In multiple myeloma (MM), both vascular endothelial (VEGF) and basic fibroblast growth factor (bFGF) promote tumor growth and survival. We have used the novel indolinone BIBF 1000 to study effects of simultaneous inhibition of VEGF, FGF and transforming growth factor-β on MM cells and their interactions with bone marrow stroma cells (BMSCs). Both, in the absence and presence of myeloma-stroma cell contacts, BIBF 1000 abrogated BMSC-derived secretion of interleukin-6 (IL-6). In addition, BIBF 1000 directly induced apoptosis in t(4;14)–positive cell lines as well as in CD138+ marrow cells from patients with t(4;14) myeloma. To a similar extent, BIBF 1000 induced apoptosis in MM.1S and MM.1R cells carrying the translocation t(14;16). In case of MM.1S and other dexamethasone-sensitive t(14;16) cell lines, BIBF 1000 and dexamethasone had additive proapoptotic effects. Induction of apoptosis by BIBF 1000 was associated with inhibition of the mitogen-activated protein kinases (MAPK) pathway in t(4;14) and inhibition of the phosphatidyl-inositol-3 kinase/AKT pathway in t(14;16) cells. Apoptotic effects did not occur in t(4;14)–or t(14;16)–positive MM cells carrying n- or k-Ras mutations. The data provide the rationale for clinical evaluation of this class of targeted kinase inhibitors in MM with focus on defined cytogenetic subgroups.
Introduction
A new generation of targeted antineoplastic agents has opened perspectives to more selective treatment of hematologic malignancies and solid cancers.1 These agents interfere with oncogenic signaling events intrinsic to the tumor cell or with survival, expansion, and spreading signals arising from the tumor environment. Along the lines of such biologically based therapies, considerable progress has been made in the treatment of multiple myeloma (MM), a malignant plasma cell disorder still considered incurable despite recent treatment advances. Novel agents in MM therapy include thalidomide, its immunomodulatory derivative CC-5013 (lenalidomide, Revlimid), the proteasome inhibitor bortezomib (Velcade, formerly PS-341), and arsenic trioxide. These drugs not only have marked antimyeloma activity but also appear suited to overcome classic drug resistance.2,3
Small molecule tyrosine kinase inhibitors represent a separate class of targeted drugs. As an outstanding example of their therapeutic potential, imatinib mesylate (Gleevec, formerly STI571) has proven remarkably effective in the treatment of chronic myeloid leukemia by inhibition of the Abl kinase that is deregulated as a consequence of the oncogenic Bcr/Abl gene fusion.4 Related compounds, designated receptor tyrosine kinase inhibitors (RTKIs), hold promise as antineoplastic agents by interference with receptor-mediated extrinsic tumor growth and survival signals. RTKIs with different receptor specificities are currently under investigation in various malignancies.
In MM, evidence accumulated over the past years has provided a solid rationale for the evaluation of RTKIs targeting receptors of vascular endothelial growth factor (VEGF) and of basic fibroblast growth factor (bFGF). Both angiogenic cytokines are expressed and secreted by myeloma cells5-7 and contribute to myeloma-associated bone marrow neovascularization.8,9 They both have been shown to stimulate bone marrow stroma cells (BMSCs) to produce interleukin-6 (IL-6),6,7 an important growth and survival factor for human MM.10-12 In turn, IL-6 enhances the production and secretion of VEGF and bFGF by myeloma cells.6,7 Besides these paracrine loops optimizing the micromilieu for MM tumors, VEGF stimulates myeloma cell proliferation and migration in an autocrine manner.13,14 Circumstantial evidence also suggests an autocrine circuit for bFGF.7 In addition, constitutively activated mutant FGF receptor 3 (FGFR3) in MM cells carrying the translocation t(4;14) functions as an oncogene that contributes to tumor progression in experimental models.15-17 Finally and most recently, it has been demonstrated that frequent overexpression of the c-maf oncogene in MM enhances VEGF secretion in cocultures of myeloma cells and BMSCs.18 Taken together, these data suggest a pivotal role for VEGF and bFGF as survival and expansion factors in human MM.
We have used a novel RTKI, the indolinone derivative BIBF 1000, that competitively binds to the ATP binding sites within the kinase domains of VEGFR1 through VEGFR3, FGFR1, FGFR3, and PDGFRα to study in vitro effects of the inhibition of VEGF and bFGF signaling on MM cells and their stromal microenvironment. The study was also aimed at assessing the antimyeloma activity of BIBF 1000 in MM cell lines with translocations of the immunoglobulin heavy-chain (IgH) locus leading to dysregulation of the oncogenic partner regions FGFR3/MMSET at 4p16.3 and c-maf at 16q23, respectively.15,19
Patients, materials, and methods
Patients
Bone marrow samples from patients with active MM and peripheral blood samples from healthy volunteers were studied. Patients and volunteers gave written informed consent prior to the sampling procedure. Bone marrow aspirate or peripheral blood sampling study protocol was approved by the Institutional Review Board of the University of Muenster.
Reagents
A 0.2 M stock solution of the investigational small molecule inhibitor BIBF 1000 synthesized in a medicinal chemistry program was prepared in DMSO. To obtain adequate working concentrations, the stock solution was diluted with ddH2O containing at least 0.005% DMSO20 (Hell-Pourmojib M, Tontsch-Grunt U, Roth G, et al., BIBF 1000: a potent orally active angiogenesis inhibitor with long lasting inhibition of the VEGFR-2 signaling pathway and broad antitumor efficacy that induces regression of established tumors, publication submitted). Dexamethasone (Dex) was purchased from Sigma-Aldrich (Munich, Germany). The phosphatidylinositol-3 kinase (PI-3K) inhibitor Ly292004 and the mitogen-activated protein kinases (MAPK) inhibitor PD98059 were obtained from Calbiochem (San Diego, CA). The pan-caspase inhibitor z-VAD-FMK was delivered by Enzyme Systems (Livermore, CA). Recombinant human VEGF165, VEGF121, transforming growth factor-β (TGF-β), tumor necrosis factor-α (TNF-α), interleukin-1β (IL-1β), IL-6, and neutralizing anti-bFGF and anti-VEGF antibodies were purchased from R&D Systems (Wiesbaden, Germany). Basic FGF was supplied from Roche Diagnostics (Mannheim, Germany). The carboxy-fluorescein diacetate succinimidyl ester (CFSE), a fluorescent impermanent membrane dye, was obtained from Renovar (Madison, WI). Annexin-V–FITC, Apo2.7, and propidium iodide (PI) were purchased from BD Sciences/Clontech (San Jose, CA), anti–CD38-phycoerythrin/cyanin5 from Coulter-Immunotech (Hamburg, Germany), and anti–CD138-fluorescein isothiocyanate from Serotec (Oxford, United Kingdom). Murine monoclonal antibodies against human CD54, CD68, CD31, and thrombomodulin came from DAKO (Glostrup, Denmark) and were used for phenotypic characterization of BMSCs. Rabbit polyclonal antibodies raised against KDR, phospho-MAPK (p44/42), phospho-STAT3 (Tyr705), and phospho-AKT (Ser473) as well as antiphosphotyrosine antibodies (mouse monoclonal anti–p-Tyr-100 and rabbit anti–p85 PI3-K binding motif, respectively) and the corresponding goat anti–rabbit- or goat anti–mouse-horseradish peroxidase conjugated secondary antibodies were obtained from Cell Signaling Technology (Beverly, MA). Rabbit polyclonal FGFR3 raised against a recombinant protein corresponding to amino acids 25 to 124 of the extracellular receptor domain was supplied from Santa Cruz Biotechnologies (Santa Cruz, CA). Monoclonal anti–β-actin (clone AC-15) came from Sigma (St Louis, MO) and 3H-thymidine (5.0 Ci [1.85 × 1010 Bq]/mmol, 1.0 mCi [37 MBq]/mL) from Amersham (Bucking-hamshire, United Kingdom).
Tyrosine kinase assays
The inhibitory activity and specificity of BIBF 1000 was determined in biochemical assays measuring autophosphorylation of receptor tyrosine kinases of VEGF receptors (VEGFRs), FGFRs, platelet-derived growth factor receptor α (PDGFRα), epidermal growth factor rector (EGFR), human epidermal growth factor receptor-2 (HER-2), insulin receptor (InsR), insulin growth factor receptor (IGFR), and hepatocyte growth factor receptor (HGFR). In addition, inhibition of autophosphorylation of the cyclin-dependent kinases CDK1, CDK2, and CDK4 by BIBF 1000 was determined. The assays were performed using recombinant clones of baculovirus contructs containing GST fusion proteins of the cytoplasmatic kinase domains of these receptors20 (Mojgan Hell-Pourmojib, Frank Hilberg, Gerald Roth, Armin Heckel, Jakobus C. A. van Meel, publication submitted, December 2005).
Immunofluorescent labeling and cell sorting
Bone marrow mononuclear cells (MNCs) from patients with active MM were separated by density gradient centrifugation using Ficoll-Paque at 1172g for 20 minutes (Pharmacia, Uppsala, Sweden). CD38high/CD138+ plasma cells were isolated from the marrow MNC fraction by fluorescence-activated cell sorting using a FacsVantage (Becton Dickinson). On reanalysis, sorted myeloma cell populations had a purity of at least 95% for the CD38high/CD138+ phenotype. CD19+/CD14- B lymphocytes (PBBLs) for control experiments were sorted from peripheral blood MNCs of healthy volunteers. CellQuest pro software (BD Sciences) was used for flow cytometric analyses.
Cell cultures
The human myeloma-derived cell lines RPMI-8226, U-266, OPM-2, NCI-H929, L363, and JJN-3 were obtained from the German Collection of Microorganisms and Cell Cultures (DSMZ, Braunschweig, Germany). KMS-11 and KMS-18 were kindly provided by T. Otsuki, Kawasaki Medical School, Okayama, Japan, the cell lines MM.1S and MM.1R by Nancy L. Krett, Robert H. Lurie Comprehensive Cancer Center, Chigago, IL, the cell lines OCI-My5 and UTMC-2 by Leif Bergsagel, Mayo Clinic, Scottsdale, AZ, and ANBL-6 by M. Chatterjee, Max-Delbrueck Zentrum, Berlin-Buch, Berlin, Germany. Cell lines and CD38high/CD138+-sorted marrow myeloma cells from patients were cultured in Roswell Park Memorial Institute (RPMI) 1640 medium with 10% fetal calf serum (FCS; Gibco-BRL, Eggenstein, Germany). Cultures of BMSCs from patients with MM (MM-BMSCs) were established from the MNC fraction of marrow aspirates according to the method of Lagneaux et al21 with minor modifications as described6,7 and maintained in MEM-alpha medium (Gibco-BRL). All culture media were supplemented with 100 U/mL penicillin, 100 μg/mL streptomycin (Biochrom, Berlin, Germany), and 2 mM l-glutamine (Gibco-BRL). Cultures were maintained at 37°C and 5% CO2.
For cytokine secretion experiments, BMSCs in passages 2 to 4 were grown in 24-well plates. Prior to stimulation with exogenous cytokines or exposure to BIBF 1000, confluent layers were starved for 12 hours (1% FCS). During the stimulation period, BMSCs were kept in serum-free conditions.
Cocultures were established using a transwell system (pore size 0.4 μm; Nunc, Wiesbaden, Germany) precluding direct cell-cell contacts, with myeloma cells seeded in the inserts at a density of 2 × 105/mL and confluent BMSCs growing on the bottom of the plates. Contact cocultures were established by directly seeding myeloma cells (2 × 105/mL) onto confluent BMSCs.
Cytokine stimulation of cell cultures and exposure to BIBF 1000
BMSC monocultures were stimulated with recombinant human VEGF165 (50 ng/mL), VEGF121 (50 ng/mL), bFGF (10 ng/mL), TGF-β (10 ng/mL), TNF-α (10 ng/mL), or IL-1β (10 ng/mL) for 72 hours in serum-free conditions with or without BIBF 1000 (0.5 μM). Transwell and contact cocultures of BMSCs and myeloma cells were run in the absence or presence of BIBF 1000 (0.125-1.0 μM, 72 hours) under serum-free conditions.
IL-6 assay
Cell culture supernatants were analyzed for IL-6 using a commercial enzyme-linked immunosorbent assay (ELISA; Quantikine; R&D Systems) with a lower detection limit of 0.7 pg/mL. Calibration curves were prepared by dilution of the IL-6 standard provided by the manufacturer. Concentrations of IL-6 are presented as picogram per milliliter corrected for 105 cells.
RNA preparation and cDNA synthesis
Total RNA was prepared using the guanidine isothiocyanate/phenol method19 (Trizol Reagent; Invitrogen, Life Technologies, Karlsruhe, Germany). Complementary DNA (cDNA) was synthesized for 1 hour at 37°C, using 1 μg total RNA, 40 U/μL RNA guard (Amersham, Pharmacia, Piscataway, NJ), 100 pmol/μL random hexamers (Amersham), 200 U M-MLV reverse transcriptase (Gibco BRL, Life Technologies, Karlsruhe, Germany), 5 × first-strand buffer (250 mM Tris-HCl, 375 mM KCl, 15 mM MgCl2, and 0.1 M DTT), 80 mM dNTPs (Amersham), and 1 μg/mL bovine serum albumin (BSA; Serva, Heidelberg, Germany).
Analysis of N-Ras and K-RAS mutations
The cDNA from myeloma cells enriched by fluorescent cell sorting was amplified by polymerase chain reaction with primers spanning the mutation hotspot codons 12, 13, and 61. The following primers were used: N-RAS forward, TTT CCC GGT CTG TGG TCC TAA AT; N-RAS reverse, CTT CGC CTG TCC TCA TGT ATT GG; K-RAS forward, CGG CTC GGC CAG TAC TCC; and K-RAS reverse, TCT TGC TAA GTC CTG AGC CTG TTT. Polymerase chain reaction (PCR) products were verified by agarose gel electrophoresis and purified. Direct cycle sequencing was performed for both strands using the same primers as for PCR reaction.
Fluorescence in situ hybridization analysis
Fluorescence in situ hybridization (FISH) was used to examine the samples for deletions of the chromosome region 13q14.3 or monosomy 13 as well as for rearrangements of the IgH gene locus. FISH was performed according to the manufacturer's instructions using commercially available probes. All probes were purchased from Abbott (Wiesbaden, Germany). The following probes have been used: LSI D13S319 (13q14.3) SpectrumOrange TM Probe, LSI 13q34 Spectrum GreenTM Probe, LSI IGH Dual Color Break Apart Rearrangement Probe, and LSI IGH/FGFR3 Dual Color Dual Fusion Translocation Probe.
Immunoblotting of receptors and signaling molecules
BMSCs were starved for 4 hours and subsequently exposed to VEGF165 (50 ng/mL), bFGF (10 ng/mL), TGF-β (10 ng/mL), TNF-α (10 ng/mL), or IL-1β (10 ng/mL) for 2 hours in the absence or presence of BIBF 1000 (39 nM to 20 μM) or the MAPK inhibitor PD98059 (20 μM). Cultured myeloma cells were starved for 4 hours prior to stimulation with IL-6 (10 ng/mL) in the absence or presence of BIBF 1000 (39 nM to 20 μM) for 5 minutes.
Thereafter, cells were harvested, washed 3 times in PBS, and lysed by RIPA-buffer (150 mM NaCl, 1% Nonidet P-40, 0.5% deoxycholate, 0.1% SDS, 50 mM Tris base, pH 8.0) supplemented with a protease inhibitor mix (phenyl-methyl-sulfonyl fluoride, leupeptin, aprotinin, β-mercaptoethanol; Boehringer Mannheim, Mannheim, Germany) and the phosphatase inhibitors NaF (25 nM; Sigma) and NaVO4 (2.5 nM; Sigma). Protein content in lysates was measured by a modified Bradford assay.22,23 Prior to electrophoresis, proteins were denaturated and boiled in Laemmli buffer.24 Protein samples were electrophoretically separated on 8% SDS-polyacrylamide gels. Prestained molecular weight markers (Bio-Rad, Munich, Germany) served for protein size control. Separated proteins were transferred from the gels onto PVDF membranes (Millipore, Billerica, MA) by a semidry blotting procedure with 0.8 mA/cm2 current for 2 hours. Subsequently, membranes were blocked with 2% milk powder (Roth, Karlsruhe, Germany) followed by hybridization at 4°C overnight using the following primary antibodies: anti-KDR, anti-phosphotyrosine, anti–phospho-MAPK (p44/p42), anti–phospho-STAT3 (Tyr705), anti–phospho-AKT (Ser473), and anti-FGFR3, respectively. Anti-β actin served as control for equal protein loading. Membranes were then incubated with the corresponding species-specific horseradish-peroxidase (HRP)–linked secondary antibodies. Finally, blots were soaked in Luminol reagent (Santa Cruz Biotechnologies) and exposed to autoradiography films (BML-1; Kodak, Stuttgart, Germany).
For immunoprecipitation of VEGFR2 (KDR), 200 μL cell lysates was incubated with anti-KDR overnight at 4°C followed by addition of protein G agarose beads for 12 hours. Samples were centrifuged, washed in lysis buffer, resuspended in sample buffer, and subsequently separated by electrophoresis. Immunoprecipitates were blotted onto PVDF membranes and hybridized with the corresponding anti-phosphotyrosine antibody. Blot signals were quantified densitometrically using BioRad Quantity One Software (Version 4.5.1).
Quantification of apoptotic cells
Myeloma cell lines were collected after 24 hours of exposure to BIBF 1000 (0.125-0.5 μM), dexamethasone (10-5 M), IL-6 (10 ng/mL), and z-VAD-FMK (100 μM), respectively, either alone or in combinations. Cells were washed in PBS, resuspended in binding buffer containing 10 mM HEPES/NaOH, 140 mM NaCl, 2.5 mM CaCl2, pH 7.4, and stained with fluoroisothiocyanate (FITC)–coupled Annexin V and PI.25 Because of the limited amount of CD138+ patient myeloma cells collected, in vitro short-term exposure (up to 24 hours) was performed only with BIBF 1000 (0.5 μM) and, if possible, with dexamethasone (10-5 M), IL-6 (10 ng/mL), or their combinations.
Flow cytometric analysis for quantification of apoptotic cells was performed using a Becton Dickinson FACSCalibur (BD Sciences). CellQuest pro software (BD Sciences) was applied for flow cytometric analyses. Data are presented as dot plots or histograms of at least 10 000 counted events per sample.
In cultured BMSCs, apoptosis was quantified by an Apo2.7 assay.26 Cells were detached from the bottom of the culture plates by short-term incubation in Versen solution (10 mM EDTA, 137 mM NaCl, 2.6 mM KCl, 8.1 mM NaH2PO4, 1.4 mM KH2PO4, 1.1 mM glucose), subsequently fixed in 1% paraformaldehyde, permeabilized, and stained with phycoerythrin (PE)–conjugated Apo2.7 antibody and costained with CD56-FITC.
Proliferation assay
Myeloma cells (5 × 104 per well) were seeded onto 96-well plates, pulsed with 0.625 μCi (0.023 MBq) 3H-thymidine per well and cultured in RPMI-1640 in the absence or presence of BIBF 1000 for 72 hours. Thereafter, cells were harvested, and 3H-thymidine incorporation was measured in a Microbeta 1450 counter (Wallac, Turku, Finland). All samples were assayed in triplicates. 3H-thymidine uptake was normalized to controls for each cell line.
Myeloma cell adhesion assay
Myeloma cells were labeled with CFSE (5 μM) in serum-free PBS for 5 minutes. Intracellular esterases are known to convert diffusible CFSE to an impermanent membrane-bound fluorescent dye by cleavage of acetate groups. CFSE is not transferred to adjacent cells.27 After intensive washing in RPMI 1640 with 10% FCS, CFSE-labeled myeloma cells (2 × 106 cells/well) were added to confluent monolayers of 105 unstained BMSCs. Adhesion cocultures were incubated in the absence or in the presence of increasing concentrations of BIBF 1000 for 24 hours. Subsequently, nonadherent cells were washed off 3 times, and the remaining adherent myeloma and stroma cells were trypsinized. Ratios of the adherent CFSE-stained myeloma cells and unstained BMSCs were quantified by flow cytometry.
Statistics
Data other than immunoblotting results are presented as individual data plots or as means plus or minus standard errors (SEs). Statistical analyses were done with SPSS package, version 12.0 (SPSS Science, Chicago, IL). Statistical significance of overall differences between multiple groups was analyzed by the Kruskal-Wallis 1-way analysis of variance. If the test was significant, pairwise comparisons were done by the multiple-comparisons' criterion. Differences between 2 independent groups were analyzed by the Mann-Whitney rank sum test. The Wilcoxon matched-pair signed rank test was used for comparison of differences within pairs.28 A P value of .05 or less was considered significant.
Results
Kinase inhibition by BIBF 1000
As determined in biochemical kinase assays and described in detail elsewhere20 (Hell-Pourmojib M, Tontsch-Grunt U, Roth G, et al., publication submitted), BIBF 1000, a novel small molecule RTKI, effectively targets VEGFR1 through VEGFR3, FGFR1 and FGFR3 as well as PDGFRα. If at all, the receptor tyrosine kinase activities of EFGR, HER-2, IGFR-1, InsR, HGFR, as well as CDK1, CDK2, and CDK4 are not substantially inhibited by BIBF 1000. The inhibitory concentration of 50% (IC50) values for the respective receptor tyrosine kinases are listed in Table 1.
Receptor tyrosine kinases inhibited by BIBF 1000
Receptor tyrosine kinase . | IC50BIBF 1000, nM . |
|---|---|
| VEGFR1 | 40 |
| VEGFR2 | 28 |
| VEGFR3 | 142 |
| FGFR1 | 43 |
| FGFR3 | 52 |
| PDGFRα | 35 |
| EGFR | > 50 000 |
| HER-2 | > 10 000 |
| IGFR1 | > 10 000 |
| InsR | > 10 000 |
| HGFR | > 10 000 |
| CDK1 | > 10 000 |
| CDK2 | > 10 000 |
| CDK4 | > 10 000 |
Receptor tyrosine kinase . | IC50BIBF 1000, nM . |
|---|---|
| VEGFR1 | 40 |
| VEGFR2 | 28 |
| VEGFR3 | 142 |
| FGFR1 | 43 |
| FGFR3 | 52 |
| PDGFRα | 35 |
| EGFR | > 50 000 |
| HER-2 | > 10 000 |
| IGFR1 | > 10 000 |
| InsR | > 10 000 |
| HGFR | > 10 000 |
| CDK1 | > 10 000 |
| CDK2 | > 10 000 |
| CDK4 | > 10 000 |
Inhibition of VEGFR2 and FGFR3 phosphorylation in cell cultures
We have previously shown that BMSCs express the angiogenic growth factor receptor VEGFR2 (KDR/Flk-1) and that receptor binding of VEGF121 and VEGF165 stimulates IL-6 release from marrow stroma cells.6 Therefore, we first tested inhibition of VEGF-dependent VEGFR2 phosphorylation by BIBF 1000 in cultures of BMSCs. Ligand-induced phosphorylation of the receptor was abrogated by BIBF 1000 at micromolar concentrations and substantially inhibited in the nanomolar concentration range (Figure 1A).
To further confirm the receptor specificity of BIBF 1000, we also studied its effects on FGFR3 tyrosine kinase phosphorylation. For these experiments, we chose the myeloma cell line OPM-2 known to overexpress the constitutively active K650E mutation of FGFR3.16 As illustrated in Figure 1B, autophosphorylation of the tyrosine kinase domain was inhibited by BIBF 1000 in a dose-dependent manner and at concentrations comparable to those inhibiting VEGFR2 phosphorylation.
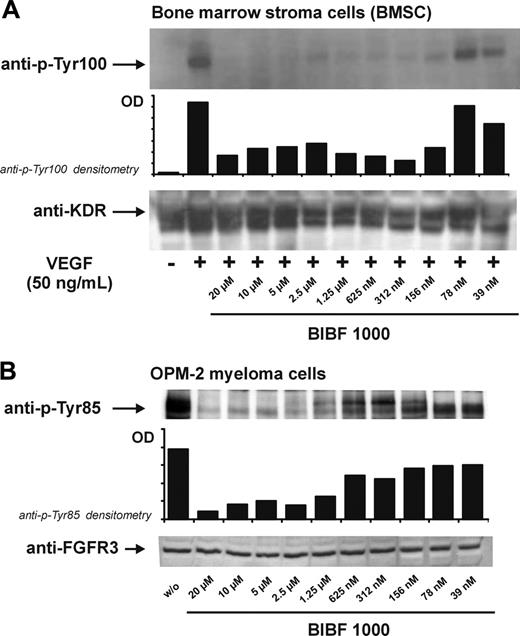
Inhibition of VEGFR2 (KDR) and FGFR3 phosphorylation by BIBF 1000. (A) Cultured marrow stroma cells were exposed to VEGF (50 ng/mL) in the presence of increasing concentrations of BIBF 1000 for 5 minutes. Dose-dependent abrogation of VEGFR2 phosphorylation (top: anti–p-Tyr100 immunoblots, densitometry) and corresponding immunoblots of total VEGFR2 (bottom) are shown. (B) OPM-2 cells known to express a constitutively active FGFR3 mutant were incubated with increasing concentrations of BIBF 1000 for 5 minutes. FGFR3 phosphorylation was inhibited in a dose-dependent manner (top: anti–p-Tyr85 immunoblots, densitometry). Bottom panel shows corresponding immunoblots of total FGFR3.

Inhibition of VEGFR2 (KDR) and FGFR3 phosphorylation by BIBF 1000. (A) Cultured marrow stroma cells were exposed to VEGF (50 ng/mL) in the presence of increasing concentrations of BIBF 1000 for 5 minutes. Dose-dependent abrogation of VEGFR2 phosphorylation (top: anti–p-Tyr100 immunoblots, densitometry) and corresponding immunoblots of total VEGFR2 (bottom) are shown. (B) OPM-2 cells known to express a constitutively active FGFR3 mutant were incubated with increasing concentrations of BIBF 1000 for 5 minutes. FGFR3 phosphorylation was inhibited in a dose-dependent manner (top: anti–p-Tyr85 immunoblots, densitometry). Bottom panel shows corresponding immunoblots of total FGFR3.
Effects of BIBF 1000 on BMSC-derived IL-6 release and related inhibition of MAPK phosphorylation
Besides VEGF and bFGF,6,7 other cytokines secreted by myeloma cells, including TGF-β, TNF-α, and IL-1β, are known to induce IL-6 release from BMSCs.29-33 This paracrine IL-6 release protects myeloma cells from apoptosis, promotes myeloma cell proliferation and survival, and confers drug resistance.10-12,34,35 In the presence of BIBF 1000 (0.5 μM), IL-6 secretion from cultured BMSCs under basal conditions and, as expected, on exposure to either VEGF121, VEGF165 or bFGF was significantly inhibited (P < .001, Figure 2A). In addition, we found that the 4-fold increased stromal IL-6 release induced by TGF-β was also strongly antagonized by BIBF 1000 (n = 4; P < .001; Figure 2B). In contrast, IL-6 release induced by TNF-α or IL-1β was not significantly reduced by BIBF 1000 (Figure 2B). The data suggest that, in addition to the targeted receptor tyrosine kinases, BIBF 1000 also interferes with paracrine TGF-β–mediated effects in the myeloma microenvironment. The viability of BMSCs was not affected by BIBF 1000 in the nanomolar concentration range (data not shown).
Because MAP kinases are final effectors of VEGF and bFGF signaling to the nucleus,36-38 secondary effects of BIBF 1000 on MAPK (p44/42) phosphorylation in BMSCs were evaluated next. Inhibition of MAPK phosphorylation by the study drug was detectable in unstimulated cultures (not shown) and was most marked in BMSCs stimulated with either VEGF or bFGF. A similar effect was observed in control experiments with the MAPK inhibitor PD98059 (Figure 2Ci,ii,vi). In BMSCs exposed to TGF-β, BIBF 1000 turned out to be almost equally potent, inhibiting MAPK phosphorylation (Figure 2Ciii). Although Sma and Mad related proteins (SMADs) are the preferred substrates and signal transducers of TGF-β receptors, there is ample evidence indicating that, in line with our observation, TGF-β may also signal through MAPK pathways.39 Consistent with the results on stimulation of IL-6 release (Figure 2A, 2B), MAPK phosphorylation in BMSCs exposed to TNF-α or IL-1β was distinctly less sensitive to equimolar concentrations of BIBF 1000 (Figure 2Civ-v). Taken together, these observations suggest that the MAPK pathway is involved in IL-6 production of marrow stroma cells induced by VEGF, bFGF, and TGF-β and that in addition to VEGF- and bFGF-, also TGF-β–induced MAPK phosphorylation can be inhibited by BIBF 1000.
![Figure 2. Effects of BIBF 1000 on stroma-derived interleukin-6 (IL-6) release and related inhibition of MAPK phosphorylation. (A-B) Effects of BIBF 1000 on bone marrow stroma cell (BMSC)–derived IL-6 release: BIBF 1000 (0.5 μM) significantly inhibited basal IL-6 release from BMSCs and IL-6 secretion induced by VEGF121, VEGF165, bFGF, and TGF-β (105.3 ± 28.1 versus 37.3 ± 16.1 [vehicle control versus BIBF 1000]; 169.2 ± 23.4 versus 45.3 ± 16.6 [VEGF121 versus VEGF121 + BIBF 1000]; 172.0 ± 22.3 versus 60.1 ± 21.9 [VEGF165 versus VEGF165 + BIBF 1000]; 185.1 ± 17.3 versus 44.7 ± 14.3 [bFGF versus bFGF + BIBF 1000]; 499.5 ± 33.8 versus 195.7 ± 36.7 3 [TGF-β versus TGF-β + BIBF 1000], respectively; P < .001). In contrast, IL-6 release on exposure to TNF-α or IL-1β was not significantly reduced (754.7 ± 122.6 versus 547.4 ± 83.0 [TNF-α versus TNF-α + BIBF 1000]; 1881.7 ± 122.3 versus 1855.8 ± 122.9 [IL-1β versus IL-1β + BIBF 1000]). IL-6 concentrations were determined in triplicates from supernatants of BMSC cultures derived from different patients with myeloma (n = 4). BMSCs were starved prior to incubation with either VEGF121, VEGF165, bFGF, TGF-β, TNF-α, or IL-1β ± BIBF 1000 and kept in serum-free conditions for 72 hours. Data are presented as means ± SE. The Wilcoxon test was used to identify differences between controls and corresponding BIBF 1000–treated BMSCs (means ± SE). (C) Inhibition of MAPK phosphorylation by BIBF 1000 in BMSCs: subconfluent BMSCs were starved for 4 hours in serum-free medium and subsequently exposed for 2 hours to either VEGF (50 ng/mL), bFGF (10 ng/mL), TGF-β (10 ng/mL), TNF-α (10 ng/mL) or IL-1β (10 ng/mL) in the absence or presence of BIBF 1000 (1, 5, or 10 μM), to BIBF alone (10 μM) or to the MAPK-inhibitor PD98059 (20 μM). Proteins were extracted, boiled, and separated by SDS-electrophoresis. Subsequent immunoblotting was performed using a rabbit polyclonal p-MAPK (p44/42) antibody and a corresponding goat anti–rabbit HRP–conjugated secondary antibody. Notably, inhibition of MAPK phosphorylation by BIBF 1000 was most effective in BMSCs exposed to VEGF, bFGF, or TGF-β. The results shown are representative of 3 independent experiments.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/107/5/10.1182_blood-2004-11-4250/2/m_zh80050692090002.jpeg?Expires=1761677921&Signature=fMb66zCEDjZdt6FQARBaONvQQRW2cGU9fGmdHMbMIJE1-Eh5T9cQ1ufIoayXuKWfOfHiiAo-GNoOQEz0WcmLqi02bNmSsh26RA6waw9~Ftx52AxcsVsZIxpxuhUcRkvDdiIKyD1yhLJuYWm9UrXngYBGFF2ixV49Znb5d0SM-eFW53DtxZIPwOKxAY4XHmqI-pvUvq6~mGwOaqjXnVF1CE7LzkYhyi31quczXV7MXoQbgN64QM21AUrCAqWhrPFTF5-DqzEis87HJf7NxFi3R2Ijb94YBnjj9ZGQBe92x9MU7npy9jLrx8zu-BjoCK3RdXMJPgplV45arMC7pn9IvQ__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
Effects of BIBF 1000 on stroma-derived interleukin-6 (IL-6) release and related inhibition of MAPK phosphorylation. (A-B) Effects of BIBF 1000 on bone marrow stroma cell (BMSC)–derived IL-6 release: BIBF 1000 (0.5 μM) significantly inhibited basal IL-6 release from BMSCs and IL-6 secretion induced by VEGF121, VEGF165, bFGF, and TGF-β (105.3 ± 28.1 versus 37.3 ± 16.1 [vehicle control versus BIBF 1000]; 169.2 ± 23.4 versus 45.3 ± 16.6 [VEGF121 versus VEGF121 + BIBF 1000]; 172.0 ± 22.3 versus 60.1 ± 21.9 [VEGF165 versus VEGF165 + BIBF 1000]; 185.1 ± 17.3 versus 44.7 ± 14.3 [bFGF versus bFGF + BIBF 1000]; 499.5 ± 33.8 versus 195.7 ± 36.7 3 [TGF-β versus TGF-β + BIBF 1000], respectively; P < .001). In contrast, IL-6 release on exposure to TNF-α or IL-1β was not significantly reduced (754.7 ± 122.6 versus 547.4 ± 83.0 [TNF-α versus TNF-α + BIBF 1000]; 1881.7 ± 122.3 versus 1855.8 ± 122.9 [IL-1β versus IL-1β + BIBF 1000]). IL-6 concentrations were determined in triplicates from supernatants of BMSC cultures derived from different patients with myeloma (n = 4). BMSCs were starved prior to incubation with either VEGF121, VEGF165, bFGF, TGF-β, TNF-α, or IL-1β ± BIBF 1000 and kept in serum-free conditions for 72 hours. Data are presented as means ± SE. The Wilcoxon test was used to identify differences between controls and corresponding BIBF 1000–treated BMSCs (means ± SE). (C) Inhibition of MAPK phosphorylation by BIBF 1000 in BMSCs: subconfluent BMSCs were starved for 4 hours in serum-free medium and subsequently exposed for 2 hours to either VEGF (50 ng/mL), bFGF (10 ng/mL), TGF-β (10 ng/mL), TNF-α (10 ng/mL) or IL-1β (10 ng/mL) in the absence or presence of BIBF 1000 (1, 5, or 10 μM), to BIBF alone (10 μM) or to the MAPK-inhibitor PD98059 (20 μM). Proteins were extracted, boiled, and separated by SDS-electrophoresis. Subsequent immunoblotting was performed using a rabbit polyclonal p-MAPK (p44/42) antibody and a corresponding goat anti–rabbit HRP–conjugated secondary antibody. Notably, inhibition of MAPK phosphorylation by BIBF 1000 was most effective in BMSCs exposed to VEGF, bFGF, or TGF-β. The results shown are representative of 3 independent experiments.
![Figure 2. Effects of BIBF 1000 on stroma-derived interleukin-6 (IL-6) release and related inhibition of MAPK phosphorylation. (A-B) Effects of BIBF 1000 on bone marrow stroma cell (BMSC)–derived IL-6 release: BIBF 1000 (0.5 μM) significantly inhibited basal IL-6 release from BMSCs and IL-6 secretion induced by VEGF121, VEGF165, bFGF, and TGF-β (105.3 ± 28.1 versus 37.3 ± 16.1 [vehicle control versus BIBF 1000]; 169.2 ± 23.4 versus 45.3 ± 16.6 [VEGF121 versus VEGF121 + BIBF 1000]; 172.0 ± 22.3 versus 60.1 ± 21.9 [VEGF165 versus VEGF165 + BIBF 1000]; 185.1 ± 17.3 versus 44.7 ± 14.3 [bFGF versus bFGF + BIBF 1000]; 499.5 ± 33.8 versus 195.7 ± 36.7 3 [TGF-β versus TGF-β + BIBF 1000], respectively; P < .001). In contrast, IL-6 release on exposure to TNF-α or IL-1β was not significantly reduced (754.7 ± 122.6 versus 547.4 ± 83.0 [TNF-α versus TNF-α + BIBF 1000]; 1881.7 ± 122.3 versus 1855.8 ± 122.9 [IL-1β versus IL-1β + BIBF 1000]). IL-6 concentrations were determined in triplicates from supernatants of BMSC cultures derived from different patients with myeloma (n = 4). BMSCs were starved prior to incubation with either VEGF121, VEGF165, bFGF, TGF-β, TNF-α, or IL-1β ± BIBF 1000 and kept in serum-free conditions for 72 hours. Data are presented as means ± SE. The Wilcoxon test was used to identify differences between controls and corresponding BIBF 1000–treated BMSCs (means ± SE). (C) Inhibition of MAPK phosphorylation by BIBF 1000 in BMSCs: subconfluent BMSCs were starved for 4 hours in serum-free medium and subsequently exposed for 2 hours to either VEGF (50 ng/mL), bFGF (10 ng/mL), TGF-β (10 ng/mL), TNF-α (10 ng/mL) or IL-1β (10 ng/mL) in the absence or presence of BIBF 1000 (1, 5, or 10 μM), to BIBF alone (10 μM) or to the MAPK-inhibitor PD98059 (20 μM). Proteins were extracted, boiled, and separated by SDS-electrophoresis. Subsequent immunoblotting was performed using a rabbit polyclonal p-MAPK (p44/42) antibody and a corresponding goat anti–rabbit HRP–conjugated secondary antibody. Notably, inhibition of MAPK phosphorylation by BIBF 1000 was most effective in BMSCs exposed to VEGF, bFGF, or TGF-β. The results shown are representative of 3 independent experiments.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/107/5/10.1182_blood-2004-11-4250/2/m_zh80050692090002.jpeg?Expires=1761677921&Signature=fMb66zCEDjZdt6FQARBaONvQQRW2cGU9fGmdHMbMIJE1-Eh5T9cQ1ufIoayXuKWfOfHiiAo-GNoOQEz0WcmLqi02bNmSsh26RA6waw9~Ftx52AxcsVsZIxpxuhUcRkvDdiIKyD1yhLJuYWm9UrXngYBGFF2ixV49Znb5d0SM-eFW53DtxZIPwOKxAY4XHmqI-pvUvq6~mGwOaqjXnVF1CE7LzkYhyi31quczXV7MXoQbgN64QM21AUrCAqWhrPFTF5-DqzEis87HJf7NxFi3R2Ijb94YBnjj9ZGQBe92x9MU7npy9jLrx8zu-BjoCK3RdXMJPgplV45arMC7pn9IvQ__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
Effects of BIBF 1000 on stroma-derived interleukin-6 (IL-6) release and related inhibition of MAPK phosphorylation. (A-B) Effects of BIBF 1000 on bone marrow stroma cell (BMSC)–derived IL-6 release: BIBF 1000 (0.5 μM) significantly inhibited basal IL-6 release from BMSCs and IL-6 secretion induced by VEGF121, VEGF165, bFGF, and TGF-β (105.3 ± 28.1 versus 37.3 ± 16.1 [vehicle control versus BIBF 1000]; 169.2 ± 23.4 versus 45.3 ± 16.6 [VEGF121 versus VEGF121 + BIBF 1000]; 172.0 ± 22.3 versus 60.1 ± 21.9 [VEGF165 versus VEGF165 + BIBF 1000]; 185.1 ± 17.3 versus 44.7 ± 14.3 [bFGF versus bFGF + BIBF 1000]; 499.5 ± 33.8 versus 195.7 ± 36.7 3 [TGF-β versus TGF-β + BIBF 1000], respectively; P < .001). In contrast, IL-6 release on exposure to TNF-α or IL-1β was not significantly reduced (754.7 ± 122.6 versus 547.4 ± 83.0 [TNF-α versus TNF-α + BIBF 1000]; 1881.7 ± 122.3 versus 1855.8 ± 122.9 [IL-1β versus IL-1β + BIBF 1000]). IL-6 concentrations were determined in triplicates from supernatants of BMSC cultures derived from different patients with myeloma (n = 4). BMSCs were starved prior to incubation with either VEGF121, VEGF165, bFGF, TGF-β, TNF-α, or IL-1β ± BIBF 1000 and kept in serum-free conditions for 72 hours. Data are presented as means ± SE. The Wilcoxon test was used to identify differences between controls and corresponding BIBF 1000–treated BMSCs (means ± SE). (C) Inhibition of MAPK phosphorylation by BIBF 1000 in BMSCs: subconfluent BMSCs were starved for 4 hours in serum-free medium and subsequently exposed for 2 hours to either VEGF (50 ng/mL), bFGF (10 ng/mL), TGF-β (10 ng/mL), TNF-α (10 ng/mL) or IL-1β (10 ng/mL) in the absence or presence of BIBF 1000 (1, 5, or 10 μM), to BIBF alone (10 μM) or to the MAPK-inhibitor PD98059 (20 μM). Proteins were extracted, boiled, and separated by SDS-electrophoresis. Subsequent immunoblotting was performed using a rabbit polyclonal p-MAPK (p44/42) antibody and a corresponding goat anti–rabbit HRP–conjugated secondary antibody. Notably, inhibition of MAPK phosphorylation by BIBF 1000 was most effective in BMSCs exposed to VEGF, bFGF, or TGF-β. The results shown are representative of 3 independent experiments.
Abrogation of IL-6 secretion by BIBF 1000 in cocultures of myeloma and marrow stroma cells
In the bone marrow microenvironment, myeloma cells induce IL-6 secretion from stroma cells through both cytokine-mediated paracrine mechanisms and cellular adhesion.6,7,29,40-42 Effects of BIBF 1000 on myeloma cell–induced IL-6 release from BMSCs were therefore studied in both transwell and contact cocultures. In both types of cocultures, IL-6 secretion was significantly higher than in monocultures of BMSCs and myeloma cells, respectively. BIBF 1000 (0.125-1.0 μM) inhibited IL-6 secretion in a dose-dependent manner in both transwell and contact cocultures of BMSCs and myeloma cell lines or myeloma cells purified from bone marrow of patients with MM (Figure 3).
Effects of BIBF 1000 on myeloma cell adhesion
It has been demonstrated that adhesion of myeloma cells to BMSCs through surface molecule interactions mediates homing, drug resistance, and protection of the malignant cells from apoptosis.43-45 Furthermore, myeloma cell adhesion induces transcription and secretion of antiapoptotic growth and survival factors such as insulin growth factor (IGF), VEGF, or IL-6.40,41,46 We therefore examined the influence of BIBF 1000 on adhesion of myeloma cell lines to cultured BMSCs. We found very significant and sensitive inhibition of stromal adherence for several myeloma cell lines, including U-266 and t(4;14)–positive OPM-2 and KMS-11. In contrast, adhesion of k-Ras–mutated RPMI-8226 cells to BMSCs was resistant to BIBF 1000 (Figure 4). The results suggest that decreased myeloma cell adhesion may contribute to the observed inhibition of IL-6 secretion by BIBF 1000 in contact cocultures with BMSCs. However, the data obtained with RPMI-8226 demonstrate that targeting paracrine signaling through VEGF, bFGF, and TGF-β even in the presence of maintained myeloma-stroma cell adherence is sufficient for relevant inhibition of IL-6 release in the myeloma microenvironment (compare Figure 3G).
Inhibition of myeloma cell proliferation
To further characterize in vitro activities of BIBF 1000, we also studied its effects on myeloma cell proliferation using a 3H-thymidine uptake assay. As illustrated in Figure 5, there was a dose-dependent inhibition of proliferation over the nanomolar concentration range of BIBF 1000. However, it was again evident that the sensitivity of myeloma cell lines varied. Although RPMI-8226, JJN-3, and ANBL-6 cells were relatively resistant (Figure 5B; white circle, gray-filled circle, and gray-filled square), U-266 and L-363 showed intermediate sensitivity with 30% to 50% inhibition of proliferation in the presence of 0.5 to 1.0 μM BIBF 1000 (Figure 5C; black-filled circle and black-filled square). Notably, most marked effects on proliferation were observed in the t(4;14)–positive, FGFR3-overexpressing KMS-11, OPM-2, and NCI-H929 cells15,16,47 (Figure 5A) and in the t(14;16)–positive, VEGFR1-expressing MM.1S and MM.1R cells (Figure 5B; black-filled circle and black-filled square).13 In these cell lines, proliferation was inhibited by 70% or greater at 1.0 μM BIBF 1000 (P < .001 for each cell line versus control without BIBF 1000, Kruskal-Wallis test).
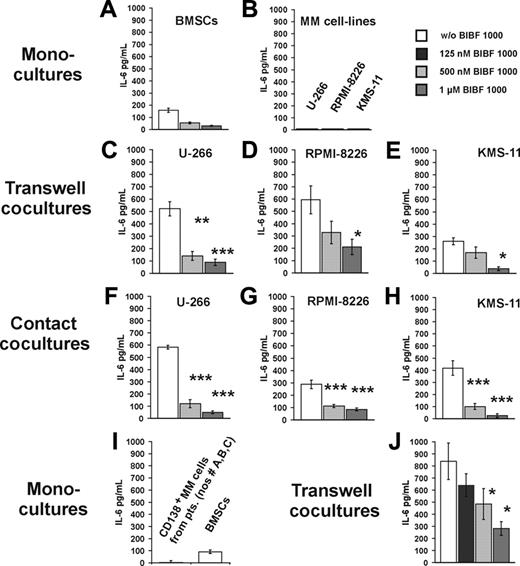
IL-6 secretion in transwell and contact cocultures of BMSCs and MM cells is significantly inhibited by BIBF 1000. (A) Control experiments in BMSC monocultures showed dose-dependent inhibition of IL-6 release by BIBF 1000 (0.5 μM). (B) Basal IL-6 secretion by myeloma cell lines ranged near or below the detection limit of the assay. (C-H) As compared with BMSC monocultures, IL-6 secretion was increased in both transwell (C-E) and contact (F-H) cocultures of BMSCs with U-266, RPMI-8226, or KMS-11 cells. Exposure to BIBF 1000 (0.125-1.0 μM) resulted in almost complete abrogation of IL-6 secretion in both types of cocultures. (I-J) In transwell cocultures of BMSCs and patient-derived CD138+-sorted MM cells, a similar increase in IL-6 secretion as compared with the respective monocultures and its dose-dependent inhibition by BIBF 1000 was observed. IL-6 concentrations were determined in serum-free supernatants of 72-hour cultures. Samples were measured in triplicates, and the results are presented as means ± SEs of at least 3 independent experiments. Significance of group differences was analyzed by the Mann-Whitney rank sum test. *P < .05, **P < .005, ***P < .001 versus controls without BIBF 1000.

IL-6 secretion in transwell and contact cocultures of BMSCs and MM cells is significantly inhibited by BIBF 1000. (A) Control experiments in BMSC monocultures showed dose-dependent inhibition of IL-6 release by BIBF 1000 (0.5 μM). (B) Basal IL-6 secretion by myeloma cell lines ranged near or below the detection limit of the assay. (C-H) As compared with BMSC monocultures, IL-6 secretion was increased in both transwell (C-E) and contact (F-H) cocultures of BMSCs with U-266, RPMI-8226, or KMS-11 cells. Exposure to BIBF 1000 (0.125-1.0 μM) resulted in almost complete abrogation of IL-6 secretion in both types of cocultures. (I-J) In transwell cocultures of BMSCs and patient-derived CD138+-sorted MM cells, a similar increase in IL-6 secretion as compared with the respective monocultures and its dose-dependent inhibition by BIBF 1000 was observed. IL-6 concentrations were determined in serum-free supernatants of 72-hour cultures. Samples were measured in triplicates, and the results are presented as means ± SEs of at least 3 independent experiments. Significance of group differences was analyzed by the Mann-Whitney rank sum test. *P < .05, **P < .005, ***P < .001 versus controls without BIBF 1000.
Induction of apoptosis in t(4;14) myeloma cells and related inhibition of the MAPK pathway
In line with the subgroup-selective effects on proliferation, we also observed significant induction of apoptosis by BIBF 1000 (0.5 μM) in the t(4;14) FGFR3-mutated cell lines KMS-11, KMS-18, OPM-2, and UTMC-2 (P < .05; Table 2).47 Additive effects on induction of apoptosis were shown when coincubating KMS-11 and OPM-2 with BIBF 1000 and dexamethasone (P ≤ .05; Table 2). As detailed in Table 2 and Figure 6, a series of t(4;14)–positive myeloma patients was also found to be sensitive to induction of apoptosis by BIBF 1000 (Table 2; Figure 6). BIBF 1000-induced apoptosis in t(4;14) myeloma cells was dose dependent, completely inhibited by the pan-caspase inhibitor z-VAD (Figure 7A) and tended to be partially antagonized by IL-6 (Table 2). Proapoptotic effects by BIBF 1000 did not occur in n-Ras–mutated t(4;14)–positive NCI-H929 (Table 2). In control experiments with L-363 and U-266 cells (Table 2; Figure 7A) as well as peripheral blood CD19+ B lymphocytes (Table 2) and CD19- lymphocytes (data not shown) from healthy donors, no proapoptotic effects of BIBF 1000 were detected. FGFR3 overexpression in OPM-2 and KMS-11 is illustrated in Figure 7B. As shown for OPM-2 and KMS-11 cells in Figure 7C-D, BIBF 1000 at concentrations effectively inducing apoptosis (0.5 μM) inhibited MAPK phosphorylation in these t(4;14) myeloma cells. MAPK phosphorylation was almost completely abrogated in the absence and partially inhibited in the presence of exogenous IL-6 in the case of OPM-2 cells. The latter finding is in line with partial prevention of BIBF 1000–induced apoptosis by IL-6 (Table 2). Indeed, the IL-6–related JAK/STAT-3 signaling cascade was not affected by BIBF 1000 (Figure 7C). Consistent with these findings on apoptosis induction by BIBF 1000, MAPK phosphorylation was not altered in n-Ras–mutated NCI-H929 (Figure 7E) or U-266 cells (Figure 7F) by the novel indolinone BIBF 1000.
Induction of apoptosis by BIBF 1000 in MM cells
. | . | . | . | Apoptotic cells . | . | . | . | . | . | . | . | . | . | . | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| MM cell . | Karyotypic abnormalities . | FGFR3 +++ . | ras mutation . | Without BIBF 1000, % . | 500 nM BIBF 1000, % . | Increase over vehicle control, % . | P* . | 500 nM BIBF 1000 + IL-6, % . | P† . | 10 μM Dex, % . | P‡ . | 500 nM BIBF 1000 + 10 μM Dex, % . | Increase over Dex, % . | P§ . | ||||||||||
| KMS-11 | t(4;14), t(14;16) | + (Y373C) | — | 17.8 ± 3.4 | 41.9 ± 8.0 | 24.0 ± 4.8 | .011 | 33.6 ± 5.8 | NS | 22.8 ± 6.1 | NS | 52.2 ± 17.9 | 29.4 ± 7.8 | .025 | ||||||||||
| KMS-18 | t(4;14) | + (G384D) | — | 10.2 ± 2.5 | 37.5 ± 14.3 | 27.3 ± 10.7 | .008 | 31.8 ± 12.1 | NS | NA | NA | NA | ||||||||||||
| OPM-2 | t(4;14) | + (K650E) | — | 13.2 ± 3.5 | 39.1 ± 8.2 | 25.9 ± 6.2 | .026 | 24.0 ± 5.3 | NS | 26.0 ± 5.8 | NS | 70.4 ± 15.1 | 44.4 ± 9.7 | .05 | ||||||||||
| UTMC-2 | t(4;14) | + (wt) | — | 32.2 ± 3.6 | 44.8 ± 2.2 | 12.6 ± 5.9 | .035 | NA | 49.5 ± 1.5 | .018 | 58.6 ± 1.3 | 9.1 ± 3.0 | NS | |||||||||||
| NCI-H929 | t(4;14) | + (wt) | + N13 | 26.2 ± 2.3 | 30.4 ± 2.9 | 4.2 ± 1.4 | NS | NA | 33.5 ± 2.5 | .043 | 41.4 ± 4.0 | 7.8 ± 2.5 | NS | |||||||||||
| Pt 1∥ | t(4;14) | + | — | 21.6 | 32.5 | 10.9 | 26.8 | 28.9 | 40.7 | 11.8 | ||||||||||||||
| Pt 2∥ | t(4;14) | + | — | 9.8 | 54.5 | 44.7 | NA | NA | NA | |||||||||||||||
| Pt 3∥ | t(4;14) | + | — | 38.5 | 49.3 | 10.8 | 28.2 | 53.6 | 64.2 | 10.6 | ||||||||||||||
| MM1.S | t(14;16) | — | — | 20.4 ± 1.7 | 47.0 ± 3.0 | 26.6 ± 3.2 | < .001 | 31.9 ± 4.2 | .027 | 53.8 ± 3.9 | .001 | 82.2 ± 2.7 | 25.2 ± 4.2 | .001 | ||||||||||
| MM1.R | t(14;16) | — | — | 25.5 ± 2.9 | 40.8 ± 5.3 | 15.3 ± 5.3 | .019 | 38.9 ± 9.1 | NS | 25.6 ± 2.4 | NS | 37.8 ± 3.8 | 12.2 ± 2.5 | .050 | ||||||||||
| ANBL-6 | t(14;16) | — | — | 8.1 ± 0.5 | 10.2 ± 1.2 | 2.1 ± 1.1 | NS | NA | 29.2 ± 0.7 | .021 | 46.9 ± 0.9 | 17.7 ± 1.1 | .021 | |||||||||||
| JJN-3 | t(14;16) | — | — | 21.6 ± 3.8 | 17.2 ± 2.6 | —4.4 ± 1.6 | NS | NA | 36.9 ± 5.8 | NS | 44.6 ± 8.4 | 7.7 ± 3.4 | NS | |||||||||||
| OCI-My5 | t(14;16) | — | — | 31.2 ± 3.8 | 32.0 ± 2.7 | 0.8 ± 1.6 | NS | NA | 46.9 ± 10.3 | NS | 55.6 ± 10.0 | 8.7 ± 0.6 | NS | |||||||||||
| RPMI-8226 | t(16;22) | — | + K12 | 8.6 ± 2.7 | 8.7 ± 3.3 | 0.1 ± 3.3 | NS | 10.9 ± 2.4 | NS | 9.5 ± 1.4 | NS | 12.3 ± 1.7 | 2.8 ± 1.7 | NS | ||||||||||
| U-266 | — | — | — | 8.7 ± 1.2 | 10.8 ± 1.4 | 2.1 ± 0.6 | NS | 10.1 ± 1.5 | NS | 9.1 ± 1.5 | NS | 12.0 ± 1.8 | 2.9 ± 0.7 | NS | ||||||||||
| L363 | — | — | + N61 | 23.2 ± 3.2 | 24.5 ± 3.8 | 1.3 ± 1.5 | NS | NA | 22.3 ± 2.9 | NS | 23.2 ± 3.4 | 0.9 ± 0.7 | NS | |||||||||||
| Pt 4∥ | — | — | — | 17.8 | 33.6 | 15.8 | NA | NA | NA | NA | ||||||||||||||
| Pt 5∥ | — | — | — | 6.6 | 37.5 | 30.9 | 14.9 | NA | NA | NA | ||||||||||||||
| Pt 6∥ | — | — | — | 17.3 | 31.6 | 14.3 | NA | 34.8 | 59.5 | 24.7 | ||||||||||||||
| Pt 7∥ | — | — | — | 31.9 | 39.1 | 7.2 | NA | 60.7 | 71.9 | 11.2 | ||||||||||||||
| Pt 8∥ | — | — | — | 23.0 | 15.8 | —7.2 | NA | 29.4 | 27.7 | —1.7 | ||||||||||||||
| Pt 9∥ | — | — | NA | 8.8 | 8.9 | 0.1 | 7.6 | NA | NA | NA | ||||||||||||||
| Pt 10∥ | — | — | — | 25.3 | 28.5 | 3.2 | NA | NA | NA | NA | ||||||||||||||
| Pt 11∥ | — | — | NA | 8.9 | 9.9 | 1.0 | NA | 9.4 | 8.3 | —1.1 | ||||||||||||||
| Pt 12∥ | — | — | — | 6.8 | 9.2 | 2.4 | NA | 41.1 | 48.7 | 7.6 | ||||||||||||||
| Pt 13∥ | — | — | NA | 6.7 | 7.4 | 0.7 | 5.9 | 14.2 | 10.2 | —4.0 | ||||||||||||||
. | . | . | . | Apoptotic cells . | . | . | . | . | . | . | . | . | . | . | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| MM cell . | Karyotypic abnormalities . | FGFR3 +++ . | ras mutation . | Without BIBF 1000, % . | 500 nM BIBF 1000, % . | Increase over vehicle control, % . | P* . | 500 nM BIBF 1000 + IL-6, % . | P† . | 10 μM Dex, % . | P‡ . | 500 nM BIBF 1000 + 10 μM Dex, % . | Increase over Dex, % . | P§ . | ||||||||||
| KMS-11 | t(4;14), t(14;16) | + (Y373C) | — | 17.8 ± 3.4 | 41.9 ± 8.0 | 24.0 ± 4.8 | .011 | 33.6 ± 5.8 | NS | 22.8 ± 6.1 | NS | 52.2 ± 17.9 | 29.4 ± 7.8 | .025 | ||||||||||
| KMS-18 | t(4;14) | + (G384D) | — | 10.2 ± 2.5 | 37.5 ± 14.3 | 27.3 ± 10.7 | .008 | 31.8 ± 12.1 | NS | NA | NA | NA | ||||||||||||
| OPM-2 | t(4;14) | + (K650E) | — | 13.2 ± 3.5 | 39.1 ± 8.2 | 25.9 ± 6.2 | .026 | 24.0 ± 5.3 | NS | 26.0 ± 5.8 | NS | 70.4 ± 15.1 | 44.4 ± 9.7 | .05 | ||||||||||
| UTMC-2 | t(4;14) | + (wt) | — | 32.2 ± 3.6 | 44.8 ± 2.2 | 12.6 ± 5.9 | .035 | NA | 49.5 ± 1.5 | .018 | 58.6 ± 1.3 | 9.1 ± 3.0 | NS | |||||||||||
| NCI-H929 | t(4;14) | + (wt) | + N13 | 26.2 ± 2.3 | 30.4 ± 2.9 | 4.2 ± 1.4 | NS | NA | 33.5 ± 2.5 | .043 | 41.4 ± 4.0 | 7.8 ± 2.5 | NS | |||||||||||
| Pt 1∥ | t(4;14) | + | — | 21.6 | 32.5 | 10.9 | 26.8 | 28.9 | 40.7 | 11.8 | ||||||||||||||
| Pt 2∥ | t(4;14) | + | — | 9.8 | 54.5 | 44.7 | NA | NA | NA | |||||||||||||||
| Pt 3∥ | t(4;14) | + | — | 38.5 | 49.3 | 10.8 | 28.2 | 53.6 | 64.2 | 10.6 | ||||||||||||||
| MM1.S | t(14;16) | — | — | 20.4 ± 1.7 | 47.0 ± 3.0 | 26.6 ± 3.2 | < .001 | 31.9 ± 4.2 | .027 | 53.8 ± 3.9 | .001 | 82.2 ± 2.7 | 25.2 ± 4.2 | .001 | ||||||||||
| MM1.R | t(14;16) | — | — | 25.5 ± 2.9 | 40.8 ± 5.3 | 15.3 ± 5.3 | .019 | 38.9 ± 9.1 | NS | 25.6 ± 2.4 | NS | 37.8 ± 3.8 | 12.2 ± 2.5 | .050 | ||||||||||
| ANBL-6 | t(14;16) | — | — | 8.1 ± 0.5 | 10.2 ± 1.2 | 2.1 ± 1.1 | NS | NA | 29.2 ± 0.7 | .021 | 46.9 ± 0.9 | 17.7 ± 1.1 | .021 | |||||||||||
| JJN-3 | t(14;16) | — | — | 21.6 ± 3.8 | 17.2 ± 2.6 | —4.4 ± 1.6 | NS | NA | 36.9 ± 5.8 | NS | 44.6 ± 8.4 | 7.7 ± 3.4 | NS | |||||||||||
| OCI-My5 | t(14;16) | — | — | 31.2 ± 3.8 | 32.0 ± 2.7 | 0.8 ± 1.6 | NS | NA | 46.9 ± 10.3 | NS | 55.6 ± 10.0 | 8.7 ± 0.6 | NS | |||||||||||
| RPMI-8226 | t(16;22) | — | + K12 | 8.6 ± 2.7 | 8.7 ± 3.3 | 0.1 ± 3.3 | NS | 10.9 ± 2.4 | NS | 9.5 ± 1.4 | NS | 12.3 ± 1.7 | 2.8 ± 1.7 | NS | ||||||||||
| U-266 | — | — | — | 8.7 ± 1.2 | 10.8 ± 1.4 | 2.1 ± 0.6 | NS | 10.1 ± 1.5 | NS | 9.1 ± 1.5 | NS | 12.0 ± 1.8 | 2.9 ± 0.7 | NS | ||||||||||
| L363 | — | — | + N61 | 23.2 ± 3.2 | 24.5 ± 3.8 | 1.3 ± 1.5 | NS | NA | 22.3 ± 2.9 | NS | 23.2 ± 3.4 | 0.9 ± 0.7 | NS | |||||||||||
| Pt 4∥ | — | — | — | 17.8 | 33.6 | 15.8 | NA | NA | NA | NA | ||||||||||||||
| Pt 5∥ | — | — | — | 6.6 | 37.5 | 30.9 | 14.9 | NA | NA | NA | ||||||||||||||
| Pt 6∥ | — | — | — | 17.3 | 31.6 | 14.3 | NA | 34.8 | 59.5 | 24.7 | ||||||||||||||
| Pt 7∥ | — | — | — | 31.9 | 39.1 | 7.2 | NA | 60.7 | 71.9 | 11.2 | ||||||||||||||
| Pt 8∥ | — | — | — | 23.0 | 15.8 | —7.2 | NA | 29.4 | 27.7 | —1.7 | ||||||||||||||
| Pt 9∥ | — | — | NA | 8.8 | 8.9 | 0.1 | 7.6 | NA | NA | NA | ||||||||||||||
| Pt 10∥ | — | — | — | 25.3 | 28.5 | 3.2 | NA | NA | NA | NA | ||||||||||||||
| Pt 11∥ | — | — | NA | 8.9 | 9.9 | 1.0 | NA | 9.4 | 8.3 | —1.1 | ||||||||||||||
| Pt 12∥ | — | — | — | 6.8 | 9.2 | 2.4 | NA | 41.1 | 48.7 | 7.6 | ||||||||||||||
| Pt 13∥ | — | — | NA | 6.7 | 7.4 | 0.7 | 5.9 | 14.2 | 10.2 | —4.0 | ||||||||||||||
Data represent means ± SE of 3 independent experiments for each type of myeloma cell lines (unless there was only a single experiment for each patient sample). PBBLs indicate peripheral blood B lymphocytes obtained from 5 healthy volunteers; Dex, dexamethasone; IL-6, interleukin-6, FGFR3, fibroblast growth factor receptor 3. Data for apoptotic PBBLs: without BIBF 1000, 4.6% ± 1.0%; with 250 nM BIBF 1000, 5.0% ± 0.5%; with 500 nM BIBF 1000, 5.1% ± 0.5%; and with 1000 nm BIBF 1000, 5.9% ± 0.7%.
NA indicates not available; NS, not significant; —, negative for t(4;14), t(14;16), and t(16;22) (column 2); FGFR3 not overexpressed (column 3); or not ras mutated (column 4).
P value of 500 nM BIBF 1000 versus vehicle control
P value of 500 nM BIBF 1000 + IL-6 versus 500 nM BIBF 1000
P value of 10 μM Dex versus vehicle control
P value of 500 nM BIBF 1000 + 10 μM Dex versus Dex alone (10 μM)
CD138+ sorted cells from patients with MM
Apoptotic effects in t(14;16) myeloma cells and inhibition of PI3-kinase/AKT signaling
In a series of t(14;16)–positive MM cell lines tested (MM.1S, MM.1R, ANBL-6, JJN-3, OCI-My5) and t(16;22)–positive RPMI-8226 BIBF 1000 showed variable effects on induction of apoptosis. Antiproliferative and proapoptotic effects of BIBF 1000 in MM.1S and MM.1R were significant and comparable to those with t(4;14). In VEGFR1-expressing MM.1S cells, proapoptotic effects of BIBF 1000 were additive to dexamethasone-induced apoptosis, partially antagonized by IL-6, and completely inhibited by the pan-caspase inhibitor z-VAD (see Table 2; Figures 5 and 8A).
In contrast, BIBF 1000 alone had no marked proapoptotic effects in ANBL-6, JJN-3, and OCI-My5. However, a consistent additive proapoptotic effect occurred in these latter cell lines by the combination of BIBF 1000 and dexamethasone (Table 2).
In contrast to t(4;14) myeloma cells, induction of apoptosis by BIBF 1000 in MM.1S cells was associated with inhibition of the PI3-kinase/AKT pathway as documented by decreased AKT phosphorylation, whereas neither MAPK nor STAT3 phosphorylation were affected (Figure 8B-C). The significance of the PI3-kinase/AKT survival pathway in t(14;16) MM.1S cells is further documented in Figure 8D, showing that similar apoptotic effects were obtained with BIBF 1000 and the PI3-kinase inhibitor Ly294002.
RPMI-8226 cells, carrying a t(16;22), were resistant to proapoptotic effects of BIBF 1000 possibly because of an additional k-Ras mutation. As expected, MAPK phosphorylation was not altered by the study drug in this cell line (data not shown).
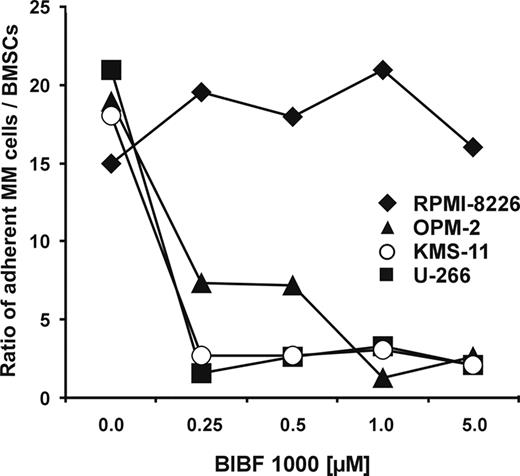
Inhibition of myeloma cell adhesion to BMSCs by BIBF 1000. Contact cocultures were performed with confluent monolayers of 1 × 105 BMSCs and 2 × 106 CFSE-prelabeled RPMI-8226, U-266, OPM-2, or KMS-11 cells, respectively. Cell cultures were incubated in the absence or in the presence of BIBF 1000 (0.25 to 5.0 μM) for 24 hours. Subsequently, nonadherent cells were washed off 3 times. The remaining adherent cells were trypsinized and quantified by flow cytometry. Data are presented as mean ratios of adherent MM cells over BMSCs from 3 independent experiments for each cell line. Analysis of significance for overall group differences was performed by the Kruskal-Wallis test (U-266, OPM-2, and KMS-11: P = .001; RPMI-8226: NS).

Inhibition of myeloma cell adhesion to BMSCs by BIBF 1000. Contact cocultures were performed with confluent monolayers of 1 × 105 BMSCs and 2 × 106 CFSE-prelabeled RPMI-8226, U-266, OPM-2, or KMS-11 cells, respectively. Cell cultures were incubated in the absence or in the presence of BIBF 1000 (0.25 to 5.0 μM) for 24 hours. Subsequently, nonadherent cells were washed off 3 times. The remaining adherent cells were trypsinized and quantified by flow cytometry. Data are presented as mean ratios of adherent MM cells over BMSCs from 3 independent experiments for each cell line. Analysis of significance for overall group differences was performed by the Kruskal-Wallis test (U-266, OPM-2, and KMS-11: P = .001; RPMI-8226: NS).
![Figure 5. Dose-dependent inhibition of myeloma cell proliferation by BIBF 1000. (A-C) Myeloma cells (5 × 104) per well were seeded onto 96-well plates and cultured in RPMI-1640 medium with or without BIBF 1000. Cells were pulsed with 0.625 μCi (0.023 MBq) 3H-thymidine per well for 72 hours. Cells were harvested, and 3H-thymidine incorporation was counted. 3H-thymidine uptake is shown as mean values of triplicate determinations from 2 independent series of experiments for each cell line. 3H-thymidine uptake was normalized to the controls without BIBF 1000. A 70% or greater inhibition of proliferation by 1.0 μM BIBF 1000 was observed in the t(4;14)–positive KMS-11, OPM-2, and NCI-H929 cells and in the t(14;16)–positive MM.1S and MM.1R cells (P < .001; BIBF 1000 [1.0 μM] versus control without BIBF 1000). BIBF 1000 (1 μM) inhibited the proliferation of U-266 and L-363 by approximately 50%, whereas it did not or only marginally influence the proliferation of the t(14;16)–positive cell lines JJN-3 and ANBL-6 as well as of the t(16;22)–positive cell line RPMI-8226.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/107/5/10.1182_blood-2004-11-4250/2/m_zh80050692090005.jpeg?Expires=1761677921&Signature=TiuF4-vHlAq99MFwJiEZG1FTjtKkLQSP7D6WGYm1C~uozYWB59Dh6od3v0MTpcToCQKU13PJ4w1FS6A-gLu2NkYxbiL7n53bMrSdaKS1ssbNYkMnWsSH8KnW-u7Ku23hlhgt-PzyCPiS93VrmTuMNwm1s8iR02dCKTpyzqfcSbpe74S3XCkhUAmqk0pRMN-NaWJ5ShHTdtOSCWmSILszEm0M2y0dZBGkNJVf12~Qx61YKZpkhCQhAXt4Ph5bA11kalQESxJwowV8y428fmTHGeNXAbpov-frHNSEUj6rTtnjtqC6HJu6rsbuJTaRAkcpers9-xwPDp3689JMKLbuvw__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
Dose-dependent inhibition of myeloma cell proliferation by BIBF 1000. (A-C) Myeloma cells (5 × 104) per well were seeded onto 96-well plates and cultured in RPMI-1640 medium with or without BIBF 1000. Cells were pulsed with 0.625 μCi (0.023 MBq) 3H-thymidine per well for 72 hours. Cells were harvested, and 3H-thymidine incorporation was counted. 3H-thymidine uptake is shown as mean values of triplicate determinations from 2 independent series of experiments for each cell line. 3H-thymidine uptake was normalized to the controls without BIBF 1000. A 70% or greater inhibition of proliferation by 1.0 μM BIBF 1000 was observed in the t(4;14)–positive KMS-11, OPM-2, and NCI-H929 cells and in the t(14;16)–positive MM.1S and MM.1R cells (P < .001; BIBF 1000 [1.0 μM] versus control without BIBF 1000). BIBF 1000 (1 μM) inhibited the proliferation of U-266 and L-363 by approximately 50%, whereas it did not or only marginally influence the proliferation of the t(14;16)–positive cell lines JJN-3 and ANBL-6 as well as of the t(16;22)–positive cell line RPMI-8226.
![Figure 5. Dose-dependent inhibition of myeloma cell proliferation by BIBF 1000. (A-C) Myeloma cells (5 × 104) per well were seeded onto 96-well plates and cultured in RPMI-1640 medium with or without BIBF 1000. Cells were pulsed with 0.625 μCi (0.023 MBq) 3H-thymidine per well for 72 hours. Cells were harvested, and 3H-thymidine incorporation was counted. 3H-thymidine uptake is shown as mean values of triplicate determinations from 2 independent series of experiments for each cell line. 3H-thymidine uptake was normalized to the controls without BIBF 1000. A 70% or greater inhibition of proliferation by 1.0 μM BIBF 1000 was observed in the t(4;14)–positive KMS-11, OPM-2, and NCI-H929 cells and in the t(14;16)–positive MM.1S and MM.1R cells (P < .001; BIBF 1000 [1.0 μM] versus control without BIBF 1000). BIBF 1000 (1 μM) inhibited the proliferation of U-266 and L-363 by approximately 50%, whereas it did not or only marginally influence the proliferation of the t(14;16)–positive cell lines JJN-3 and ANBL-6 as well as of the t(16;22)–positive cell line RPMI-8226.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/107/5/10.1182_blood-2004-11-4250/2/m_zh80050692090005.jpeg?Expires=1761677921&Signature=TiuF4-vHlAq99MFwJiEZG1FTjtKkLQSP7D6WGYm1C~uozYWB59Dh6od3v0MTpcToCQKU13PJ4w1FS6A-gLu2NkYxbiL7n53bMrSdaKS1ssbNYkMnWsSH8KnW-u7Ku23hlhgt-PzyCPiS93VrmTuMNwm1s8iR02dCKTpyzqfcSbpe74S3XCkhUAmqk0pRMN-NaWJ5ShHTdtOSCWmSILszEm0M2y0dZBGkNJVf12~Qx61YKZpkhCQhAXt4Ph5bA11kalQESxJwowV8y428fmTHGeNXAbpov-frHNSEUj6rTtnjtqC6HJu6rsbuJTaRAkcpers9-xwPDp3689JMKLbuvw__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
Dose-dependent inhibition of myeloma cell proliferation by BIBF 1000. (A-C) Myeloma cells (5 × 104) per well were seeded onto 96-well plates and cultured in RPMI-1640 medium with or without BIBF 1000. Cells were pulsed with 0.625 μCi (0.023 MBq) 3H-thymidine per well for 72 hours. Cells were harvested, and 3H-thymidine incorporation was counted. 3H-thymidine uptake is shown as mean values of triplicate determinations from 2 independent series of experiments for each cell line. 3H-thymidine uptake was normalized to the controls without BIBF 1000. A 70% or greater inhibition of proliferation by 1.0 μM BIBF 1000 was observed in the t(4;14)–positive KMS-11, OPM-2, and NCI-H929 cells and in the t(14;16)–positive MM.1S and MM.1R cells (P < .001; BIBF 1000 [1.0 μM] versus control without BIBF 1000). BIBF 1000 (1 μM) inhibited the proliferation of U-266 and L-363 by approximately 50%, whereas it did not or only marginally influence the proliferation of the t(14;16)–positive cell lines JJN-3 and ANBL-6 as well as of the t(16;22)–positive cell line RPMI-8226.
Induction of apoptosis by BIBF 1000 in patient myeloma cells
Apoptosis was quantified flow cytometrically by annexin V/propidium iodide staining in freshly isolated and subsequently CD138+-sorted cells derived from 12 patients with active MM with or without t(4;14) (Table 2). In line with the data from t(4;14)–positive cell lines, we found consistent induction of apoptosis by BIBF 1000 (500 nM) in CD138+-sorted marrow cells from the 3 patients with t(4;14)–positive myeloma (Table 2). Again, BIBF 1000–induced apoptosis was partially antagonized by exogenous IL-6 (Table 2). In addition, we observed additive effects of BIBF 1000 and dexamethasone in 2 of those patients (Table 2; Figure 6).
Apart from these findings in myeloma cells with defined cytogenetic abnormalities, BIBF 1000 induced apoptosis to a variable extent in CD138+ marrow cells from 4 of 10 patients with active MM carrying neither the t(4;14) nor the t(14;16) translocations (Table 2).
Discussion
BIBF 1000 is a novel indolinone-derived RTKI primarily targeting VEGFR1 through VEGFR3, FGFR1 and FGFR3, and PDGFRα kinases. From our results it can be inferred that BIBF 1000 also interferes with a paracrine signaling pathway triggered by TGF-β, another key cytokine in myeloma-marrow stroma interactions.32 The data presented herein delineate 2 types of in vitro effects of BIBF 1000 in myeloma that both have implications for understanding the tumor biology and for clinical applications of RTKIs in this heterogeneous disease.48-50
First and irrespective of the type of myeloma cells studied, BIBF 1000 strongly inhibited stroma-derived IL-6 release in the myeloma microenvironment. This effect of BIBF 1000 can in partbe attributed to inhibition of paracrine signals through VEGF, bFGF, and TGF-β.6,7,32 In addition, it may also be a consequence of the variably decreased adherence of myeloma to stroma cells (compare Figure 4).29,40-42 However, for the first time, the data obtained with BIBF 1000 demonstrate that inhibition of paracrine mechanisms significantly reduces microenvironmental IL-6 production both in the absence (U-266, KMS-11) or presence (RPMI-8226) of conserved myeloma cell adherence to marrow stroma cells. These findings extend previous reports on decreased IL-6 production in myeloma-stroma cell cocultures exposed to investigational RTKIs targeting VEGFRs, such as PTK787/ZK222584 or GW654652.51,52 Furthermore, given the importance of IL-6 as a tumor growth and resistance factor in MM,10-12 it can be speculated that BIBF 1000 may have indirect proapoptotic effects in MM in vivo through inhibition of paracrine IL-6 circuits and should also be suited to revert IL-6–mediated conventional drug resistance.
The second type of BIBF 1000–mediated effects comprises inhibition of proliferation and direct induction of myeloma cell apoptosis. In this respect, the sensitivity of myeloma cells toward BIBF 1000 varied considerably and was related to subgroups of myeloma cells with defined cytogenetic abnormalities. The antiproliferative activity of BIBF 1000 appeared to be most marked in, though not restricted to, myeloma cell lines carrying the translocations t(4;14) or t(14;16).
A similar subgroup preference was evident for the proapoptotic effects of BIBF 1000. Strong antiproliferative and proapoptotic activities of BIBF 1000 and almost complete inhibition of stroma adherence were observed in cell lines with t(4;14) and different activating mutations of FGFR3 (OPM-2, KMS-11, and KMS-18) as well as wild-type FGFR3 (UTMC-2) and in freshly isolated CD138+ myeloma cells from the marrow of patients with t(4;14)–positive MM (Table 2). The proapoptotic effects were paralleled by interruption of receptor signaling through MAPK (Figure 7C-D). These findings and the marked selectivity of the drug effects strongly support the notion that FGFR3 overexpression and mutation is a dominant transforming event in this type of myeloma cells. In this respect, our results with BIBF 1000 in FGFR3-mutated myeloma cell lines are in line with recently reported effects of PD173074 and CHIR-258, experimental RTKIs also targeting FGFRs.47,53
Notably, proapoptotic effects by BIBF 1000 did not occur in n-Ras–mutated t(4;14)–positive NCI-H929 cells (Figure 7E), implicating that the presence of mutated Ras in MM cells constitutively activates MAPK signaling independent from upstream receptor tyrosine kinase (RTK) activity.
Furthermore, induction of apoptosis by BIBF 1000 was only partially antagonized by adding exogenous IL-6 at saturating concentrations. Thus, abnormal signaling through the FGFR3 pathway appears to be crucial for tumor cell survival in these cell lines, whereas the IL-6 signal is dispensable and can only partly sustain cell survival in the absence of deregulated FGFR3 signaling. The observations demonstrate a hierarchy of survival pathways in this myeloma subgroup that may have implications for therapeutic targeting.
![Figure 6. Induction of apoptosis by BIBF 1000 in patient myeloma cells. Quantification of apoptosis by flow cytometry in CD138+-sorted cells from the marrow of a patient with t(4;14)–positive myeloma, [pt 3]). Increased early/late apoptosis by BIBF 1000, dexamethasone, and their combination is depicted in the top row. The bottom row shows partial reversal of apoptosis by IL-6. Percentage quantification of early apoptotic cells (annexin V–positive and propidium iodide [PI]–negative; bottom right quadrants) or late apoptotic cells (annexin V–positive and PI-positive; top right quadrants) as indicated in dot plot panels.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/107/5/10.1182_blood-2004-11-4250/2/m_zh80050692090006.jpeg?Expires=1761677921&Signature=BHEMK9HuC17piRseqfJt7F2CrGqA2od4v~dIdqcdAaZpTggol4qF23yPxKSiSeO-9vIUUSQxQmAoy~Yacdo9M~W122KEYiJmILCYTp~~sgbwRga4UAH~wIeJULymrRyTN~TI3z0KFl3J6Xinrwl1qKxyypdT8NxKsvNSLCSkrzrzcnzoTUx9VEGzQnbQyqQLamUhaSoy3U2nbd~dzgEmDPrSXA66aDYncKHNxxCBd3Ze4~qZbC80-GPmTT0Q7NsFx9cpBvx7wGF1BKRjZ6B5EbFgveu4QNM7H7XEgDwHmHyw1GZLDZ4qEwfYstFUV~hucW3qFK3ugsZ4xXvWosnXTg__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
Induction of apoptosis by BIBF 1000 in patient myeloma cells. Quantification of apoptosis by flow cytometry in CD138+-sorted cells from the marrow of a patient with t(4;14)–positive myeloma, [pt 3]). Increased early/late apoptosis by BIBF 1000, dexamethasone, and their combination is depicted in the top row. The bottom row shows partial reversal of apoptosis by IL-6. Percentage quantification of early apoptotic cells (annexin V–positive and propidium iodide [PI]–negative; bottom right quadrants) or late apoptotic cells (annexin V–positive and PI-positive; top right quadrants) as indicated in dot plot panels.
![Figure 6. Induction of apoptosis by BIBF 1000 in patient myeloma cells. Quantification of apoptosis by flow cytometry in CD138+-sorted cells from the marrow of a patient with t(4;14)–positive myeloma, [pt 3]). Increased early/late apoptosis by BIBF 1000, dexamethasone, and their combination is depicted in the top row. The bottom row shows partial reversal of apoptosis by IL-6. Percentage quantification of early apoptotic cells (annexin V–positive and propidium iodide [PI]–negative; bottom right quadrants) or late apoptotic cells (annexin V–positive and PI-positive; top right quadrants) as indicated in dot plot panels.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/107/5/10.1182_blood-2004-11-4250/2/m_zh80050692090006.jpeg?Expires=1761677921&Signature=BHEMK9HuC17piRseqfJt7F2CrGqA2od4v~dIdqcdAaZpTggol4qF23yPxKSiSeO-9vIUUSQxQmAoy~Yacdo9M~W122KEYiJmILCYTp~~sgbwRga4UAH~wIeJULymrRyTN~TI3z0KFl3J6Xinrwl1qKxyypdT8NxKsvNSLCSkrzrzcnzoTUx9VEGzQnbQyqQLamUhaSoy3U2nbd~dzgEmDPrSXA66aDYncKHNxxCBd3Ze4~qZbC80-GPmTT0Q7NsFx9cpBvx7wGF1BKRjZ6B5EbFgveu4QNM7H7XEgDwHmHyw1GZLDZ4qEwfYstFUV~hucW3qFK3ugsZ4xXvWosnXTg__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
Induction of apoptosis by BIBF 1000 in patient myeloma cells. Quantification of apoptosis by flow cytometry in CD138+-sorted cells from the marrow of a patient with t(4;14)–positive myeloma, [pt 3]). Increased early/late apoptosis by BIBF 1000, dexamethasone, and their combination is depicted in the top row. The bottom row shows partial reversal of apoptosis by IL-6. Percentage quantification of early apoptotic cells (annexin V–positive and propidium iodide [PI]–negative; bottom right quadrants) or late apoptotic cells (annexin V–positive and PI-positive; top right quadrants) as indicated in dot plot panels.
In MM.1S and MM.1R cells, characterized by translocation t(14;16) (IgH/c-maf), VEGFR1 expression and sensitivity to autocrine stimulation by VEGF,13,18,51 similar antiproliferative and proapoptotic effects by BIBF 1000 were observed. Of note, despite the finding that BIBF 1000 alone had no marked effect in a series of other t(14;16)–positive cell lines, a consistent additive proapoptotic effect occurred in these cells (ANBL-6, JJN-3, OCI-My5) on exposure to BIBF 1000 and dexamethasone. In t(14;16) MM.1S cells, induction of apoptosis by BIBF 1000 appeared to be related to the inhibition of the PI3-kinase/AKT pathway, indicating its association with inhibition of VEGFR signaling. Recently, MM.1S have been shown to be protected from apoptosis through VEGF-induced up-regulation of Mcl-1, an antiapoptotic member of the Bcl-2 family.54 Therefore, the observed effects of BIBF 1000–mediated interruption of VEGFR signaling in this cell line were not unexpected. As with t(4;14) cell lines, induction of apoptosis by BIBF 1000 in MM.1S cells was only partly prevented by exogenous IL-6, although IL-6 receptor signaling through the JAK/STAT cascade was not impaired. This finding again points to a hierarchy of survival pathways that may characterize this type of myeloma cells. In line with the findings in t(4;14)–positive MM, k-Ras–mutated t(16;22)–positive RPMI-8226 cells were resistant to BIBF 1000 treatment even in the presence of dexamethasone.
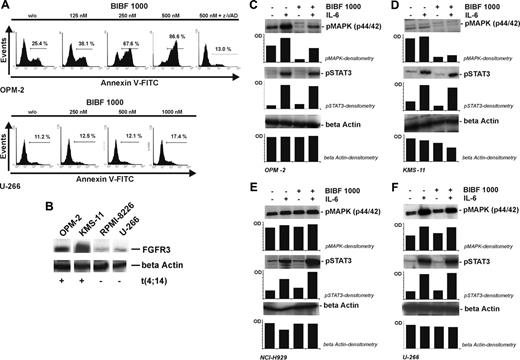
BIBF 1000–induced apoptosis in t(4;14)–positive myeloma cells and related inhibition of MAPK (p44/42) phosphorylation. (A) Dose-dependent induction of apoptosis in t(4;14)–positive, FGFR3-overexpressing OPM-2 cells by BIBF 1000 (125-500 nM, top). Induction of apoptosis was completely reverted by the pan-caspase-inhibitor z-VAD-FMK (100 μM). No significant BIBF 1000-induced apoptosis occurred in U-266 cells serving as a t(4;14)–negative control (bottom). (B) FGFR3 protein expression in t(4;14)–positive OPM-2 and KMS-11 versus t(4;14)–negative RPMI-8226 and U-266 cells. Representative immunoblots are shown. (C) Inhibition of MAPK phosphorylation in t(4;14) OPM-2 cells. Constitutive and IL-6–induced MAPK phosphorylation were almost completely or partially inhibited by BIBF 1000, whereas STAT3 phosphorylation was not affected. (D) Inhibition of MAPK phosphorylation in t(4;14) KMS-11 cells. Results were similar to those obtained with OPM-2 cells. (E-F) Neither MAPK nor STAT3 phosphorylation were altered by BIBF 1000 in N-Ras mutated t(4;14)–positive NCI-H929 cells (E) and in t(4;14)–negative U-266 cells (F). In panels C through F, myeloma cell lines were starved for 2 hours and exposed to IL-6 (10 ng/mL) and/or BIBF 1000 (500 nM) for 5 minutes prior to extraction of proteins by RIPA-buffer containing protease and phosphatase inhibitors.

BIBF 1000–induced apoptosis in t(4;14)–positive myeloma cells and related inhibition of MAPK (p44/42) phosphorylation. (A) Dose-dependent induction of apoptosis in t(4;14)–positive, FGFR3-overexpressing OPM-2 cells by BIBF 1000 (125-500 nM, top). Induction of apoptosis was completely reverted by the pan-caspase-inhibitor z-VAD-FMK (100 μM). No significant BIBF 1000-induced apoptosis occurred in U-266 cells serving as a t(4;14)–negative control (bottom). (B) FGFR3 protein expression in t(4;14)–positive OPM-2 and KMS-11 versus t(4;14)–negative RPMI-8226 and U-266 cells. Representative immunoblots are shown. (C) Inhibition of MAPK phosphorylation in t(4;14) OPM-2 cells. Constitutive and IL-6–induced MAPK phosphorylation were almost completely or partially inhibited by BIBF 1000, whereas STAT3 phosphorylation was not affected. (D) Inhibition of MAPK phosphorylation in t(4;14) KMS-11 cells. Results were similar to those obtained with OPM-2 cells. (E-F) Neither MAPK nor STAT3 phosphorylation were altered by BIBF 1000 in N-Ras mutated t(4;14)–positive NCI-H929 cells (E) and in t(4;14)–negative U-266 cells (F). In panels C through F, myeloma cell lines were starved for 2 hours and exposed to IL-6 (10 ng/mL) and/or BIBF 1000 (500 nM) for 5 minutes prior to extraction of proteins by RIPA-buffer containing protease and phosphatase inhibitors.
![Figure 8. BIBF 1000 induced apoptosis in t(14;16)–positive MM.1S cells and related inhibition of the phosphatidyl-inositol-3 kinase (PI3-K)/AKT pathway. (A) BIBF 1000 induced apoptosis in native t(14;16)–positive MM.1S cells and had additive apoptotic effects in the presence of Dex (compare percentages of annexin V-positive, PI-negative cells, lower right quadrants). Apoptosis induced by BIBF 1000 was partially antagonized by IL-6 at saturating concentrations (10 ng/mL) and completely prevented by the pan-caspase inhibitor z-VAD. Percentage quantification of early apoptotic cells (annexin V–positive and PI-negative; bottom right quadrants) or late apoptotic cells (annexin V–positive and PI-positive; top right quadrants) as indicated in dot plot panels. (B) BIBF 1000 had no effect on MAPK or STAT3 phosphorylation in MM.1S cells. (C) The PI3-K/AKT pathway was inhibited by BIBF 1000 both in the absence and presence of IL-6 as shown by decreased phosphorylation of AKT. (D) BIBF 1000 (0.5 μM) and the PI3-K inhibitor Ly294002 (10 μM) had similar effects on the induction of early and late apoptosis in MM.1S cells (annexin V–positive [+ve], PI-negative [-ve], and annexin V–positive, PI-positive cells, respectively; means ± SE).](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/107/5/10.1182_blood-2004-11-4250/2/m_zh80050692090008.jpeg?Expires=1761677921&Signature=YTubE4P1N3b57nMTFTshhKvpF0mQ3xbf0y0gkwQ0p8ksPUmW7Kfbv4O98uGQpGKyKhUl92JRBTSdoCQfH9c~MI9aen2nYfERj7nv6ddiCUkbs88ntyYSS9pq4ggmQPb8UoQRXAuz1ZbE4j02qUhXNUmGtUlWx5TEbZsdQLQchy5h9~CtOTUzdpfj2522n13iNVVV~N5BP93l-WuA~O~KC9gFuY~LdQIiMbQPBKP9-YA05N8i2G9GzKJCSvfyljg0JRXGR4ledwrLfeqg9HwOVryIPn9SsenxDmrTS9VDBIguz-FQvFGoA3mmPZiN8BwEbUOfaAp4xZVWu-ilpwOFFQ__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
BIBF 1000 induced apoptosis in t(14;16)–positive MM.1S cells and related inhibition of the phosphatidyl-inositol-3 kinase (PI3-K)/AKT pathway. (A) BIBF 1000 induced apoptosis in native t(14;16)–positive MM.1S cells and had additive apoptotic effects in the presence of Dex (compare percentages of annexin V-positive, PI-negative cells, lower right quadrants). Apoptosis induced by BIBF 1000 was partially antagonized by IL-6 at saturating concentrations (10 ng/mL) and completely prevented by the pan-caspase inhibitor z-VAD. Percentage quantification of early apoptotic cells (annexin V–positive and PI-negative; bottom right quadrants) or late apoptotic cells (annexin V–positive and PI-positive; top right quadrants) as indicated in dot plot panels. (B) BIBF 1000 had no effect on MAPK or STAT3 phosphorylation in MM.1S cells. (C) The PI3-K/AKT pathway was inhibited by BIBF 1000 both in the absence and presence of IL-6 as shown by decreased phosphorylation of AKT. (D) BIBF 1000 (0.5 μM) and the PI3-K inhibitor Ly294002 (10 μM) had similar effects on the induction of early and late apoptosis in MM.1S cells (annexin V–positive [+ve], PI-negative [-ve], and annexin V–positive, PI-positive cells, respectively; means ± SE).
![Figure 8. BIBF 1000 induced apoptosis in t(14;16)–positive MM.1S cells and related inhibition of the phosphatidyl-inositol-3 kinase (PI3-K)/AKT pathway. (A) BIBF 1000 induced apoptosis in native t(14;16)–positive MM.1S cells and had additive apoptotic effects in the presence of Dex (compare percentages of annexin V-positive, PI-negative cells, lower right quadrants). Apoptosis induced by BIBF 1000 was partially antagonized by IL-6 at saturating concentrations (10 ng/mL) and completely prevented by the pan-caspase inhibitor z-VAD. Percentage quantification of early apoptotic cells (annexin V–positive and PI-negative; bottom right quadrants) or late apoptotic cells (annexin V–positive and PI-positive; top right quadrants) as indicated in dot plot panels. (B) BIBF 1000 had no effect on MAPK or STAT3 phosphorylation in MM.1S cells. (C) The PI3-K/AKT pathway was inhibited by BIBF 1000 both in the absence and presence of IL-6 as shown by decreased phosphorylation of AKT. (D) BIBF 1000 (0.5 μM) and the PI3-K inhibitor Ly294002 (10 μM) had similar effects on the induction of early and late apoptosis in MM.1S cells (annexin V–positive [+ve], PI-negative [-ve], and annexin V–positive, PI-positive cells, respectively; means ± SE).](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/107/5/10.1182_blood-2004-11-4250/2/m_zh80050692090008.jpeg?Expires=1761677921&Signature=YTubE4P1N3b57nMTFTshhKvpF0mQ3xbf0y0gkwQ0p8ksPUmW7Kfbv4O98uGQpGKyKhUl92JRBTSdoCQfH9c~MI9aen2nYfERj7nv6ddiCUkbs88ntyYSS9pq4ggmQPb8UoQRXAuz1ZbE4j02qUhXNUmGtUlWx5TEbZsdQLQchy5h9~CtOTUzdpfj2522n13iNVVV~N5BP93l-WuA~O~KC9gFuY~LdQIiMbQPBKP9-YA05N8i2G9GzKJCSvfyljg0JRXGR4ledwrLfeqg9HwOVryIPn9SsenxDmrTS9VDBIguz-FQvFGoA3mmPZiN8BwEbUOfaAp4xZVWu-ilpwOFFQ__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
BIBF 1000 induced apoptosis in t(14;16)–positive MM.1S cells and related inhibition of the phosphatidyl-inositol-3 kinase (PI3-K)/AKT pathway. (A) BIBF 1000 induced apoptosis in native t(14;16)–positive MM.1S cells and had additive apoptotic effects in the presence of Dex (compare percentages of annexin V-positive, PI-negative cells, lower right quadrants). Apoptosis induced by BIBF 1000 was partially antagonized by IL-6 at saturating concentrations (10 ng/mL) and completely prevented by the pan-caspase inhibitor z-VAD. Percentage quantification of early apoptotic cells (annexin V–positive and PI-negative; bottom right quadrants) or late apoptotic cells (annexin V–positive and PI-positive; top right quadrants) as indicated in dot plot panels. (B) BIBF 1000 had no effect on MAPK or STAT3 phosphorylation in MM.1S cells. (C) The PI3-K/AKT pathway was inhibited by BIBF 1000 both in the absence and presence of IL-6 as shown by decreased phosphorylation of AKT. (D) BIBF 1000 (0.5 μM) and the PI3-K inhibitor Ly294002 (10 μM) had similar effects on the induction of early and late apoptosis in MM.1S cells (annexin V–positive [+ve], PI-negative [-ve], and annexin V–positive, PI-positive cells, respectively; means ± SE).
Notably, normal CD19+ B lymphocytes, CD19- lymphocytes as well as L363 and U-266 cells were insensitive to BIBF 1000 treatment with respect to apoptosis induction over the range of concentrations tested, indicating that merely toxic effects of the study drug can be excluded.
Taken together, our data provide the rationale for currently ongoing phase 1 clinical evaluation of this novel class of indolinone derivatives. They also suggest that future clinical trials on RTKIs with similar target profiles should be designed to detect reversal of resistance to classic antimyeloma agents (eg, dexamethasone) that may be achieved through the inhibition of IL-6 production in the myeloma-marrow microenvironment. Potential clinical applications should particularly focus on, but not be limited to, cytogenetically defined myeloma subgroups, such as those with t(4;14) or t(14;16) in which myeloma survival and stroma interactions appear to be driven, at least in part, by VEGFR or mutant FGFR signaling. Finally, the Ras mutational status may also have implications for treatment with this class of targeted drugs.
Prepublished online as Blood First Edition Paper, November 8, 2005; DOI 10.1182/blood-2004-11-4250.
Supported by research funding from Boehringer Ingelheim Pharma GmbH & Co. KG (G.B. and J.K.).
Several of the authors (F.H., G.M., G.J.R., and M.S.) are employed by Boehringer Ingelheim Austria GmbH and/or Boehringer Ingelheim Pharma GmbH and Co. KG, whose product was studied in the present work.
G.B. and M.K. contributed equally to this work.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
D.W. contributed experiments to fulfill requirements for her MD thesis.
We thank Takemi Otsuki from Kawasaki Medical School, Okayama, Japan, for kindly providing the KMS-11 and KMS-18 myeloma cell lines and Nancy L. Krett, Robert H Lurie Comprehensive Cancer Center, Chigago, IL, for supplying MM.1S and MM.1R cell lines. We thank Leif Bergsagel, Mayo Clinic, Scottsdale, AZ, for supplying the OCI-My5 and UTMC-2 lines and Manik Chatterjee, Max-Delbrueck Zentrum, Berlin-Buch, Berlin, Germany, for kindly providing the ANBL-6 myeloma cell line. We thank Christina Burhoi for excellent technical assistance.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal