Abstract
The receptor tyrosine kinase EphB4 and its ligand EphrinB2 play a crucial role in vascular development during embryogenesis. The soluble monomeric derivative of the extracellular domain of EphB4 (sEphB4) was designed as an antagonist of EphB4/EphrinB2 signaling. sEphB4 blocks activation of EphB4 and EphrinB2; suppresses endothelial cell migration, adhesion, and tube formation in vitro; and inhibits the angiogenic effects of various growth factors (VEGF and bFGF) in vivo. sEphB4 also inhibits tumor growth in murine tumor xenograft models. sEphB4 is thus a therapeutic candidate for vascular proliferative diseases and cancer.
Introduction
Differentiation of mesodermal cells to angioblasts occurs with simultaneous commitment to either arterial or venous lineage. Angioblasts spontaneously aggregate, proliferate, and differentiate to form endothelial tubes of each lineage. Independently developing arterial and venous vascular networks eventually join to form the original cardiovascular loop in the process of vasculogenesis.1-3 Sprouting of new vessels from this primary complex, or angiogenesis, is mediated by growth factors that induce endothelial cell (EC) proliferation, migration, and assembly, followed closely by the recruitment of perivascular cells, including smooth muscle cells, and remodeling of the extracellular matrix.4,5 A number of EC-specific receptor tyrosine kinases have been identified that play important roles in the early development of blood vessels and formation of the cardiovascular system, and include VEGF receptors and Tie-1 and Tie-2 receptors.5-11 More recently, Eph receptors and their ligands have been shown to play a critical role in the development and maturation of the cardiovascular system.11-13
The Ephs and Ephrins together comprise the largest of the receptor tyrosine kinase subfamilies (with 14 receptors and 8 ligands) and are subdivided into EphA and EphB categories based on sequence homologies and binding properties to Ephrin ligands. EphA receptors bind to glycosylphosphatidylinositol (GPI)–anchored Ephrin ligands (EphrinA subfamily), whereas EphB receptors bind Ephrin ligands that contain transmembrane and cytoplasmic domains (EphrinB subfamily).14 The extracellular domain of Eph receptors consists of a ligand-binding (globular or G) and a cysteine-rich (C) domain followed by 2 fibronectin III-like repeats (F1 and F2). The intracellular domain contains an autoinhibitory tyrosine in the juxtamembrane region, followed by a kinase domain, sterile α and PDZ-binding motifs.15,16
Eph receptor tyrosine kinases and their Ephrin ligands regulate a diverse array of cellular functions such as cell migration, repulsion, and adhesion, but lack effects on cell proliferation.9,17-21 These functions are dependent on bidirectional signals between cells expressing receptors and cells expressing ligands, which, for uniformity of communication, are termed “forward” and “reverse” signaling, respectively.6,9,11,21-25 EphrinB2 is specifically expressed in arterial angioblasts and endothelial and perivascular mesenchymal cells, whereas EphB4 is expressed in ECs belonging to the venous lineage only.12,26-28 EphrinB2 is the sole ligand for EphB4, although EphrinB2 can induce and be induced by several EphB receptor members.29,30 Targeted disruption of either EphB4 or EphrinB2 results in early lethality in the developing embryo as a result of arrest in angiogenesis but not vasculogenesis.12,26,28,31 However, recent studies indicate that inactivation of reverse signaling (mouse EphrinB2 knock-out and knock-in of C-terminal truncated EphrinB2) allows angiogenesis to occur in an orderly, sequenced manner.32
On binding, the receptor and ligand on adjacent cells undergo dimerization and clustering, which are required for further activation. This feature led us to test whether the monomeric form of the extracellular domain of EphB4 will function as an antagonist of EphB4-EphrinB2 interaction. Specifically, we hypothesized that the soluble extracellular domain of EphB4 (sEphB4) will not itself activate EphrinB2 and also block phosphorylation of EphB4 and EphrinB2 on cells when stimulated by clustered EphrinB2 or EphB4, respectively. Pursuant to these biochemical effects, sEphB4 was predicted to modulate the migration of ECs and maturation to form tubelike structures in response to growth factor stimuli in biologic assays in vitro and in vivo. Lastly, such effects of sEphB4 would retard angiogenesis as a whole and tumor growth, in particular. Recombinant soluble extracellular domain of EphB4, containing the globular ligand-binding subdomain, indeed fulfills these expectations and is thus a candidate for formal investigation in human diseases in which vascular proliferation has a significant contribution.
Materials and methods
Expression constructs
Two soluble proteins encoding the entire extracellular portion of EphB4 (sEphB4) and its N-terminus truncated analog (CF2) were generated. To produce the entire extracellular domain of EphB4 (soluble EphB4 [sEphB4]), the encoding fragment was amplified from full-length human EphB4 cDNA by polymerase chain reaction (PCR) using TACTAGTCCGCCATGGAGCTCCGGGTGCTGCT as a direct and TGCGGCCGCTTAATGGTGATGGTGATGATGCTGCTCCCGCCAGCCCTCGCTCTCAT as a reverse oligo primer. To generate the CF2, N-terminal truncated analog of sEphB4 in which the G-region is deleted (Δ amino acids 13-183), we used a multistep cloning strategy. First, 2 overlapping fragments encoding for native leader peptide and CF2 region were obtained separately by PCR amplification. For this purpose, 2 pairs of oligo primers were used: TACTAGTCCGCCATGGAGCTCCGGGTGCTGCT as a direct and CAGCTGAGTTTCCAATTTTGTGTTC as a reverse primer for leader peptide-containing fragment and GAACACAAAATTGGAAACTCAGCTGACTGTGAACCTGAC as a direct and GCGGCCGCCCTGCTCCCGCCAGCCCTCGCT as a reverse primer for CF2 region. A BstX/EcoRV-digested DNA fragment encoding for sEphB4 protein was used as the template. As a second step, an equimolar mixture of obtained fragments was used as a template for PCR amplification with TACTAGTCCGCCATGGAGCTCCGGGTGCTGCT and GCGGCCGCCCTGCTCCCGCCA-GCCCTCGCT oligo primers. The resulting 1107-bp DNA fragment encoded for both native leader peptide and CF2 region. This CF2 DNA fragment was TA cloned into the mammalian expression vector pEF6/V5-His-TOPO (Invitrogen, Carlsbad, CA), followed by digestion with NotI and self-ligation to allow in-frame fusion to V5 and His-tag.
The vector for producing secreted human EphrinB2-alkaline phosphatase (EphrinB2-AP) was constructed by PCR amplification of human EphrinB2 cDNA using primers TAAAGCTTCCGCCATGGCTGTGAGAAGGGAC and TAGGATCCTTCGGAACCGAGGATGTTGTTCCC primer pair. The resulting fragment was digested with HindIII and BamHI, and subcloned into HindIII/BglII-digested pAPTag2 vector (GenHunter, Basgvukke, TN). In each case, inserts in expression vectors were verified by complete sequencing.
Antibodies and other reagents
Anti-EphB4 monoclonal antibodies (mAbs) mAb265 and mAb138 were raised in mice against human sEphB4 protein. The antiphosphotyrosine antibody 4G10 was from Upstate Biotechnology (Lake Placid, NY), anti-EphrinB2 antibody from R&D Systems (Minneapolis, MN), anti-CD31 (M20) from Santa Cruz Biotechnology (Santa Cruz, CA), monoclonal anti-Ki-67 from Dako (Carpenteria, CA), and antihuman Fc from Jackson Laboratories (Bar Harbor, ME). PDGF and Ephrin-B2/Fc chimeric proteins were from R&D Systems and human IgG Fc fragment from Jackson Laboratories.
Expression and purification of EphB4-derived recombinant proteins
To produce EphB4 recombinant proteins, cultured human embryonic kidney cells HEK293T were transfected with the corresponding plasmid construct using Lipofectamine 2000 reagent (Invitrogen). Twelve to 16 hours after transfection, growth medium (DMEM + 10% fetal bovine serum) was aspirated and cells were washed once with serum-free DMEM and replaced with serum-free DMEM. Conditioned medium containing the secreted proteins was harvested 72 to 96 hours later, clarified by centrifugation, and used for purification on Ni-NTA agarose (Qiagen, Valencia, CA).
The purity and quantity of the recombinant proteins was tested by sodium dodecyl sulfate–polyacrylamide gel electrophoresis (SDS-PAGE) with Coomassie blue, Western blotting, and UV spectroscopy. Purified proteins were dialyzed against 20 mM Tris-HCl, 0.15 M NaCl, pH 8, and stored at –70°C.
Ligand-binding assay
To test EphrinB2-binding properties of the expressed and purified proteins, 10 μL Ni-NTA-agarose beads (Qiagen) were incubated in microcentrifuge tubes with 1 to 50 ng His-tagged proteins diluted in 0.2 mL binding buffer (20 mM Tris-HCl, 0.15 M NaCl, 0.1% bovine serum albumin, pH 8). After incubation for 30 minutes on a shaking platform, beads were washed twice with 1.4 mL binding buffer, followed by application of 200 ng EphrinB2-AP fusion protein in a final volume of 0.2 mL. Binding was performed for 45 minutes on a shaking platform and tubes were centrifuged and washed twice with 1.4 mL binding buffer. The amount of precipitated AP was measured colorimetrically at 405 nm after application of p-nitrophenyl phosphate. The experiment was repeated 3 times.
Immunoprecipitation and cell-based EphB4 and EphrinB2 tyrosine kinase assay
Typically, human umbilical vein endothelial cells (HUVECs) were grown in 60-mm dishes until 100% confluence and were treated with either clustered EphrinB2-Fc at 1 μg/mL in RPMI for 10 minutes to activate EphB4 receptor or Fc fragment alone as a negative control. To study the effect of different derivatives of soluble EphB4 proteins on EphB4 receptor activation, sEphB4 or CF2 was premixed with EphrinB2-Fc at a 1:5 molar ratio and incubated for 20 minutes followed by application to cells for 10 minutes.
After stimulation, media was aspirated and cells were immediately harvested with protein extraction buffer containing 20 mM Tris-HCl, pH 7.4, 150 mM NaCl, 1% (vol/vol) Triton X-100, 1 mM EDTA, 1 mM PMSF, 1 mM sodium vanadate, pH 7.8. Protein extracts were clarified by centrifugation at 18 000 g for 20 minutes at 4°C. Clarified protein samples were incubated overnight with protein A-agarose precoated with mAb138. The precipitated immune complexes were washed twice with the same extraction buffer containing 0.1% Triton X-100, solubilized in 1 × SDS-PAGE loading buffer and separated on 10% SDS-PAGE. For EphB4 receptor activation studies, electroblotted membrane was probed with anti-p-Tyr–specific antibody 4G10 at 1:1,000 dilution followed by application of protein G-HRP conjugate (Sigma, St Louis, MO) at 1:5000 dilution.
For EphrinB2 phosphorylation assay, 293T cells were transiently cotransfected with EphrinB2-Myc tag and EphB4 plasmid DNAs. Transfected cells were either treated or nontreated with sEphB4 at a final concentration of 5 μg/mL for 24 hours. Cell lysates were prepared as described and immunoprecipitation was performed with anti-Myc antibody. Phosphorylated EphrinB2 was detected by p-Tyr–specific antibody 4G10 and EphrinB2 was detected by anti-EphrinB2 antibody. All experiments were performed in triplicate and repeated 3 times.
Cell culture
Normal HUVECs and human umbilical arterial ECs (HUAECs) were obtained from Cambrex (Walkersville, MD) and maintained in EGM2-supplemented medium (Invitrogen). For all experiments, HUVECs were used at passages 4 or below and collected from a confluent dish. SCC-15 and MCF-7 cell lines were obtained from American Type Culture Collection (Manassas, VA) and cultured under recommended conditions.
EC tube formation assay
Matrigel (250 μL; BD Biosciences, Palo Alto, CA) was placed in each well of an ice-cold 24-well plate. The plate was allowed to sit at room temperature for 15 minutes then incubated at 37°C for 30 minutes to permit Matrigel to solidify. HUVECs were prepared in EGM2 medium at a concentration of 1 × 105 cells/mL. The test protein was prepared at twice the desired concentration (5 concentration levels) in the same medium. Cells (500 μL) and protein (500 μL) were mixed and 200 μL of this suspension was placed in duplicate on the polymerized Matrigel. After 6-hour and 24-hour incubations, triplicate pictures were taken for each concentration using a Bioquant Image Analysis system (Bioquant, Nashville, TN). Length of cords formed and number of junctions were compared among various groups. Experiments were performed in triplicate and repeated twice.
Cell adhesion assay
HUAECs were grown to 80% confluence and varying concentrations of sEphB4 were added to the supernatant overnight. Cells were prepared in EGM2MV medium at a density of 2 × 104 cells/mL and 250 μL cells were added to Matrigel-coated 96-well plates in the presence or absence of 10 ng/mL PDGF-B. Fifteen minutes later, the supernatant was removed and wells washed with PBS. Fresh medium was added with 5 mg/mL MTT and absorbance measured in 2 hours as in cell viability assay.
Cell migration assay
Chemotaxis of HUVECs to VEGF- or bFGF-containing solution was assessed using a modified Boyden chamber, transwell membrane filter inserts in 24-well plates, 6.5 mm in diameter, 8 μm pore size, 10 μm thick, Matrigel-coated polycarbonate membranes (BD Biosciences). Cell suspensions of HUVECs (2 × 105 cells/mL) in 200 μL medium were seeded in the upper chamber and 70 nM sEphB4 added simultaneously with stimulant (10-20 ng/mL VEGF or bFGF) to the lower compartment of the chamber. After incubation for 4 hours at 37°C, the upper surface of the filter was scraped with swab and filters were fixed and stained with Diff Quick. Ten random fields at × 40 magnification were counted and the results expressed as mean number of cells. Negative nonstimulated control values were subtracted from stimulated control and protein-treated sample values, and the data were plotted as mean migrated cell plus or minus SD.
Cell viability assay
HUVECs, HUAECs, or cancer cell lines were plated in a 48-well plate at a density of 1 × 104 cells/well in a total volume of 500 μL. Medium was changed after cells attached and triplicate samples were treated with sEphB4 or CF2 (100 μL) as described in “Results.” Cell viability was assessed by MTT as described previously.33
Murine Matrigel plug angiogenesis assay
In vivo angiogenesis was assayed in mice as growth of blood vessels from subcutaneous tissue into a Matrigel plug containing the test sample. Matrigel rapidly forms a solid gel at body temperature, trapping the factors to allow slow release and prolonged exposure to surrounding tissues. Matrigel (8.13 mg/mL, 0.5 mL) in liquid form at 4°C was mixed with vehicle alone (PBS containing 0.25% BSA) or VEGF (180 ng/ml) or bFGF (90 ng/mL), in the presence of vehicle, sEphB4 or CF2. Matrigel (0.5 mL) was injected into the abdominal subcutaneous tissue of female Balb/C nu/nu mice (6 weeks old) along the peritoneal midline. There were 5 mice in each group. Animals were cared for in accordance with institutional and National Institutes of Health guidelines. On day 6, mice were humanely killed and plugs were recovered and processed for histology. Typically, the overlying skin was removed, and gels were cut out by retaining the peritoneal lining for support, fixed in 10% buffered formalin in PBS, and embedded in paraffin. Sections (5 μm) were cut and stained with Masson trichrome stain and examined under a light microscope; Masson trichrome stains Matrigel blue and ECs purple. The experiment was repeated twice with similar results. The vascularized area in each section was calculated using Scion Image Analysis Software (Fredrick, MD).
Immunohistochemistry and immunofluorescence
Sections (5 μm) of formalin-fixed paraffin-embedded tissues were deparaffinized and hydrated. Antigen epitope retrieval was performed by boiling slides in 10 mM sodium citrate buffer (pH 8.5) at 80°C for 20 minutes. Endogenous peroxidase activity was blocked by incubation in 3% H2O2 in PBS for 10 minutes, followed by blocking of nonspecific binding sites with SuperBlock blocking buffer (Pierce, Rockford, IL) for 1 hour both at room temperature. Sections were incubated with primary antibody overnight at 4° C and, after 3 washes in PBS, with appropriate secondary antibody for 1 hour at room temperature. Antibody binding was localized with ABC staining kit form Vector Laboratories (Burlingame, CA) according to the manufacturer's instructions and peroxidase activity detected using DAB substrate solution (Vector Laboratories). Sections were counterstained with Harris hematoxylin for 45 seconds, dehydrated, and mounted in xylene. Routine negative controls included deletion of primary and secondary antibody and substitution of normal IgG isotope for primary antibody. When using mouse anti–human Ki-67 antibody, the MOM kit (Vector Laboratories) was used to block nonspecific binding to mouse tissue. The number of cells staining positive was counted by a blinded observer in 5 random × 40 fields and compared by Student t test.
Fluorescent immunocytochemical analysis was performed in a similar fashion to detect the expression level of EC-specific markers including CD31, EphrinB2, and EphB4 (mAb138). Appropriate fluorescein-conjugated secondary antibodies (Sigma-Aldrich, St Louis, MO) were used and nuclei were counterstained with 4′, 6-diamidino-2-phenylindole dihydrochloride hydrate (DAPI). Slides were mounted with Vectashield antifade mounting solution (Vector Laboratories) and images obtained using an Olympus AX70 fluorescence microscope and Spot v2.2.2 (Diagnostic Instruments, Sterling Heights, MI) digital imaging system. Apoptosis was detected in deparaffinized sections of animal tumors by TdT-mediated dUTP nick-end labeling (TUNEL) assay using the in situ cell death detection kit (Roche, Piscataway, NJ) according to manufacturer's instructions.
Mouse corneal micropocket assay
Mouse corneal micropocket assay (experiment performed in triplicate) was performed as detailed by Kenyon et al.34 Briefly, Hydron pellets (polyhydroxyethylmethacrylate [polyHEMA], Interferon Sciences, New Brunswick, NJ) containing either 90 ng bFGF (R&D Systems) or 180 ng VEGF (R&D Systems) and 40 μg sucrose aluminum sulfate (Sigma) were prepared. Using an operating microscope, a stromal linear keratotomy was made with a surgical blade (Bard-Parker no. 15) parallel to the insertion of the lateral rectus muscle in an anesthetized mouse. An intrastromal micropocket was dissected using a modified von Graefe knife (30 mm). A single pellet was implanted and advanced toward the temporal corneal limbus (within 0.7 ± 0.1 mm for bFGF pellets and 0.5 ± 0.1 mm for VEGF pellets). Erythromycin ointment was then applied to the operated eye to prevent infection and decrease surface irregularities. The subsequent vascular response was measured extending from the limbic vasculature toward the pellet. Data and clinical photos presented here were obtained on day 4 after pellet implantation.
Murine tumor xenografts
Tumor cells (5 × 106) SCC-15 (head and neck squamous cell carcinoma cell line) or MCF-7 (human breast cancer cell line) were implanted subcutaneously in bilateral flanks of male athymic BalbC nu/nu mice (6-8 weeks old), 5 mice/group. For assessing local effects of sEphB4, tumor cells were mixed with Matrigel (1:1 vol/vol; BD Biosciences) with or without 70 nM sEphB4. For assessing systemic effects, sEphB4 CF2, or PBS alone were injected intravenously daily, beginning on day 3 at a dose of 4 mg/kg. Tumor volume was measured 3 times a week estimated as 0.52 × a × b2, where a and b are the largest and smallest lengths of the palpable tumor. The Student t test was used to compare tumor volumes, with P < .05 being considered significant. This experiment was done in triplicate with similar results in each experiment. Animals were humanely killed and tumors and normal organs harvested after 3 weeks. Harvested tissue was fixed in formalin for paraffin embedding and histologic analysis. All procedures were approved by our Institutional Animal Care and Use Committee and performed in accordance with the Animal Welfare Act regulations.
Results
Monomeric sEphB4 antagonizes forward and reverse signaling
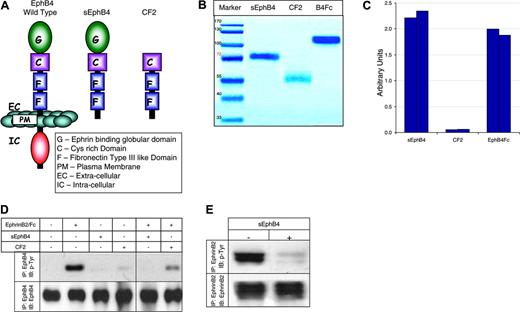
The extracellular domain of EphB4 contains an N-terminal ligand-binding globular domain followed by a cysteine-rich domain and 2-fibronectin type III repeats. To identify the subdomain organization of the ectopic part of EphB4 necessary for the antiangiogenic activity, 2 deletion variants of EphB4 were produced and tested (Figure 1A): soluble EphB4 (sEphB4) protein, containing all 4 extracellular domains of the receptor, and its truncated variant lacking the ligand-binding domain (CF2). Both C-terminally His-tagged proteins were expressed in transiently transfected cultured mammalian cells and affinity purified to homogeneity from the conditioned media on Ni2+-chelate resin. The proteins migrate on SDS-PAGE higher than suggested by their predicted molecular weights of 57.8 kDa (sEphB4) and 41.5 kDa (CF2), due to glycosylation (Figure 1B).
To confirm that the purified sEphB4 recombinant protein was fully functional, equal amounts of sEphB4 and CF2 (10 pmol), as well as commercially available murine EphB4-Fc chimeric protein, were immobilized on beads. Equal aliquots of these beads (1% or 0.1 pmol) were used in an EphrinB2-AP–binding assay (Figure 1C), whereas the remaining beads were analyzed on SDS-PAGE (Figure 1B). As expected, sEphB4 and murine EphB4-Fc, but not CF2, bind EphrinB2-AP fusion protein as measured by AP enzymatic activity.
Structure and biochemical properties. (A) Schematic illustration of truncated soluble proteins. (B) SDS-PAGE. Coomassie staining of EphB4-derived soluble proteins. (C) Binding of EphrinB2-AP fusion protein to EphB4 soluble proteins immobilized on Ni-NTA agarose beads. Results of 2 independent experiments are shown for each protein. Experiments were repeated 3 times (D) Tyrosine phosphorylation of EphB4 receptor in HUVECs in response to stimulation with EphrinB2-Fc (15 minutes) in the absence or presence of EphB4-derived soluble proteins. (E) Tyrosine phosphorylation of EphrinB2 by EphB4 in the presence of sEphB4. 293T cells transiently transfected with full-length EphrinB2 and EphB4 expression vector were cocultured (15 minutes) with or without sEphB4 for 24 hours (see “Monomeric sEphB4 antagonizes forward and reverse signaling” for details). Total amount of EphrinB2 by Western blot (bottom panel) and phosphorylation status of EphrinB2 after immunoprecipitation (IP) (top panel) are shown. Experiments were repeated twice.
Structure and biochemical properties. (A) Schematic illustration of truncated soluble proteins. (B) SDS-PAGE. Coomassie staining of EphB4-derived soluble proteins. (C) Binding of EphrinB2-AP fusion protein to EphB4 soluble proteins immobilized on Ni-NTA agarose beads. Results of 2 independent experiments are shown for each protein. Experiments were repeated 3 times (D) Tyrosine phosphorylation of EphB4 receptor in HUVECs in response to stimulation with EphrinB2-Fc (15 minutes) in the absence or presence of EphB4-derived soluble proteins. (E) Tyrosine phosphorylation of EphrinB2 by EphB4 in the presence of sEphB4. 293T cells transiently transfected with full-length EphrinB2 and EphB4 expression vector were cocultured (15 minutes) with or without sEphB4 for 24 hours (see “Monomeric sEphB4 antagonizes forward and reverse signaling” for details). Total amount of EphrinB2 by Western blot (bottom panel) and phosphorylation status of EphrinB2 after immunoprecipitation (IP) (top panel) are shown. Experiments were repeated twice.
Recombinant sEphB4 and CF2 proteins were also tested for their ability to block EphrinB2-induced EphB4 phosphorylation in HUVECs (Figure 1D). Stimulation of HUVECs with EphrinB2-Fc fusion protein leads to a rapid induction of tyrosine phosphorylation of the EphB4 receptor. However, preincubation of the ligand with sEphB4, and to a lesser extent CF2, suppresses subsequent EphB4 activation, whereas sEphB4 and CF2 alone do not affect EphB4 phosphorylation status (Figure 1D left).
sEphB4 was also tested for its ability to block EphB4-induced phosphorylation of EphrinB2. 293T cells were transiently transfected with EphB4 or EphrinB2. Expression of transfected protein along with the lack of expression of both proteins in the parent cell line was confirmed by Western blotting (data not shown). EphB4- and EprinB2-expressing 293T cell lines when cocultured induced phosphorylation of EphrinB2, which was blocked by the addition of sEphB4 (Figure 1E).
Effects of sEphB4 on HUVECs in vitro
Studies were performed to determine whether sEphB4 affects EC attachment, migration/invasion, proliferation, and tubule formation. HUVECs were plated on standard Matrigel-coated wells and treated with either sEphB4 or CF2 in the presence or absence of growth factors (see “Materials and methods”). sEphB4 inhibited tube formation (Figure 2A) in a dose-dependent manner (Figure 2B), whereas CF2 had no effect (Figure 2A). sEphB4, even at the lowest dose tested (2 nM), showed decrease in both the length of tubes and the number of junctions, with maximum effect seen at the highest concentration of 70 nM (Figure 2C). sEphB4 maintained its inhibitory activity regardless of the growth factors used in the assay.
sEphB4 inhibits tubule formation on Matrigel in vitro. (A) HUVECs were cultured on standard Matrigel in growth factor-stimulated conditions with either sEphB4 or CF2 proteins for 24 hours. Shown are representative pictures from triplicate wells repeated twice. (B) Dose response. HUVECs were cultured as described in panel A with sEphB4 at indicated concentrations. (C) Quantitative analysis of data from panel B for tube length and the number of junctions in sEphB4-treated HUVECs (Bioquant Image Analysis; mean from triplicate wells in 2 repetition experiments). (D) sEphB4 inhibits invasion of HUVECs (top right) compared to cells treated with CF2 (bottom left) or no treatment (top left). Data are presented as number of invading cells ± SE from duplicate wells in 2 experiments (bottom right). (E) sEphB4 inhibits PDGF-stimulated attachment of HUAECs to Matrigel over 5 minutes and 15 minutes. Shown are number of attached cells as a percentage of control in triplicate wells; experiment was repeated twice. Photomicrographs in panels A, B, and D were taken using a Nikon Coolpix 5000 camera (Nikon, Tokyo, Japan) and a Carl Zeiss Invertoskop microscope (Zeiss, Goettingen, Germany) with a 4 ×/0.12 NA objective and 10 × eyepiece.
sEphB4 inhibits tubule formation on Matrigel in vitro. (A) HUVECs were cultured on standard Matrigel in growth factor-stimulated conditions with either sEphB4 or CF2 proteins for 24 hours. Shown are representative pictures from triplicate wells repeated twice. (B) Dose response. HUVECs were cultured as described in panel A with sEphB4 at indicated concentrations. (C) Quantitative analysis of data from panel B for tube length and the number of junctions in sEphB4-treated HUVECs (Bioquant Image Analysis; mean from triplicate wells in 2 repetition experiments). (D) sEphB4 inhibits invasion of HUVECs (top right) compared to cells treated with CF2 (bottom left) or no treatment (top left). Data are presented as number of invading cells ± SE from duplicate wells in 2 experiments (bottom right). (E) sEphB4 inhibits PDGF-stimulated attachment of HUAECs to Matrigel over 5 minutes and 15 minutes. Shown are number of attached cells as a percentage of control in triplicate wells; experiment was repeated twice. Photomicrographs in panels A, B, and D were taken using a Nikon Coolpix 5000 camera (Nikon, Tokyo, Japan) and a Carl Zeiss Invertoskop microscope (Zeiss, Goettingen, Germany) with a 4 ×/0.12 NA objective and 10 × eyepiece.
We then performed an in vitro invasion assay to measure the ability of HUVECs to degrade basement membrane and migrate toward a growth factor stimulus. sEphB4, but not CF2, significantly inhibited both bFGF and VEGF-induced migration by nearly 50% compared to control (Figure 2D). We then wished to assess if sEphB4 had an effect on EC attachment to basement membrane. To this end, HUAECs grown to 80% confluence were incubated with varying doses of sEphB4 overnight. Equal numbers of cells were allowed to plate on Matrigel-coated wells in the presence of 10 ng/mL PDGF-BB. PDGF-BB increased EC attachment 2-fold at 15 minutes. sEphB4 effectively inhibited this response in a dose-dependent fashion (Figure 2E). At a dose of 300 ng/mL, sEphB4 inhibited EC attachment by over 80% compared to control nonstimulated cells. A similar effect was observed at 5 minutes as well. Lastly, sEphB4 was found to have no detectable cytotoxic effect on HUVECs as assessed by MTT assay (data not shown).
sEphB4 inhibits vascularization of Matrigel plugs in vivo
To further demonstrate that sEphB4 can directly inhibit angiogenesis in vivo, we performed a murine Matrigel plug experiment. Matrigel supplemented with bFGF, VEGF, or both, with and without sEphB4, was injected into the ventral abdominal subcutaneous tissue of Balb/C nu/nu mice. Plugs without growth factors had virtually no vascularization after 6 days (Figure 3A top left). In contrast, plugs supplemented with bFGF or VEGF or growth factor along with CF2 (70 nM) had extensive vascularization and vessels with open lumen containing red cells throughout the plug (Figure 3A top and bottom right). Plugs containing 70 nM sEphB4 along with the growth factors had markedly reduced vascularization (Figure 3A bottom left) comparable to plugs without growth factor (Figure 3B). Furthermore, histologic examination showed decreased vessel staining in sEphB4-treated plugs. Fluorescence immunohistochemistry for CD31 confirms the endothelial origin of these cells, whereas the presence of EphB4+ and EphrinB2+ cells indicates that, as expected, both arterial and venous ECs migrate into the Matrigel in response to the growth factors (Figure 3C left panels). sEphB4 treatment reduced the numbers of CD31+, EphB4+, and EphrinB2+ as well (Figure 3C right panels). We quantitated the area of vascularization in 7 random blinded high-power fields using Spot v2.2.2 digital imaging system. sEphB4 treatment inhibited CD31+ vessels by 78%, EphB4+ vessels by 84%, and EphrinB2+ vessels by 64%.
sEphB4 inhibits angiogenesis in murine Matrigel and corneal micropocket assays. (A) Matrigel solution was injected subcutaneously into Balb/C nu/nu mice. After 6 days, plugs were removed and processed in paraffin. Individual sections were stained with Masson trichrome and representative photographs at × 20 magnification from triplicate plugs in 2 independent experiments are shown. Top left shows section of a Matrigel plug without any added proteins; top right, section of plug containing VEGF only; bottom left, section of plug with sEphB4 and VEGF; and bottom right, section of plug with CF2 and VEGF. Photomicrographs were taken with a Nikon Coolpix 5000 camera on a Nikon Eclipse E400 microscope with a 4 ×/0.13 NA objective and a 10 × eyepiece. (B) Quantitation of vascularized area averaged (± SEM) from all plugs (Scion Image software). (C) Immunofluorescent staining of dissected plugs (with or without sEphB4) with PECAM, EphB4, and EphrinB2 antibodies. (D) sEphB4 was added to Hydron polymer and sucralfate with or without bFGF and inserted into a micropocket in corneas of Balb/C mice in triplicate. Shown is neovascular response on day 4 after implantation: Hydron with bFGF (left), with no growth factor added (center), and growth factor and sEphB4 (right). (E) Quantitation of vascularization averaged (± SEM) over all corneas in each group.
sEphB4 inhibits angiogenesis in murine Matrigel and corneal micropocket assays. (A) Matrigel solution was injected subcutaneously into Balb/C nu/nu mice. After 6 days, plugs were removed and processed in paraffin. Individual sections were stained with Masson trichrome and representative photographs at × 20 magnification from triplicate plugs in 2 independent experiments are shown. Top left shows section of a Matrigel plug without any added proteins; top right, section of plug containing VEGF only; bottom left, section of plug with sEphB4 and VEGF; and bottom right, section of plug with CF2 and VEGF. Photomicrographs were taken with a Nikon Coolpix 5000 camera on a Nikon Eclipse E400 microscope with a 4 ×/0.13 NA objective and a 10 × eyepiece. (B) Quantitation of vascularized area averaged (± SEM) from all plugs (Scion Image software). (C) Immunofluorescent staining of dissected plugs (with or without sEphB4) with PECAM, EphB4, and EphrinB2 antibodies. (D) sEphB4 was added to Hydron polymer and sucralfate with or without bFGF and inserted into a micropocket in corneas of Balb/C mice in triplicate. Shown is neovascular response on day 4 after implantation: Hydron with bFGF (left), with no growth factor added (center), and growth factor and sEphB4 (right). (E) Quantitation of vascularization averaged (± SEM) over all corneas in each group.
sEphB4 inhibited corneal neovascularization
To further investigate the antiangiogenic activity of sEphB4 in vivo, we studied the effect of sEphB4 on bFGF- or VEGF-induced neovascularization in the mouse cornea. Growth factor–supplemented Hydron pellets implanted into a corneal micropocket induced angiogenesis (Figure 3D left). Neovascularization was markedly inhibited in mouse cornea with implanted pellets containing bFGF and sEphB4, in sharp contrast to the effect seen in corneas with pellets containing bFGF alone (Figure 3D right and left, respectively). Both the length of tubes and the number of junctions were significantly decreased in response to sEphB4 (Figure 3E). Similarly, the VEGF-induced vascular response was also significantly inhibited by sEphB4 (data not shown).
sEphB4 inhibits the growth of human tumors in athymic mice
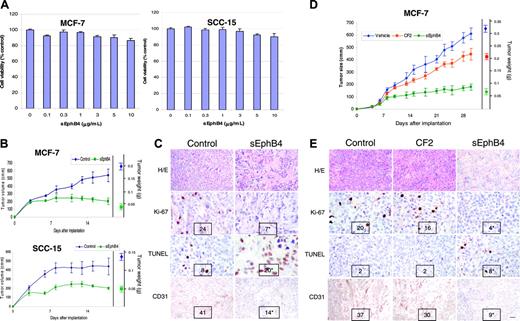
Several tumor cell lines express EphB4 and increasing evidence indicates that EphB4 signaling may provide survival signals to these cells.35,36 To determine the effect of sEphB4 on the survival of tumor cells in vitro, tumor cells were cultured in the presence of varying concentrations of sEphB4. sEphB4 had no effect on tumor cell viability in vitro in all cell lines tested, including EphB4-expressing MCF-7, SCC-15, B16, PC3, and EphrinB2-expressing SLK cell lines (Figure 4A and data not shown).
Given the ability of sEphB4 to profoundly affect angiogenesis in vivo, we speculated that sEphB4 can inhibit tumor growth in vivo. We therefore examined the activity of sEphB4 in vivo in tumor xenograft models. MCF-7 (human breast carcinoma cell line) and SCC-15 (human squamous cell carcinoma of the head and neck) cells were premixed with Matrigel-containing vehicle or sEphB4 and implanted subcutaneously. Tumors in the control groups grew steadily over the treatment period, whereas xenografts supplemented with sEphB4 exhibited a significantly reduced growth rate (Figure 4B). MCF-7 tumor xenografts had a 62% reduction in final tumor volume and an 80% reduction in final tumor weight, whereas SCC-15 xenografts had a 54% and 64% reduction, respectively. Similar results were obtained in 2 additional independent experiments. Histologic analysis of tumors harvested at the time the animals were humanely killed (Figure 4C) showed large areas of necrosis as assessed by hematoxylin and eosin in the sEphB4-treated tumors. Tumor cell proliferation measured by Ki-67 staining was reduced by 70% in sEphB4-treated SCC-15 xenografts, with a 2.5-fold increase in apoptosis. In addition, sEphB4-treated tumor implants were markedly less vascularized (66% reduction) as assessed by CD31+ vessels in tumor sections. Similar results were obtained in MCF-7 tumor xenografts (data not shown). Ki-67 staining was reduced by 65%, with a 4-fold increase in apoptosis and a 75% reduction in tumor microvasculature.
We next studied the effect of systemic administration of sEphB4 on tumor growth. SCC-15 and MCF-7 tumor xenograft-bearing mice were treated with intravenous sEphB4 at a daily dose of 10 mg/kg starting 4 days after tumor implantation. Administration of sEphB4 reduced MCF-7 tumor volume by over 70% and tumor weight by 82%, whereas CF2 inhibited tumor growth by 25% (Figure 4D). sEphB4 administration was well tolerated with no significant effect on body weight or the general well being of the animals, as determined by the absence of lethargy, intermittent hunching, tremors, or disturbed breathing patterns. Examination of vital organs (lung, liver, kidney, heart) obtained when the animals were humanely killed showed no changes on histologic examination in sEphB4-treated mice (data not shown). sEphB4 reduced the number of proliferating tumor cells by 80%, increased apoptosis 4-fold, and diminished microvascular density by 75%. Similarly, SCC-15 tumor volume was reduced with sEphB4 treatment by 50% and tumor weight by 65% (data not shown). Proliferating cell number fell by 70%, apoptosis increased 2.8-fold, and tumor microvasculature was decreased by 77% (data not shown).
sEphB4 inhibits the tumor growth in a murine tumor xenograft model. (A) 1 × 104 MCF-7 and SCC-15 cells were grown in triplicate in the presence of increasing concentrations of sEphB4 for 72 hours. Cell viability was assessed by MTT assay. The experiment was repeated twice. (B) Mice (n = 6/group) were given implants with 5 × 106 SCC-15 or MCF-7 cells in a Matrigel preparation with PBS or sEphB4 (70 nM) and tumor volumes measured 3 times a week. Three weeks after implantation, tumors were harvested and weighed (right axis). Tumor volumes were significantly smaller in the sEphB4 arm, beginning on day 7 of measurement. The experiment was repeated twice. (C) Representative histology (hematoxylin and eosin) of SCC-15 tumors is shown. Tumor proliferation was assessed by immunohistochemical detection of Ki-67 protein and apoptosis by TUNEL. Microvasculature was assessed by CD31 immunohistochemistry. Number of cells staining positive was averaged over 5 random 40 × fields by a blinded observer and is shown at the bottom of each photomicrograph. (D) For assessing the effect of systemic administration of sEphB4, mice (n = 6/group) were given implants of tumor cells subcutaneously and treated with sEphB4 or CF2 (10 mg/kg/d) or an equal volume of PBS intravenously starting on day 4. Tumor volumes and weights were assessed as in panel B. Tumor volumes were significantly smaller in the sEphB4 arm compared to animals treated with PBS and CF2. (E) Histologic analysis of harvested MCF-7 tumors was performed as in panel C. All values are expressed as mean ± SEM. *P < .01 by Student t test. Bar in bottom right panel in E represents 100 μm in hematoxylin-eosin section, 60 μm in Ki-67 and apoptosis sections, and 75 μm in CD31 sections. Photomicrographs in panels C and E were taken using a Nikon Coolpix 5000 camera and a Nikon Eclipse E400 microscope with a 10 × eyepiece. Magnification was as follows: top row, 4 ×/0.13 NA; middle rows, 40 ×/0.75 NA; and bottom rows, 20 ×/0.5 NA objectives.
sEphB4 inhibits the tumor growth in a murine tumor xenograft model. (A) 1 × 104 MCF-7 and SCC-15 cells were grown in triplicate in the presence of increasing concentrations of sEphB4 for 72 hours. Cell viability was assessed by MTT assay. The experiment was repeated twice. (B) Mice (n = 6/group) were given implants with 5 × 106 SCC-15 or MCF-7 cells in a Matrigel preparation with PBS or sEphB4 (70 nM) and tumor volumes measured 3 times a week. Three weeks after implantation, tumors were harvested and weighed (right axis). Tumor volumes were significantly smaller in the sEphB4 arm, beginning on day 7 of measurement. The experiment was repeated twice. (C) Representative histology (hematoxylin and eosin) of SCC-15 tumors is shown. Tumor proliferation was assessed by immunohistochemical detection of Ki-67 protein and apoptosis by TUNEL. Microvasculature was assessed by CD31 immunohistochemistry. Number of cells staining positive was averaged over 5 random 40 × fields by a blinded observer and is shown at the bottom of each photomicrograph. (D) For assessing the effect of systemic administration of sEphB4, mice (n = 6/group) were given implants of tumor cells subcutaneously and treated with sEphB4 or CF2 (10 mg/kg/d) or an equal volume of PBS intravenously starting on day 4. Tumor volumes and weights were assessed as in panel B. Tumor volumes were significantly smaller in the sEphB4 arm compared to animals treated with PBS and CF2. (E) Histologic analysis of harvested MCF-7 tumors was performed as in panel C. All values are expressed as mean ± SEM. *P < .01 by Student t test. Bar in bottom right panel in E represents 100 μm in hematoxylin-eosin section, 60 μm in Ki-67 and apoptosis sections, and 75 μm in CD31 sections. Photomicrographs in panels C and E were taken using a Nikon Coolpix 5000 camera and a Nikon Eclipse E400 microscope with a 10 × eyepiece. Magnification was as follows: top row, 4 ×/0.13 NA; middle rows, 40 ×/0.75 NA; and bottom rows, 20 ×/0.5 NA objectives.
Discussion
EphrinB2 is the only ligand for EphB4 receptor and both proteins are essential for the development of the cardiovascular system. EphrinB2 is expressed in angioblasts destined to mature into arterial ECs and perivascular smooth muscle cells, whereas EphB4 defines venous lineage at the same step. However, neither protein is essential for this commitment because targeted disruption of either protein allows vasculogenesis to occur. Loss of either protein leads to arrest of angiogenesis leading to early embryonic lethality. Membrane localization of both proteins and, thus, signaling via cell-cell contact, with resultant dimerization and clustering of proteins on respective cells is required for optimal signaling. Dimeric or multimeric forms of the extracellular domain of EphrinB2 can replicate this effect leading to EphB4 receptor activation. Similarly, dimeric or multimeric forms of the extracellular domain of EphB4 can replicate this effect leading to EphrinB2 activation. We hypothesized that the soluble form of EphB4 (sEphB4) consisting of the extracellular domain, especially the N-terminus globular ligand-binding domain, would have an opposite function such that it would not only fail to induce phosphorylation of EphrinB2, but would also function as dominant negative, thus blocking forward and reverse signaling. sEphB4 indeed does not phosphorylate EphrinB2 and blocks ligand-induced EphB4 phosphorylation and receptor-induced ligand activation. Pursuant to the biochemical results, biologic studies fulfill the promise that sEphB4 blocks organization and migration of ECs to form tubules, which are the principal events leading to maturation of newly forming vessels. Importantly, sEphB4 exerts similar effects in angiogenesis models in vivo, and further, significantly inhibits tumor growth as well. Another recombinant protein, CF2, which lacks the globular ligand-binding domain, was also tested. Interestingly, this protein partially inhibits EphB4 phosphorylation by EphrinB2 and also has a small inhibitory effect on tumor growth in vivo. Given that this protein does not inhibit ligand binding, the observed effects may occur due to the binding of CF2 to endogenous EphB4 to interrupt receptor clustering and form functionless heterodimers. Such an event has been suggested with EphA receptors.37
Angiogenesis involves the recruitment and attachment of ECs at sites of vascularization and their proliferation and organization into tubelike structures. We show here that sEphB4 interrupts 2 of these 3 processes—prevents EC attachment to extracellular matrix proteins and their organization to form tubelike structures. EphB4, like other Eph receptors, may also interact with other cell membrane proteins as exemplified by the interaction of EphB2 with glutamate receptors38-41 and possibly other yet unidentified interactions. It is conceivable that sEphB4 competes not only with EphrinB2/EphB4 interactions but also other extracellular protein interactions. Along these lines, we show that sEphB4 can inhibit PDGF-induced EC attachment. Eph receptor signaling has been shown to modulate focal adhesion kinases42 and modulates several members of the integrin family of proteins.43 These proteins are also targets of PDGF-R signaling. Thus, interruption of EphB4 signaling by sEphB4 may modulate integrin-matrix interaction, similar to that seen with disruption of EphB1 activation43 and thus reduce cell attachment and tube formation.
Loss of EphB4 in the embryo permits the primary vascular complex to develop. However, the vessels fail to mature, such that all vessels have small and similar luminal diameter, and lack communication between arterial and venous capillary networks. We demonstrate similar effects with sEphB4 in vivo. Growth factors such as bFGF or VEGF or a combination of factors when impregnated in Matrigel recruit ECs and produce large, branching vessels integrated into the circulation with red blood cells in the lumen and degradation of surrounding matrix. Rudimentary vessel-like structures seen in sEphB4-containing Matrigel plugs indicates inhibition of effective EC migration resulting in few, short vessel-like structures with narrow or no lumen, lacking red cells. sEphB4 antagonizes equally well the angiogenic response to several different vascular growth factors including VEGF and bFGF. This is consistent with the role of EphB4-EphrinB2 in embryonic vessel maturation, which is common to several proangiogenic stimuli. sEphB4 is also highly effective in laser injury-induced models of choroidal neovascularization in which numerous factors are induced simultaneously.44
Tumor growth and metastasis are dependent on angiogenesis via release of various proangiogenic factors. Maturation of tumor vessels is dependent on EphB4-EphrinB2 interactions and thus subject to modulation. Tumor growth of 2 distinct epithelial tumor types was markedly reduced in response to sEphB4 with a profound inhibition in tumor microvasculature. Thus, sEphB4 significantly influences tumor-induced vascular response and secondarily, tumor growth. Tumor xenografts also showed reduced proliferation and increased apoptosis following sEphB4 treatment. EphB4 and EphrinB2 are not known to influence cell proliferation or cell survival and sEphB4 does not have an impact on tumor cell survival in vitro. Hence, these effects on tumor growth are likely secondary to reduced blood supply. The possibility that EphB4, EphrinB2, or other EphrinB2-activating Eph receptors, when expressed in tumor cells, provide survival signals and thus subject to modulation needs further investigation. Similarly, it is well known that stroma plays a critical role in the growth and progression of tumors. It is also likely that sEphB4 modulates the interaction between tumor cells and stroma or tumor cells and matrix proteins released from the stroma. Lastly, tumor growth is modulated by the innate immune system even in athymic mice. The role played by tumor cell-expressed EphB4 or EphrinB2 on innate immune responses has not yet been investigated.
A recent report by Martiny-Baron et al45 showed that interruption of EphB4-EphrinB2 signaling can inhibit angiogenesis and tumor growth. Our studies add further mechanistic insights into these effects and further establish sEphB4 as a candidate for therapy in vascular proliferative disorders and cancers. First, we provide documentation that sEphB4 interrupts ligand-induced receptor phosphorylation and receptor-induced ligand phosphorylation. Further, sEphB4 inhibits EC attachment and tube formation in vitro in the absence of exogenous EphB4, regardless of the growth factor that is driving angiogenesis, data that better reflect the known effects of EphB4 knockdown in the embryo. We extend these findings to show that sEphB4 can similarly inhibit angiogenesis in vivo in different models of adult neovascularization. Lastly, our tumor studies in different tumor types using systemic administration of sEphB4 demonstrate that sEphB4 can reach tumor tissue and hence has potential for clinical application.
In summary, the monomeric form of sEphB4 ectodomain displays inhibitory effects on the following: (1) forward and reverse signaling by EphB4 and EphrinB2; (2) EC adhesion, migration, and tube formation; (3) growth factor- or tumor-derived angiogenesis; and (4) tumor growth accompanied by reduced proliferation and increased apoptosis. sEphB4 is thus a candidate for therapeutic assessment in angioproliferative disorders such as macular degeneration and cancer.
Prepublished online as Blood First Edition Paper, December 1, 2005; DOI 10.1182/blood-2005-04-1655.
Supported in part by National Institutes of Health grant RO1CA79218 (P.S.G.).
N.K., V.K., R.R., L.L., and S.Z. are employees of Vasgene Therapeutics, Inc, and P.S.G. holds stock in Vasgene Therapeutics, Inc.
N.K. and V.K. contributed equally to the manuscript.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
We wish to thank Drs D. Lynne Smith and David Quinn for critical review of the manuscript.



This feature is available to Subscribers Only
Sign In or Create an Account Close Modal