Abstract
Interleukin-2 (IL-2) is historically known as a T-cell growth factor. Accumulating evidence from knockout mice suggests that IL-2 is crucial for the homeostasis and function of CD4+CD25+ regulatory T cells in vivo. However, the impact of administered IL-2 in an immune intact host has not been studied in rodents or humans. Here, we studied the impact of IL-2 administration on the frequency and function of human CD4+CD25hi T cells in immune intact patients with melanoma or renal cancer. We found that the frequency of CD4+CD25hi T cells was significantly increased after IL-2 treatment, and these cells expressed phenotypic markers associated with regulatory T cells. In addition, both transcript and protein levels of Foxp3, a transcription factor exclusively expressed on regulatory T cells, were consistently increased in CD4 T cells following IL-2 treatment. Functional analysis of the increased number of CD4+CD25hi T cells revealed that this population exhibited potent suppressive activity in vitro. Collectively, our results demonstrate that administration of high-dose IL-2 increased the frequency of circulating CD4+CD25hi Foxp3+ regulatory T cells. Our findings suggest that selective inhibition of IL-2-mediated enhancement of regulatory T cells may improve the therapeutic effectiveness of IL-2 administration. (Blood. 2006;107:2409-2414)
Introduction
Interleukin-2 (IL-2) is a cytokine that is produced primarily by recently activated T cells to support the growth and expansion of T cells.1 However, administration of IL-2 into immune competent patients with metastatic melanoma and renal cell carcinoma results in 15% to 20% objective clinical responses, with 7% complete long-term responders.2 The impact of IL-2 administration on T-cell populations in vivo is poorly understood. In mice, lack of IL-2 or its signaling components leads to lymphoproliferative diseases, suggesting that IL-2 is essential for the development and maintenance of CD4+CD25+ regulatory T cells in vivo.3,4 Because regulatory T cells can impede antitumor immune responses5,6 and their function is dependent on IL-2,7,8 it is possible that IL-2 administration may impede antitumor immune responses through activation of regulatory T cells.
Human regulatory T cells, characterized as CD4+CD25+ T cells with suppressive function, are found in peripheral blood.9-11 These cells typically express cytolytic T-lymphocyte-associated antigen 4 (CTLA-4), glucocorticoid-induced tumor-necrosis factor receptor-related protein (GITR), and CD45RO,10,12,13 albeit these markers can also be expressed on recently activated T cells. More recently, a novel transcription factor, Foxp3, was demonstrated to be exclusively expressed by regulatory T cells14,15 and not by activated T cells.16 Therefore, Foxp3 expression can be used as a surrogate molecular marker for quantifying regulatory T cells in peripheral blood.
In the current study, we investigated the impact of high-dose IL-2 administration on the CD4 T-cell population in patients with metastatic melanoma and renal cell cancer. We report that following IL-2 treatment, the proportion of CD4+CD25hi T cells expressing Foxp3 was significantly increased in peripheral blood. These cells phenotypically resembled regulatory T cells and exhibited suppressive function in vitro. These findings provide evidence that IL-2 administration alters the homeostasis of CD4+CD25hiFoxp3+ regulatory T cells in cancer patients and suggest that elimination of these cells may lead to improved responses to IL-2 therapy.
Patients and methods
Patients and PBMC samples
Patients with metastatic melanoma or renal cell carcinoma who were refractory to standard therapy were referred to the Surgery Branch, National Cancer Institute (Bethesda, MD). Each patient received high-dose bolus IL-2 (720 000 IU/Kg) intravenously every 8 hours as tolerated but not to exceed 15 doses.2 Except for patient 6, who received 7 doses, patients received 9 to 11 doses. These patients had not previously been treated with IL-2. Peripheral blood mononuclear cells (PBMCs) were obtained by leukapheresis prior to the start of IL-2 treatment (designated as PRE), during the rebound phase (designated as REB), and 17 to 21 days following the initial start of the treatment (designated as POST). PBMCs were isolated by Ficoll gradient separation and either used freshly or cryopreserved until analyzed. The protocol was approved by the Institutional Review Board of the National Cancer Institute. Informed consent was obtained from all patients.
Antibodies, reagents, and media
The following mAbs specific for human surface antigens were purchased from BD Pharmingen (San Diego, CA): fluorescein isothiocyanate (FITC)-conjugated anti-CD3 (SK7 clone), anti-CD27 (M-T271), and anti-CD69 (FN50 clone); phycoerythrin (PE)-conjugated anti-CD25 (2A3 clone) and anti-CTLA (BNI3 clone); and allophycocyanin-conjugated anti-CD4 (RPA-T4), anti-CD8 (RPA-T8), and anti-CD25 (2A3 clone). FITC-conjugated anti-GITR (110416 clone) antibody was purchased from R&D Systems (Minneapolis, MN). Biotin-conjugated anti-human Foxp3 antibody (PCH101 clone) and its isotype control antibody were purchased from eBioscience (San Diego, CA). Recombinant human IL-2 was supplied by Chiron (Emeryville, CA). Human T cells were cultured in complete medium consisting of RPMI 1640 (Gibco, Carlsbad, CA) supplemented with 10% heat-inactivated human AB serum (Gemini Bio-products, Cala-basa, CA); 100 U/mL penicillin and 100 μg/mL streptomycin (Biofluids, Camarillo, CA); 25 mM HEPES buffer (Biofluids), 2 mM l-glutamine (Biofluids), and 50 μM 2-mercaptoethanol (Gibco).
CD4+ T-cell enrichment
CD4 T cells were negatively enriched using Miltenyi CD4 T-Cell Isolation Kit II (Miltenyi Biotec, Auburn, CA) with modifications to the manufacturer's instructions. To achieve a consistently high CD4 purity rate for quantitative reverse transcriptase-polymerase chain reaction (RT-PCR) analysis, the amount of cocktail antibody, antibiotin MicroBeads, and incubation time were increased by 25%, and cells were eluted on an LD column. The CD4 T-cell purity rate was consistently higher than 97%. For CD4+CD25hi T-cell isolation, PBMCs were negatively enriched for CD4 T cells using Miltenyi CD4 T-Cell Isolation Kit II according to the manufacturer's instructions. Purified CD4 T cells were stained with anti-CD25 PE (2A3 clone) at a concentration of 5 μL/1 × 106 cells for 30 minutes on ice, washed, and subsequently incubated with anti-PE MicroBeads (Miltenyi Biotec) according to the manufacturer's instructions. The CD4+CD25hi fraction was collected by eluting the cells first through an LS column and then through a magnetic separation (MS) column to further enrich for CD4+CD25hi T cells. The purity of CD4+CD25hi fractions ranged from 85% to 91%. The CD4+CD25- fraction was additionally purified over an LD column. For isolation of CD4+CD25hi and CD4+CD25- subsets using fluorescence-activated cell-sorter (FACS), PBMCs were enriched for CD4 T cells as described above and stained with PE-conjugated anti-CD25 (2A3 clone), allophycocyanin-conjugated anti-CD4, and FITC-conjugated anti-CD3 antibodies for 30 minutes on ice, washed, and run on FACSVantage DiVa sorter (BD Biosciences, San Jose, CA).
Immunosuppression assays
CD4CD25- T cells (5 × 104 cells/well) were cocultured with CD4CD25+ T cells (5 × 104 cells/well, 1:1 ratio) with 1 μg/mL anti-CD3 antibody (OKT3) in the presence of irradiated (3500 Rad) T-depleted PBMCs (2.5 × 105 cells/well) in a 96-well flat-bottom plate. Accessory cells were isolated by negative selection of PBMCs incubated with anti-CD3 Dynabeads antibody (Dynal Biotech, Oslo, Norway) according to the manufacturer's instructions. Proliferation was assessed by [3H]thymidine (1 μCi [0.037 MBq] per well) incorporation pulsed on day 4 and quantified 18 hours later using a liquid scintillation counter. For FACS-sorted suppression assay, irradiated fresh allogeneic T-depleted PBMCs (5 × 105 cells/well) were used, and cultures were pulsed on day 6 and harvested 18 hours later.
Flow cytometry analysis
Cells were resuspended in staining buffer (phosphate-buffered saline [PBS] containing 3% fetal bovine serum [FBS]) and stained with fluorochrome-conjugated antibodies specific for humans. PBMCs were first blocked with mouse Ig (Caltag Laboratories, Burlingame, CA) for 15 to 30 minutes at room temperature prior to surface staining. Stained cells were subsequently washed in staining buffer twice and briefly stained with propidium iodide (PI) for nonviable cell exclusion prior to analyzing in a FACSCalibur (Becton Dickinson, San Jose, CA). Intracellular staining of CTLA-4 was performed by using the Cytofix/Cytoperm Kit from BD Pharmingen. Intracellular staining for Foxp3 protein was performed by using fixation and permeabilization buffers provided by the Foxp3 kit (eBioscience) according to the manufacturer's instructions, followed by visualization with streptavidin PE antibody.
Expression analysis by real-time RT-PCR
To determine gene expression, total RNA was extracted from CD4+-enriched cell lysates using RNeasy Mini Kit (QIAGEN, Valencia, CA) according to the manufacturer's instructions. cDNA was synthesized using oligo-dT-primers and ThermoScript RT as reverse transcriptase (Invitrogen, Carlsbad, CA). Expression of Foxp3 was measured using Assays-On-Demand primers and probes (PE Applied Biosystems, Foster City, CA). β-actin was used as an endogenous reference gene and was quantified using primers and probes as previously described.17 Standard curves were generated from serial dilutions of purified plasmid DNA encoding the respective genes with a linear regression R greater than 0.99 and used to quantify mRNA copy numbers for each sample.
Statistical analysis
Statistical significance of the observed phenotypic differences as well as Foxp3 transcript levels in PRE, REB, and POST were assessed by paired 2-tailed Student t tests. Bonferroni adjustment was used to correct for the effect of multiple comparisons by multiplying the P values by 3. For suppression assays, 2-sample t test with equal variance was used to calculate P values. P values less than .05 were considered significant. The data had normal distribution.
Results
IL-2 administration altered the absolute lymphocyte counts
In the current study, we evaluated the impact of administration of high-dose IL-2 on 8 patients with metastatic melanoma or renal cancer who had received this treatment for the first time. The median age of the patients was 46.5 years, and the range was 18 to 63 years (Table 1). None of the patients had received prior IL-2 therapy. Other prior treatments are shown in Table 1. Most patients tolerated 9 to 11 doses of high-dose IL-2, however, none of them had a clinical response to this therapy.
Patient characteristics
Patient . | Age/sex . | Cancer type . | Prior immunotherapy . | No. IL-2 doses . |
|---|---|---|---|---|
| 1 | 55/M | Melanoma | None | 11 |
| 2 | 58/M | Melanoma | MART vaccine | 11 |
| 3 | 63/M | Melanoma | None | 10 |
| 4 | 40/M | Melanoma | Interferon-α | 9 |
| 5 | 35/M | Melanoma | MART/gp100 vaccine | 9 |
| 6 | 59/F | Kidney | None | 7 |
| 7 | 44/M | Kidney | Anti-CTLA-4 | 9 |
| 8 | 18/M | Melanoma | Interferon-α | 9 |
Patient . | Age/sex . | Cancer type . | Prior immunotherapy . | No. IL-2 doses . |
|---|---|---|---|---|
| 1 | 55/M | Melanoma | None | 11 |
| 2 | 58/M | Melanoma | MART vaccine | 11 |
| 3 | 63/M | Melanoma | None | 10 |
| 4 | 40/M | Melanoma | Interferon-α | 9 |
| 5 | 35/M | Melanoma | MART/gp100 vaccine | 9 |
| 6 | 59/F | Kidney | None | 7 |
| 7 | 44/M | Kidney | Anti-CTLA-4 | 9 |
| 8 | 18/M | Melanoma | Interferon-α | 9 |
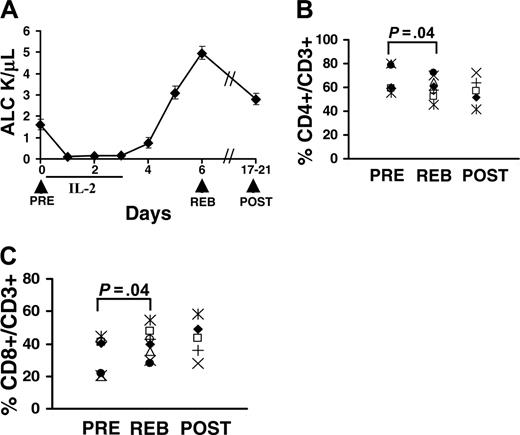
To evaluate any changes in the number of circulating T cells, the absolute lymphocyte count (ALC) was measured daily during the therapy. We found that IL-2 administration resulted in an immediate and significant reduction in the ALC, a nearly 15-fold drop compared with pretreatment ALC levels (Figure 1A). Although the mechanism of IL-2-induced lymphopenia is not clearly understood, it is presumed to be mediated by margination or leakage of lymphocytes. The lymphopenia was transient and persisted only during the duration of the IL-2 administration (Figure 1A). Immediately following the last dose of IL-2, the ALC increased significantly and rebounded to a level 3-fold higher than the pretreatment levels (Figure 1A, REB vs PRE, respectively). The ALC gradually decreased over the next 2 weeks but remained higher than pretreatment levels (Figure 1A, POST vs PRE). Even though the absolute lymphocyte counts had increased during the rebound phase (Figure 1A), the overall percentage of CD4 T cells in the CD3 T-cell population was slightly decreased in the rebound compared with pretreatment (Figure 1B, REB vs PRE, P = .04), while the percent of CD8 T cells per CD3 T cells slightly increased (Figure 1C, REB vs PRE, P = .04). The median for the percent CD4 T cells was 66.2% (range, 55%-80%, n = 8) for PRE, 59.9% (range, 45%-72%, n = 8) for REB, and 57.2% (range, 42%-72%, n = 5) for POST. Similarly, the median for percent CD8 T cells was 33.8% (range, 20%-45%, n = 8) for PRE, 40.1% (range, 28%-55%, n = 8) for REB, and 42.8% (range, 28%-58%, n = 5) for POST.
Administration of high-dose IL-2 alters lymphocyte counts. (A) Patients received 3 daily doses of high-dose IL-2 as tolerated (total of 7-11 doses per patient). Patients were leukapheresed prior to the start of treatment on day 0 (PRE), during the peak of lymphocytosis or rebound (REB), and about 2 to 3 weeks after therapy (POST), as indicated by the arrows. Absolute lymphocyte counts (ALCs) were measured daily. The percent of CD4+ (B) and CD8+ (C) T cells per CD3+ T-cell population was quantified for 8 patients by staining PBMCs with anti-CD4 and anti-CD3 antibodies. The dot plots were gated on live (propidium idodide negative, PI-) CD3+ T cells. For CD8 T cells, CD3+CD4- T cells were considered as CD8 T cells in some experiments. Each symbol represents one patient. Leukaphereses samples for POST were available only for 5 patients. P values were determined using paired t test and adjusted for multiple comparisons.
Administration of high-dose IL-2 alters lymphocyte counts. (A) Patients received 3 daily doses of high-dose IL-2 as tolerated (total of 7-11 doses per patient). Patients were leukapheresed prior to the start of treatment on day 0 (PRE), during the peak of lymphocytosis or rebound (REB), and about 2 to 3 weeks after therapy (POST), as indicated by the arrows. Absolute lymphocyte counts (ALCs) were measured daily. The percent of CD4+ (B) and CD8+ (C) T cells per CD3+ T-cell population was quantified for 8 patients by staining PBMCs with anti-CD4 and anti-CD3 antibodies. The dot plots were gated on live (propidium idodide negative, PI-) CD3+ T cells. For CD8 T cells, CD3+CD4- T cells were considered as CD8 T cells in some experiments. Each symbol represents one patient. Leukaphereses samples for POST were available only for 5 patients. P values were determined using paired t test and adjusted for multiple comparisons.
IL-2 administration increased the frequency of CD4+CD25+ T cells
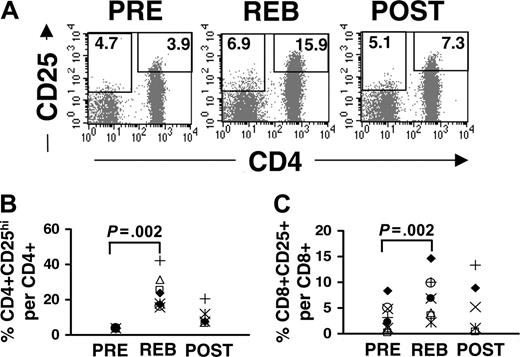
Although the percentage of CD8 T cells in the CD3 T-cell population was increased in rebound more than CD4 T cells, expression of the IL-2R α-chain (CD25) was increased more profoundly on CD4 than CD8 T cells after IL-2 therapy (Figure 2A). Given that CD25 is continuously expressed on human CD4 T cells, and there is no exclusive surface marker for human regulatory T cells, it is difficult to distinguish between CD4+CD25+ regulatory T cells and recently activated T cells expressing CD25. Therefore, we gated on the top 4% of the CD4+CD25+ population (designated as CD4+CD25hi) isolated prior to IL-2 treatment (PRE), since this population is highly enriched for regulatory T cells,11,13 and we used this gate to evaluate the frequency of CD4+CD25hi T cells in rebound and post-treatment (Figure 2A, right box). There was a substantial increase in the percentage of CD4 T cells that were CD25hi (Figure 2A, REB, 15.9%; mean, 23.7%; range, 15.9%-41.9%; n = 8) as well as a modest increase in the mean fluorescence intensity of this population in rebound compared with pretreatment (Figure 2A, PRE, 3.9%). In general, the proportion of CD4+CD25hi T cells in total CD4 T cells was increased nearly 6-fold in rebound compared to pretreatment (P = .002, paired t test; n = 8). At 2 to 3 weeks after treatment (POST), the percent of CD4+CD25hi T cells in the CD4 T-cell population was reduced compared with the rebound phase but remained above pretreatment levels (Figure 2A,B). In contrast to CD4 T cells, CD8 T cells (CD3+CD4-) did not express high levels of CD25 expression, even at rebound (Figure 2A, REB). However, a small number of CD8 T cells expressed low levels of CD25 (4.7%) that were modestly increased (6.9%) following IL-2 treatment (Figure 2A, PRE vs REB, respectively; the left box represents the gate for CD8+CD25+ and was drawn based on the isotype control antibody). The overall proportion of CD25+ T cells in the CD8 T-cell population was increased in rebound (P = .002, paired t test; Figure 2A, left box, and Figure 2C) yet not to the same level of intensity as CD4+CD25hi T cells. Furthermore, both the percent of CD4+CD25hi T cells and the level of intensity of CD25 expression on these cells were increased significantly in the peripheral blood even during the lymphopenia (days 2-3, data not shown), indicating that the induction of CD25 expression precedes lymphocytosis. These results collectively demonstrate that administration of high-dose IL-2 leads to a substantial increase in the frequency of circulating CD4+CD25hi T cells.
Induction of CD25 expression by T cells following IL-2 administration. Cryopreserved PBMCs were triple-stained with allophycocyanin-conjugated anti-CD4, PE-conjugated anti-CD25, and FITC-conjugated anti-CD3 antibodies. (A) The dot plots were gated on PI-CD3+ T cells. The gate for CD4+CD25hi T cells was drawn based on the top 4% of CD4+ T cells expressing high levels of CD25 in the peripheral blood of each patient prior to IL-2 therapy (PRE). The same gate was used for rebound and post-treatment samples for each patient. The number represents the percentage of CD4+CD25hi T cells per total CD4 T cells. The gate for CD8+CD25+ (CD4-CD3+) was based on the isotype control antibody, and the number represents the percentage of CD8+CD25+ T cells per total CD8 T cells. The percent induction of CD25 expression of CD4 (B) and CD8 (C) T cells in 8 patients were quantified by using the gate for CD4+CD25hi and for CD8+CD25+ T cells, respectively. To enhance for consistency and accuracy, PBMCs from PRE, REB, and POST for each patient were stained and analyzed at the same time. Leukaphereses samples for POST were available only for 5 patients. Each symbol represents one patient. The P values were calculated using paired t test and adjusted for multiple comparisons.
Induction of CD25 expression by T cells following IL-2 administration. Cryopreserved PBMCs were triple-stained with allophycocyanin-conjugated anti-CD4, PE-conjugated anti-CD25, and FITC-conjugated anti-CD3 antibodies. (A) The dot plots were gated on PI-CD3+ T cells. The gate for CD4+CD25hi T cells was drawn based on the top 4% of CD4+ T cells expressing high levels of CD25 in the peripheral blood of each patient prior to IL-2 therapy (PRE). The same gate was used for rebound and post-treatment samples for each patient. The number represents the percentage of CD4+CD25hi T cells per total CD4 T cells. The gate for CD8+CD25+ (CD4-CD3+) was based on the isotype control antibody, and the number represents the percentage of CD8+CD25+ T cells per total CD8 T cells. The percent induction of CD25 expression of CD4 (B) and CD8 (C) T cells in 8 patients were quantified by using the gate for CD4+CD25hi and for CD8+CD25+ T cells, respectively. To enhance for consistency and accuracy, PBMCs from PRE, REB, and POST for each patient were stained and analyzed at the same time. Leukaphereses samples for POST were available only for 5 patients. Each symbol represents one patient. The P values were calculated using paired t test and adjusted for multiple comparisons.
IL-2 enhanced expression of GITR and CTLA-4 on CD4+CD25+ T cells but not on CD8 T cells
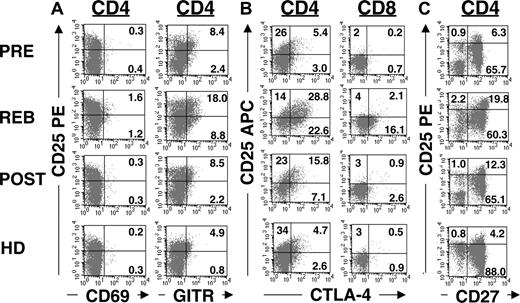
To phenotypically characterize CD4+CD25+ T cells generated following IL-2 treatment, we analyzed the expression of CTLA-4 and GITR markers associated with regulatory T cells. Prior to IL-2 treatment, the CD4+CD25+ T cells expressed basal levels of GITR (Figure 3A, PRE, 8.4%; mean, 3.5%; range, 0.02%-8.4%; n = 6) and CTLA-4 (Figure 3B, PRE, 5.4%; mean, 5.8%; range, 4.8%-7.0%; n = 5) consistent with previous findings that regulatory T cells constitutively express these markers.12,13 Following IL-2 therapy, both GITR (Figure 3A, REB, 18%; mean, 13.4%; range, 6%-18%; n = 6) and CTLA-4 (Figure 3B, REB, 28.8%; mean, 23.3%; range, 14%-30%; n = 5) expressions were enhanced on CD4+CD25+ T cells nearly 4-fold. This induction was detected predominantly on CD4+CD25+ T cells, although subtle changes were also detected on CD4+CD25int T cells (Figure 3A,B, upper right versus lower right quadrants) or CD8 T cells (Figure 3B, righthand column). Although the increase in the expression of GITR and CTLA-4 in rebound was highly significant (P = .003 and P = .008, respectively; paired t test), their expressions were reduced 3 weeks later (POST) and were comparable to pretreatment levels. In contrast to the induction of GITR and CTLA-4 expression, CD69 expression was not detected at significant levels on CD4 T cells during rebound (Figure 3A, lefthand column). Given that CTLA-4 and GITR can be expressed on regulatory T cells as well as recently activated effector CD4 T cells, they cannot reliably distinguish regulatory T cells. However, lack of CD69 expression on the CD4+CD25+ T cells implied that these cells were not recently activated cells. Moreover, addition of IL-2 to purified CD4 T cells in vitro was sufficient to up-regulate CD25 but not CD69 in the absence of T-cell receptor (TCR) stimulation (data not shown). Even though induction of CD25 is typically associated with TCR activation and expression of CD69 in T cells, IL-2 is sufficient to up-regulate CD25 expression on T cells expressing the high-affinity IL-2 receptor (ie, CD4+CD25+ T cells), because IL-2 is known to up-regulate its own receptor.18 Therefore, the increased numbers of CD4+CD25hi T cells in the rebound phase were probably not due to recent TCR activation but rather to IL-2-mediated expansion of this population.
Up-regulation of GITR and CTLA-4 following IL-2 administration. (A) Purified CD4 T cells or PBMCs were stained with allophycocyanin-conjugated anti-CD4, PE-conjugated anti-CD25, and FITC-conjugated anti-CD69, anti-GITR, or (C) anti-CD27 antibodies. Dot plots were gated on CD4+ T cells. (B) Purified CD4 T cells or PBMCs were stained initially with either FITC-conjugated anti-CD4 or anti-CD8 antibodies with allophycocyanin-conjugated anti-CD25 antibody, followed by intracellular staining with PE-conjugated anti-CTLA-4 antibody. The dot plots were gated on CD4+ or CD8+ T cells, respectively. The quadrants for CD69, GITR, CD27, and CTLA-4 were based on the isotype control antibodies as well as T cells that lack expression of these markers. This result is representative of 6 patients and 3 healthy donors.
Up-regulation of GITR and CTLA-4 following IL-2 administration. (A) Purified CD4 T cells or PBMCs were stained with allophycocyanin-conjugated anti-CD4, PE-conjugated anti-CD25, and FITC-conjugated anti-CD69, anti-GITR, or (C) anti-CD27 antibodies. Dot plots were gated on CD4+ T cells. (B) Purified CD4 T cells or PBMCs were stained initially with either FITC-conjugated anti-CD4 or anti-CD8 antibodies with allophycocyanin-conjugated anti-CD25 antibody, followed by intracellular staining with PE-conjugated anti-CTLA-4 antibody. The dot plots were gated on CD4+ or CD8+ T cells, respectively. The quadrants for CD69, GITR, CD27, and CTLA-4 were based on the isotype control antibodies as well as T cells that lack expression of these markers. This result is representative of 6 patients and 3 healthy donors.
More recently, expression of CD27 on CD4+CD25hi T cells has been suggested as a useful marker to distinguish regulatory CD4 T cells from effector T cells.19 CD27 is a member of the tumor necrosis factor (TNF) receptor family and is typically expressed on naive and subsets of memory T cells but not on terminally differentiated effector T cells.20,21 We found that peripheral CD4+CD25hi T cells predominantly expressed CD27 prior to IL-2 therapy (Figure 3C, PRE, 6.3%; mean, 4.5%; range, 2.6%-6.3%; n = 5) comparable to the frequency found in healthy donors (Figure 3C, HD, 4.2%; mean, 5.0%; range, 4.2%-6.1%; n = 3). Following IL-2 administration, the frequency of CD4+CD25hi CD27+ T cells (Figure 3C, upper right quadrant) was significantly (P = .008) increased in rebound (Figure 3C, REB, 19.8%; mean, 20.2%; range, 15.6%-26.6%; n = 5). Although the frequency of CD4+CD25hiCD27+ T cells was reduced over time (Figure 3C, POST, 12.3%; mean, 9.3%; range, 5.5%-12.3%; n = 4), it remained above the pretreatment level (P = .04). Together, these phenotypic results signify that IL-2 administration led to a substantial increase in the frequency of CD4 T cells that express phenotypic markers associated with regulatory T cells.
IL-2 increased the frequency of CD4+CD25+Foxp3+ T cells in peripheral blood. (A) RNA was extracted from highly purified CD4 T cells (> 97%-99%) isolated from PBMCs and collected through leukapheresis of 8 patients pretreatment (PRE), during rebound phase (REB), and 2 to 3 weeks after treatment (POST). Foxp3 levels were quantified using real-time RT-PCR and normalized for endogenous β-actin. The P value was calculated using paired t test and adjusted for multiple comparisons. (B) To determine Foxp3 expression per cell, cryopreserved PBMCs from patients or healthy donors were initially stained with allophycocyanin-conjugated anti-CD25, FITC-conjugated anti-CD4 or anti-CD8, and PerCP-conjugated anti-CD3 antibodies, followed by intracellular staining for Foxp3 protein. The dot plots were gated on CD3+CD4+ T cells. The quadrants for Foxp3 were based on the isotype control antibody. This result is representative of 6 patients and 3 healthy donors.
IL-2 increased the frequency of CD4+CD25+Foxp3+ T cells in peripheral blood. (A) RNA was extracted from highly purified CD4 T cells (> 97%-99%) isolated from PBMCs and collected through leukapheresis of 8 patients pretreatment (PRE), during rebound phase (REB), and 2 to 3 weeks after treatment (POST). Foxp3 levels were quantified using real-time RT-PCR and normalized for endogenous β-actin. The P value was calculated using paired t test and adjusted for multiple comparisons. (B) To determine Foxp3 expression per cell, cryopreserved PBMCs from patients or healthy donors were initially stained with allophycocyanin-conjugated anti-CD25, FITC-conjugated anti-CD4 or anti-CD8, and PerCP-conjugated anti-CD3 antibodies, followed by intracellular staining for Foxp3 protein. The dot plots were gated on CD3+CD4+ T cells. The quadrants for Foxp3 were based on the isotype control antibody. This result is representative of 6 patients and 3 healthy donors.
IL-2 increased the frequency of CD4+CD25+Foxp3+ T cells in peripheral blood
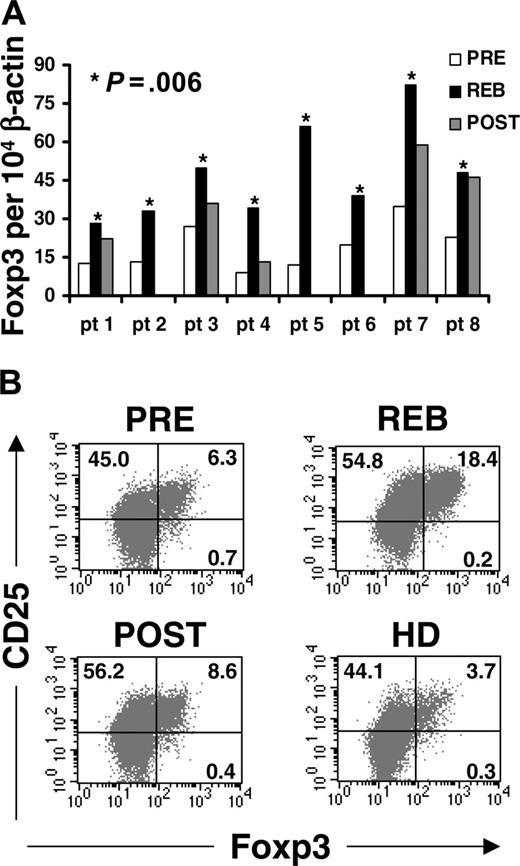
Although there are no reliable surface markers exclusively expressed on regulatory T cells, expression of the forkhead transcription factor, Foxp3, can be used as a molecular marker for regulatory T cells.14-16,22 We thus examined mRNA levels of Foxp3 in highly purified CD4 T cells before and after IL-2 treatment. We found that the mRNA levels of Foxp3 in CD4 T cells were consistently increased in the rebound phase compared with pretreatment levels (P = .006, paired t test) in all 8 patients (Figure 4A, REB vs PRE). Compared with rebound, the levels of Foxp3 in purified CD4 T cells decreased 3 weeks after treatment (POST); however, its overall level remained above the pretreatment level in 3 of the 5 patients (Figure 4A).
Although RT-PCR analysis gave a quantitative measure of Foxp3 transcripts in the CD4 T-cell population, it was limited to quantifying mRNA levels per population and not protein levels per cell. Therefore, we used intracellular staining for Foxp3 protein to estimate the frequency of Foxp3-expressing CD4 T cells before and after IL-2 treatment. We found that IL-2 increased the frequency of CD4+CD25+ T cells expressing Foxp3 (Figure 4B, upper right quadrant, 6.3% in PRE, compared with 18.4% in REB). The mean increase in the frequency of CD4+CD25+Foxp3+ T cells in the rebound (mean 18.8%; range, 14.0-24.5; n = 6) was highly significant (P = .001, 2-tailed t test) compared with pretreatment levels (mean, 5.6%; range, 4.3-6.3) (Table 2). The frequency of circulating CD4+CD25+Foxp3+ T cells dropped to levels comparable to pretreatment levels at 3 weeks after treatment (Figure 4B, 6.3% PRE vs 8.6% POST; Table 2, PRE; mean, 5.6%; range, 4.3-6.3; n = 6 versus POST; mean, 10.1%; range, 7.3-12.2; n = 4 for POST samples). Interestingly, the frequency of CD4+CD25+Foxp3+ T cells in the healthy donors (mean, 3.9%; range, 3.3-4.7, in 3 healthy individuals) was lower than in the patients with metastatic cancer prior to treatment (Figure 4B, 3.7% in HD vs 6.3% in PRE, respectively), however, the sample numbers were not large enough to draw conclusions. The CD4+ Foxp3+ T cells primarily expressed CD25 at high levels, however, there was a small percentage of CD4+Foxp3+ T cells that expressed low levels of CD25 prior to treatment and in healthy adults (Figure 4B, lower right quadrant, 0.7% in PRE and 0.3% in HD, respectively). The expression of CD25 on CD4+Foxp3+ T cells was increased by IL-2 in rebound (REB, MFI, 525; mean, 482; range, 405-628) and reduced to pretreatment levels (PRE, MFI, 230; mean, 252; range, 228-290) at 3 weeks after treatment (POST, MFI, 226; mean, 228; range, 190-256), similar to healthy adults (HD, MFI 280; mean, 278; range, 269-284) (Figure 4B, upper right quadrants). Collectively, these findings clearly demonstrate that IL-2 therapy significantly increased the frequency of circulating CD4+CD25+Foxp3+ T cells in the peripheral blood of cancer patients.
The frequency of CD4+CD25+Foxp3+ T cells
Patient . | PRE, % . | REB, % . | POST, % . |
|---|---|---|---|
| 1 | 6.3 | 18.4 | 8.6 |
| 3 | 6.3 | 24.5 | 12.2 |
| 4 | 4.3 | 14.0 | 7.3 |
| 5 | 5.3 | 22.2 | NA* |
| 6 | 5.9 | 14.4 | NA* |
| 8 | 5.7 | 19.4 | 12.4 |
Patient . | PRE, % . | REB, % . | POST, % . |
|---|---|---|---|
| 1 | 6.3 | 18.4 | 8.6 |
| 3 | 6.3 | 24.5 | 12.2 |
| 4 | 4.3 | 14.0 | 7.3 |
| 5 | 5.3 | 22.2 | NA* |
| 6 | 5.9 | 14.4 | NA* |
| 8 | 5.7 | 19.4 | 12.4 |
Leukapheresis sample was not available.
CD4+CD25hi T cells isolated after IL-2 treatment were suppressive in vitro
The hallmark of regulatory T cells is their ability to suppress immune responses as measured in vitro by inhibiting proliferation of CD4+CD25- responder T cells. Thus, we sought to examine the suppressive ability of this increased number of CD4+CD25hi T cells isolated from the rebound phase (REB). Purified CD4 T cells were highly enriched for CD4+CD25hi and CD4+CD25- T cells using a modified protocol (described in “Patients and methods”). To determine the percentage of CD4+CD25hi T cells, we used the gate that corresponds to the top 4% of CD4+CD25hi T cells in PRE and applied the same gate in rebound, which was 15.9% for this patient (Figure 5A, Unfractionated). Following bead separation, the CD4+CD25hi fraction was more than 91% pure (Figure 5A, CD4+CD25hi). The purity of the CD4+CD25hi fraction was comparable to FACS sort separation (84%, data not shown). Although the CD4+CD25- fraction contained negligible amounts of CD4+CD25hi T cells (0.03%), it contained cells expressing low levels of CD25 (Figure 5A, CD4+CD25-). However, the mRNA levels of Foxp3 were 46-fold lower in the CD4+CD25- fraction relative to the CD4+CD25hi fraction (data not shown), indicating that Foxp3+ regulatory T cells were confined to the latter fraction, as also demonstrated in Figure 4B (REB). Compared with the vigorous proliferation by CD4+CD25- T cells, CD4+CD25hi T cells isolated from rebound were hypoproliferative to anti-CD3 stimulation (Figures 5B-D; CD25- vs CD25hi) as previously reported,11 and they significantly inhibited the proliferation of CD4+CD25- responder T cells at a 1:1 ratio in 3 different patients. In fact, small numbers of CD4+CD25hi T cells isolated from rebound (REB) were sufficient to significantly suppress the proliferation of responder cells (1:100 or 1:30 ratio of CD25hi: CD25-, Figure 5B, P = .01, and Figure 5C, P = .0008, respectively). It is notable that cryopreserved T cells are not as robust as fresh T cells in responding to TCR stimulation, which may explain the overall lower proliferation shown in Figure 5D compared with Figures 5B and 5C. Collectively, the functional data demonstrate that the CD4+CD25hi T-cell subset, the population that was significantly expanded by the administration of high-dose IL-2, exhibited a strong suppressive function.
CD4+CD25hi T cells from rebound phase were suppressive in vitro. To fractionate CD4+CD25hi and CD4+CD25- T cells, purified CD4 T cells isolated from rebound were stained with anti-CD25 PE and subsequently with anti-PE MicroBeads and isolated over magnetic columns as described in “Patients and methods.” We developed and optimized this procedure to consistently enrich for CD4+CD25hi T cells without FACS sorting. (A) The dot plots were gated on CD3+CD4+ T cells and represent the initial starting CD4 T-cell population (Unfractionated) and the subsequent purified CD4CD25 subsets. The numbers represent the percentage of CD4+CD25hi T cells for each fraction. (B, C) Freshly isolated CD4+CD25- T cells (50 000 cells/well) were cocultured alone (CD25-) or with CD4+CD25hi T cells at different ratios and stimulated with anti-CD3 and irradiated T-depleted PBMCs (250 000 cells/well). Proliferation was assessed by [3H]thymidine incorporation pulsed on day 4 and harvested 18 hours later. The results represent the average [3H]thymidine incorporation (CPM) from 5 replicate wells per culture with calculated standard error of the mean. The P values were calculated using 2-sample t test. (D) CD4 T cells were initially purified from cryopreserved PBMCs collected during rebound from another patient, and stained with PE-conjugated anti-CD25, allophycocyanin-conjugated anti-CD4, and FITC-conjugated anti-CD3 antibodies, and subsequently separated into CD4+CD25hi and CD4+CD25- subsets using FACSVantage DiVa sorter. The result is the mean of [3H]thymidine incorporation in triplicate cultures with standard error of the mean, pulsed on day 6 and harvested 18 hours later.
CD4+CD25hi T cells from rebound phase were suppressive in vitro. To fractionate CD4+CD25hi and CD4+CD25- T cells, purified CD4 T cells isolated from rebound were stained with anti-CD25 PE and subsequently with anti-PE MicroBeads and isolated over magnetic columns as described in “Patients and methods.” We developed and optimized this procedure to consistently enrich for CD4+CD25hi T cells without FACS sorting. (A) The dot plots were gated on CD3+CD4+ T cells and represent the initial starting CD4 T-cell population (Unfractionated) and the subsequent purified CD4CD25 subsets. The numbers represent the percentage of CD4+CD25hi T cells for each fraction. (B, C) Freshly isolated CD4+CD25- T cells (50 000 cells/well) were cocultured alone (CD25-) or with CD4+CD25hi T cells at different ratios and stimulated with anti-CD3 and irradiated T-depleted PBMCs (250 000 cells/well). Proliferation was assessed by [3H]thymidine incorporation pulsed on day 4 and harvested 18 hours later. The results represent the average [3H]thymidine incorporation (CPM) from 5 replicate wells per culture with calculated standard error of the mean. The P values were calculated using 2-sample t test. (D) CD4 T cells were initially purified from cryopreserved PBMCs collected during rebound from another patient, and stained with PE-conjugated anti-CD25, allophycocyanin-conjugated anti-CD4, and FITC-conjugated anti-CD3 antibodies, and subsequently separated into CD4+CD25hi and CD4+CD25- subsets using FACSVantage DiVa sorter. The result is the mean of [3H]thymidine incorporation in triplicate cultures with standard error of the mean, pulsed on day 6 and harvested 18 hours later.
Discussion
Although IL-2 has been approved by the Food and Drug Administration for the treatment of patients with metastatic melanoma and renal cell carcinoma, only 15% to 20% of these patients experience a clinical response, with 7% complete long-term responders.2 We report here that administration of high-dose IL-2 resulted in a nearly 6-fold increase in the frequency of CD4+CD25hi T cells in the peripheral blood, compared with the pretreatment levels. These cells expressed phenotypic markers associated with regulatory T cells and suppressed the proliferation of CD4+CD25- responder T cells. Moreover, CD4 T cells isolated after IL-2 administration exhibited significantly higher expression of Foxp3, a surrogate molecular marker for regulatory T cells, since its expression is limited to regulatory T cells14,15 and not to activated or naive T cells.16 Furthermore, IL-2 administration led to a profound increase (nearly 4-fold) in the frequency of circulating Foxp3-expressing CD4 T cells in peripheral blood. Therefore, our results demonstrate that administration of high-dose IL-2 alters the homeostasis of regulatory CD4 T cells in an immune intact setting.
The autoimmunity generated in mice deficient in IL-2 or IL-2 receptor signaling components has suggested that IL-2 is essential for survival and function of regulatory CD4 T cells.23 The current report provides the first demonstration of the in vivo expansion of CD4+CD25hi regulatory T cells by administered IL-2 under an immune intact setting. Conversely, in vivo neutralization of IL-2 in mice reduces the number of CD4+CD25+Foxp3+ regulatory T cells, resulting in autoimmunity.24 Furthermore, IL-2 was found to be critical for CD4+CD25+ regulatory T cells not only to function in vitro7 but also to expand in large numbers in cultures.25,26 Taken together, these results strengthen our findings that IL-2 administration leads to expansion of regulatory T cells in vivo.
The mechanism of IL-2-mediated tumor regression in the small number of patients (15%-20%) who respond to IL-2 therapy remains unclear2 ; however, all of the patients analyzed in this report had tumor progression following the completion of their IL-2 treatment. Since IL-2 is a growth factor for any cell that expresses the high-affinity IL-2R complex, it also can activate effector T cells expressing CD25 as well as CD4+CD25+ regulatory T cells. Therefore, the balance between activation and expansion of tumor-reactive effector T cells relative to regulatory T cells by IL-2 may determine the clinical outcome for a patient.
In conclusion, our study demonstrates that the administration of high-dose IL-2 mediates the in vivo expansion of regulatory T cells in humans. Our findings also suggest that IL-2-mediated expansion of regulatory T cells may contribute to the lack of objective response to IL-2 therapy in more than 80% of patients with melanoma and renal cell carcinoma. Activated regulatory T cells may hinder generation of antitumor immune responses, especially considering that many tumor antigens are self-antigens. Depletion of regulatory T cells may enhance the ability of IL-2 to elicit antitumor immune responses in cancer patients.
Prepublished online as Blood First Edition Paper, November 22, 2005; DOI 10.1182/blood-2005-06-2399.
Supported in part by the Intramural Research Program of the National Institutes of Health, National Cancer Institute, Center for Cancer Research.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
The authors would like to thank P. Antony and P. Robbins for critical reading of this manuscript, Y. Li for his assistance with cloning Foxp3 plasmid, A. Mixon and S. Farid for flow cytometry, W. Telford for FACS sorting, S. Topalian and J. Aerts for providing β-actin plasmid, and D. White and S. Steinberg for statistical consulting.
The authors have no conflicting financial interests.

![Figure 5. CD4+CD25hi T cells from rebound phase were suppressive in vitro. To fractionate CD4+CD25hi and CD4+CD25- T cells, purified CD4 T cells isolated from rebound were stained with anti-CD25 PE and subsequently with anti-PE MicroBeads and isolated over magnetic columns as described in “Patients and methods.” We developed and optimized this procedure to consistently enrich for CD4+CD25hi T cells without FACS sorting. (A) The dot plots were gated on CD3+CD4+ T cells and represent the initial starting CD4 T-cell population (Unfractionated) and the subsequent purified CD4CD25 subsets. The numbers represent the percentage of CD4+CD25hi T cells for each fraction. (B, C) Freshly isolated CD4+CD25- T cells (50 000 cells/well) were cocultured alone (CD25-) or with CD4+CD25hi T cells at different ratios and stimulated with anti-CD3 and irradiated T-depleted PBMCs (250 000 cells/well). Proliferation was assessed by [3H]thymidine incorporation pulsed on day 4 and harvested 18 hours later. The results represent the average [3H]thymidine incorporation (CPM) from 5 replicate wells per culture with calculated standard error of the mean. The P values were calculated using 2-sample t test. (D) CD4 T cells were initially purified from cryopreserved PBMCs collected during rebound from another patient, and stained with PE-conjugated anti-CD25, allophycocyanin-conjugated anti-CD4, and FITC-conjugated anti-CD3 antibodies, and subsequently separated into CD4+CD25hi and CD4+CD25- subsets using FACSVantage DiVa sorter. The result is the mean of [3H]thymidine incorporation in triplicate cultures with standard error of the mean, pulsed on day 6 and harvested 18 hours later.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/107/6/10.1182_blood-2005-06-2399/4/m_zh80060692710005.jpeg?Expires=1769086233&Signature=osOE3kHlhrF-3xnGJCmptyN-uHOD-3~KVebUdyvX5B86JRhSp-7pJ3rYHOPHxfnhVvln7c7GP7PyGZehbdLhwZtHRxBvenkE4KwKr0hEURsU75cI065OYqnsto81wGzdXNAcDiDDK-WhLB4dmnl2SRXtgnTuVgyB2vW~o9UFFpk5cq-hKysODNc0Bq73DWu2sFCK31I4e9y1OKLDLgVG5zt1tkG5i7Nt2mIwchb~9g1sw7QSZmeiBrr5gE9RQs52bA6pxBY8dIJqegaKTqXxy-sLvrA2fJAS9ywCQI2g0o4R8swdg-hfDSyIX2TAMVrGBGz~uasp-4nbLpR4LRXcjg__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
This feature is available to Subscribers Only
Sign In or Create an Account Close Modal