Abstract
In multiple myeloma (MM), circulating endothelial cells (CECs) represent a vascular marker of angiogenesis and may reflect tumor mass. In this report, we showed that, in 5 MM patients with 13q14 deletion, CECs carried the same chromosome aberration as the neoplastic plasma cells (11%-32% of CECs with 13q14 deletion). Most of the CECs displayed immunophenotypic features of endothelial progenitor cells as they expressed CD133, a marker gradually lost during endothelial differentiation and absent on mature endothelial cells. To the contrary, in 3 patients with monoclonal gammopathy of undetermined significance and 13q14 deletion, CECs were cytogenetically normal and had a mature immunophenotype. In MM CECs, immunoglobulin genes were clonally rearranged. These findings suggest a possible origin of CECs from a common hemangioblast precursor that can give rise to both plasma cells and endothelial cells and point to a direct contribution of MM-derived CECs to tumor vasculogenesis and possibly to the spreading and progression of the disease. (Blood. 2006;107:2531-2535)
Introduction
Several studies have shown that bone marrow-derived endothelial cells (ECs) may contribute to tumor angiogenesis,1-3 and that in the peripheral blood of cancer patients there is an increased amount of circulating ECs (CECs)4 that may participate to vessel formation.5 Recent data also showed that microvascular ECs in B-cell lymphomas are in part tumor-related, reflecting a novel aspect of tumor angiogenesis.6
All together, these observations suggest that tumors can elicit the sprouting of new vessels from existing capillaries through the secretion of angiogenic factors7 and that, in some cases, cancer cells can also mimic the activities of ECs by participating in the formation of vascular-like networks.6,8
In multiple myeloma (MM), the proliferation and survival of neoplastic plasma cells is regulated by microenvironmental bone marrow factors and, to this extent, neoangiogenesis is thought to have a key role in the pathogenesis and progression of the disease.9 In patients with MM, CEC levels are higher than in controls and correlate positively with serum M protein and β2-microglobulin, therefore representing a vascular marker which reflects tumor mass and prognosis.10 To clarify whether CECs in MM are tumor derived, we studied 5 patients with MM and 3 patients with monoclonal gammopathy of undetermined significance (MGUS) with 13q14 deletion and sought to characterize immunomagnetically sorted CECs by immunophenotypic analyses, fluorescence in situ hybridization (FISH), and molecular genetic studies.
Patients, materials, and methods
Patients
These patients represent consecutive cases with 13q14 deletion seen at our institution between January 2000 and June 2004. The patients were studied by FISH on morphologically intact bone marrow cells as previously reported11 using a commercially available 13q14 probe in combination with a chromosome 10-centromeric probe as control, and a 14q32 immunoglobulin H (IgH) probe (Vysis, Downers Grove, IL; distributed by Abbott, Rome, Italy). Five patients had MM (3 patients at diagnosis, 2 at relapse), and 3 patients had MGUS. All the patients carried the 13q14 deletion in 52% to 68% of neoplastic plasma cells. As controls, 5 MM patients without 13q14 deletions were analyzed. All patients gave written informed consent to an ethics committee-approved protocol.
CEC isolation and characterization
CECs were isolated, with some modifications, as previously described.12 Briefly, 20 mL peripheral blood was obtained at enrollment and CECs were isolated by immunomagnetic sorting, according to manufacturer's instructions, with Dynabeads Pan Mouse IgG (Dynal A.S., Oslo, Norway) by a 2-step approach. In order to eliminate hematopoietic cells that are CD45 positive and to isolate CECs that are CD45 negative, we first performed a negative selection with Dynabeads coated with anti-CD45 (clone 2D1; Becton Dickinson, Milan, Italy). CD45-negative cells were subsequently subjected to a positive selection with Dynabeads coated with anti-CD146 antibody (clone P1H12; Becton Dickinson, Milan, Italy). CD146 (also known as MCAM, MUC18, S-endo-1, and Mel-CAM9) is an antigen expressed almost exclusively on endothelial cells, the exception being stroma cells, smooth muscle cells, follicular dendritic cells, and some tumor cell lines.13 Its absence on hematopoietic cells makes CD146 a helpful reagent to specifically discriminate endothelium from hematopoietic tissues.13 For further characterization, immunomagnetically sorted cells were then stained with Ulex Europeus lectin 1 (UEA-1; Dako, Milan Italy) followed by FITC-conjugated swine anti-rabbit immunoglobulins (Dako) as a secondary reagent, and in double-staining experiments with the following antibodies: rabbit anti-VEGFR-2 (Santa Cruz Biotechnology, Santa Cruz, CA) followed by FITC-conjugated swine anti-rabbit immunoglobulins (Dako) as secondary reagent in combination with murine anti-human von Willebrand factor (VWF) antibody (Dako) followed by TRITC-conjugated rabbit anti-mouse immunoglobulins (Dako); FITC-conjugated rabbit anti-CD144 antibody (vascular endothelial [VE]-cadherin; Serotec, Oxford, United Kingdom) in combination with murine anti-human VWF antibody followed by TRITC-conjugated rabbit anti-mouse immunoglobulins as a secondary reagent; murine anti-human VWF antibody followed by FITC-conjugated rabbit anti-mouse immunoglobulins (Dako) as a secondary reagent in combination with PE-conjugated murine anti-CD138 antibody (clone BB4; Beckman Coulter, Milan, Italy); murine anti-human VWF antibody followed by FITC-conjugated rabbit anti-mouse immunoglobulins as a secondary reagent in combination with PE-conjugated murine anti-CD38 antibody (clone HB7; Becton Dickinson); and FITC-conjugated murine anti-CD45 (clone 2D1) in combination with PE-conjugated anti-CD14 (clone MoP9; Becton Dickinson). Evaluation of immunophenotypic results was performed using a Nikon fluorescence-equipped microscope with a charge-coupled black-and-white camera device and appropriate hardware and software (Cytovision System, Applied Imaging; distributed by Nikon Italy, Florence, Italy).
FISH analysis on CECs
The following probes were used simultaneously in dual-color experiments: a Spectrum orange LSI D13S25 DNA probe to the band 13q14.3 and a Spectrum green CEP 10 alpha satellite DNA probe to the centromere band region 10p11.1-q11.1 of human chromosome 10 (Vysis). A detailed description of the technique has been reported previously.12 Briefly, immunomagnetically sorted CECs were washed twice in 4 × saline sodium citrate (SSC; Vysis) for 5 minutes each, subsequently dehydrated in an ethanol alcohol series (70%, 85%, and 100%), and air-dried. The slides were prewarmed on a hot plate and then immersed in a 70% formamide 2 × SSC solution at 72°C for 5 minutes and dehydrated again with an alcohol series at -20 °C. Each probe (10 μL) was added to each slide and covered with a coverslip. Rubber cement was used to seal the edges, and the slides were incubated overnight at 37°C in a moist chamber. Posthybridization washes included baths at 73°C in 0.4 × SSC/0.3% NP-40 (Vysis) for 5 minutes, and at room temperature in 2 × SSC/0.1% NP-40, without intermittent agitation. Cells were subsequently stained with PE-conjugated murine anti-CD133 antibody (AC133; Miltenyi Biotec, Bergisch Gladbach, Germany) according to the manufacturer's instructions. Evaluation of FISH results was performed using a Nikon fluorescence-equipped microscope with a charge-coupled black-and-white camera device and appropriate hardware and software. To prevent data misinterpretation because of inefficient hybridization, only those areas with more than 80% of cells showing 2 control signals were analyzed. As controls, we used CECs obtained in the same way from 5 MM patients without 13q14 deletion. To ensure better visualization and reproduction of the images, pseudocolours were attributed to the background, the cells, and the signals.
Immunoglobulin gene rearrangements and polymerase chain reaction
Polymerase chain reaction (PCR) was performed in 2 MM patients with 13q14 deletion for whom cell material was available. Plasma cells of the same patients isolated at the time of diagnosis were used as a positive control. To rule out contaminating plasma cells giving false-positive results, we evaluated the sensitivity of our method by analyzing negative samples with decreasing percentages of clonal plasma cell contaminations (15%, 1%, 0.1%, and 0.01%). In these experimental conditions that, due to the low number of CECs that can be isolated from the peripheral blood, are characterized by the lowest amount of DNA allowed for the method, 1% or more of contaminating plasma cells were necessary to give positive results (Figure S1; see the Supplemental Figure link at the top of the online article on the Blood website). VH genes were amplified from genomic DNA. DNA was extracted from cryopreserved cells using Trizol reagent (Life Technologies, Paisley, Scotland). We performed a set of 8 family-specific PCRs to isolate the clonally expressed VHDHJH. The sense primers were from the leader regions (LVH1: 5′-ATG GAC TGS ACC TGG AGV ATC C-3′; LVH1-46: 5′-GTC TTC TGC TTG CTG GCT GTA G-3′; LVH2: 5′-CAC RCT CCT GCT GCT GAC CA-3′; LVH3a: 5′-GCT GGG TTT TCC TTG TTG C-3′; LVH3b: 5′-ATG GAG TTK GGR CTG AGC TG-3′; LVH4: 5′-ATG AAR CAC CTG TGG TTY TTC CT-3′; LVH5: 5′-CCT CCT CCT GGC TGT TCT C-3′; and LVH6: 5′-CTG TCT CCT TCC TCA TCT TCC-3′). In all the reactions, the same antisense JH degenerate primer was employed (JH deg: 5′-CTY ACC TGA RGA GAC RGT GAC C-3′). The 30 μL reactions (10 pM of each primer, 1.5 mM MgCl2, and 100 μM of each dNTP and 1U Ampliterm Hot Start DNA polymerase in supplied buffer (Fisher Scientific, Hampton, NH) were cycled 35 times at an annealing temperature of 60°C. PCR products were spin-column purified (Promega, Madison, WI) and directly sequenced using the automated sequencer. Sequence alignment was to the Entrez database (National Center for Biotechnology Information [NCBI] Bethesda, MD) and to the V Base (MRC Center for Protein Engineering, Cambridge, United Kingdom).
Results
Patients
Demographic and principal clinical characteristics of the 8 patients with 13q14 deletion (5 MM and 3 MGUS) are reported in Table 1.
Demographic and principal clinical characteristics of patients
Patient no. . | Age, y/sex . | Diagnosis . | Stage . | M comp, g/L . | β-2M, mg/L . | CRP, mg/L . | CD133+ CECs/total CECs (%) . | CECs with 13q14 del/total CECs (%) . | CD133+ CECs with 13q14 del/CECs with 13q14 del (%) . |
|---|---|---|---|---|---|---|---|---|---|
| 1 | 63/M | MM IgG/κ | IA | 12.0 | 4.4 | 9.6 | 183/200 (91.5) | 22/200 (11) | 18/18 (100) |
| 2 | 68/F | MM IgA/κ | IIIA | 13.9 | 5.0 | 25.9 | 168/200 (84) | 32/200 (16) | 32/32 (100) |
| 3 | 70/M | MM IgG/λ | IA | 28.0 | 2.7 | 0.4 | 185/200 (92.5) | 26/200 (13) | 20/22 (90.9) |
| 4 | 62/F | MM IgG/κ | IIIA | 12.1 | 3.1 | 0.9 | 158/200 (79) | 36/200 (18) | 36/36 (100) |
| 5 | 44/M | MM IgG/λ | IIIA | 30.1 | 2.5 | 8.9 | 168/200 (84) | 64/200 (32) | 62/64 (96.9) |
| MGUS | |||||||||
| 6 | 49/F | IgG/κ | NA | 12.8 | 1.5 | 0.3 | 16/200 (8) | 6/200 (3) | NA |
| MGUS | |||||||||
| 7 | 47/F | IgG/κ | NA | 11.8 | 1.4 | 1.9 | 18/170 (10.6) | 7/170 (4.1) | NA |
| MGUS | |||||||||
| 8 | 61/F | IgA/κ | NA | 5.7 | 2.1 | 1.5 | 21/155 (13.5) | 6/155 (3.9) | NA |
Patient no. . | Age, y/sex . | Diagnosis . | Stage . | M comp, g/L . | β-2M, mg/L . | CRP, mg/L . | CD133+ CECs/total CECs (%) . | CECs with 13q14 del/total CECs (%) . | CD133+ CECs with 13q14 del/CECs with 13q14 del (%) . |
|---|---|---|---|---|---|---|---|---|---|
| 1 | 63/M | MM IgG/κ | IA | 12.0 | 4.4 | 9.6 | 183/200 (91.5) | 22/200 (11) | 18/18 (100) |
| 2 | 68/F | MM IgA/κ | IIIA | 13.9 | 5.0 | 25.9 | 168/200 (84) | 32/200 (16) | 32/32 (100) |
| 3 | 70/M | MM IgG/λ | IA | 28.0 | 2.7 | 0.4 | 185/200 (92.5) | 26/200 (13) | 20/22 (90.9) |
| 4 | 62/F | MM IgG/κ | IIIA | 12.1 | 3.1 | 0.9 | 158/200 (79) | 36/200 (18) | 36/36 (100) |
| 5 | 44/M | MM IgG/λ | IIIA | 30.1 | 2.5 | 8.9 | 168/200 (84) | 64/200 (32) | 62/64 (96.9) |
| MGUS | |||||||||
| 6 | 49/F | IgG/κ | NA | 12.8 | 1.5 | 0.3 | 16/200 (8) | 6/200 (3) | NA |
| MGUS | |||||||||
| 7 | 47/F | IgG/κ | NA | 11.8 | 1.4 | 1.9 | 18/170 (10.6) | 7/170 (4.1) | NA |
| MGUS | |||||||||
| 8 | 61/F | IgA/κ | NA | 5.7 | 2.1 | 1.5 | 21/155 (13.5) | 6/155 (3.9) | NA |
M comp indicates M component; β-2M, β2-microglobulin; CRP, C-reactive protein; and NA, not applicable.
CEC isolation and characterization
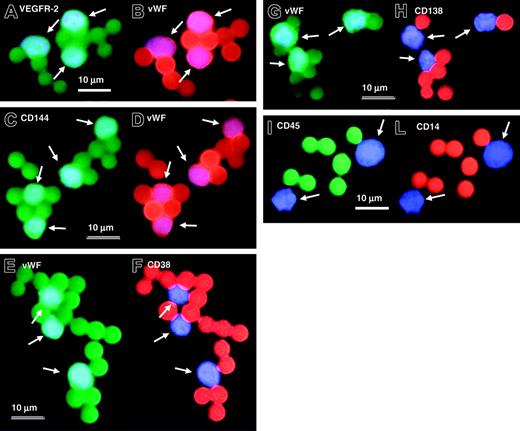
In all experiments more than 95% of immunomagnetically sorted cells were of endothelial origin as demonstrated by phenotypic analyses (Table 2; Figure 1). In patients with the 13q14 deletion, the mean percentage of CD45+ cells after immunomagnetic selection was 0.39% (range, 0.0%-1.0%). CD14 was expressed in 0.1% of all immunomagnetically sorted CECs (range, 0.0%-0.5%; Table 2). The great majority of immunomagnetically sorted CECs coexpressed VEGFR-2 and VWF (mean value, 98.1%; range, 96.0%-100%; Figure 1A-B), and CD144 and VWF (mean value, 98.25%; range, 95.5%-100%; Figure 1C-D). UEA-1 lectin-positive staining was present in 97.1% of all CECs (range, 96.2%-98.5%). Very few immunomagnetically sorted CECs coexpressed VWF and the plasma cell-associated antigens CD138 (mean value, 0.17%; range, 0.0%-0.5%; Figure 1E-F) and CD38 (mean value, 0.25%; range, 0.0%-0.5%; Figure 1G-H).
Immunophenotypic characterization of CECs in MM and MGUS patients with 13q14 deletion
Patient no. . | UEA-1+ CECs/total CECs (%) . | VEGFR-2+ VWF+ CECs/total CECs (%) . | CD144+ VWF+ CECs/total CECs (%) . | VWF+ CD138+ CECs/total CECs (%) . | VWF+ CD38+ CECs/total CECs (%) . | CD45+ cells/total CECs (%) [CD45+ CD14−/CD45+ CD14+] . |
|---|---|---|---|---|---|---|
| 1 | 195/200 (97.5) | 198/200 (99.0) | 197/200 (98.5) | 1/200 (0.5) | 1/200 (0.5) | 0/200 (0.0) [0/0] |
| 2 | 193/200 (96.5) | 196/200 (98.0) | 195/200 (97.5) | 0/200 (0.0) | ND | 2/200 (1.0) [2/0] |
| 3 | 195/200 (97.5) | 197/200 (98.5) | 199/200 (99.5) | 0/200 (0.0) | ND | 1/200 (0.5) [1/0] |
| 4 | 197/200 (98.5) | 200/200 (100) | 200/200 (100) | 0/200 (0.0) | 1/200 (0.5) | 0/200 (0.0) [0/0] |
| 5 | 194/200 (97.0) | 196/200 (98.0) | 197/200 (98.5) | 1/200 (0.5) | 0/200 (0.0) | 1/200 (0.5) [0/1] |
| 6 | 194/200 (97.0) | 195/200 (97.5) | ND | 0/170 (0.0) | ND | 0/180 (0.0) [0/0] |
| 7 | 154/160 (96.25) | ND | 172/180 (95.5) | 0/158 (0.0) | ND | 1/140 (0.7) [1/0] |
| 8 | 152/158 (96.2) | 144/150 (96.0) | ND | ND | 0/145 (0.0) | ND |
Patient no. . | UEA-1+ CECs/total CECs (%) . | VEGFR-2+ VWF+ CECs/total CECs (%) . | CD144+ VWF+ CECs/total CECs (%) . | VWF+ CD138+ CECs/total CECs (%) . | VWF+ CD38+ CECs/total CECs (%) . | CD45+ cells/total CECs (%) [CD45+ CD14−/CD45+ CD14+] . |
|---|---|---|---|---|---|---|
| 1 | 195/200 (97.5) | 198/200 (99.0) | 197/200 (98.5) | 1/200 (0.5) | 1/200 (0.5) | 0/200 (0.0) [0/0] |
| 2 | 193/200 (96.5) | 196/200 (98.0) | 195/200 (97.5) | 0/200 (0.0) | ND | 2/200 (1.0) [2/0] |
| 3 | 195/200 (97.5) | 197/200 (98.5) | 199/200 (99.5) | 0/200 (0.0) | ND | 1/200 (0.5) [1/0] |
| 4 | 197/200 (98.5) | 200/200 (100) | 200/200 (100) | 0/200 (0.0) | 1/200 (0.5) | 0/200 (0.0) [0/0] |
| 5 | 194/200 (97.0) | 196/200 (98.0) | 197/200 (98.5) | 1/200 (0.5) | 0/200 (0.0) | 1/200 (0.5) [0/1] |
| 6 | 194/200 (97.0) | 195/200 (97.5) | ND | 0/170 (0.0) | ND | 0/180 (0.0) [0/0] |
| 7 | 154/160 (96.25) | ND | 172/180 (95.5) | 0/158 (0.0) | ND | 1/140 (0.7) [1/0] |
| 8 | 152/158 (96.2) | 144/150 (96.0) | ND | ND | 0/145 (0.0) | ND |
ND indicates not done.
FISH analysis on CECs
FISH analysis showed that, in patients with MM, a significant proportion of CECs was tumor derived because they harbored the 13q14 deletion as observed in neoplastic plasma cells. The fraction of CECs showing the 13q14 deletion averaged 18% (range, 11%-32%; 200 cells observed in each case) while in 5 MM patients without 13q14 deletion the percentage of CECs with 13q14 deletion averaged 2.6% (range, 2.0%-3.5%; P = .008 for the comparison with CECs in patients with 13q14 deletion, Mann Whitney U test; 200 cells observed in each case). In 3 MGUS patients with the 13q14 deletion, the number of CECs with the 13q14 deletion averaged 3.7% (range, 3.0%-4.1%; P = .036 for the comparison with CECs in MM patients with 13q14 deletion, Mann Whitney U test). No statistical difference was observed between MM patients without 13q14 deletion and patients with MGUS concerning the percentage of CECs with 13q14 deletion. In MM patients with 13q14 deletion, compared with MGUS patients, the majority of CECs presented features of endothelial progenitor cells (EPCs) because they expressed CD133, a marker gradually lost during EC differentiation and absent in mature ECs (86.2% CD133+ CECs in MM patients with the 13q14 deletion vs 10.7% CD133+ CECs in MGUS patients; P = .025, Mann Whitney U test; Table 1). Overall, 97.7% of MM CECs with 13q14 deletion were CD133 positive (Figure 2; Table 1).
Immunophenotypic characterization of immunomagnetically sorted CECs by means of Dynabeads in MM patients with 13q14 deletion. In simultaneous double fluorescence staining experiments, CECs coexpress VEGFR-2 (positive green staining for anti-VEGFR-2 antibody) (A) and VWF (positive red staining for anti-VWF monoclonal antibody) (B), and CD144 (positive green staining for anti-CD144 monoclonal antibody) (C) and VWF (positive red staining for anti-VWF monoclonal antibody) (D). Immunomagnetically sorted CECs do not coexpress VWF (positive green staining for anti-VWF monoclonal antibody) (E,G) with plasma cell-associated markers CD38 (negative red staining for anti-CD38 monoclonal antibody) (F) and CD138 (negative red staining for anti-CD138 monoclonal antibody) (H). In patients with MM, immunomagnetically sorted CECs do not express CD45 (negative green staining for anti-CD45 monoclonal antibody) (I) and CD14 (negative red staining for anti-CD14 monoclonal antibody) (L). Note that several Dyna-beads are attached to the cells and that beads present red or green staining depending on the filter used for fluorescence analysis. White arrows indicate CECs: cell nuclei have been counterstained with DAPI II (blue staining). Scale bars equal 10 μm.
Immunophenotypic characterization of immunomagnetically sorted CECs by means of Dynabeads in MM patients with 13q14 deletion. In simultaneous double fluorescence staining experiments, CECs coexpress VEGFR-2 (positive green staining for anti-VEGFR-2 antibody) (A) and VWF (positive red staining for anti-VWF monoclonal antibody) (B), and CD144 (positive green staining for anti-CD144 monoclonal antibody) (C) and VWF (positive red staining for anti-VWF monoclonal antibody) (D). Immunomagnetically sorted CECs do not coexpress VWF (positive green staining for anti-VWF monoclonal antibody) (E,G) with plasma cell-associated markers CD38 (negative red staining for anti-CD38 monoclonal antibody) (F) and CD138 (negative red staining for anti-CD138 monoclonal antibody) (H). In patients with MM, immunomagnetically sorted CECs do not express CD45 (negative green staining for anti-CD45 monoclonal antibody) (I) and CD14 (negative red staining for anti-CD14 monoclonal antibody) (L). Note that several Dyna-beads are attached to the cells and that beads present red or green staining depending on the filter used for fluorescence analysis. White arrows indicate CECs: cell nuclei have been counterstained with DAPI II (blue staining). Scale bars equal 10 μm.
Immunoglobulin gene rearrangement
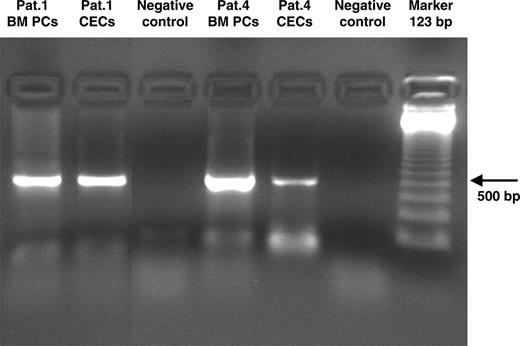
It was possible to demonstrate by PCR analysis in 2 patients (patient 1 and patient 4) with available cells that immunoglobulin genes were clonally rearranged in MM CECs (Figure 3). Sequence analysis showed that MM CECs carried the same immunoglobulin rearrangement as plasma cells (Patient 1: VH3-11, D1-26, JH6; Patient 4: VH2-5, D3-3, JH4).
FISH analysis of CECs in patients with MM. Multicolour FISH analysis of immunomagnetically sorted CECs by means of Dynabeads shows 1 CEC (bottom left) with a 13q14 deletion (1 red signal, bottom left arrow) and a normal diploid copy number of chromosome 10 (2 green signals), and 1 cytogenetically normal CEC (top right) with 2 red (top 2 arrows) and 2 green signals. In patients with MM, most CECs are EPCs because they express CD133, a marker gradually lost during EC differentiation (positive red staining for PE-conjugated murine anti-CD133 monoclonal antibody). Note that several Dynabeads are attached to the cells. Scale bar equals 10 μm.
FISH analysis of CECs in patients with MM. Multicolour FISH analysis of immunomagnetically sorted CECs by means of Dynabeads shows 1 CEC (bottom left) with a 13q14 deletion (1 red signal, bottom left arrow) and a normal diploid copy number of chromosome 10 (2 green signals), and 1 cytogenetically normal CEC (top right) with 2 red (top 2 arrows) and 2 green signals. In patients with MM, most CECs are EPCs because they express CD133, a marker gradually lost during EC differentiation (positive red staining for PE-conjugated murine anti-CD133 monoclonal antibody). Note that several Dynabeads are attached to the cells. Scale bar equals 10 μm.
Discussion
It has been shown that in patients with MM, ECs differ markedly from umbilical vein ECs, their quiescent counterpart, with regard to the secretion of growth factors, growth properties, genetic profile, and structural features.14 Recent findings also showed that in patients with MM, the level of CECs, which comprise mature ECs and EPCs, were higher than in controls and correlated positively with serum M protein and β2-microglobulin, thereby representing a vascular marker that reflects tumor mass and prognosis.10 In addition, a correlation was documented between the level of CECs/EPCs and response to thalidomide treatment,10 suggesting an antiangiogenetic mechanism of thalidomide action.
This is the first demonstration that, in MM patients with the 13q14 deletion, a significant proportion of CECs was tumor derived because they carried the same chromosome aberration as the neoplastic plasma cells and presented the same immunoglobulin gene rearrangement as MM plasma cells. In addition, we showed that most CECs presented EPC features as they expressed CD133, a marker gradually lost during endothelial differentiation and absent on mature endothelial cells. On the contrary, in patients with MGUS and 13q14 deletions, CECs were cytogenetically normal and had a mature immunophenotype.
Due to the very low number of CECs in peripheral blood,5,12 in order to avoid contamination by nonendothelial cells we used a dual-step immunomagnetic sorting by means of CD45 and CD146 antibodies to isolate CECs. By using immunomagnetic sorting in combination with CD45, we first eliminated all hematopoietic cells, which are CD45 positive, without affecting the endothelial cell component, which is characteristically CD45 negative. We then sorted CECs by means of CD146, an antigen expressed almost exclusively on ECs. Its absence on hematopoietic cells makes CD146 a helpful reagent to specifically discriminate endothelial from hematopoietic cells.13 To confirm the endothelial commitment of these sorted cells, we then performed additional phenotypic studies with antibodies recognizing endothelial and plasma cell antigens. CECs expressed UEA-1 lectin, VWF, CD144, and VEGFR-2. On the contrary, CD138 and CD38, 2 plasma cell-associated antigens, were not expressed on sorted CECs. In conclusion, the immophenotypic data are consistent with the endothelial identity of these cells.
Different mechanisms may be proposed as a possible explanation for our findings.15 First, ECs and MM plasma cells may derive from the same multipotent hemangioblast precursor cell, as suggested by evidence coming from studies in patients with chronic myeloid leukemia16 and from the observation that most MM CECs display immunophenotypic features of EPCs. According to this hypothesis, in patients with MM, angiogenic factors secreted into the microenvironment could actively recruit bone marrow hemangioblast precursor cells and induce them to differentiate into plasma cells and EPCs, with both displaying the same rearrangement of immunoglobulins. These EPCs could then enter blood circulation and contribute to neovasculogenesis and tumor dissemination. Alternatively, the ECs that carry the genetic lesion of MM plasma cells may have arisen, under the influence of microenvironmental angiogenic factors, through a process of dedifferentiation of a cell already committed to the lymphoid lineage into a cell with EPC characteristics, followed by a redifferentiation into a terminally differentiated EC.17 Disguised plasma cells may then mimic functional CECs and contribute to tumor neovasculogenesis.18 To this extent, however, it has been shown that VEGFR-2 is the only marker shared by CECs and plasma cells, and that MM plasma cells are negative at mRNA and protein levels for most of endothelial cell markers including factor VIII-related antigen, VE-cadherin, and UEA-1.14 Cell fusion, in our study, seems unlikely because the fusion of MM plasma cells and ECs should result in a tetraploid karyotype and, in our patients with MM, all CECs contained a normal diploid copy number of chromosome 10. It seems also unlikely that, as observed in solid tumors, our findings could reflect an inherent cytogenetic instability of tumor endothelial cells, because FISH results were not consistent with the heterogeneous cytogenetic profile of ECs observed in solid tumors.19
Immunoglobulin gene rearrangements in immunomagnetically sorted CECs in MM patients with 13q14 deletion. PCR analysis demonstrated that in MM CECs, immunoglobulin genes were clonally rearranged. Sequence analysis showed that MM CECs carried the same immunoglobulin rearrangement as bone marrow plasma cells (Patient 1: VH3-11, D1-26, JH6; Patient 4: VH2-5, D3-3, JH4) BM PCs indicate bone marrow plasma cells.
Immunoglobulin gene rearrangements in immunomagnetically sorted CECs in MM patients with 13q14 deletion. PCR analysis demonstrated that in MM CECs, immunoglobulin genes were clonally rearranged. Sequence analysis showed that MM CECs carried the same immunoglobulin rearrangement as bone marrow plasma cells (Patient 1: VH3-11, D1-26, JH6; Patient 4: VH2-5, D3-3, JH4) BM PCs indicate bone marrow plasma cells.
In addition, the results presented in this paper, by showing that only a subset of CECs harbors the 13q14 deletion, suggest that in patients with MM the neoplastic microenvironment determines an activation of the vasculogenic potential that includes both neoplastic and nonclonal bone marrow-derived CECs with EPC features. However, from the observation that MM CECs represent only a minor component of all CECs with EPC features, it is possible to speculate that neoplastic CECs might act as a sort of bridgehead on which more numerous and possibly more specialized and functional active nonclonal bone marrow-derived EPCs could actively differentiate in mature vessels and contribute to tumor neovascularization and spreading. In contrast, patients with MGUS and 13q14 deletion do not have peripheral blood CECs with 13q14 deletion and most CECs have the features of mature endothelial cells, suggesting that in these patients the neoplastic angiogenic switch has not yet occurred and that the vasculogenic potential of the bone marrow microenvironment is still limited. Additional studies are warranted to clarify, on bioptical samples of patients with MM, the actual contribution of MM-derived endothelial cells with specific cytogenetic aberrations to tumor neovasculogenesis and spreading.
In conclusion, our findings suggest that in patients with MM CECs are in part tumor related and have EPC features. These CECs may contribute to tumor neovasculogenesis and possibly to the spreading and progression of the disease. The study of CECs may have important implications not only for the understanding of MM-specific biological aspects but also for the translation of new antiangiogenic therapies to the clinic.20
Prepublished online as Blood First Edition Paper, November 29, 2005; DOI 10.1182/blood-2005-04-1768.
Supported by MURST (60%), an AIRC regional grant, MIUR PRIN, and MIUR FIRB.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.


This feature is available to Subscribers Only
Sign In or Create an Account Close Modal