It has been shown that in vivo and in vitro treatment with G-CSF induces the generation of low-density granulocytes (LDGs), which copurify with PBMCs and inhibit IFN-γ production by human T cells. These results prompted us to postulate an immunomodulatory role for LDGs in acute graft-versus-host disease (aGVHD). Here it is shown that in the mouse experimental model, in vivo and in vitro G-CSF treatment generates LDGs capable of inhibiting 80% of T-cell IFN-γ production. To assess the role of these LDGs in aGVHD, lethally irradiated (C57BL/6 × BALB/c) F1 hosts were reconstituted with T cell–depleted bone marrow cells plus nylon wool–purified spleen cells from G-CSF–treated (G-NWS) or –nontreated (NWS) C57BL/6 donors. Recipients of G-NWS had a 75% survival rate in contrast to a rate of 25% in the NWS recipients. The protective effect was completely abolished, and the mortality rate was 100% if donor-cell infusion was treated with anti-Gr1. Moreover, if LDGs were infused with NWS, full protection of aGVHD was observed, and no signs of disease were evidenced by mortality rate, weight loss, or histopathology of target organs. These results revealed the unexpected immunosuppressive capacity of G-CSF based on the generation of LDGs, leading to the possibility of using these cells as inhibitors of aGVHD.
Introduction
Allogeneic hematopoietic stem cell transplantation (HSCT) is widely used to treat a series of hematologic diseases. Unfortunately, this treatment is associated with serious adverse effects, of which acute graft-versus-host disease (aGVHD) is the most important. In most studies,1-5 the incidence of grade II-IV aGVHD in recipients of HLA-matched, related transplantation ranges from 30% to 40%, and the mortality rate can reach 30%.
The pathophysiology of aGVHD depends on the presence of T lymphocytes in the incoming graft; it can be completely abrogated by T-cell depletion. Elimination of this lymphocyte subpopulation results in an increased incidence of bone marrow failure and, in patients with malignant diseases, higher relapse rates.6,7 Because T-cell depletion is not a solution, finding different ways to moderate the T-cell response has been a major goal in HSCT.
Some unexpected results were observed in HSCT outcome when G-CSF–mobilized peripheral blood stem cell transplantation (PBSCT) was compared with bone marrow transplantation (BMT). In addition to accelerated engraftment,8-10 the incidence of aGVHD has been surprisingly similar in both procedures1,9,11,12 despite the 10 times greater number of CD3+ cells in PBSCT than in BMT. These results suggest that G-CSF can functionally moderate potentially hazardous T cells.
Inhibitory effects of G-CSF mediated by monocytes involving IL-10, IL-12, or NO production have been described.13-16 Effects on dendritic cell function have also been observed, with G-CSF inducing increased numbers of DC2.17 With regard to direct analysis of T-cell function, the generation of IL-10+ regulatory cells18 and deviation to the inhibitory type 2 profile, with increased IL-4 production, have been suggested in some studies,19-21 whereas other studies20,22-25 demonstrate the inhibition of T-cell proliferation and lymphokine production by G-CSF–treated peripheral blood mononuclear cells in the absence of a clear type 2 response.
It had been previously shown that G-CSF may indeed inhibit human T-cell function.26 PBMCs purified from healthy persons on Ficoll-Hypaque density gradient is composed primarily of lymphocytes and a small number of monocytes. However, low-density cells from the peripheral blood of G-CSF–treated donors carry high numbers of low-density granulocytes (LDGs) that copurify with the mononuclear cell fraction. These LDGs inhibit the production of IL-4 and IFN-γ by CD3+ cells measured ex vivo by flow cytometry.24,26 LDGs are short lived. After 48 hours in culture, they undergo apoptosis, and T-cell IFN-γ production is reverted. Furthermore, after in vitro treatment of high-density granulocytes with G-CSF, LDGs become low-density cells and acquire the capacity to inhibit IFN-γ production in the T lymphocytes of healthy, untreated donors. In fact, the actual numbers of lymphokine (IL-4 and γ-IFN)–producing cells per kilogram injected into patients receiving PBSCT and BMT were the same.26 These results can explain the reported clinical findings1,9,11 of similar incidences of aGVHD in patients treated with PBSCT or BMT.
Given that the literature demonstrates lower incidences of aGVHD in humans and mice when donors are treated with G-CSF,19,20 that PBSCT is rich in LDG inhibitory cells, and that G-CSF can inhibit lymphokine production ex vivo and in vitro, we suggest an inhibitory role for G-CSF–treated granulocytes in aGVHD.
In this report, using the C57BL/6 into (C57BL/6 × BALB/c) F1 radiation chimeras as an aGVHD experimental model, we show that the protective role of G-CSF depended on granulocyte activity and that the coinjection of in vitro–generated low-density granulocytes and donor T cells from C56BL/6 mice completely abolished acute disease.
Materials and methods
Animals
Female A/J (H-2a), C57BL/6 (H-2b), and (C57BL/6 × BALB/c) F1 (H-2b × H-2d) mice were bred in the animal facility at Instituto Nacional de Câncer (Rio de Janeiro, Brazil). Animals used as hosts in the transplantation protocols were at least 10 weeks old. All other animals were 8 to 10 weeks old. All mice were housed in sterilize micro-isolator cages and were handled according to our institutional guidelines.
In vivo and in vitro G-CSF treatment
Recombinant human G-CSF (rhG-CSF) was purchased from Laboratórios Biosintética (São Paulo, SP, Brazil). For in vivo treatment, mice were treated with subcutaneous injections of rhG-CSF (1.2 μg/g body weight) daily for 5 days. For in vitro treatment, high-density granulocytes were cultured in RPMI (Sigma Chemical, St Louis, MO), with 10% FCS (Gibco, Carlsbad, CA) and 200 ng/mL rhG-CSF for 18 hours.
High- and low-density granulocytes
Bone marrow and spleen cell suspensions were submitted to Ficoll-Hypaque Plus (Sigma Chemical) density gradient separation (d = 1.077).27 High-density granulocytes (HDGs) were obtained from the lower fraction. For in vivo infusions, high-density cells obtained from bone marrow suspensions were used. Low-density granulocytes for in vitro or in vivo experiments were obtained after treatment of HDGs with G-CSF. Briefly, cells were cultured at a concentration of 5 × 106 per well in 24-well plates (Corning, Corning, NY) with 200 ng/mL G-CSF for 15 hours at 37°C in RPMI (Sigma Chemical) and with 10% FCS (Gibco). They were then submitted to a second Ficoll-Hypaque Plus (Sigma Chemical) gradient, and cells in the low-density fraction were isolated. For in vivo–obtained LDGs, spleen cells from G-CSF–treated mice were submitted to Ficoll-Hypaque Plus (Sigma Chemical) density gradient, and the low-density fraction was collected. Granulocyte analysis was performed by cytology and flow cytometry, as indicated.
Cytospin slide preparation
Cells (104) were cytocentrifuged over conventional glass slides and stained with the use of a Hemacolor Kit (Merck, Rio de Janeiro, Brazil) according to the manufacturer's protocols.
Cell culture for intracellular lymphokine detection
Mesenteric lymph node (MLN) cells were activated in vitro with 1 μg/mL anti-CD3 (clone 145.2C11) for 24 hours. After that, they were washed twice and plated at a final cell concentration of 2 × 106 cells per well in a 24-well plate (Corning). Preactivated MLN cells were cultured for 4 hours with 20 nM PMA (Calbiochem, San Diego, CA), 1 μM ionomycin (Calbiochem), and 3 μM monensin (Sigma Chemical) at 37°C, in 5% CO2 atmosphere, in the presence or absence of granulocytes (granulocyte-lymphocyte ratio, 5:1) and 1000 U/mL bovine catalase (Sigma Chemical), as indicated. After coculture of MLN cells and LDGs, lymphocytes were checked for viability using annexin V and PI.28
Intracellular staining
After in vitro stimulation, cells were fixed with 2% paraformaldehyde at 37°C for 5 minutes. After washing with PBS/2% normal mouse serum (NMS), cells were permeabilized with saponin 0.3% (Sigma Chemical) and incubated with CD5-FITC (BD PharMingen, San Diego, CA) and IFN-γ-PE (BD PharMingen) for 30 minutes at room temperature. Samples were then washed in the presence of saponin and resuspended in PBS/2% NMS for cytometric analysis (Cell Quest software; FACScalibur, BD PharMingen). Specificity controls were performed as previously described.23
Surface immunostaining
Cells were washed in ice-cold PBS and incubated in PBS/2% NMS for 5 minutes to block Fc receptors. PE–anti-CD3 or SCA1 (BD PharMingen), FITC–anti–Gr-1, anti-CD11b, or anti-CD14 (BD PharMingen) antibodies were incubated for 30 minutes on ice. Cells were washed twice in ice-cold PBS/2% NMS and subsequently collected using a FACScalibur (BD PharMingen) flow cytometer. All data were analyzed on Cell Quest software (BD PharMingen).
Detection of intracellular reactive oxygen species
To measure intracellular ROS, dihydrorhodamine 123 (DHR) (Invitrogen–Molecular Probes, Carlsbad, CA) was used. This is an uncharged, nonfluorescent dye that passively diffuses across most cell membranes, where it is oxidized to cationic green fluorescent rhodamine 123 by reaction with hydrogen peroxide. Hydrogen peroxide activity was measured after 5-minute incubation with 20 nM PMA plus 5-minute incubation with 45 μM DHR. LDGs and MLN cell coincubation was performed in the presence or absence of 1000 U/mL bovine catalase (Sigma Chemical), and lymphocyte ROS was identified with Cy-Chrome–anti-CD4/CD8 (BD PharMingen). All samples were collected using a FACScalibur (BD PharMingen) flow cytometer. All data were analyzed with Cell Quest software (BD PharMingen).
Cell depletion
For T cell–depleted BM (BMTD), BM cells (1 × 108) were incubated with anti-CD4 (GK1.5 hybridoma supernatant) and anti-CD8 (53-6.7 hybridoma supernatant) in RPMI (Sigma Chemical) with 20% rabbit serum (RS) as a source of complement for 30 minutes at 37°C. Cells were washed once and reincubated with RPMI/20% RS for another 30 minutes. After the incubation period, cells were washed and checked for the presence of CD3+ cells by flow cytometry. For granulocyte depletion, 1 × 108 spleen cells from rhG-CSF–treated donors were incubated with anti-Gr1 (20% of a Gr1 hybridoma supernatant) in RPMI/20% RS, as described for T cells. After depletion, cell suspensions were checked for the presence of granulocytes by cytologic analysis and flow cytometry. In both cases depletion reached 100% efficiency. For cytometric evaluation of cell depletion efficiency, Gr1-FITC (RB6-8C5 clone; BD PharMingen) was used at 250 μg/mL (100 times more than the concentration normally used to stain cells) to compete with unlabeled monoclonal antibody. Competition controls showed 100% staining of Gr1+ cells when compared with Gr1-FITC–stained cells only, in the absence of the unlabeled antibody (data not shown).
BMT and aGVHD induction
Hosts received filtered acidic drinking water (pH, 2.5-3.0) starting 2 weeks before irradiation and continuing for the next 4 weeks. One day before transplantation, (C57BL/6 × BALB/c) F1 recipients received 850 cGy total body irradiation (TH780C irradiator with a cobalt Co 60 [60Co] source). For all experiments, 5 × 106 BMTD cells plus 5 × 106 nonadherent nylon wool spleen (NWS)29 cells from C57BL/6 donors were injected intravenously into (C57BL/6 × BALB/c) F1 recipients. NWS cells were from normal donors (NWS) or donors treated for 5 days with rhG-CSF (G-NWS), depleted or not depleted of granulocytes with anti-Gr1 mAb and complement. In the different protocols, T-cell infusion was normalized based on CD3 counts. When indicated, HDGs or LDGs were coinjected with BMTD and NWS.
Assessment of GVHD
The severity of GVHD was assessed by the 3 most important parameters: weight loss, histopathology of terminal ileum, and death. Before irradiation, mice were tagged and weighed. After transplantation, their weights were evaluated every 2 days. Histopathologic examination was performed 20 days after transplantation. All samples were fixed with formalin and embedded in paraffin. Tissue sections were stained with hematoxylin and eosin and evaluated by light microscopy. For survival curves, mice were inspected daily.
Image acquisition
Images shown in Figures 1, 3, 4, and 5 were obtained with an Olympus BX-41 microscope (Olympus, Melville, NY) and 100 ×/1.4 NA, 40 ×/0.65 NA, and 20 ×/0.4 NA objectives. Photographs were taken with a digital Olympus C-7070/7.1 megapixel camera, using the HQ mode (3072 × 2304 width/height), and were saved as JPEG files. Images were processed with Adobe Photoshop CS software (Adobe Systems, San Jose, CA).
Statistical analysis
Results
In vivo and in vitro treatment with G-CSF generates low-density granulocytes in mice
To evaluate in vivo generation of low-density granulocytes (LDGs), C57BL/6 mice were treated for 5 days with subcutaneous injections of rhG-CSF. After that period, their splenocytes were centrifuged over a Ficoll-Hypaque density gradient. The low-density fraction was analyzed to evaluate the percentage of granulocytes by flow cytometry and cytologic examination. It can be seen in Figure 1A that 20% of all cells are granulocytes. In contrast, untreated animals have basically mononuclear cells (lymphocytes and monocytes, with virtually no granulocytes) in the low-density fraction (data not shown). These results are in accordance with our previous observations with human peripheral blood from G-CSF–treated donors.26 When these low-density cells are gated on SSC high-density cells, 90% are Gr1+ and do not stain for CD11b or CD14 (Figure 1A, lower panels). Of these, 25% are immature Gr1+SCA1+ and 72% are mature Gr1+SCA1- granulocytes (Figure 1D, right panel).
To access LDG generation after in vitro treatment with G-CSF, high-density granulocytes were obtained from spleen cell suspension as the high-density cell fraction after Ficoll-Hypaque gradient centrifugation. These high-density cells were cultivated with rhG-CSF for 15 hours, after which a second gradient was performed to obtain low-density cells. The low-density fraction recovered 90% of the cells from the starting population of HDGs and was composed of 95% granulocytes and completely devoid of erythrocytes. These data indicate that low-density granulocytes were not a minor subpopulation (Figure 1B). These in vitro–generated LDGs were Gr1+, CD11b-, and CD14- (Figure 1B, bottom panels). Similarly, 89% of LDGs were generated when high-density cells from bone marrow samples were treated with G-CSF in vitro (Figure 1C). More than 90% of the in vitro–generated LDGs were Gr1+ SCA1-, a phenotype compatible with mature cells (Figure 1D, left panel).
LDGs inhibit IFN-γ production by T cells in mice
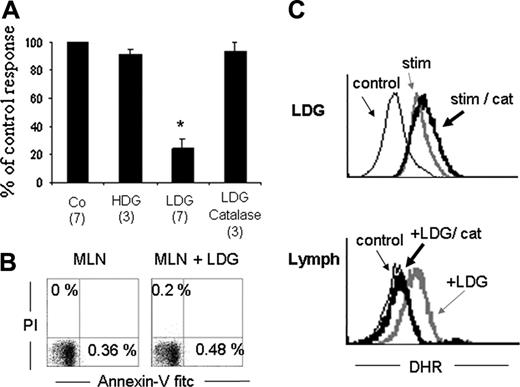
After confirming that in mice, high-density granulocytes (HDGs) turn into LDGs after G-CSF treatment, we sought to determine whether these LDGs could inhibit T-cell IFN-γ production.26 Mesenteric lymph node cells (MLNs) were isolated and activated for 15 hours with soluble anti-CD3. Activated T cells were then cocultured for 5 hours with LDGs, and intracellular detection of IFN-γ was studied by flow cytometry. Figure 2A shows that LDGs inhibit up to 80% of IFN-γ–producing cells, whereas no inhibitory effect was observed if T cells were cultured in the presence of HDGs. The inhibitory effect was not caused by a toxic effect of LDGs over T cells because no annexin V+ cells were detected after the culture period (Figure 2B). To confirm our previous data with human cells showing that LDGs inhibit IFN-γ T-cell production by a mechanism dependent on hydrogen peroxide, MLNs were cocultivated with LDGs in the presence of catalase.26 As can be seen, the inhibition of IFN-γ production induced by LDGs was reverted in the presence of catalase (Figure 2A). Catalase had no direct effect on IFN-γ production by T cells (data not shown).
In vivo and in vitro treatment with rhG-CSF generates LDGs. C57BL/6 mice were treated with subcutaneous rhG-CSF injections, and cytospin slides were prepared with the low-density fraction (Ficoll-Hypaque gradient) of spleen cells. Hematoxylin and eosin (H&E) staining (A, top panels, 40 × and 100 × magnification). Low-density cells were stained as indicated and analyzed by flow cytometry, gated or not on SSC × FSC plots, as indicated (A, bottom panels). High-density spleen cells from C57BL/6 mice were treated in vitro with rhG-CSF for 15 hours, after which they where passed over a Ficoll-Histopaque Plus density gradient, and the low-density fraction was collected and stained with H&E (B, top panels, 40 × and 100 × magnification) The same population was also stained with the indicated antibodies and analyzed by flow cytometry ungated population (B, bottom panels). Bone marrow HDCs were treated with G-CSF, after which, the low-density cells were obtained and stained with anti-Gr1 antibody. Data shown were obtained from the total, not the gated, population (C). BM LDGs generated in vitro (not gated) and spleen LDGs generated in vivo (gated on high SSC) were analyzed for the presence of immature cells using Sca-1– and Gr1-specific mAb (D). Results are representative of 5 different experiments.
In vivo and in vitro treatment with rhG-CSF generates LDGs. C57BL/6 mice were treated with subcutaneous rhG-CSF injections, and cytospin slides were prepared with the low-density fraction (Ficoll-Hypaque gradient) of spleen cells. Hematoxylin and eosin (H&E) staining (A, top panels, 40 × and 100 × magnification). Low-density cells were stained as indicated and analyzed by flow cytometry, gated or not on SSC × FSC plots, as indicated (A, bottom panels). High-density spleen cells from C57BL/6 mice were treated in vitro with rhG-CSF for 15 hours, after which they where passed over a Ficoll-Histopaque Plus density gradient, and the low-density fraction was collected and stained with H&E (B, top panels, 40 × and 100 × magnification) The same population was also stained with the indicated antibodies and analyzed by flow cytometry ungated population (B, bottom panels). Bone marrow HDCs were treated with G-CSF, after which, the low-density cells were obtained and stained with anti-Gr1 antibody. Data shown were obtained from the total, not the gated, population (C). BM LDGs generated in vitro (not gated) and spleen LDGs generated in vivo (gated on high SSC) were analyzed for the presence of immature cells using Sca-1– and Gr1-specific mAb (D). Results are representative of 5 different experiments.
LDGs inhibit T cell IFN-γ production in an H2O2-dependent manner. (A) MLN cells were activated with anti-CD3 mAb for 15 hours. Activated MLN cells were cultured alone (Co) or with HDGs, LDGs, or LDGs plus catalase (1000 U/mL) and stimulated with PMA, ionomycin, and monensin for the last 5 hours. Cells were stained with anti–IFN-γ and anti-CD5 to assess IFN-γ–producing T cells (CD5 gated). Results are expressed as percentage of control response. The percentage of IFN-γ–producing cells in the controls ranges from 9% to 17% of all T cells. Numbers in parentheses indicate the number of experiments. Bars represent standard error. *P < .05. (B) MLNs cultured or not cultured with LDGs, as in panel A, were assessed for viability with annexin V and PI staining. Data presented correspond to lymphocytes gated by SSC and FSC parameters. (C) Hydrogen peroxide intracellular content measured with DHR on LDGs (top panel) or lymphocytes (bottom panel). LDGs were cultured in the absence (stim) or presence of catalase (stim/cat) or were left unstained (control). Anti-CD4/CD8 gated, preactivated MLN cells were cultured alone (control), in the presence of LDGs without (LDG) or with (LDG/cat) catalase. Shown are histograms for LDGs alone or lymphocytes. Note that lymphocytes fluoresce with DHR only in the presence of LDGs.
LDGs inhibit T cell IFN-γ production in an H2O2-dependent manner. (A) MLN cells were activated with anti-CD3 mAb for 15 hours. Activated MLN cells were cultured alone (Co) or with HDGs, LDGs, or LDGs plus catalase (1000 U/mL) and stimulated with PMA, ionomycin, and monensin for the last 5 hours. Cells were stained with anti–IFN-γ and anti-CD5 to assess IFN-γ–producing T cells (CD5 gated). Results are expressed as percentage of control response. The percentage of IFN-γ–producing cells in the controls ranges from 9% to 17% of all T cells. Numbers in parentheses indicate the number of experiments. Bars represent standard error. *P < .05. (B) MLNs cultured or not cultured with LDGs, as in panel A, were assessed for viability with annexin V and PI staining. Data presented correspond to lymphocytes gated by SSC and FSC parameters. (C) Hydrogen peroxide intracellular content measured with DHR on LDGs (top panel) or lymphocytes (bottom panel). LDGs were cultured in the absence (stim) or presence of catalase (stim/cat) or were left unstained (control). Anti-CD4/CD8 gated, preactivated MLN cells were cultured alone (control), in the presence of LDGs without (LDG) or with (LDG/cat) catalase. Shown are histograms for LDGs alone or lymphocytes. Note that lymphocytes fluoresce with DHR only in the presence of LDGs.
To further characterize the mechanism by which LDGs inhibit T-cell activation, we next assessed the production of hydrogen peroxide by LDGs and its diffusion into lymphocytes. Dihydrorhodamine 123 (DHR) was used as an indicator of intracellular hydrogen peroxide content. DHR is a nonpolar, nonfluorescent substrate for hydrogen peroxide that becomes fluorescent after oxidation. LDGs were cultured with PMA in the presence of DHR, with or without catalase. Figure 2C shows that activated LDGs do contain hydrogen peroxide and that this content is not modified in the presence of catalase (top panel). On the other hand, lymphocytes only become fluorescent by oxidizing DHR when cultured in the presence of LDGs, suggesting that LDG-derived peroxide diffuses into the lymphocytes. These results are reinforced by the inhibitory action of catalase, which inhibits DHR oxidation, lowering the fluorescence to control levels on the lymphocyte population (lower panel) but not in the granulocytes (top panel).
Protective effect of G-CSF–treated spleen in the aGVHD experimental model is mediated by granulocytes
It has been previously shown that nonadherent NWS cells from G-CSF–treated mice were unable to induce severe aGVHD in the mouse model.19 To determine whether this inhibitory action was dependent on the in vivo–generated LDG, we took advantage of the C57BL/6 into (C57BL/6 × BALB/c)F1 aGVHD experimental model. The model consists of injecting BMTD cells together with NWS from C57BL/6 mice, as a source of alloreactive T cells, into lethally irradiated F1 hosts.
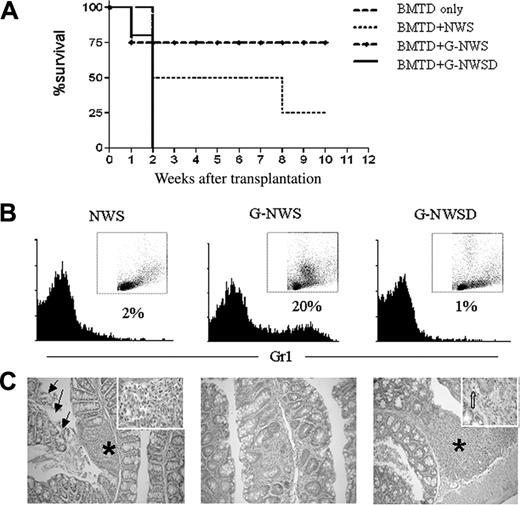
When tested in the in vivo model for aGVHD, G-NWS were not as potent as NWS in the induction of aGVHD, confirming previously published data.19 The percentage of surviving animals was higher (75%) in the recipients of G-NWS than in the recipients of NWS (20%) (Figure 3A). To determine whether the observed inhibition depended on granulocytes, we first checked the cellular composition of nonadherent purified NWS cells from control animals (NWS) and G-CSF–treated donors (G-NWS) (Figure 3B). Surprisingly, G-NWS contained 20% of Gr1+ cells, indicating that granulocytes were not completely retained in the nylon wool column. If in vivo G-CSF–treated granulocytes do indeed play a role in protecting G-NWS recipients from aGVHD, eliminating granulocytes from this cell population should abolish the observed protective effect. To this end, G-NWS cells were treated with anti-Gr1 mAb and complement to deplete granulocytes. Figure 3B shows that antibody and complement treatment completely eliminated Gr1+ cells, which had a high SSC profile. The G-NWS Gr1–depleted population (G-NWSD) was then used as aGVHD inducers. Indeed, the inhibitory effect was completely abolished, confirming a regulatory role of granulocytes on aGVHD (Figure 3A). Remarkably, more aggressive disease is induced by this protocol, with mortality rates reaching 100% within the first 2 weeks.
Protective effect of NWS from G-CSF–treated donors over aGVHD is abolished in the absence of granulocytes. (C57BL/6 × BALB/c) F1 mice were lethally irradiated and received T cell–depleted BM alone (BMTD only) or together with nylon wool spleen (NWS) cells, NWS cells from G-CSF–treated donor (G-NWS), or NWS cells from G-CSF–treated donor mice after granulocyte depletion (G-NWSD). (A) Percentages of surviving animals. Log rank: P = .05 between G-NWS and G-NWSD and between BMTD and G-NWS. (B) NWS from C57BL/6 control, G-NWS, or G-NWSD were stained with anti-Gr1 FITC, as indicated in “Materials and methods.” Inset: SSC × FSC dot plots of the same histogram samples (note the high SSC cells in G-NWS that are not present in G-NWSD). (C) Histopathology of the small intestine. Moderate inflammatory infiltrate in the lamina propria (asterisk and inset) associated with extensive epithelial cell sloughing (arrows) of NWS (left panel); villous atrophy and mild inflammatory infiltrate in lamina propria of G-NWS (middle panel) and marked mucosal atrophy, luminal cell debris, and accumulation of inflammatory cells in lamina propria (asterisk) of G-NWSD (right panel). Inset: Apoptotic crypt epithelial cell (broad arrow). Original magnification, 100 × (left panel); 200 × (middle and right panels); 1000 × (inset). Data are from 1 of 2 representative experiments (with 4 to 5 animals/group) for panels A and C and 1 of 5 representative experiments for panel B.
Protective effect of NWS from G-CSF–treated donors over aGVHD is abolished in the absence of granulocytes. (C57BL/6 × BALB/c) F1 mice were lethally irradiated and received T cell–depleted BM alone (BMTD only) or together with nylon wool spleen (NWS) cells, NWS cells from G-CSF–treated donor (G-NWS), or NWS cells from G-CSF–treated donor mice after granulocyte depletion (G-NWSD). (A) Percentages of surviving animals. Log rank: P = .05 between G-NWS and G-NWSD and between BMTD and G-NWS. (B) NWS from C57BL/6 control, G-NWS, or G-NWSD were stained with anti-Gr1 FITC, as indicated in “Materials and methods.” Inset: SSC × FSC dot plots of the same histogram samples (note the high SSC cells in G-NWS that are not present in G-NWSD). (C) Histopathology of the small intestine. Moderate inflammatory infiltrate in the lamina propria (asterisk and inset) associated with extensive epithelial cell sloughing (arrows) of NWS (left panel); villous atrophy and mild inflammatory infiltrate in lamina propria of G-NWS (middle panel) and marked mucosal atrophy, luminal cell debris, and accumulation of inflammatory cells in lamina propria (asterisk) of G-NWSD (right panel). Inset: Apoptotic crypt epithelial cell (broad arrow). Original magnification, 100 × (left panel); 200 × (middle and right panels); 1000 × (inset). Data are from 1 of 2 representative experiments (with 4 to 5 animals/group) for panels A and C and 1 of 5 representative experiments for panel B.
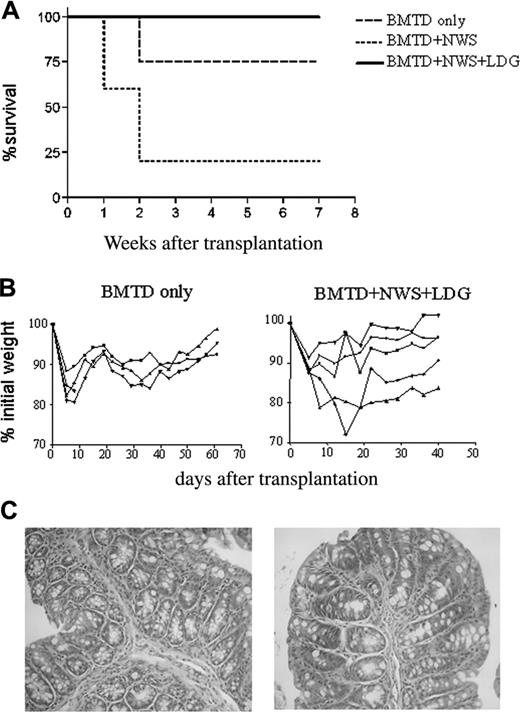
In vitro–generated LDGs completely inhibit acute GVHD. (C57BL/6 × BALB/c) F1 mice were lethally irradiated, reconstituted with BMTD only, BMTD plus NWS, or NWS+LDG. (A) Percentages of surviving animals. Log rank: P = .05 between BMTD and NWS and P = .01 between NWS+LDG and NWS. (B) Weight loss for BMTD and BMTD+NWS+LDG groups. Twice a week, mice were weighed and the results plotted as the percentage of initial weight. (C) Histopathology of the small intestine. Mucosal atrophy with preservation of simple columnar epithelium of the villi are observed without significant inflammatory infiltrates in lamina propria in right and left panels. Crypts exhibit regenerative changes, with typical goblet. Original magnifications, 100 × (left panel), 200 × (right panel). Results are representative of 2 experiments with 4 to 5 mice per group.
In vitro–generated LDGs completely inhibit acute GVHD. (C57BL/6 × BALB/c) F1 mice were lethally irradiated, reconstituted with BMTD only, BMTD plus NWS, or NWS+LDG. (A) Percentages of surviving animals. Log rank: P = .05 between BMTD and NWS and P = .01 between NWS+LDG and NWS. (B) Weight loss for BMTD and BMTD+NWS+LDG groups. Twice a week, mice were weighed and the results plotted as the percentage of initial weight. (C) Histopathology of the small intestine. Mucosal atrophy with preservation of simple columnar epithelium of the villi are observed without significant inflammatory infiltrates in lamina propria in right and left panels. Crypts exhibit regenerative changes, with typical goblet. Original magnifications, 100 × (left panel), 200 × (right panel). Results are representative of 2 experiments with 4 to 5 mice per group.
In addition, histopathologic data of the small intestine show villous atrophy and mild inflammatory infiltrates in G-NWS recipients compared with NWS control mice (Figure 3C). When granulocytes were depleted from the G-NWS transplantation product (G-NWSD), more severe disease was observed in the histologic analysis with marked mucosal atrophy, luminal cell debris, and accumulation of inflammatory infiltrate in the lamina propria (Figure 3C).
Granulocytes treated in vitro with G-CSF inhibit aGVHD
Considering that in vivo G-CSF–treated granulocytes had indeed provided protection from aGVHD, we next sought to determine whether administration of in vitro–generated LDGs would also efficiently inhibit acute disease. We treated high-density granulocytes in vitro with G-CSF, recovered the cells in the low-density fraction, and coinjected them with NWS from healthy donors (NWS+LDG). According to our prediction, the NWS+LDG group showed 100% survival, whereas BMTD and control NWS displayed, respectively, 75% and 25% survival rates (Figure 4A). Because the survival of animals treated with granulocytes was not in itself an indication of complete disease inhibition, we examined weight loss as a clinical parameter. An initial decrease in weight was seen as the result of the conditioning protocol. However, NWS+LDG mice started gaining weight 15 days after transplantation, though not as fast as the BM control group (Figure 4B). After 2 months, they had completely recovered to their initial weights and survived up to 11 months (data not shown). More impressive, histopathologic analysis clearly correlated with the survival curve and clinical data—that is, animals that received G-CSF–treated granulocytes showed a pattern similar to that of control animals (recipients receiving BMTD only) with preservation of simple columnar epithelium without significant inflammatory infiltrates (Figure 4C).
Inhibitory effect of granulocytes depends on low-density granulocytes and major histocompatibility complex granulocyte/T-cell compatibility
Granulocytes are widely used in the clinical setting to treat neutropenia. They are obtained as HDGs from unrelated donors, requiring compatibility only for the ABO blood group but not for major histocompatibility complex (MHC) antigens. We tested whether HDGs or third-party MHC-incompatible granulocytes could inhibit aGVHD. HDGs were obtained from C57BL/6 bone marrow, as described in “Materials and methods.” As third-party LDGs, HDGs from bone marrow of A/J (H-2a) mice were obtained, treated with rhG-CSF in vitro, and purified as the low-density fraction after Ficoll-Hypaque gradient centrifugation.
As shown earlier, no major histopathologic abnormalities were observed when LDGs syngeneic to the donor C57BL/6 NWS were infused (Figure 5A). However, when allogeneic third-party LDGs from A/J mice were used, histopathologic signs of aGVHD were easily detected (Figure 5B). The inability of the MHC-incompatible granulocytes to inhibit aGVHD could be a consequence of recognition and destruction of these granulocytes by the C57BL/6 allogeneic T cells and not a need for cooperation under MHC restriction. In addition, syngeneic HDGs could not inhibit aGVHD when cotransplanted with NWS (Figure 5C). These results strongly suggest that the protective effect depends on low-density granulocytes and could not be exerted by non–G-CSF treated high-density cells.
Inhibition of GVHD by granulocytes requires activation and histocompatibility. Irradiated (C57BL/6 × BALB/c) F1 receptors mice received BMTD and T cells from C57BL/6 mice plus LDGs from C57BL/6 mice (A), allogeneic third-party LDGs from A/J mice (B), or non–G-CSF–treated HDGs from C57BL/6 mice (C). After 22 days the animals were killed and the intestines were submitted to histopathologic analysis. Intense inflammatory foci in lamina propria is observed in A/J LDGs (B) and HDGs recipient (C) with mononuclear cells (B, inset) and mixed inflammatory infiltrate (*C, inset) associated with crypt cell apoptosis (arrows). Original magnifications, 40 × (A-C); 400 × (B, inset); 1000 × (C, inset). Results are representative of 1 of 5 experiments.
Inhibition of GVHD by granulocytes requires activation and histocompatibility. Irradiated (C57BL/6 × BALB/c) F1 receptors mice received BMTD and T cells from C57BL/6 mice plus LDGs from C57BL/6 mice (A), allogeneic third-party LDGs from A/J mice (B), or non–G-CSF–treated HDGs from C57BL/6 mice (C). After 22 days the animals were killed and the intestines were submitted to histopathologic analysis. Intense inflammatory foci in lamina propria is observed in A/J LDGs (B) and HDGs recipient (C) with mononuclear cells (B, inset) and mixed inflammatory infiltrate (*C, inset) associated with crypt cell apoptosis (arrows). Original magnifications, 40 × (A-C); 400 × (B, inset); 1000 × (C, inset). Results are representative of 1 of 5 experiments.
Discussion
Patients who undergo PBSCT receive 10 times more T lymphocytes than those who undergo BMT. Somewhat unexpected are the clinical reports showing that the incidence of aGVHD is the same in both groups of patients.1,5,9,11 The fact that T cells present in PBSCT are not as efficient in causing acute disease as the ones present in bone marrow infusion had pointed to a possible immunoregulatory role for G-CSF over the adaptive immune system. Indirect effects, probably mediated by monocytes13-16 and dendritic cells,17 were hypothesized either as a diminution of T-cell function mediated by monocytes16,22 or as immune deviation in response to DC2 stimulation.17,19 In addition, we have shown that PBMCs from G-CSF–treated donors are enriched in LDGs that copurify with mononuclear cells26 and are inhibitory for T-cell lymphokine secretion in in vitro studies.24,26 We asked whether the LDGs could really be responsible for the low incidence of aGVHD observed in PBSCT in comparison with BMT considering the numbers of T cells infused and, if so, whether they could be used as immune modulators.
To determine the answers, it was necessary to translate into the mouse system the results previously obtained from human donors.26 We did this by treating mice in vivo with rhG-CSF and by activating granulocytes in vitro with the same drug. In all parameters analyzed, mouse and human cells behaved similarly: (1) the low-density fraction of spleens from G-CSF–treated donors were enriched in granulocytes (LDGs); (2) high-density granulocytes (HDGs) turned into low-density cells (LDGs) after in vitro treatment with G-CSF; (3) LDGs inhibited IFN-γ production by T cells in vitro; and (4) inhibition was reverted by catalase. After ensuring that mouse and human cells responded similarly to G-CSF, we set the classic parent (C57BL/6) into the (C57BL/6 × BALB/c) F1 aGVHD experimental model. Using this same model, other authors19,20 had shown that nonadherent NWS cells from G-CSF–treated donors (G-NWS) were not as potent aGVHD inducers as control NWS. G-NWS contains approximately 20% of Gr1+ cells, confirming the presence of granulocytes and suggesting that these populations could be responsible for the observed protection in G-NWS recipients in contrast to NWS recipients. Differences in the number of T cells infused could be ruled out because all infusions were immunophenotyped with anti-CD3, and the actual number of T cells was calculated and normalized before infusion.
We propose that mature low-density granulocytes, generated in response to G-CSF treatment, are actually the cells responsible for the inhibitory effect. In fact, disease protection is abolished when granulocytes are depleted from G-NWS infusion. The increase in disease severity, confirmed by histopathologic examination, was surprising. This effect is perhaps the result of the elimination of a small number of granulocytes normally present in the nonadherent NWS cells. It had been shown in an in vitro proliferative assay that spleen granulocytes are able to inhibit T-cell responses.30 It is known that a fraction of monocytes directly related to inflammatory activity are Gr1+.31 However, we do not believe that Gr1+ monocytes played an important role in our system because monocytes were retained in the nylon wool column and no Gr1+ CD14+ cells could be detected in the experimental system used.
To confirm the immunosuppressive effect of G-CSF–activated granulocytes over aGVHD, we asked whether in vitro G-CSF–treated granulocytes could protect from acute disease. In fact, when in vitro–generated LDGs were injected together with NWS, there was no sign of acute disease, either by clinical or histopathologic examination. This effect was dependent on LDGs; inhibition was not observed with HDGs not treated with G-CSF. Moreover, in the absence of histocompatibility, no inhibition was observed. The need for histocompatibility was probably related to impaired survival of allogeneic LDGs in the presence of residual host and the infused donor T cells and did not depend on MHC restriction on the interaction between granulocytes and T cells.
Others32-34 have shown an immunosuppressive role of immature granulocytes. In our experiments this did not seem to be the case. The inhibitory LDGs used were mature granulocytes; only a small number (less than 2%) were SCA1+Gr1+ progenitors. Moreover, the LDGs did not express myelomonocytic progenitors markers such as CD11b or exclusive monocyte markers such as CD14.35-37 These data indicate that the LDGs reported herein are indeed mature granulocytes.
The mechanism by which LDGs inhibit the development of aGVHD must reside in the first phase of the disease, as defined by Ferrara and Antin.38 The first phase is defined as T-cell independent but important in determining the second phase, in which T-cell activation takes place. We know that the half-life of G-CSF–treated granulocytes is not longer than 48 hours26,39 and that T-cell activity is reverted on granulocyte elimination or peroxide inhibition.24,26 In vivo, after 48 hours, the granulocyte inhibitory effect is diminished and T cells are then licensed to become effectors. However, it has been shown that the injection of T cells 4 days after irradiation and bone marrow infusion results in a 60% decrease in disease incidence.40 This can be interpreted as a fail-safe time frame in which the animals can still recover from the acute inflammation generated by the conditioning regimen. Moreover, it had been shown that the intensity of the initial inflammation, measured as the amount of TNF-α detected in the serum, is directly proportional to disease severity.41,42 As a parallel, in our case, the inhibition of T-cell activity by G-CSF–treated granulocytes for the initial 2 to 3 days should have been enough to constrain the disease. Given that immune cells will migrate to inflammatory sites,43 granulocytes and T cells should colocalized, ensuring the local effect in the mucosal surfaces.
The role of commensal flora and mucosal immunity is well established in aGVHD. In humans, polymorphism of NOD2/CARD15, an important molecule in mucosal immunity and inflammation in epithelial cells and monocytes, was shown to be an important risk factor for aGVHD and an independent variable when 1-year transplantation-related mortality (TRM) was evaluated.44 In experimental models, LPS translocation leading to macrophage activation had been shown as extremely important in the initiation of aGVHD.41,42,45 T-cell activation in MLNs and Peyer patches was shown to occur early after transplantation,46 and Peyer patches were reported as essential to the activation of GVHD-specific T cells.47 Intestinal microflora alterations by probiotic microorganisms had been shown beneficial to aGVHD, diminishing the number of bacterial translocations to MLNs and the incidence and severity of disease.48 LDGs could act on the intestinal microflora, avoiding translocation of high numbers of intestinal bacteria into the host and contributing to the inhibition of the “first-phase” inflammation. When intestinal damage is not evident, increases in activated granulocyte numbers should not be beneficial. In fact, in donor lymphocyte infusion (DLI), the use of infusion products from G-CSF–treated donors does not show any effect on GVHD development,49 and the conditioning regimen is mild and does not lead to extensive mucosal damage.
Studies of BMT in humans have shown that the incidence of aGVHD is lower in patients who undergo transplantation with G-CSF–primed BM than in patients who undergo the standard BMT protocols in the absence of priming.2,50 Although these reports do not address the presence of LDGs in primed human bone marrow infusion, it is plausible that the inhibitory effect observed in the clinical trials was the result of the presence of G-CSF–treated granulocytes in the infusion, which would inhibit aGVHD but not cGVHD.2,50 Fortunately, this inhibitory effect present in the PBSCT and primed BMT did not seem to affect the graft-versus-leukemia effect because higher relapse rates had not been reported in either case.
Whatever the mechanisms by which the LDGs inhibit aGVHD, our data support the interpretation that it operates during the early phase of disease and that it could guide new protocols based on the use of LDGs as a tool for inhibiting aGVHD.
Prepublished online as Blood First Edition Paper, October 25, 2005; DOI 10.1182/blood-2005-08-3239.
Supported by grants from Conselho Nacional de Desenvolvimento Cientifico e Technológico (CNPq), Fundaçao Carlos Chagas Filho de Amparo à Pesquisa do Estado do Rio de Janeiro (FAPERJ), and Fundaçao Ary Frauzino/Instituto Nacional de Câncer (FAF/INCA). R.B.A. is an Undergraduate Fellow supported by the 2005 Programa Aristides Pacheco Leão, Academia Brasileira de Ciências, Brazil.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
We thank Dr Daniel Tabak for suggestions and critical reading of the manuscript and Dr Mary Evelyn Flowers for discussions and encouragement. We also thank Dr Décio Lerner and Dr Rita de Cássia Tavares for discussions about GVHD in human patients. Finally, we thank all members of Bonomianos Group and MEDEX/INCa for suggestions and discussions, especially João Paulo B. Monteiro for critical review.



This feature is available to Subscribers Only
Sign In or Create an Account Close Modal