Epstein-Barr virus (EBV) induces CD95 expression and the CD95 gene (FAS) is regulated by NF-κB, STAT1, and/or p53. To understand the contribution of these factors in the regulation of CD95 by EBV in lymphoblastoid cell lines (LCLs), we cloned dominant-active IκBα, active (STAT1α) and inactive (STAT1β) forms of STAT1, p53, a dominant-negative mutant of LMP1, and wild-type LMP1 into a novel double-inducible episomal vector, pRT-1. These plasmids were stably transfected either into wild-type LCLs or EREB2-5 cells, an LCL with an estrogen-regulatable EBNA2 protein. Inhibition of LMP1 signaling decreased expression of CD95, whereas overexpression of LMP1 markedly increased it. Induction of the latency III program in EREB2-5 cells correlated with activation of NF-κB, STAT1, and p53. CD95 expression was regulated by these 3 transcriptional systems. STAT1 and p53 activation were secondary to NF-κB activation. CD95 surface expression sensitized EBV-infected B cells to the induction of CD95-mediated apoptosis. In vitro inhibition of CD95-CD95 ligand interaction was found to reverse T-cell killing of EBV-infected B cells. Therefore, LMP1 activation of NF-κB sensitizes infected B cells to CD95-mediated apoptosis and renders EBV latency III–immortalized B cells susceptible to elimination by the immune system, contributing to the establishment of a host/virus equilibrium.
Introduction
Epstein-Barr virus (EBV) was first reported to be associated with Burkitt lymphoma (BL). EBV is able to immortalize B cells both in vitro and in vivo. EBV is also the etiologic agent of infectious mononucleosis, a benign viral disease of young adults that regresses spontaneously. In most cases, primary EBV infection is asymptomatic. Epidemiologic and serologic studies showed that EBV is widely distributed in humans around the world and that more than 95% of adults have been infected by EBV in their past. The ability to grow EBV-infected B-cell lines from peripheral blood mononuclear cells (PBMCs) of EBV-seroconverted healthy subjects leads to the conclusion that this virus persists as a clinically silent infection for the lifetime of the infected host.1 Nevertheless, it is of note that the virus remains continuously active in immunocompetent individuals even though at very low levels.2
The EBV genome consists of a 180-kb double-stranded DNA that remains stable in the cell nucleus in an episomal form. In vitro immortalization of B cells by EBV is due to the expression of a restricted set of viral genes, the so-called latency III program, coding for latent membrane proteins (LMP1, LMP2a, LMP2b), Epstein-Barr nuclear antigens (EBNA1, 2, 3A, 3B, 3C, LP), and small noncoding RNAs (EBERs), and leads to the establishment of lymphoblastoid cell lines (LCLs).3 Among these genes, EBNA1 is responsible for episomal maintenance of the EBV genome.4 EBNA2 alone is responsible for the expression of the entire set of genes of the latency III program.5 EBNA2 reroutes the Notch pathway by targeting RBP-jkappa.6 EBNA2 is also the main viral transactivator of the BNLF1 gene coding for LMP1. LMP1 mimics a constitutively activated receptor of the TNF receptor family through aggregation of TRAF/TRADD molecules.7,8 In vivo, the latency III program is expressed in newly infected B-blasts during primary EBV infection.9 Most of these newly EBV-infected B-blasts are killed by cytotoxic T cells. However, some EBV-infected B-blasts are considered to follow the normal process of B-cell maturation until the B-cell memory stage.10 The B-cell memory compartment constitutes the EBV reservoir in humans. EBV is almost silent in its reservoir.10-12
The immune response against EBV is associated with a dramatic oligoclonal increase in EBV-specific CD8 cytotoxic T cells during the initial phase of primary EBV infection.13-15 Cytotoxic T cells may prevent in vitro de novo infection of B cells.16,17 In Purtilo syndrome, the defect of SH2D1A/SAP gene alters T-cell signaling in response to antigen.18 The SAP signaling pathway plays a key role in the cytolytic activity of T cells against EBV-positive targets,19 and Purtilo syndrome is characterized by fatal infectious mononucleosis after primo-infection.18 Emergence of EBV-associated lymphoma in patients with acquired T-cell immunodeficiencies such as HIV infection or posttransplantation immunosuppressive regimens provided strong evidence for the key role of the immune system in the control of EBV burden.20,21 Infusion of cytotoxic T cells may prevent EBV-induced lymphoma in allogeneic transplant recipients.22 EBV-associated lymphoproliferations and severe chronic active EBV infections with increased EBV genomes and EBV antibody titers have been reported in cases of congenital atypical lymphoproliferative syndromes (ALPSs), which indirectly suggest the role of CD95-CD95 ligand interaction in T-cell killing of EBV-infected B cells.23
CD95 is a member of the family of death receptors that initiate apoptosis by recruiting Fas-associated death domain protein (FADD), procaspase-8, procaspase-10, and cellular FLICE-like inhibitory protein to the death-inducing signaling complex (DISC), which forms after binding to the cognate ligand (CD95L).24,25 Various inducers of CD95 expression have been reported including NF-κB activators (TNFα, CD40),26,27 STAT1 activators (IFNγ),27 and p53 activators (chemotoxic agents).28,29 The CD95 promoter is known to harbor binding sites for NF-κB,30 STAT1,31,32 and p53.28,29 Expression of CD95 is also increased in EBV latency III–immortalized B cells.33 LMP1 has been reported to induce CD95 in BL cells,34 and this result was confirmed by cDNA array.35
In this work, we have studied the viral and cellular determinants responsible for regulation of CD95 expression. We have also analyzed the apoptotic function of CD95 in EBV latency III–immortalized LCLs. Our results support a model according to which LMP1 activation of NF-κB during the EBV latency III program renders the infected cells susceptible to their future CD95-mediated T-cell killing.
Materials and methods
Plasmid constructs and cDNA
The pRT-1–inducible vector was derived from the previously described CKR516 vector36 in which the Eμ-RSV promoter was replaced by a Eμ-chicken-beta-actin promoter (Eμ-CAG promoter). The EBNA1 gene was added to favor maintenance of the vector as an episome also in EBV-negative cells. The EGFP marker was replaced by an inactive truncated version of the NGF-R lacking the cytoplasmic domain (NGFR-t). The bidirectional tetracycline-inducible promoter drives the expression of both, NGFR-t and the cDNA of interest. Complete description of these vectors is done elsewhere.37 The cDNAs that were cloned into the SfiI sites coded for IκBαS32,36A (constitutively active form of IκBα),38 STAT1α (natural active isoform of STAT1), STAT1β (natural inactive isoform of STAT1),36 LMP1CT (a dominant-negative mutant of LMP139 ), and LMP1wt. We have also cloned the cDNA coding for p53wt from the BL2 cell line into a Topo vector (Invitrogen, Cergy-Pontoise, France) after polymerase chain reaction (PCR) amplification of its full-length mRNA (p53sense: 5′-GATTGGCCAGACTGCCTTC-3′; p53antisense: 5′-CAAGGGTTCAAAGACCCAAA-3′). After verifying its entire sequence, this cDNA was cloned into the SfiI sites of the pRT-1 vector.
Cell culture, treatment, transfection, and cell sorting
The lymphoblastoid cell line PRI, the EBV-negative Burkitt lymphoma cell line BL2, and the EREB2-5 cell line were cultured in RPMI 1640 medium (Gibco BRL-Life Technologies, Cergy-Pontoise, France) supplemented with 10% tetracycline-free decomplemented fetal calf serum (FCS; Clontech, Palo Alto, CA) and standard concentration of antibiotics. EREB2-5 cells were grown in the presence of 1 μM estradiol.6 Treatment of cells with BAY11-7082 (E-3-(4-methylphenylsulfonyl)-2-propenenitrile, BAY11, purchased from Calbiochem [San Diego, CA] and reconstituted in dimethyl sulfoxide [DMSO]) was performed as described.40
Stable transfection of cells and hygromycin selection were performed as described.36,38 Induction was stable after 4 weeks of selection. Varying with transfection, 60% to 90% of the cells became NGFR-t positive after 24-hour treatment with 1 μg/mL doxycyline.
Where indicated, NGFR-t–expressing cells were purified following the manufacturer's protocol (Miltenyi Biotech Macs microbeads, Auburn, CA): Briefly, viable cells were isolated by Ficoll-Paque Plus density gradient centrifugation (Eurobio, Les Ulis, France). Cells were divided in 2 batches, one treated with doxycyline for 36 hours at 1 μg/mL, and one without doxycyline. Both batches were then submitted in parallel to cell purification. Incubation was performed for 30 minutes with 1 μg mAb anti-NGFR (BD Pharmingen, San Diego, CA) for 106 doxycyline-treated cells, or without primary antibody for untreated cells. Cell separation was performed with 20 μL goat anti–mouse IgG magnetic beads (Miltenyi Biotech Macs microbeads) for 107 cells. Purified cells were resuspended in complete medium with or without doxycyline overnight. Then, where indicated, cells were treated for an additional 24 hours with either 100 UI/mL TNFα (Invitrogen), 250 UI/mL IFNγ (Roche Diagnostics, Meylan, France), 30 μM fludarabine (Schering, Lys-lez-Lannoy, France), anti-CD95 agonist BG27 mAb (Diaclone, Besançon, France) diluted at 1/50, anti-CD95–neutralizing BD29 mAb at 1/50 (Diaclone), or anti-CD95 ligand–neutralizing mAb BR17 (Diaclone) at 1/50.
Flow cytometry, annexin V labelings, and DNA content
Induction of NGFR-t expression was assessed by flow cytometry on an XL Beckman Coulter apparatus (Beckman Coulter, Villepinte, France) after indirect labeling with the anti-NGFR mAb (BD Pharmingen) diluted at 1/20, and a RPE-Cy5–conjugated goat anti–mouse IgG (Dako, Glostrup, Denmark). The apoptosis rate was measured using the annexin V–FITC kit (Beckman Coulter Immunotech, Marseille, France) according to the manufacturer's instructions. Direct labelings of CD2, CD3, CD4, CD5, CD7, CD8, CD19, CD25, and CD95 antigens were performed using PE-conjugated 39C1.5 mAb, PE-Cy5–conjugated UCHT1 mAb, PE-conjugated 13B8.2 mAb, FITC-conjugated BL1a mAb, PE-conjugated 8H8.1 mAb, FITC-conjugated B9.11 mAb, PE-Cy7–conjugated J4.119 mAb, FITC-conjugated B1.49.9 mAb, and PE-conjugated UB2 mAb, respectively, according to standard procedures recommended by the manufacturer (Beckman Coulter Immunotech).
DNA content of cells was assessed with the DNA Prep Kit (Beckman Coulter Immunotech), according to standard procedures recommended by the manufacturer.
Mixed lymphoproliferative reaction
Mixed lymphoproliferative reaction (MLR) was performed after purification of T cells from PBMCs of 3 healthy volunteer donors, using an anti-CD3 mAb (UCHT1 clone) using the same procedure described for purification of NGFR-t–positive cells. Purification was assessed by triple labelings for CD3, CD4, CD8, and for CD3, CD19, CD5. Levels of remaining monocytes and B cells were always less than 2%. Thirty million purified T cells were incubated with the same number of LCL cells (PRI cell line) irradiated at 75 Gy. Activation of T cells was assessed 3 days later by triple labelings for CD3, CD19, and CD25, and counting T cells expressing the CD25 marker. After 5 days of stimulation, activated T cells were purified using an anti-CD2 mAb followed by magnetic bead separation. Activated T cells were incubated for 24 hours with their targets (PRI cells) at a ratio of 4 T cells to 1 target cell. Induction of B-cell apoptosis was assessed by colabeling with FITC-conjugated annexin V and Pe-Cy7–conjugated anti-CD19. Inhibition of T-cell–mediated B-cell apoptosis was performed by preincubation of B cells with the antagonist BD29 anti-CD95 mAb or preincubation of activated T cells with the antagonist BR17 anti-CD95 ligand mAb for 15 minutes at room temperature.
Western blotting
Western blotting was performed as described elsewhere.36 The following antibodies were used: anti-CD95 (C-20 rabbit polyclonal; Santa Cruz Biotechnology, San Diego, CA) at 1/500, anti-STAT1 (clone 9H2; Cell Signaling, Beverly, MA) at 1/500, anti–phosphotyrosine-701 STAT1 (rabbit polyclonal; Cell Signaling) at 1/500, and anti-p53 (DO-7 clone; DAKO) at 1/500. Revelation was performed with the corresponding horseradish peroxidase–conjugated secondary antibody (Goat antimouse or Goat anti-rabbit at 1/5000 from Bio-Rad, Hercules, CA) followed by chemiluminescence reaction (ECL; Amersham, Orsay, France) and autoradiography (Xomat R films; Kodak, Rochester, NY). Quantification of the signal and normalization to Ponceau Red staining was performed with the Optiquant software (Hewlett Packard Instrument, Palo Alto, CA).
Quantitative RQ-PCR
Extraction of RNA and quantitative real-time (RQ)–PCR was performed as described.36,41 We defined, as reference RNA, a pool of RNAs extracted from different tonsils, lymph nodes, and spleen with benign reactive follicular hyperplasia. RNA levels for the CD95 (FAS) and A20 genes were quantified in parallel with the different RNA extracts and the RNA pool on an ABI PRISM 7000 automat using the TaqManR Assay on demand gene expression reference system (Applied Biosystem, http//www.appliedbiosystem.com; product reference nos. Hs00531110-m1 and Hs00234713-m1). The Abl1 gene was used as a reference gene for the control of amplification (product reference no. Hs00245443-m1). All amplification steps were performed in duplicate. The calculated relative gene expression level was equal to 2-DDCT, where DDCT is the delta delta CT and CT the cycle threshold, as previously described.36,41
Electrophoretic mobility shift assay (EMSA)
Results
Relationship between CD95 and LMP1 expression in EBV latency III–immortalized B cells
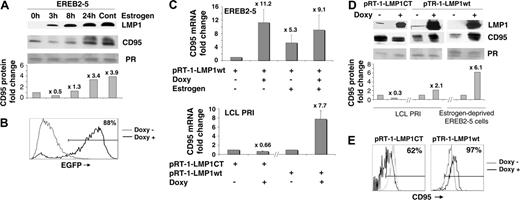
CD95 expression has been reported to be induced in EBV-infected B cells,33 raising the question of its regulation in these cells. In EREB2-5 cells in which the latency III program is under the control of an estrogen-regulatable EBNA2 protein,6 expression of CD95 was markedly induced by addition of estrogen (Figure 1A). This induction was preceded by induction of LMP1, the major EBV-transforming protein and a direct target gene product of EBNA2 (Figure 1A). Of importance, CD95 expression was also induced at the mRNA level (Figure 1C, upper panel), suggesting a transcriptional regulation of the gene.
Relationship between LMP1 and CD95 expression. (A) Kinetics of LMP1 and CD95 expression by Western blot in EBV latency III EBNA2-regulatable EREB2-5 cells: EREB2-5 cells were starved for estrogen for 48 hours to arrest the EBV latency III program. Readdition of estrogen reinduced EBNA2 activity and subsequent expression of the whole set of latency III viral genes. 0h indicates estrogen deprived; 3h, 8h, 24h, and cont indicate 3, 8, and 24 hours, and continuous estrogen exposition, respectively. Ponceau red staining of an 18-kDa band is shown (PR). After background subtraction, the CD95 signal was normalized to Ponceau red signal. Fold changes in CD95 protein were then calculated using the Optiquant system and are presented in the histogram. Fold change values are indicated at the top of each bar when compared with the estrogen-deprived condition. (B) Flow cytometry detection of LMP1CT induction in LCL cells transfected with pRT-1-LMP1CT after treatment of the cells with 1 μg/mL doxycycline for 36 hours. The percentage of EGFP-positive cells is indicated in the upper-right corner of the histogram. (C) CD95 mRNA expression by real-time RT-PCR (fold changes are indicated at the top of each bar when compared with the untreated condition) in EREB2-5 cells stably transfected with the pRT1-LMP1wt vector, in the absence (-) or presence (+) of doxycycline or estrogen (upper panel) and in LCL cells stably transfected either with pRT1-LMP1CT coding for a dominant-negative form of LMP1 or with the pRT1-LMP1wt–inducible vector (lower panel). Error bars correspond to the standard deviation of 3 experiments. (D) LMP1 and CD95 protein expression by Western blot in LCL and estrogen-deprived EREB2-5 cells stably transfected either with the pRT1-LMP1CT vector or with the pRT1-LMP1wt vector, in the absence (-) or presence (+) of doxycycline. As in Figure 1A, the CD95 signal was normalized to Ponceau red (PR) signal, and fold changes in CD95 protein are presented in the histogram. (E) Expression of surface membrane CD95 by flow cytometry in LCL cells transfected with either pRT1-LMP1CT or pRT1-LMP1wt vector. Percentage of positive cells for CD95 in the doxycycline-treated condition is indicated in the upper-right corner of the histogram.
Relationship between LMP1 and CD95 expression. (A) Kinetics of LMP1 and CD95 expression by Western blot in EBV latency III EBNA2-regulatable EREB2-5 cells: EREB2-5 cells were starved for estrogen for 48 hours to arrest the EBV latency III program. Readdition of estrogen reinduced EBNA2 activity and subsequent expression of the whole set of latency III viral genes. 0h indicates estrogen deprived; 3h, 8h, 24h, and cont indicate 3, 8, and 24 hours, and continuous estrogen exposition, respectively. Ponceau red staining of an 18-kDa band is shown (PR). After background subtraction, the CD95 signal was normalized to Ponceau red signal. Fold changes in CD95 protein were then calculated using the Optiquant system and are presented in the histogram. Fold change values are indicated at the top of each bar when compared with the estrogen-deprived condition. (B) Flow cytometry detection of LMP1CT induction in LCL cells transfected with pRT-1-LMP1CT after treatment of the cells with 1 μg/mL doxycycline for 36 hours. The percentage of EGFP-positive cells is indicated in the upper-right corner of the histogram. (C) CD95 mRNA expression by real-time RT-PCR (fold changes are indicated at the top of each bar when compared with the untreated condition) in EREB2-5 cells stably transfected with the pRT1-LMP1wt vector, in the absence (-) or presence (+) of doxycycline or estrogen (upper panel) and in LCL cells stably transfected either with pRT1-LMP1CT coding for a dominant-negative form of LMP1 or with the pRT1-LMP1wt–inducible vector (lower panel). Error bars correspond to the standard deviation of 3 experiments. (D) LMP1 and CD95 protein expression by Western blot in LCL and estrogen-deprived EREB2-5 cells stably transfected either with the pRT1-LMP1CT vector or with the pRT1-LMP1wt vector, in the absence (-) or presence (+) of doxycycline. As in Figure 1A, the CD95 signal was normalized to Ponceau red (PR) signal, and fold changes in CD95 protein are presented in the histogram. (E) Expression of surface membrane CD95 by flow cytometry in LCL cells transfected with either pRT1-LMP1CT or pRT1-LMP1wt vector. Percentage of positive cells for CD95 in the doxycycline-treated condition is indicated in the upper-right corner of the histogram.
Since (1) LMP1 induction by EBNA2 preceded CD95 induction, (2) LMP1 is the main NF-κB activator in latency III EBV–immortalized B cells, and (3) the regulatory region of the CD95 gene harbors NF-κB binding sites, we first attempted to study the role of LMP1 in CD95 induction by EBV. To this end, we used a novel double-inducible episomal vector, named pRT-1, as an expression vector (“Materials and methods” and Bornkamm et al37 ). To inhibit LMP1 signaling, we also used a new dominant-negative mutant of LMP1, called LMP1CT, consisting of EGFP fused in frame with the carboxyterminal end of LMP1.39 An advantage of this dominant form of LMP1 is its specificity against LMP1 signaling39 combined with the ability to follow expression of LMP1CT both by Western blot and flow cytometry due to its EGFP moiety. The cDNA of LMP1CT was cloned into pRT-1 and stably transfected into the LCL PRI. After 6 weeks of hygromycin selection, transfected cells were treated for 36 hours with doxycyline. More than 80% of cells became positive for EGFP (Figure 1B). EGFP induction paralleled a marked induction of LMP1CT at the protein level (Figure 1D). The decrease in TRAF1 expression after induction of LMP1CT reflects inhibition of LMP1 signaling due to a decrease in NF-κB activation (Wang et al44 and not shown). Induction of LMP1CT was associated with a decrease in CD95 expression at both RNA (Figure 1C, lower panel) and protein (Figure 1D) levels, as well as by flow cytometry (Figure 1E).
We then cloned the LMP1 wild-type cDNA (LMP1wt) into pRT-1 and stably transfected it into both LCL and EREB2-5 cells. Induction of LMP1wt was associated with an increase in CD95 expression in EBV latency III–immortalized B cells at both RNA and protein levels as well as by flow cytometry (Figure 1C [lower panel] and 1D). Induction of LMP1wt in estrogen-deprived EREB2-5 cells (ie, EBNA2 and LMP1-negative EBV-positive B cells) was also associated with an increase in CD95 expression (Figure 1C [upper panel] and 1D). Altogether, these results show that LMP1 up-regulates CD95 expression in EBV-immortalized B cells.
Relationship between CD95 expression and NF-κB, STAT1, and p53 activation in EBV-immortalized B cells
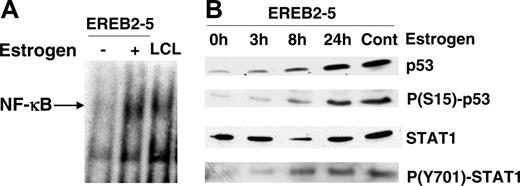
Three main transcriptional systems have been reported to regulate CD95 gene expression, NF-κB, STAT1, and p53. Therefore, we looked at their activation following induction of the latency III program in EREB2-5 cells (Figure 2). As expected, in EBV latency III–infected B cells,7,45 NF-κB binding activity was induced after treatment of EREB2-5 cells with estrogen (Figure 2A). We also found an induction of p53 protein expression (Figure 2B). Moreover, phosphorylation of p53 on serine 15 paralleled induction of the p53 protein, reflecting its activation during the EBV latency III program. STAT1 protein levels decreased shortly after addition of estrogen and reincreased at later time points. Activation of STAT1, reflected by its phosphorylation at tyrosine 701, continuously increased after estrogen induction of EREB2-5 cells (Figure 2B). Therefore, the 3 transcriptional systems, NF-κB, p53, and STAT1, are activated during the induction of the EBV latency III proliferation program. Additionally, treatment of EBV-negative BL2 cells with known inducers of NF-κB, STAT1, and p53, such as TNFα, IFNγ, or fludarabine, respectively, was associated with an increase in CD95 expression at both protein and RNA levels (data not shown).
To demonstrate the role of NF-κB, STAT1, and p53 in the regulation of CD95, we cloned a series of cDNAs coding for dominant-active or dominant-negative variants of these transcription factors into the pRT-1–inducible vector, including IκBαS32,36A, STAT1β, STAT1α, and p53wt. Stably transfected PRI LCL cells for each of these conditional expression constructs were established and investigated. Induction of IκBαS32,36A was associated with a decrease in both NF-κB binding activity (Feuillard et al38 and not shown) and CD95 mRNA expression (Figure 3A, upper panel). The decrease in CD95 protein expression by Western blot was only marginal but paralleled variations of CD95 mRNA (Figure 3A, middle panel). This Western blot result may be due to a weak sensitivity of this technique for detection of small variations of CD95 protein levels since surface membrane CD95 expression was decreased by flow cytometry in IκBαS32,36A-expressing cells (Figure 3A, lower panel). Treatment of pRT-1-STAT1β–transfected cells with IFNγ, a specific STAT1 activator, markedly induced CD95 mRNA expression in the absence of doxycycline, yet, induction of CD95 by IFNγ was completely abolished by overexpression of STAT1β, the natural dominant-negative isoform of STAT1 (Figure 3B, upper panel). Induction of CD95 by IFNγ was only partially abolished at the protein level by STAT1β (Figure 3B, lower panel), suggesting a posttranscriptional regulation of CD95 expression in these conditions. Treatment of pRT-1-STAT1α–transfected cells with both doxycycline and IFNγ markedly induced CD95 mRNA expression (data not shown). Treatment of pRT-1-P53wt–transfected cells with fludarabine or with doxycycline also induced CD95 mRNA and CD95 protein expression (Figure 3C). These results show that the 3 transcriptional factors, NF-κB, STAT1, and p53, participate in the regulation of CD95 gene expression in LCL cells.
Induction of NF-κB, STAT1, and p53 activation in latency III–induced EREB2-5 cells. (A) NF-κB binding activity in estrogen-deprived (-) or estrogen-treated (+) EREB2-5 cells and in LCL cells by EMSA. (B) Kinetics of p53 protein expression, serine 15 p53 phosphorylation, STAT1 protein expression, and tyrosine 701 STAT1 phosphorylation in EREB2-5 cells. 0h indicates estrogen-deprived; 3h, 8h, 24h, and cont indicate 3, 8, and 24 hours, and continuous estrogen exposition, respectively.
Induction of NF-κB, STAT1, and p53 activation in latency III–induced EREB2-5 cells. (A) NF-κB binding activity in estrogen-deprived (-) or estrogen-treated (+) EREB2-5 cells and in LCL cells by EMSA. (B) Kinetics of p53 protein expression, serine 15 p53 phosphorylation, STAT1 protein expression, and tyrosine 701 STAT1 phosphorylation in EREB2-5 cells. 0h indicates estrogen-deprived; 3h, 8h, 24h, and cont indicate 3, 8, and 24 hours, and continuous estrogen exposition, respectively.
Since NF-κB is the main transcriptional system regulated by LMP1, we attempted to evaluate the NF-κB dependence of p53 and STAT1 activation. We found that inhibition of NF-κBbyIκBαS32,36A was associated with a marked decrease in both STAT1 protein and tyrosine 701 STAT1 phosphorylation (Figure 4A), as well as a decrease in serine 15 phosphorylation of p53 (Figure 4B). These results suggest that activation of both STAT1 and p53 is dependent on NF-κB activation in these cells. We then assessed the effect of NF-κB inhibition on STAT1 and p53 signaling by treatment of cells with the inhibitor Bay11 in the presence of either IFNγ or fludarabine. Treatment with Bay11 alone decreased expression of CD95 in parental PRI LCL cells in the same order of magnitude as induction of IκBαS32,36A in pRT-1-IκBαS32,36A–transfected cells (compare Figure 4C with Figure 3A). CD95 mRNA levels were markedly increased after IFNγ treatment (Figure 4C). This effect was significantly reduced in the presence of Bay11 (Figure 4A). Fludarabine treatment induced a moderate increase of CD95 mRNA levels in parental PRI cells (compare Figure 4C and 3C), an effect that was also affected by Bay11 treatment (Figure 4C). Altogether, these results indicate that NF-κB is likely to control CD95 expression both directly and indirectly via secondary STAT1 and p53 activation and that regulation of CD95 by either STAT1 or p53 inducers may depend on NF-κB.
CD95 targeting in EBV latency III–immortalized B cells
In a next step, we wanted to assess the function of CD95 in LCL cells. We first wanted to link LMP1-induced CD95 expression with CD95-mediated apoptosis. We analyzed the induction of CD95-mediated apoptosis in LCL cells stably transfected with either pRT-1-LMP1CT or pRT-1-LMPwt. Treatment with the agonistic mAb BG27 against CD95 clearly induced apoptosis of LCL PRI cells, whereas the anti-CD95–neutralizing mAb BD29 had virtually no effect (Figure 5). Induction of LMP1CT, the dominant-negative form of LMP1, had a slight effect on the level of apoptosis in LCL PRI cells, an effect detectable mainly at 48 hours that more than likely reflected the inhibition of LMP1 signaling by LMP1CT. LMP1CT overexpression was associated with a decrease in CD95-mediated apoptosis, probably related to a decrease in the density of surface membrane CD95 molecules. LMP1wt induction was associated with an increase in spontaneous apoptosis of transfected cells (Figure 5), reflecting the known toxicity of LMP1.46 Overexpression of LMP1wt was associated with a marked increase in CD95-mediated apoptosis at 48 hours (Figure 5), suggesting that LMP1-induced CD95 expression is associated with sensitization of cells to CD95-mediated apoptosis. These results show that the CD95 apoptosis pathway is functional in EBV latency III–immortalized B cells. Moreover, this suggests that susceptibility of LCL cells to CD95-mediated apoptosis is related to the surface membrane density of CD95 molecules, a parameter that is under the control of LMP1.
Relationship between CD95 expression and NF-κB, STAT1, or p53 activation in LCL cells. (A) CD95 expression in LCL cells transfected with pRT-1-IκBαS32,36A–inducible vector pretreated with (+) or without (-) doxycycline for 36 hours and additionally treated with TNFα for 24 hours or left untreated. Upper panel: CD95 mRNA expression by real-time RT-PCR (fold changes are indicated at the top of each bar compared with the untreated condition; error bars correspond to the standard deviation of 3 experiments). Middle panel: CD95 expression by Western blot. As in Figure 1A, the CD95 signal was normalized to Ponceau red (PR) signal and fold changes in CD95 protein are presented in the histogram. Lower panel: Expression of surface membrane CD95 by flow cytometry in LCL cells transfected with pRT1-IκBαS32,36A. Percentage of CD95+ cells is given in the upper-left corner of the histogram for NGFR-positive (IκBαS32,36A expressing) cells and in the upper-right corner of the histogram for NGFR-negative cells. (B) CD95 expression in LCL cells transfected with pRT-1-STAT1β–inducible vector pretreated with (+) or without (-) doxycycline for 36 hours and additionally treated with IFNγ for 24 hours or left untreated. Upper panel: CD95 mRNA expression by real-time RT-PCR (fold changes are indicated at the top of each bar compared with the untreated condition; error bars correspond to the standard deviation of 3 experiments). Lower panel: Expression of CD95 and STAT1 by Western blot. As in Figure 1A, the CD95 signal was normalized to Ponceau red (PR) signal and fold changes in CD95 protein are presented in the histogram. (C) CD95 expression in LCL cells transfected with pRT-1-p53wt vector pretreated with (+) or without (-) doxycycline for 36 hours and additionally treated with fludarabine for 24 hours or left untreated. Upper panel: CD95 mRNA expression by real-time RT-PCR (fold changes are indicated at the top of each bar compared with the untreated condition; error bars correspond to the standard deviation of 3 experiments). Lower panel: Expression of CD95 and p53 by Western blot. As in Figure 1A, the CD95 signal was normalized to Ponceau red (PR) signal and fold changes in CD95 protein are presented in the histogram. Note that protein loading was decreased in the doxycycline-treated condition, probably due to increase of spontaneous apoptosis of the cells (not shown).
Relationship between CD95 expression and NF-κB, STAT1, or p53 activation in LCL cells. (A) CD95 expression in LCL cells transfected with pRT-1-IκBαS32,36A–inducible vector pretreated with (+) or without (-) doxycycline for 36 hours and additionally treated with TNFα for 24 hours or left untreated. Upper panel: CD95 mRNA expression by real-time RT-PCR (fold changes are indicated at the top of each bar compared with the untreated condition; error bars correspond to the standard deviation of 3 experiments). Middle panel: CD95 expression by Western blot. As in Figure 1A, the CD95 signal was normalized to Ponceau red (PR) signal and fold changes in CD95 protein are presented in the histogram. Lower panel: Expression of surface membrane CD95 by flow cytometry in LCL cells transfected with pRT1-IκBαS32,36A. Percentage of CD95+ cells is given in the upper-left corner of the histogram for NGFR-positive (IκBαS32,36A expressing) cells and in the upper-right corner of the histogram for NGFR-negative cells. (B) CD95 expression in LCL cells transfected with pRT-1-STAT1β–inducible vector pretreated with (+) or without (-) doxycycline for 36 hours and additionally treated with IFNγ for 24 hours or left untreated. Upper panel: CD95 mRNA expression by real-time RT-PCR (fold changes are indicated at the top of each bar compared with the untreated condition; error bars correspond to the standard deviation of 3 experiments). Lower panel: Expression of CD95 and STAT1 by Western blot. As in Figure 1A, the CD95 signal was normalized to Ponceau red (PR) signal and fold changes in CD95 protein are presented in the histogram. (C) CD95 expression in LCL cells transfected with pRT-1-p53wt vector pretreated with (+) or without (-) doxycycline for 36 hours and additionally treated with fludarabine for 24 hours or left untreated. Upper panel: CD95 mRNA expression by real-time RT-PCR (fold changes are indicated at the top of each bar compared with the untreated condition; error bars correspond to the standard deviation of 3 experiments). Lower panel: Expression of CD95 and p53 by Western blot. As in Figure 1A, the CD95 signal was normalized to Ponceau red (PR) signal and fold changes in CD95 protein are presented in the histogram. Note that protein loading was decreased in the doxycycline-treated condition, probably due to increase of spontaneous apoptosis of the cells (not shown).
Relationship between NF-κB activation and STAT1 or p53 activation in LCL cells. (A) Expression of STAT1 protein and levels of STAT1 tyrosine 701 phosphorylation in LCL cells transfected with pRT-1-IκBαS32,36A–inducible vector treated with (+) or without (-) doxycycline for 36 hours. (B) Expression of p53 protein and levels of p53 serine 15 phosphorylation in LCL cells transfected with pRT-1-IκBαS32,36A–inducible vector and pretreated with (+) or without (-) doxycycline for 36 hours. (C) CD95 mRNA expression by real-time RT-PCR (fold changes are indicated at the top of each bar compared with the untreated condition; error bars correspond to the standard deviation of 2 experiments) in LCL cells treated or not with the NF-κB inhibitor Bay11 in the presence or the absence of either IFNγ or fludarabine*.
Relationship between NF-κB activation and STAT1 or p53 activation in LCL cells. (A) Expression of STAT1 protein and levels of STAT1 tyrosine 701 phosphorylation in LCL cells transfected with pRT-1-IκBαS32,36A–inducible vector treated with (+) or without (-) doxycycline for 36 hours. (B) Expression of p53 protein and levels of p53 serine 15 phosphorylation in LCL cells transfected with pRT-1-IκBαS32,36A–inducible vector and pretreated with (+) or without (-) doxycycline for 36 hours. (C) CD95 mRNA expression by real-time RT-PCR (fold changes are indicated at the top of each bar compared with the untreated condition; error bars correspond to the standard deviation of 2 experiments) in LCL cells treated or not with the NF-κB inhibitor Bay11 in the presence or the absence of either IFNγ or fludarabine*.
Induction of CD95-mediated apoptosis depends on LMP1 in LCL cells. pRT-1-LMP1CT (light gray bars) and pRT-1-LMP1wt (dark gray bars) transfected cells were pretreated with (+) or without (-) doxycycline for 36 hours. Cells were maintained ± doxycycline and additionally treated with the anti-CD95 agonistic mAb BG27 or with the CD95-neutralizing mAb BD29 for 24 hours, 48 hours, or left untreated. Apoptosis was quantified by flow cytometry, measuring the percentage of sub-G1 cells after cell staining with the propidium iodide dye of DNA.
Induction of CD95-mediated apoptosis depends on LMP1 in LCL cells. pRT-1-LMP1CT (light gray bars) and pRT-1-LMP1wt (dark gray bars) transfected cells were pretreated with (+) or without (-) doxycycline for 36 hours. Cells were maintained ± doxycycline and additionally treated with the anti-CD95 agonistic mAb BG27 or with the CD95-neutralizing mAb BD29 for 24 hours, 48 hours, or left untreated. Apoptosis was quantified by flow cytometry, measuring the percentage of sub-G1 cells after cell staining with the propidium iodide dye of DNA.
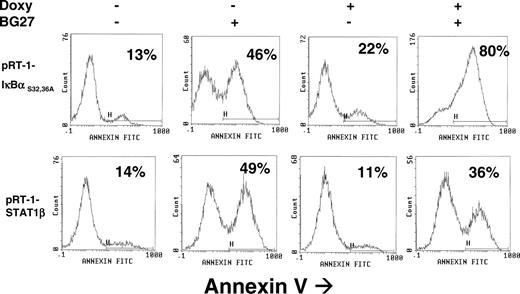
Induction of CD95-mediated apoptosis in LCL cells. pRT-1-IκBαS32,36A–transfected (upper panel) or pRT-1-STAT1β–transfected (lower panel) cells were pretreated with (+) or without (-) doxycycline for 36 hours. Cells were maintained ± doxycycline and additionally treated with the anti-CD95 agonistic mAb BG27 for 24 hours or left untreated. Percentage of annexin V–positive cells is indicated in each graph. Results were confirmed by quantification of sub-G1 peak of cells (not shown).
Induction of CD95-mediated apoptosis in LCL cells. pRT-1-IκBαS32,36A–transfected (upper panel) or pRT-1-STAT1β–transfected (lower panel) cells were pretreated with (+) or without (-) doxycycline for 36 hours. Cells were maintained ± doxycycline and additionally treated with the anti-CD95 agonistic mAb BG27 for 24 hours or left untreated. Percentage of annexin V–positive cells is indicated in each graph. Results were confirmed by quantification of sub-G1 peak of cells (not shown).
We then analyzed the induction of CD95-mediated apoptosis in LCL cells stably transfected either with pRT-1-IκBαS32,36A or pRT-1-STAT1β. In the absence of doxycyline, treatment of cells with the agonistic monoclonal antibody BG27 against CD95 resulted in nearly 50% apoptosis induction (Figure 6). In pRT-1-IκBαS32,36A stably transfected cells, inhibition of NF-κB by doxycyline pretreatment markedly sensitized cells to CD95-mediated apoptosis (Figure 6). As previously shown (Figure 3A), inhibition of NF-κB marginally or moderately decreased CD95 expression at the protein level by Western blot or by flow cytometry. It is of note that inhibition of NF-κB was also associated with a decrease in TRAF1 and CIAP2 gene expression (not shown), both involved in protection against apoptosis by NF-κB.44 Therefore, the consequence of NF-κB inhibition was to sensitize LCL cells to CD95-mediated apoptosis. In pRT-1-STAT1β stably transfected cells, overexpression of STAT1β, the inhibitor of STAT1α, resulted in a weak but detectable decrease in CD95-mediated apoptosis (36% versus 49%), suggesting that STAT1α activation is likely to favor this apoptotic pathway.
As a first attempt to demonstrate a possible functional link between the CD95 activation pathway and T-cell–mediated homeostasis of EBV-immortalized B cells, we performed in vitro MLR in order to test the role of CD95-CD95 ligand interaction in assays among the strongest cytotoxic killing reaction that can be induced in vitro. To this end, EBV-immortalized B cells were incubated with cytotoxic T cells presensitized to their cellular targets. Most EBV-infected B cells became apoptotic after 18-hour coculture with T cells. B-cell apoptosis was inhibited when B cells were preincubated with the neutralizing antibody BD29 against CD95 (Figure 7) or when T cells were preincubated with the BR17 antibody against CD95 ligand (Figure 7). These results clearly suggest a role for CD95-CD95 ligand interaction in the induction of T-cell–mediated killing of LCL cells. At this stage, we cannot attribute a specific role for CD8 cytotoxic T cells or, more temptingly, for CD4 T cells known to be key players in the killing of EBV-infected cells and for inducing CD95 signaling of target cells.17,47
Discussion
In this report, we present a comprehensive study of the regulation of CD95 in EBV latency III–immortalized B cells. Our results show that LMP1 is able to control CD95 expression through the activity of NF-κB, STAT1, and p53 and that STAT1 and p53 are under the control of NF-κB. Furthermore, we show that CD95 expression allows T cells to kill EBV-immortalized B cells.
Induction of T-cell killing of EBV-immortalized LCL cells. A mixed lymphocyte reaction was performed by incubating T cells with their targets (LCL cells) in the presence (+) or absence (-) of the anti-CD95–neutralizing mAb BD29 or anti-CD95 ligand–neutralizing mAb BR17. Anti-CD95 BD29 mAb and anti-CD95 ligand BR17 mAb were preincubated for 15 minutes at room temperature with LCL cells or purified primed T cells, respectively, before coincubation of T cells with their targets. Control indicates LCL cells incubated with an irrelevant anti-NGFR antibody; LCL + primed T cells, LCL cells incubated with T cells primed against their target by coculture with the same LCL cells irradiated at 75 Gy for 5 days.
Induction of T-cell killing of EBV-immortalized LCL cells. A mixed lymphocyte reaction was performed by incubating T cells with their targets (LCL cells) in the presence (+) or absence (-) of the anti-CD95–neutralizing mAb BD29 or anti-CD95 ligand–neutralizing mAb BR17. Anti-CD95 BD29 mAb and anti-CD95 ligand BR17 mAb were preincubated for 15 minutes at room temperature with LCL cells or purified primed T cells, respectively, before coincubation of T cells with their targets. Control indicates LCL cells incubated with an irrelevant anti-NGFR antibody; LCL + primed T cells, LCL cells incubated with T cells primed against their target by coculture with the same LCL cells irradiated at 75 Gy for 5 days.
To perform this study, we used a conditional expression system in a systematic manner in order to control the activity of a given factor that has been putatively involved in the control of CD95 expression. To control the whole EBV latency III program, we have used the EREB2-5 cell line harboring an estrogen-regulatable EBNA2 protein.6 To control the activity of LMP1, NF-κB, STAT1, and p53, we constructed a novel inducible episomal vector, called pRT-1, that carries all the elements for conditional gene expression including the gene of interest on one EBV-derived episomally replicating plasmid,37 including the rtTA2s-M2 cassette48-50 and a bidirectional doxycycline-regulatable promoter that allowed simultaneous expression of the gene of interest and a second gene (eg the truncated NGF receptor gene [NGFR-t]) used as surrogate marker. Using these versatile vectors, we were able to control LMP1, NF-κB, p53, and STAT1 activity in both EREB2-5 cells and the LCL PRI, providing useful tools for the understanding of CD95 regulation in EBV-infected B cells. Of interest, we found that contribution of LMP1 to the regulation of CD95 expression was higher than that of each of the 3 transcriptional factors individually, which may likely correspond to a synergistic or cooperative interaction between these factors in the regulation of the CD95 promoter.
EBV is one of the most powerful transforming agents of B cells in vivo and in vitro. This immortalization potential is counterbalanced by the host capacity to elaborate a vigorous cytotoxic immune response against the virus. The final result is the establishment of a host/virus equilibrium so that EBV persists in the circulating memory B-cell compartment in a silent form throughout life.12,51 EBV has coevolved with its human host and is thus excellently adapted. Strikingly, it has been suggested that the selective pressure exerted on the virus has favored its immunogenicity.52 The role of cytotoxic T cells in the control of the EBV burden is well established. Primary EBV infection is associated with both natural killer (NK) and T-cell cytotoxic responses against the virus, with a tremendous expansion of EBV-specific T-cell clones.13,14 Furthermore, CD4+ T lymphocytes have a key role for CD95-mediated killing of EBV latency III–immortalized B cells.47,53 EBV-specific T cells may prevent immortalization of cord blood cells by the virus.17 Immunosuppressive regimens following organ transplantation are responsible for EBV-associated lymphoproliferative disorders that may regress when immunosuppression is decreased.54 Posttransplantation lymphoproliferative disorders (PTLDs) are preceded by an increase in EBV burden, and injection of donor lymphocytes results in a marked reduction of EBV-infected cells and the prevention of PTLDs.22
Not only T-cell defects but also defects in induction and execution of apoptosis may lead to emergence of EBV-associated lymphoproliferative disease. This is illustrated by the frequency of EBV-associated lymphomas in ataxia telangiectasia syndrome, which corresponds to secondary mutations of ATM resulting in a defect of p53 activation in the case of DNA strand breaks.55-57 Atypical lymphoproliferative syndrome (ALPS), a recently described entity, is a genetic disorder that involves the Fas-Fas ligand pathway of cell death, which was first reported to be related to CD95 germ-line mutations.58,59 ALPS may be associated with chronic active EBV infection in children, a disease caused by a defect of cytotoxic T lymphocyte (CTL) and NK killing.23 The risk of developing non-Hodgkin and Hodgkin lymphomas in ALPS is 14 and 51 times higher, respectively, than in healthy subjects.60 A significant proportion of these tumors is associated with EBV, and it has been speculated that a defect in the CD95-mediated pathway for killing of virally infected cells might allow EBV to persist.60
Expression of CD95 in EBV latency III–immortalized B cells has been reported,33 and a role for LMP1 in the regulation of CD95 expression has been suggested.34 In vivo, both cellular expression and serum concentration of CD95 are markedly increased in patients with infectious mononucleosis.61 The regulatory region of the CD95 gene has been characterized and has revealed its putative capacity to integrate various signals, including NF-κB, STAT1, and/or p53 activation.26-28,30 Agents known to activate these 3 transcriptional systems such as TNFα, IFNγ, or genotoxic agents are known to induce CD95 expression.24 Our results clearly show that EBV-induced CD95 expression is related to LMP1 expression. Our results also argue for the fact that both STAT1 and p53 activation depends on NF-κB activation for regulation of C95 expression, raising the question of the relationship between NF-κB activation by LMP1 and STAT1 or p53 activation.
The signaling pathway of STAT1 activation in EBV latency III–immortalized B cells has been a matter of controversy. A direct interaction between LMP1 and the JAK3 kinase has been described,62 but was not confirmed by other reports.63 A recent report shows that most STAT1 activity in EBV-infected B cells is related to an autocrine loop through the secretion of IFNγ, an effect that is under the control of NF-κB.64
Regulation of p53 activity in EBV-infected B cells is the result of a complex interplay between various cellular factors. LMP1 induces both antiapoptotic and proapoptotic genes, including p53 target genes.35 C-myc, which is induced by EBNA2,65 contributes to the regulation of TP53 gene expression.66 P53 induction and activation during infection of B cells by EBV also depends on NF-κB.67 STAT1 activation is likely to favor p53 activation.36,68 Conversely, induction of p53-mediated apoptosis is inhibited in EBV-infected cells by A20 protein,69 a p53 inhibitor that is a target gene of NF-κB.45 The p65/RelA subunit of NF-κB also decreases p53 transcriptional activity by competing for CBP/p300 protein.70,71 Therefore, EBV latency III–immortalized B cells harbor both proapoptotic and antiapoptotic activated factors, with reciprocal inhibition and/or activation between them, resulting in a dynamic equilibrium that is likely to be evolutive according to the microenvironment, being able to lead the infected cell to apoptosis or proliferation.
Altogether, our results indicate that LMP1 is responsible for CD95 up-regulation through the activation of NF-κB activation. Moreover, our results suggest that NF-κB also controls STAT1 activation and contributes to p53 activation, and that both STAT1 and p53 activation positively regulate CD95 expression in LCL cells. Both p53 and STAT1 favor apoptosis, whereas NF-κB is antiapoptotic. The CD95-mediated apoptosis pathway is functional and is likely to participate in T-cell killing of EBV latency III–immortalized B cells. These results, with some from the literature, show that EBV both protects infected cells against apoptosis through NF-κB activation and renders them susceptible to future CD95-mediated apoptosis.
In conclusion, our results support the notion that the EBV latency III program, in addition to B-cell immortalization, activates a cellular program that sensitizes the infected cells to their elimination by T cells. These results suggest that EBV induction of CD95 plays a key role in host control of EBV-immortalized B cells in vivo, and stresses the importance of secondary genetic events in the emergence of EBV-associated lymphoproliferative disorders.72
Prepublished online as Blood First Edition Paper, November 29, 2005; DOI 10.1182/blood-2005-05-2053.
Supported by Cancéropôle Grand-Sud-Ouest, Ligue Nationale contre le Cancer, Conseil Régional du Limousin, and Association pour la Recherche sur le Cancer. UMR CNRS is laboratoire labellisé Ligue Nationale contre le Cancer. C.L.C. is supported by Ligue Nationale contre le Cancer, comité de la Corrèze. I.Y.-M. is supported by Conseil Régional du Limousin, and G.W.B., by Deutsche Forschungsgemeinschaft (DFG, SFB455).
C.L.C. performed research and analyzed data; I.Y.-M. performed research, contributed vital new reagents, and analyzed data; E.A., J.C., and G.W.B. contributed vital new reagents and analyzed data; and J.F. designed research, performed research, contributed vital new reagents, analyzed data, and wrote the paper.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
We would like to thank A. Allegraud and J. L. Faucher from Laboratoire d'Hématologie CHU Dupuytren for technical assistance in cell culture and flow cytometry, respectively. We thank Dr Jenny Cook, EA 3839, Faculté de Médecine de Limoges, for English rereading. We also thank the 2 anonymous reviewers for their helpful suggestions.





This feature is available to Subscribers Only
Sign In or Create an Account Close Modal