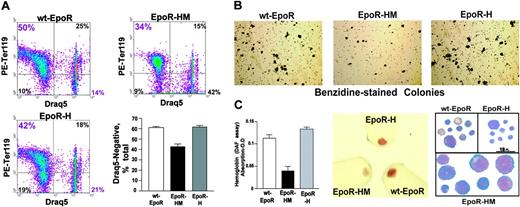
The limited signal transduction capacities observed for EpoR-HM in primary erythroblasts prompted additional follow-up biofunctional investigations. Compromised erythropoiesis often induces elevated Epo production. Epo levels in EpoR-HM, EpoR-H, and wt-EpoR mice, therefore, were first assessed. RT-PCR analysis of renal Epo transcript levels provided high sensitivity and reproducibility, and was used. In EpoR-HM mice, Epo levels were elevated on average to 1.9-fold above wt-EpoR controls. By direct comparison, levels in EpoR-H mice were decreased to approximately 60% of wild-type. These differences were uniformly observed in all mice assayed (n = 3 per group) and were significant at a level of P < .01 (Table 2). Second, Epo-induced reticulocyte production in response to Epo dosing was assayed. Mice were injected twice with Epo (2.5 U/g), and reticulocytes were assayed on day 5. Levels in EpoR-HM mice were diminished several fold compared with wild-type congenic controls and with EpoR-H mice (Figure 4A). Third, late-stage development of EpoR-HM and EpoR-H erythroblasts was analyzed in vitro. In brief, this involved expansion for 3 days, followed by culture in a differentiation medium containing transferrin, insulin, and Epo. At 40 hours of culture, maturation was assessed quantitatively based on CD71 and Ter119 marker expression, plus side- and forward-angle light scatter. Of interest (and despite the inclusion of Epo at a nonlimiting 2.5-U/mL concentration), EpoR-HM cells faltered in their maturation. Defects in late-stage differentiation first were observed in analyses of CD71highTer119pos cell formation (Figure 4Bi, left panels) in parallel with a 2.5-fold or higher disadvantage in the formation of low forward-angle light scatter, late-stage erythroblasts (Figure 4Bi-ii). For EpoR-H erythroblasts, a detectable attenuation of Ter119 marker expression also was observed. Progression to low forward angle light scatter (FALS) populations, however, was essentially normal. Apparent defects in EpoR-HM erythroblast differentiation were characterized further based on DRAQ5 staining, hemoglobinization, and cytomorphology (Figure 5). For differentiating EpoR-HM erythroblasts, frequencies of immature DRAQ5pos cells were increased over EpoR-H and wt-EpoR erythroblasts, while EpoR-HM Ter119posDRAQ5neg enucleated cells were correspondingly decreased (Figure 5A). In addition, direct benzidine staining of differentiating erythroblasts as well as diaminofluorene assays of lysed cells revealed 2.4-fold or higher defects in EpoR-HM hemoglobinization, and attenuated differentiation of EpoR-HM erythroblasts was also visually obvious in pelleted cells and cytospin preparations (Figure 5B-C).
Faltered Epo-induced reticulocyte formation in EpoR-HM mice and attenuated maturation of EpoR-HM erythroblasts in vitro. (A) Deficient reticulocyte production in Epo-treated EpoR-HM mice. At 1 and 24 hours, Epo was administered to wt-EpoR, EpoR-HM, and EpoR-H mice (2.5 U/g). On day 5, induced levels of reticulocytes were assayed. Illustrated are representative flow cytometric profiles of thiazole orange staining, together with mean reticulocyte values (± SE) (n = 4 wt-EpoR, EpoR-HM, and EpoR-H mice per group). (B) Attenuated formation of low FALS CD71pos Ter119pos EpoR-HM erythroblasts in vitro. Bone marrow–derived erythroid progenitor cells were expanded for 3 days in SP34-EX medium and were then transferred to differentiation medium (containing Epo, insulin, and transferrin). At 40 hours of culture, frequencies of maturing CD71highTer119pos erythroblasts were analyzed by flow cytometry (Bi, left panels). Maturation was also assessed based on transitions to low side-angle and forward-angle light scatter populations (Bi, right panels). In panel Bii, defects in this transition for EpoR-HM erythroblasts are graphically summarized. The numbers in quadrants (clockwise from bottom left) indicate the percentage of CD71negTer119neg, Ter119negCD71pos, and CD71posTer119high cells among total live-gated cells. Plotted values are mean values plus or minus SE; n = 3 wt-EpoR, EpoR-HM, and EpoR-H mice per group, representative of 3 experiments.
Faltered Epo-induced reticulocyte formation in EpoR-HM mice and attenuated maturation of EpoR-HM erythroblasts in vitro. (A) Deficient reticulocyte production in Epo-treated EpoR-HM mice. At 1 and 24 hours, Epo was administered to wt-EpoR, EpoR-HM, and EpoR-H mice (2.5 U/g). On day 5, induced levels of reticulocytes were assayed. Illustrated are representative flow cytometric profiles of thiazole orange staining, together with mean reticulocyte values (± SE) (n = 4 wt-EpoR, EpoR-HM, and EpoR-H mice per group). (B) Attenuated formation of low FALS CD71pos Ter119pos EpoR-HM erythroblasts in vitro. Bone marrow–derived erythroid progenitor cells were expanded for 3 days in SP34-EX medium and were then transferred to differentiation medium (containing Epo, insulin, and transferrin). At 40 hours of culture, frequencies of maturing CD71highTer119pos erythroblasts were analyzed by flow cytometry (Bi, left panels). Maturation was also assessed based on transitions to low side-angle and forward-angle light scatter populations (Bi, right panels). In panel Bii, defects in this transition for EpoR-HM erythroblasts are graphically summarized. The numbers in quadrants (clockwise from bottom left) indicate the percentage of CD71negTer119neg, Ter119negCD71pos, and CD71posTer119high cells among total live-gated cells. Plotted values are mean values plus or minus SE; n = 3 wt-EpoR, EpoR-HM, and EpoR-H mice per group, representative of 3 experiments.
Based on the observed hyperactivation of ERKs in EpoR-HM erythroblasts, experiments also were performed to test the extent to which U0126 inhibition of MEK1,241,42 might impact on Epo receptor–dependent erythroblast differentiation (especially as supported by EpoR-HM). In pilot experiments, U0126 at 10 μM was observed to have little effect on expansion (but at 20 μM detectably affected viability, data not shown). EpoR-HM, EpoR-H, and wt-EpoR erythroblasts, therefore, were expanded in the presence of 10 μM U0126 and then shifted to differentiation medium. Upon differentiation, U0126 proved to essentially correct dysregulated EpoR-HM erythroblast maturation as revealed first by clear decreases in forward-angle light scatter (Figure 6A). As assayed based on Ter119 and CD71 marker expression, U0126 also promoted the maturation of a subpopulation of EpoR-HM Ter119posCD71low erythroblasts (Figure 6B). In contrast, U0126 at this dose had no significant effects on the maturation of wt-EpoR erythroblasts (or on EpoR-H erythroblasts, data not shown). Dose dependency of this U0126 effect on EpoR-HM erythroblasts also is illustrated (Figure 6C). The capacity of U0126 to inhibit EpoR-mediated ERK activation also was assessed directly in expanded wt-EpoR, EpoR-HM, and EpoR-H erythroblasts. At the 10-μM dosage used in bioresponse assays, U0126 proved to effectively inhibit the ability of each EpoR form to activate ERKs as analyzed in cytokine-withdrawal and Epo-stimulated format (Figure 6D). For comparison, possible effects of the MAPK-p38 inhibitor SB202190 and JNK inhibitor SB600125 on wt-EpoR, EpoR-HM, and EpoR-H differentiation were tested. At concentrations of 0.25, 1, and 4 μM (SB202190) and 1 and 15 μM (SB600125), no significant effects were observed (data not shown).
Sustained DRAQ5 positivity, decreased hemoglobinization, and altered cytomorphology of maturing EpoR-HM erythroblasts. (A) Bone marrow–derived erythroid progenitor cells were expanded in SP34-EX medium and subsequently were cultured in differentiation medium for 40 hours. Frequencies of DRAQ5negTer119pos erythroblasts then were determined. (B) In parallel, cultures were analyzed for hemoglobinization (benzidine-positive colonies). (C) Hemoglobin levels in maturing wt-EpoR, EpoR-HM, and EpoR-H erythroblasts were also assayed using diaminofluorene (DAF) and by visualization of pelleted cells. For EpoR-HM erythroblasts, apparently immature morphologies were observed in cytospin preparations (right panel). Micrograph images were visualized using an Olympus IX70 microscope equipped with a 100×/1.3 oil-immersion objective lens (Olympus, Melville, NY). Immersion oil no. 16212 from Cargille Labs (Cedar Grove, NJ) was used with the lens. A Zeiss Axiocam 412312 camera and Axio-vision 4.1 software (Zeiss, Thornwood, NY) were used to capture and process images. In dotplots, the numbers in quadrants (clockwise from bottom left) indicate the percentage of Ter119negDraq5neg, Ter119highDraq5neg, Ter119highDraq5high, and Ter119negDraq5pos cells among total live-gated cells. In the bar graphs, plotted values are mean values plus or minus SE; n = 4 wt-EpoR, EpoR-HM, and EpoR-H mice.
Sustained DRAQ5 positivity, decreased hemoglobinization, and altered cytomorphology of maturing EpoR-HM erythroblasts. (A) Bone marrow–derived erythroid progenitor cells were expanded in SP34-EX medium and subsequently were cultured in differentiation medium for 40 hours. Frequencies of DRAQ5negTer119pos erythroblasts then were determined. (B) In parallel, cultures were analyzed for hemoglobinization (benzidine-positive colonies). (C) Hemoglobin levels in maturing wt-EpoR, EpoR-HM, and EpoR-H erythroblasts were also assayed using diaminofluorene (DAF) and by visualization of pelleted cells. For EpoR-HM erythroblasts, apparently immature morphologies were observed in cytospin preparations (right panel). Micrograph images were visualized using an Olympus IX70 microscope equipped with a 100×/1.3 oil-immersion objective lens (Olympus, Melville, NY). Immersion oil no. 16212 from Cargille Labs (Cedar Grove, NJ) was used with the lens. A Zeiss Axiocam 412312 camera and Axio-vision 4.1 software (Zeiss, Thornwood, NY) were used to capture and process images. In dotplots, the numbers in quadrants (clockwise from bottom left) indicate the percentage of Ter119negDraq5neg, Ter119highDraq5neg, Ter119highDraq5high, and Ter119negDraq5pos cells among total live-gated cells. In the bar graphs, plotted values are mean values plus or minus SE; n = 4 wt-EpoR, EpoR-HM, and EpoR-H mice.
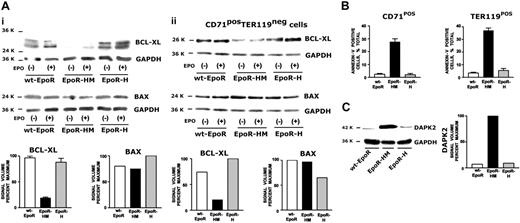
EpoR-H and EpoR-HM erythroblast survival potential, and Bcl-x, Bax, and DAPK-2 expression
Based on Epo's primary role as an antiapoptotic factor,43 possible differences among EpoR-HM, EpoR-H, and wt-EpoR erythroblast survival and Bcl-xl, Bax, and DAPK-2 expression were analyzed (Figure 7). In expanded EpoR-HM erythroblasts, Bcl-xl levels proved to be decreased several fold (Figure 7Ai). Bax expression levels, by comparison, were similar for each EpoR allele. In repeated analyses in Ter119-depleted CD71high wt-EpoR, EpoR-HM, and EpoR-H populations, findings were similar (Figure 7Aii). The extent to which altered EpoR-HM signaling capacities might correlate with compromised erythroblast survival potential also was assessed. In these experiments, Kitpos cells first were isolated (by magnetic activated cell sorting [MACS]) from bone marrow preparations prior to expansion in SP34-EX medium. This provided improved flow cytometric discrimination of stepwise development. Costaining with annexin-V revealed increased stage-specific staining of relatively late-stage EpoR-HM CD71highKitneg as well as differentiated Ter119pos erythroblasts compared directly with wt-EpoR and EpoR-H cells (Figure 7B), and this was despite sustained exposure to high-dose Epo (2.5 U/mL). In part via gene-profiling analyses of purified developmentally staged erythroblasts, our laboratory recently reported on predominant late-stage erythroid expression of the proapoptotic death-associated protein kinase-2 (DAPK-2).44 In expanded wt-EpoR, EpoR-HM, and/or EpoR-H progenitors, possible differential expression of DAPK-2 was assessed. Of interest, DAPK-2 levels proved to be significantly elevated selectively in EpoR-HM erythroblasts (Figure 7C). This observation is consistent with the decreased survival potential of EpoR-HM cells (and with possible roles for EpoR PY343, and Stat5, in down-modulating DAPK-2).
Discussion
As revealed through transgenic mouse models, the EpoR is essential for erythroblast formation,45 and its conditional hematopoietic expression is sufficient for normal overall development.45 Crystallographic and structural analyses have provided detailed insight into Epo-mediated dimerization of its receptor2,46 and conformation-dependent mechanisms of Jak2 activation.3 Epo signal transduction studies also have gone far to define a broad, yet select set of factors and associated pathways that mediate and/or modulate Epo's actions as a clinically important antianemia agent.47,48 The present work uses mice with knocked-in minimal EpoR alleles and primary marrow-derived erythroblasts to further define core signal transduction events that are integral to Epo receptor function.
A minimal model for EpoR action is one whereby Jak2 fulfills a central role and supports Epo action independently from EpoR PY modulating effects. This model is predicted by the in vivo erythropoietic capacity of EpoR-HM and has previously been framed in cell line models. Specifically, EpoR-HM has been reported in 32D cells to induce Bcl-x and c-Myc expression,49,50 and to support ERK activation at wild-type levels.51 In the present investigations, we analyzed EpoR-HM's capacity in primary erythroblasts to activate Jak2, Stat5, and Stat5-target genes (Bcl-x and Myc; see “Discussion”). We also examined possible EpoR-HM regulation of Akt, p70S6-kinase, p60Src, p38-MAPK, JNKs, and ERKs. Jak2 activation profiles were essentially normal, and in the absence of PY343, no significant induction of Stat5 or Stat5-target genes was detected. In addition, EpoR-HM essentially failed to activate JNKs or p70S6-kinase, and was substantially compromised in its ability to simulate Akt (as well as p60-Src). As observed for the wt-EpoR (and EpoR-H), EpoR-HM modestly stimulated p38-MAPK. The major intact (and selectively altered) response pathway for EpoR-HM, however, involved ERKs. This finding (ie, EpoR-HM hyperactivation of ERKs) was unexpected, and prompts considerations of, first, how the EpoR might couple to ERKs and, second, what signals ERK might provide to developing primary erythroblasts.
Mek1,2 inhibition reverses EpoR-HM erythroblast stage-specific differentiation defects. (A) Bone marrow–derived wt-EpoR, EpoR-HM, and EpoR-H erythroid progenitor cells were cultured for 72 hours in SP34-EX medium containing U0126 (± 10 μM). Expanded erythroblasts then were differentiated (in transferrin, BSA, and insulin-containing medium) with Epo at 2.5 U/mL and U0126 (± 10 μM). At 40 hours of culture, frequencies of high forward-angle light scatter erythroblasts were assayed. Note the reversal of differentiation defects in EpoR-HM erythroblasts as illustrated by U0126-induced decreases in forward scatter (cell size). (B) U0126 reversal of EpoR-HM erythroblast differentiation defects as analyzed by Ter119 and CD71 marker expression. At 40 hours of differentiation, EpoR-HM erythroblasts also exhibited significantly decreased frequencies of Ter119pos erythroblasts specifically within a subpopulation of maturing cells with decreased CD71 expression. U0126 reversed this defect (but had no significant effects on control wt-EpoR cells). (C) U0126 dose-dependent reversal of EpoR-HM erythroblast differentiation defects also was observed based on U0126-dependent increases in frequencies of Ter119pos erythroblasts. (D) U0126 inhibition of ERK1,2 activation in primary wt-EpoR, EpoR-HM, and EpoR-H erythroblasts. The capacity of U0126 to inhibit the Epo-stimulated activation of ERKs was confirmed directly by exposing expanded, Ter119-depleted erythroblast preparations to ± 10 μM U0126. In scatterplots, the numbers in quadrants (clockwise from bottom left) indicate the percentage of CD71negTer119neg, Ter119negCD71pos, CD71posTer119high, and Ter119highCD71neg cells among total live-gated cells. Boxes in each top right quadrant indicate the percentage of CD71highTer119high (top) and CD71lowTer119high (bottom) populations.
Mek1,2 inhibition reverses EpoR-HM erythroblast stage-specific differentiation defects. (A) Bone marrow–derived wt-EpoR, EpoR-HM, and EpoR-H erythroid progenitor cells were cultured for 72 hours in SP34-EX medium containing U0126 (± 10 μM). Expanded erythroblasts then were differentiated (in transferrin, BSA, and insulin-containing medium) with Epo at 2.5 U/mL and U0126 (± 10 μM). At 40 hours of culture, frequencies of high forward-angle light scatter erythroblasts were assayed. Note the reversal of differentiation defects in EpoR-HM erythroblasts as illustrated by U0126-induced decreases in forward scatter (cell size). (B) U0126 reversal of EpoR-HM erythroblast differentiation defects as analyzed by Ter119 and CD71 marker expression. At 40 hours of differentiation, EpoR-HM erythroblasts also exhibited significantly decreased frequencies of Ter119pos erythroblasts specifically within a subpopulation of maturing cells with decreased CD71 expression. U0126 reversed this defect (but had no significant effects on control wt-EpoR cells). (C) U0126 dose-dependent reversal of EpoR-HM erythroblast differentiation defects also was observed based on U0126-dependent increases in frequencies of Ter119pos erythroblasts. (D) U0126 inhibition of ERK1,2 activation in primary wt-EpoR, EpoR-HM, and EpoR-H erythroblasts. The capacity of U0126 to inhibit the Epo-stimulated activation of ERKs was confirmed directly by exposing expanded, Ter119-depleted erythroblast preparations to ± 10 μM U0126. In scatterplots, the numbers in quadrants (clockwise from bottom left) indicate the percentage of CD71negTer119neg, Ter119negCD71pos, CD71posTer119high, and Ter119highCD71neg cells among total live-gated cells. Boxes in each top right quadrant indicate the percentage of CD71highTer119high (top) and CD71lowTer119high (bottom) populations.
Mechanisms of EpoR-mediated ERK activation are incompletely defined, but previous cell line studies have outlined several EpoR PY–dependent routes. This includes a PI3-kinase dependent pathway52 ; a recently described PY479 plus PLC-gamma–dependent route53 ; an Shc plus Grb2 pathway to mSos and Ras54 ; a CrkL plus C3G–coupled route55 ; as well as a SOCS-3–mediated mechanism involving PY-SOCS box sequestration of GAP.56 In contrast, the presently observed strong activation of ERKs via EpoR-HM in primary erythroblasts suggests that PY sites may be nonessential for ERK activation. Candidate factors that might couple EpoR-HM (and perhaps the wt-EpoR) to ERKs are presently undefined, but G-proteins as well as PKCs stimulate MAPK modules57,58 and represent 2 sets of potentially Epo-regulated candidate effectors. Based on the substantial in vivo activity exerted by EpoR-HM, such Epo-regulated pathways should be of interest to define. In addition, a specific loss of PY343 signaling is associated with EpoR-HM–mediated ERK hyperactivation. By speculation, this effect might involve decreased MAPK phosphatase action. Mkp7 haploinsufficiency, for example, has been associated with BCR-ABL–induced proliferation,59 while increased MKP-1 activity is associated with preadipocyte differentiation.60
Biofunctional roles that Epo-regulated ERKs might play in developing erythroblasts also merit consideration. In context-specific settings, ERK signaling can affect differentiation, survival, and proliferation (reviewed in Roux and Blenis61 and Wada and Penninger62 ). However, ERKs are most commonly activated via growth factor receptors.61 Moreover, ERKs have been shown in primary erythroid cells to be the prime effector of H-Ras–induced transformation.63 It is suggested, therefore, that ERK hyperactivation in EpoR-HM erythroblasts may inappropriately enforce proliferation at the expense of differentiation. This case also is supported by restoration of wild-type differentiation profiles for EpoR-HM erythroblasts upon exposure to the MEK1,2 inhibitor U0126 (Figure 6). It is noted, however, that chronic activation of ERKs also can induce apoptosis,64 and this response also might contribute to the elevated apoptosis observed among maturing EpoR-HM cells. Finally, for EpoR-HM, 2 additional responses described previously in cell line models are c-Myc and Bcl-x transcript induction.49,50 In the present system, c-Myc (and n-Myc) induction by Epo was analyzed. Each was up-modulated approximately 1.5-fold uniformly in EpoR-HM, EpoR-H, and wt-EpoR erythroblasts (D.M.W., unpublished results, August 2005). This therefore was not a strong or differential response. For Bcl-x, possible regulation via EpoR alleles is discussed below.
Altered Bcl-xl and DAPK-2 expression in EpoR-HM erythroblasts. (Ai) Levels of Bcl-xl in expanded wt-EpoR, EpoR-HM, and EpoR-H erythroblasts were assayed (by Western blotting) at 2 time points—directly following cytokine withdrawal and at 30 minutes of Epo exposure. Note the decreased Bcl-xl levels in EpoR-HM erythroblasts. For comparison, Bax levels also were analyzed. (Aii) Bcl-xl expression in wt-EpoR, EpoR-HM, and EpoR-H erythroblasts also was analyzed in Ter119-depleted, CD71high erythroblasts. (B) Defective survival of EpoR-HM CD71highKitneg erythroblasts and rescue of survival potential by PY343 in EpoR-H. Kitpos progenitor cells were prepared from wt-EpoR, EpoR-HM, and EpoR-H bone marrow and were expanded in SP34-EX media. At day 3 of culture, CD71 and Ter119 marker expression was assayed and cells were costained with annexin-V. Relative frequencies of annexin-V–positive cells among CD71pos subpopulations of EpoR-HM, EpoR-H, and wt-EpoR erythroblasts are graphed. Expanded cells also were shifted to differentiation medium, and frequencies of annexin-V and Ter119–copositive cells were analyzed. (C) Elevated DAPK-2 expression in EpoR-HM erythroblasts. Death-associated protein kinase-2 (DAPK-2) expression in wt-EpoR, EpoR-HM, and EpoR-H erythroblasts was assayed by Western blotting (and digital densitometry). Note the several fold increase in DAPK-2 levels in EpoR-HM erythroblasts (representative of 3 independent experiments). For Bcl-xl, plotted quantitation values are mean values plus or minus SE; n = 3 wt-EpoR, EpoR-HM, and EpoR-H mice per group, x = 2 experiments. For annexin-V staining, plotted values are mean values plus or minus SE; n = 3 wt-EpoR, EpoR-HM, and EpoR-H mice per group, x = 4 experiments.
Altered Bcl-xl and DAPK-2 expression in EpoR-HM erythroblasts. (Ai) Levels of Bcl-xl in expanded wt-EpoR, EpoR-HM, and EpoR-H erythroblasts were assayed (by Western blotting) at 2 time points—directly following cytokine withdrawal and at 30 minutes of Epo exposure. Note the decreased Bcl-xl levels in EpoR-HM erythroblasts. For comparison, Bax levels also were analyzed. (Aii) Bcl-xl expression in wt-EpoR, EpoR-HM, and EpoR-H erythroblasts also was analyzed in Ter119-depleted, CD71high erythroblasts. (B) Defective survival of EpoR-HM CD71highKitneg erythroblasts and rescue of survival potential by PY343 in EpoR-H. Kitpos progenitor cells were prepared from wt-EpoR, EpoR-HM, and EpoR-H bone marrow and were expanded in SP34-EX media. At day 3 of culture, CD71 and Ter119 marker expression was assayed and cells were costained with annexin-V. Relative frequencies of annexin-V–positive cells among CD71pos subpopulations of EpoR-HM, EpoR-H, and wt-EpoR erythroblasts are graphed. Expanded cells also were shifted to differentiation medium, and frequencies of annexin-V and Ter119–copositive cells were analyzed. (C) Elevated DAPK-2 expression in EpoR-HM erythroblasts. Death-associated protein kinase-2 (DAPK-2) expression in wt-EpoR, EpoR-HM, and EpoR-H erythroblasts was assayed by Western blotting (and digital densitometry). Note the several fold increase in DAPK-2 levels in EpoR-HM erythroblasts (representative of 3 independent experiments). For Bcl-xl, plotted quantitation values are mean values plus or minus SE; n = 3 wt-EpoR, EpoR-HM, and EpoR-H mice per group, x = 2 experiments. For annexin-V staining, plotted values are mean values plus or minus SE; n = 3 wt-EpoR, EpoR-HM, and EpoR-H mice per group, x = 4 experiments.
In EpoR-H erythroblasts, the selective restoration of PY343 restored efficient Epo activation of Stat5 (and also increased p60-Src activation). As described, ERK activation also was down-modulated compared with EpoR-HM (to approximately wt-EpoR levels). JNK and p70S6 kinases, however, remained essentially uncoupled, and Akt activation also was inefficient (as observed for EpoR-HM). Based on these results, enhanced bioactivities of EpoR-H are suggested to depend primarily upon Stat5 activation, and the actions of select Stat5-target genes within developing erythroblasts. Five genes that previously have been suggested in cell line models to comprise Epo and Stat5-regulated genes were assessed for Epo regulation in EpoR-H, EpoR-HM, and wt-EpoR erythroblasts (Pim1, oncostatin-M, Socs3, Cis1, and Bclx). With the exception of Bcl-x, each was induced at least 5-fold by Epo via EpoR-H and the wt-EpoR, but not via EpoR-HM. SOCS-3 and Cis-1 are known to provide negative feedback, in part via inhibition of Jak2 at activated EpoR complexes.22,65 Pim-1 and oncostatin-M, in contrast, may well act in positive modes. Pim kinases recently have been shown to act in parallel with mTOR to promote hematopoietic progenitor cell survival.66 Oncostatin-M, by comparison, is a pleiotropic cytokine that is expressed primarily by macrophage and activated T cells. Of interest, however, disruption of oncostatin-M receptor-beta expression limits bone marrow erythroid progenitor cell formation.67 Whether erythroblast-derived oncostatin-M might act via autocrine routes, or perhaps on neighboring macrophages in blood islands, is under investigation. Finally, present findings for Epo-regulated Bcl-x expression are negative ones, and therefore should be conservatively interpreted. Two points to be considered here, nonetheless, are, first, that Epo (and Stat5) may not act to strongly or rapidly up-regulate Bcl-x gene transcription. In recent studies by Rhodes et al68 this possibility also has been raised. Second, decreases in Bcl-xl levels presently observed in EpoR-HM erythroblasts therefore may only correlate with decreased Epo-dependent survival potential.
With further regard to Stat5, the ability of PY343 (and Stat5) signals in EpoR-H to restore wild-type erythroblast development (vs faltered development for EpoR-HM) predicts that erythroid defects should exist in Stat5-deficient mice. In phenylhydrazine-treated Stat5a,b–/– mice, faltered splenic erythropoiesis has been characterized,69 and certain studies also have outlined deficient embryonic erythropoiesis.33 Spontaneous (erythro)splenomegaly, however, has been reported to involve immune cell bystander effects.30 In addition, the Stat5 exon targeting strategy used may allow for expression of at least partially functional Stat5 proteins. The recent development of mice with floxed Stat5a and b alleles70 offers an alternate future approach to Stat5 action mechanisms (ie, erythroid lineage–specific disruption). In these mice, conditional Stat5 disruption at the oocyte stage results in embryonic anemia.70
Finally, and compared with the wt-EpoR, PY-deficient EpoR-HM and EpoR-H alleles faltered in their abilities to support Epo induction of Akt, p70S6-kinase, and JNKs. For Akt and p70S6-kinase, this likely reflects uncoupling from PI3-kinase.37,38 By comparison, less is known concerning Epo and EpoR regulation of JNKs or roles played by JNKs during erythropoiesis. Epo activation of JNK1 and JNK2 has been observed in SKT6 and HCD57 cell lines. In SKT6 cells, JNK activation was linked to Epo-dependent differentiation.71 In HCD57 cells, antisense and inhibitor-based knock-down of JNKs was observed to limit proliferation.72 JNK2 deficiency, however, appears to increase erythroid progenitor cell proliferative potential.73 In primary erythroblasts, Epo appears to activate at least 2 JNK isoforms, but this proved to depend upon EpoR carboxyl-terminal PY sites beyond PY343. Factors that link the EpoR to MEK4/7 and JNK pathways remain to be discovered, as do the contributions of this typically stress-activated module to erythropoiesis.
Overall, the present studies reveal that core Jak2-mediated, Epo receptor PY–independent signals for erythroblast formation are associated primarily with PY-null EpoR-HM activation of ERKs, and are essentially uncoupled from Stat5, Stat1, Stat3, JNK kinases, or p70S6-kinase pathways. Whether ERK activation is necessary and sufficient for EpoR-HM action nonetheless is uncertain, and it is possible that other lateral pathways are engaged (eg, IRS1,2 and/or Gab1,2).20,74 Such possibilities are under investigation, as are possible contributions of residual low-level Akt activation. It is clear, however, that U0126 attenuation of ERK hyperactivation corrects a late-stage developmental defect in maturing EpoR-HM erythroblasts, and this result fortifies the notion that ERK activation is functionally consequential. In addition, EpoR PY343 signaling is shown to be essential for Stat5 activation, and Stat5-stimulated events are shown to rescue wild-type (or higher) levels of Epo bioresponses in vitro and in vivo. Here, it is possible that EpoR PY343 stimulates additional pathways beyond Stat5. However, this does not appear to include Stat1 or Stat3, p70S6-kinase, JNKs, or Akt. EpoR-H's activity (and that of the wt-EpoR), therefore, may more likely depend upon the actions of one or more key Stat5-target genes in developing erythroblasts. Two candidate targets are Pim1 and oncostatin M, but others are being actively sought using the presently developed primary erythroblast system.
Prepublished online as Blood First Edition Paper, December 6, 2005; DOI 10.1182/blood-2005-02-0684.
Supported by NIH grants HL44491 and P20 RR18789.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
The authors thank the following collaborators for their generous indicated contributions: Dr James Ihle (St Jude Children's Research Hospital, Memphis, TN) for provision of EpoR-H and EpoR-HM mice, and Jane Mitchell (MMCRI) for expert assistance with flow cytometry and FACS analysis.


This feature is available to Subscribers Only
Sign In or Create an Account Close Modal