It has been suggested that human cytomegalovirus (HCMV) evades the immune system by infecting and paralyzing antigen-presenting cells. This view is based mainly on studies of dendritic cells (DCs) obtained after culture of monocytes (moDCs). It is contradicted by the asymptomatic course of HCMV infection in healthy persons, indicating that other key antigen-presenting cells induce an efficient immune response. Here we show that HCMV activates CD11c+ DCs and plasmacytoid DCs (PDCs). In contrast to moDCs, CD11c+ DCs and PDCs produced interferon (IFN) type 1 when exposed to HCMV. Autocrine IFN type 1 partially protected CD11c+ DCs against infection, whereas PDCs were resistant to HCMV even when IFN type 1 activity was inhibited. HCMV exposure induced the maturation of CD11c+ DCs by IFN type 1-dependent and -independent mechanisms. Importantly, CD11c+ DCs infected by inhibiting IFN type 1 activity retained full capacity to stimulate T cells. Renal transplant recipients receiving immunosuppressive treatment had lower frequencies of CD11c+ DCs and PDCs in blood than did healthy controls. The results show that HCMV activates the immune system by interacting with CD11c+ DCs and PDCs and that recipients of renal transplants have low frequencies of these cell types in blood.
Introduction
Human cytomegalovirus (HCMV) infection represents a major clinical problem in immunocompromised persons, including transplant recipients and AIDS patients. Prevalence of HCMV seropositivity ranges between 50% and 90% in healthy adults; after primary infection, the virus establishes lifelong latency in the host.1 Usually, CMV in immunocompromised patients is caused by the reactivation of latent virus. A number of studies have suggested that the virus may aggravate immunodeficiency by interfering with antigen presentation.2-7 In these publications, dendritic cells (DCs) have been generated from monocytes or CD34+ bone marrow progenitor cells after 5 to 12 days of cytokine-supplemented culture. Such monocyte-derived DCs (moDCs) or bone marrow-derived Langerhans cells are highly potent stimulators of T-cell activation. Exposure of these DCs to HCMV leads to functional paralysis of the cells, causing impaired T-cell activation.2-7 Several mechanisms for this immunosuppression have been suggested. First, major histocompatibility complex (MHC) class I and class II and costimulatory molecules are down-regulated, resulting in impaired antigen presentation and increased susceptibility to natural killer cell-mediated lysis.2-8 Second, a virally induced, soluble immunosuppressive factor released by mature moDCs has been postulated by several groups and was recently identified as CD83.5,7 Third, infection of immature moDCs is lytic and leads to cell death.7
Studies of HCMV effects on moDCs and Langerhans cells have shed light on mechanisms for viral immune evasion. However, the results showing paralysis of antigen presentation also raise questions as to how HCMV can be so effectively controlled in the immunocompetent host. Most primary infections induce minimal symptoms, and there is no clear evidence for clinically relevant immunosuppression.1 Reactivation of the latent virus is believed to happen frequently,9,10 but it proceeds asymptomatically in a healthy host, indicating a successful immune response. Indeed, an impressively large part of the T-cell repertoire is HCMV specific in seropositive persons. Labeling with MHC class I tetramers in complex with HCMV peptides has shown that 1% to 4% of all CD8+ T cells are specific for HCMV proteins.11,12 In view of the low pathogenicity of HCMV in immunocompetent persons, paralysis of key antigen-presenting cells by the virus seems unlikely.
Elegant studies in mice have elucidated mechanisms for successful immunity to murine CMV (MCMV).13 After injection of virus into mice, a small subset of DCs, termed plasmacytoid dendritic cells (PDCs), produce high levels of IFN-α, IL-12, and TNF-α. The rapid innate response is followed by maturation of several well-defined DC subsets in the mouse spleen and initiation of a strong MCMV-directed T-cell response. Frequencies of infected DCs were generally low and were restricted to a subset expressing CD8α. These results show a high degree of specialization between murine DCs with regard to their roles in MCMV immunity and cell type-dependent heterogeneity in susceptibility to infection.
The discrepancy between the suppressive and the stimulatory effects of CMV on human and murine DCs, respectively, could be species related. CMVs are highly adapted to their hosts.1 Therefore, it may be difficult to compare results obtained with human cells and animal models. Another possibility is that moDCs are not representative of the DCs that combat HCMV in humans. The 2 major types of DCs that have been isolated from human blood and lymphoid organs14-16 are not derived from monocytes but from dedicated bone marrow progenitors.15,17 Human CD123hi (IL-3Rαhi) PDCs15,16 share a multitude of characteristics with their murine counterparts,18,19 though important differences in functions and phenotype have also been demonstrated. For example, only murine PDCs produce high levels of IL-12.20,21 The human CD11c+ DCs do not have a clear murine counterpart, but they share several functional properties with subsets of CD11chi murine DCs.22 The murine and human CD11c+ DCs also appear to develop through similar developmental pathways.17,23
Because moDCs are readily infected and paralyzed by HCMV, we hypothesized that other DC types initiate an innate immune response and retain T cell-stimulatory capacity to fight the virus in immunocompetent persons. We examined the possibility that PDCs and CD11c+ DCs serve these functions. We also determined whether frequencies of PDCs and CD11c+ DCs were reduced in recipients of transplanted organs who are at high risk for active HCMV infection.
Materials and methods
Media, cell lines, and cytokines
RPMI 1640 with HEPES (N-2-hydroxyethylpiperazine-N′-2-ethanesulfonic acid) and l-glutamine (PAA Laboratories GmbH, Pasching, Austria) was supplemented with 10% pooled heat-inactivated HCMV-seronegative human serum (PS) or 10% heat-inactivated fetal calf serum (FCS) (Gibco, Invitrogen, Paisley, Scotland), penicillin 50 U/mL, and streptomycin 50 μg/mL (Sigma Chemical, St Louis, MO), referred to as RPMI 10% PS and RPMI 10% FCS, respectively. For production and titration of stock virus, low-passage (less than 20) human embryonic (HE) fibroblasts (National Institute of Public Health, Oslo, Norway) were propagated in minimal essential medium (MEM; Gibco) with 10% heat-inactivated, endotoxin- and Mycoplasma-free FCS. After the addition of virus, cells were maintained in MEM supplemented with 2% FCS, l-glutamine (0.3 mg/mL), gentamicin (40 μg/mL), amphotericin B (2.5 μg/mL), and penicillin G (6 μg/mL). HE cells were routinely screened for Mycoplasma with DNA staining with bisbenzimides (Behring Werke AG, Marburg, Germany). The following recombinant human cytokines were used: granulocyte macrophage-colony-stimulating factor (GM-CSF) (Leukomax; Sandoz, Basel, Switzerland), interleukin-4 (IL-4; R&D Systems, Minneapolis, MN), interleukin-3 (IL-3; Peprotech, Rocky Hill, NJ), tumor necrosis factor-α (TNF-α; R&D Systems), prostaglandin E2 (PGE2; Sigma Chemical) and IFN-α (IntronA; Schering-Plough International, Kenilworth, NJ).
Antibodies
The following mouse monoclonal antibodies (mAbs) directly conjugated with fluorescein isothiocyanate (FITC), phycoerythrin (PE), peridinin chlorophyll protein (PerCP), or allophycocyanin (APC) were used: anti-CD83 PE, anti-CD11c PE, anti-CD16 PE, anti-CD19 PE, anti-CD56 PE, anti-HLA-DR PerCP, anti-CD11c APC, and anti-HLA-ABC APC (BD Biosciences, San Jose, CA); anti-CD3 PE and anti-CD14 PE (Diatec.com, Oslo, Norway), anti-CD123 APC (Miltenyi Biotec, Bergish Gladbach, Germany); anti-immediate early antigen (IEA) FITC (clone 8B1.2; Chemicon International, Temecula, CA). Mouse isotype controls were immunoglobulin G2a (IgG2a) FITC, IgG1/IgG2a PE (Diatec.com); IgG2a PerCP, IgG1 APC (BD Biosciences), and IgG1 (Sigma Chemical). The following unconjugated mAbs were used: anti-human IFN receptor (R) αβ (CD118) (clone MMHAR-2; PBL Biomedical Laboratories, Piscataway, NJ) and anti-IEA (clone E13; Argene Biosoft, Varilhes, France). Polyclonal antibodies included rabbit anti-human IFN-α and rabbit anti-human IFN-β (PBL Biomedical Laboratories). AffiniPure goat anti-mouse (GAM) IgG (H + L) Cy3 (Jackson ImmunoResearch Laboratories, West Grove, PA) and GAM IgG1 PE (Southern Biotechnology Associates, Birmingham, AL) were used as secondary antibodies.
Viruses
Low-passage (fewer than 5) clinical isolates of HCMV, HCMV2006 and TB40/E (an endothelial cell-adapted strain kindly provided by Dr C. Sinzger, University of Tübingen, Germany), were used.24 Both strains were propagated in HE cells at low virus-to-cell ratios to diminish production of defective particles. Supernatants were harvested following an extensive cytopathogenic effect, centrifuged briefly at 400g to remove cell debris, and then centrifuged at 48 000g for 90 minutes. The pellet was placed on a potassium tartrate-glycerol gradient prepared in 0.05 M Tris-HCl, pH 7.4, and 0.1 M NaCl and was centrifuged at 125 000g for 60 minutes. The band containing purified virus was harvested, medium was added, and purified virions were pelleted at 48 000g for 90 minutes. The virus was resuspended in medium, titrated in low-passage HE cells, and frozen in aliquots at -70°C (titer 1 × 107 - 5 × 107 pfu/mL). TB40/E stocks were passaged 3 times on HE cells. Mock preparations were treated the same way.
Isolation and generation of cells
Buffy coats were obtained from healthy blood donors from Ullevaal University Hospital Blood Bank (Oslo, Norway). Peripheral blood mononuclear cells (PBMCs) were isolated by density gradient centrifugation (Lymphoprep; Axis-Shield, Oslo, Norway). Dendritic cells and CD14+ monocytes were then isolated in the following order: (1) PDCs (BDCA-4 cell isolation kit), (2) CD11c+ DCs (BDCA-1 DC isolation kit), and (3) CD14+ monocytes (CD14 microbeads). All kits were used according to the manufacturer's (Miltenyi Biotec) recommendations, with some modifications to obtain highly purified cell populations.25 This procedure yielded a purity of 98.98% ± 0.14% (n = 17) for the PDCs (CD123+CD11c-HLA-DR+lineage-) (Figure 1A). Lineage markers were CD3, CD14, CD16, CD19, CD20, and CD56. Only 0.23% ± 0.08% were CD11c+, HLA-DR+, reflecting minimal contamination of CD11c+ DCs. A purity of 98.68% ± 0.37% (n = 17) was demonstrated for CD11c+ DCs (CD11c+CD123-HLA-DR+lineage-) (Figure 1B). Only 0.37% ± 0.09% were CD123+, HLA-DR+, reflecting minimal contamination of PDCs. The purity of CD14+ monocytes was always higher than 96% (not shown).
Immature moDCs (imoDCs) were generated from CD14+ monocytes by culture of 106 cells/mL in 6-well plates (Costar; Corning, Corning, NY) in 3 mL RPMI, 10% FCS with GM-CSF (100 ng/mL), and IL-4 (50 ng/mL) for 7 days. On days 2 and 4, 1 mL medium was replaced with an equivalent volume of fresh medium. To generate mature moDCs (mmoDCs), imoDCs were cultured for 2 additional days with GM-CSF, IL-4, TNF-α (20 ng/mL), and PGE2 (1 μg/mL).
For isolation of CD4+ or CD8+ T cells, peripheral blood was drawn in ACD vacutainers (BD Biosciences) from healthy HCMV-negative (serology and PCR) persons. Cells were isolated from PBMCs with CD4 or CD8 Dynabeads in combination with Detachabead reagent (Dynal Biotech, Oslo, Norway) to more than 99% purity for both cell types. Flow cytometric analysis confirmed virtually no contaminating DCs, monocytes, natural killer cells, or B cells in the T-cell fraction. T cells were resuspended in RPMI 10% PS before use in allostimulation experiments.
Peripheral blood was drawn in EDTA (ethylenediaminetetraacetic acid) vacutainers (BD Biosciences) from patients who had undergone renal transplantation and who were negative for HCMV pp65 or had 2 consecutive positive pp65 test results, indicating the absence or presence of active HCMV infection, respectively. Samples were taken 0 to 3 months after transplantation. Patients were treated with immunosuppressants, including prednisolone, cyclosporin A, and mycophenolate mofetil. Control peripheral blood samples were taken from healthy volunteers. Experiments were approved by the regional ethics committee (Regional Committee for Medical Research Ethics, Region South), and written consent was provided in accordance with the Declaration of Helsinki.
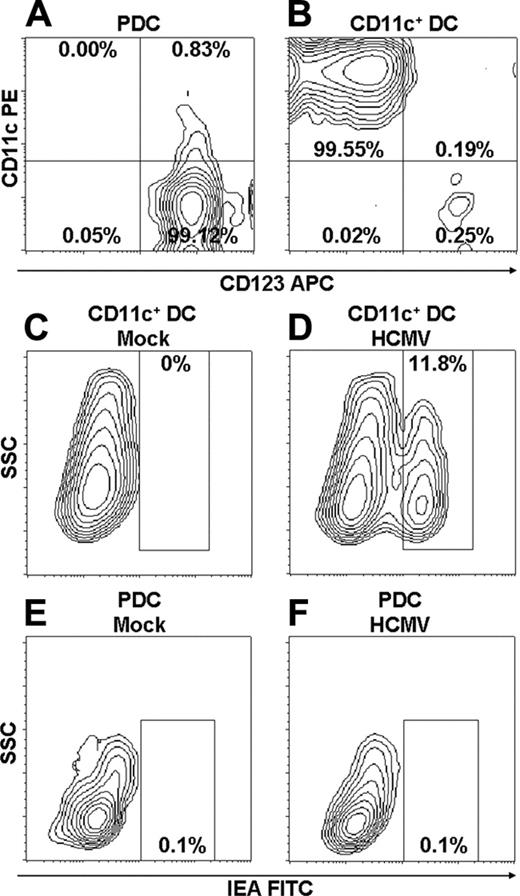
CD11c+ DCs are infected at low frequencies with HCMV, whereas PDCs are resistant. Purity of PDCs (A) and CD11c+ DCs (B) after multiple rounds of positive selection using MACS beads was analyzed by flow cytometry. Results of 1 of 17 representative experiments are shown. Mean purity of 98.98% ± 0.59% and 98.68% ± 1.51% were obtained for PDCs and CD11c+ DCs, respectively (n = 17; separate donors). For details, see “Materials and methods.” PDCs and CD11c+ DCs were incubated with mock (C, E) or TB40/E (MOI, 10) (D, F) and were cultured for 84 hours. Infection of DCs was detected as cells positive for IEA by intracellular staining and was analyzed by flow cytometry (D, F). Results of 1 of 8 representative experiments are shown (separate donors).
CD11c+ DCs are infected at low frequencies with HCMV, whereas PDCs are resistant. Purity of PDCs (A) and CD11c+ DCs (B) after multiple rounds of positive selection using MACS beads was analyzed by flow cytometry. Results of 1 of 17 representative experiments are shown. Mean purity of 98.98% ± 0.59% and 98.68% ± 1.51% were obtained for PDCs and CD11c+ DCs, respectively (n = 17; separate donors). For details, see “Materials and methods.” PDCs and CD11c+ DCs were incubated with mock (C, E) or TB40/E (MOI, 10) (D, F) and were cultured for 84 hours. Infection of DCs was detected as cells positive for IEA by intracellular staining and was analyzed by flow cytometry (D, F). Results of 1 of 8 representative experiments are shown (separate donors).
Infection
DC subsets (2 × 105/well) were incubated in RPMI 10% FCS for 84 hours in Costar 96-well plates (Corning) with TB40/E (at a multiplicity of infection [MOI] of 10-20), HCMV2006 (MOI, 5-35), or mock. GM-CSF (20 ng/mL) or IL-3 (10-30 ng/mL) was added to promote survival of CD11c+ DCs and PDCs, respectively. Where indicated, an IFN-blocking cocktail was added to the cultures at final concentrations of 5000 neutralization U/mL for anti-IFN-α, 2000 neutralization U/mL for anti-IFN-β, and 20 μg/mL for anti-IFNR-αβ (CD118).26 In some experiments, exogenous IFN-α was added at a concentration of 10 ng/mL.
Immunofluorescence microscopy
DC subsets were harvested and washed, and cytospins were prepared (5 × 104 cells/100 μL). Slides were dried at room temperature, acetone fixed (20 minutes), and stored at -70°C until use. Cytospins were incubated with anti-IEA mAb (1.5 μg/mL) or isotype-matched control mAb (10 μg/mL) for 60 minutes, washed, labeled with GAM IgG Cy3 (2.2 μg/mL) for 60 minutes, and counterstained with Hoechst nuclear stain (5 minutes). Immunostained slides were examined with a fluorescence microscope (E800; Nikon, Tokyo, Japan).
Flow cytometry and detection of infection
DC subsets were harvested, washed, and incubated with 20% mouse serum (10 minutes); this was followed by surface staining with mAbs (30 minutes). For detection of infection, cells were fixed in 1% paraformaldehyde (4 hours) and permeabilized in PBS containing 1% Tween 20 (Sigma Chemical) overnight. After wash and incubation with 20% mouse serum (10 minutes), cells were stained intracellularly with anti-IEA FITC. The entire staining procedure was performed at 4°C.
Percentages of DCs among PBMCs from patients who had undergone renal transplantation and from healthy controls were determined as lineage-, HLA-DRhi cells that were either CD11c+ or CD123+. Numbers of viable cells were assessed by relating cells that stained negatively with the DNA dye propidium iodide (Sigma Chemical) to a known number of added particles (Trucount beads; BD Biosciences). All cell analyses were performed using a FACSCalibur (BD Biosciences) flow cytometer.
Cytokine detection
Interleukin-1β, IL-6, IL-8, IL-10, TNF-α, IL-12p70, and IFN-γ in culture supernatants were measured using TH1/TH2 and inflammation cytokine cytometric bead array (CBA) and BD CBA software (BD PharMingen, San Diego, CA), as recommended by the manufacturer. Conventional ELISA was used to measure levels of IFN-α secreted by 2 × 105 cells (sensitivity, 4.8 pg/mL) (Bender Medsystems, Vienna, Austria).
Mixed leukocyte reaction
CD11c+ DCs were incubated with virus or mock, as indicated, and resuspended in RPMI 10% PS. Carboxyfluorescein diacetate succinidyl ester (CFSE)-labeled (Molecular Probes, Eugene, OR) purified CD4+ or CD8+ T cells (105) were cultured in triplicate with indicated numbers of live CD11c+ DCs in RPMI 10% PS at titrated doses in 96-well, U-bottom plates. On day 5, cells were stained with CD3 APC and CD11c PE. Accumulated proliferation of T cells was assessed by flow cytometry as CFSElow cells in percentages of CD3+CD11c- cells. Dead cells were excluded on the basis of low values for forward and side scatter.
Statistical analysis
GraphPad Prism version 4.03 (GraphPad Software, San Diego, CA) was used. Data are presented as mean ± SEM. Paired t test was used for all figures except Figure 7C, for which unpaired t test was used. Two-tailed P values less than .05 were considered significant.
Results
CD11c+ DCs can be infected at low frequencies with HCMV, whereas PDCs are resistant to infection
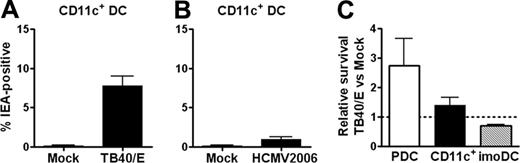
We first investigated whether CD11c+ DCs and PDCs could be infected with the 2 clinical isolates TB40/E and HCMV2006. Dendritic cell populations of very high purity (Figure 1A-B) were incubated with virus or mock preparations. A population of immediate early antigen (IEA)27 -positive cells, indicating infection, was readily identified by flow cytometric analysis on day 3.5 of culture (Figure 1D). Mean frequencies of IEA-positive CD11c+ DCs were 7.8% and 1% for TB40/E and HCMV2006, respectively (Figure 2A-B). PDCs were resistant to HCMV infection, with frequencies of IEA-positive cells lower than 0.2% (Figure 1F) even at the highest MOI. These results were confirmed by comparable numbers of cells staining positively for IEA assessed by immunofluorescence microscopy (not shown). Compared with those reported for moDCs, the low rates of infection of CD11c+ DCs and PDCs did not result from the inability of the virus preparations to infect human cells. Separate experiments showed frequencies of 25% and 15% IEA-positive cells among imoDCs and mmoDCs, respectively (not shown). In addition, CD11c+ DCs were infected at 4-fold higher frequencies under certain conditions (see “CD11c+ DCs are protected against HCMV infection by autocrine production of IFN-α”).
Surprisingly, exposure to HCMV enhanced the viability of CD11c+ DCs and PDCs by factors of 1.44 and 2.75, respectively, as determined by flow cytometric counting of propidium iodide-negative cells (Figure 2C). In contrast, HCMV reduced the number of viable control imoDCs to 0.69 of that in mock cultures (n = 7; P < .05; Figure 2C). The data show that PDCs are resistant to HCMV infection, whereas CD11c+ DCs are infected at low frequencies. Furthermore, the virus seems to provide survival stimuli to both cell types, in contrast to the lytic effect exerted on imoDCs.
CD11c+ DCs are infected at low frequencies by TB40/E and HCMV2006, and survival of CD11c+ DCs and PDCs is not decreased after HCMV exposure. HCMV strains TB40/E (A; n = 8; separate donors; MOI, 10-20) or HCMV2006 (B; n = 4; separate donors; MOI, 5-35) or mock were added to CD11c+ DCs, and cells were cultured for 84 hours. The fraction of IEA-positive cells was determined by flow cytometry, as described in Figure 1, and was found to be significantly higher with TB40/E than with mock (A; P < .001). (C) Viable PDCs, CD11c+ DCs, and control imoDCs were counted by flow cytometry as propidium iodide-negative events and were compared with a known number of added beads, allowing absolute cell numbers to be calculated. Numbers of viable HCMV-exposed DCs were divided by numbers of viable mock-exposed cells. Relative numbers are shown, and all mean ± SEM are indicated (n = 3, n = 7, and n = 7 for PDCs, CD11c+ DCs, and imoDCs, respectively; separate donors). The relative number of viable HCMV-exposed CD11c+ DCs was significantly higher than of HCMV-exposed imoDCs (P < .05).
CD11c+ DCs are infected at low frequencies by TB40/E and HCMV2006, and survival of CD11c+ DCs and PDCs is not decreased after HCMV exposure. HCMV strains TB40/E (A; n = 8; separate donors; MOI, 10-20) or HCMV2006 (B; n = 4; separate donors; MOI, 5-35) or mock were added to CD11c+ DCs, and cells were cultured for 84 hours. The fraction of IEA-positive cells was determined by flow cytometry, as described in Figure 1, and was found to be significantly higher with TB40/E than with mock (A; P < .001). (C) Viable PDCs, CD11c+ DCs, and control imoDCs were counted by flow cytometry as propidium iodide-negative events and were compared with a known number of added beads, allowing absolute cell numbers to be calculated. Numbers of viable HCMV-exposed DCs were divided by numbers of viable mock-exposed cells. Relative numbers are shown, and all mean ± SEM are indicated (n = 3, n = 7, and n = 7 for PDCs, CD11c+ DCs, and imoDCs, respectively; separate donors). The relative number of viable HCMV-exposed CD11c+ DCs was significantly higher than of HCMV-exposed imoDCs (P < .05).
PDCs and CD11c+ DCs produce substantial amounts of IFN-α when stimulated with HCMV, whereas imoDCs do not
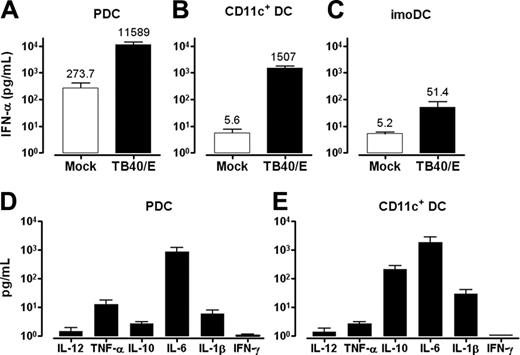
HCMV induces secretion of IFN-α from human leukocytes, but the cellular sources have not been defined.28 Data in Figure 3A show that PDCs produced high amounts of IFN-α in response to TB40/E, with a mean production of 11 589 pg/mL. Similar results were obtained with HCMV2006 (mean, 30 358 pg/mL). Surprisingly, CD11c+ DCs also produced substantial levels of IFN-α in response to TB40/E, with a mean of 1507 pg/mL (Figure 3B). This amounted to 13% of that observed in PDC cultures. Autocrine production of IFN-α in the absence of virus was also higher for PDCs than for CD11c+ DCs (mean values, 273.7 pg/mL and 5.6 pg/mL, respectively). The frequencies of PDCs in preparations of CD11c+ DCs were lower than 0.4%, excluding the possibility that IFN-α was derived from this subset. IFN-α production by CD11c+ DCs did not simply reflect a general ability of leukocytes to produce IFN-α in response to HCMV; rather, minimal amounts of IFN-α were detected in cultures of imoDCs exposed to TB40/E (mean, 51.4 pg/mL; Figure 3C). Thus, the 2 major DC populations in human blood share the ability to produce high levels of IFN-α on challenge with HCMV.
We examined whether HCMV-exposed PDCs and CD11c+ DCs produced other cytokines involved in DC maturation or T-cell activation (Figure 3D-E). Both cell types produced IL-6 (mean values, 849 pg/mL and 1813 pg/mL for PDCs and CD11c+ DCs, respectively), and CD11c+ DCs secreted low levels of IL-10 (mean, 212 pg/mL). Only negligible amounts of IL-1β, TNF-α, IL-12 p70, and IFN-γ were detected. No cytokines were detected in the absence of HCMV (not shown).
CD11c+ DCs are protected against HCMV infection by autocrine production of IFN-α
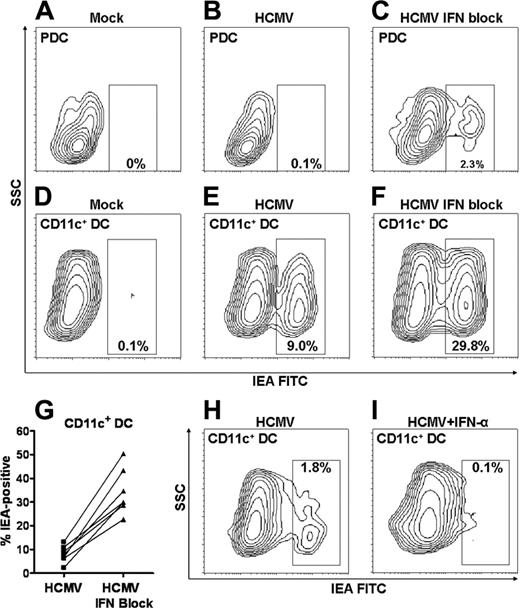
We hypothesized that the secretion of IFN type 1 might protect PDCs and CD11c+ DCs from HCMV infection. Cells were cultured with HCMV in the presence or absence of a mixture of antibodies neutralizing IFN-α and IFN-β and blocking IFNR-αβ (CD118) (hereafter referred to as IFN-blocking cocktail). The frequency of IEA-positive PDCs was increased from, on average, 0.10% ± 0.05% to 1.16% ± 0.56% in the presence of the IFN-blocking cocktail, as analyzed by flow cytometry (n = 3; Figure 4A-C). These data were confirmed by immunofluorescence microscopy in 4 additional donors. Interestingly, the frequency of IEA-positive CD11c+ DCs increased, on average, 4.2-fold in the presence of the IFN-blocking cocktail (mean HCMV, 8.03%; mean HCMV + IFN-blocking, 34.23%; Figure 4D-G). As many as 50% IEA-positive cells were observed. Blocking of IFN type 1 did not change the viability of the CD11c+ DCs (n = 4; not shown). IFN-α concentrations in supernatants were reduced to nondetectable levels by blocking antibodies, and control antibodies did not affect the degree of infection (not shown). Furthermore, adding exogenous IFN-α abolished infection of the CD11c+ DCs (Figure 4H-I). Thus, CD11c+ DCs resist HCMV infection by autocrine production of IFN-α, whereas PDCs appear to use other mechanisms. These results also document that the low rates of infection of CD11c+ DCs in Figure 2 were not caused by defective virus preparations.
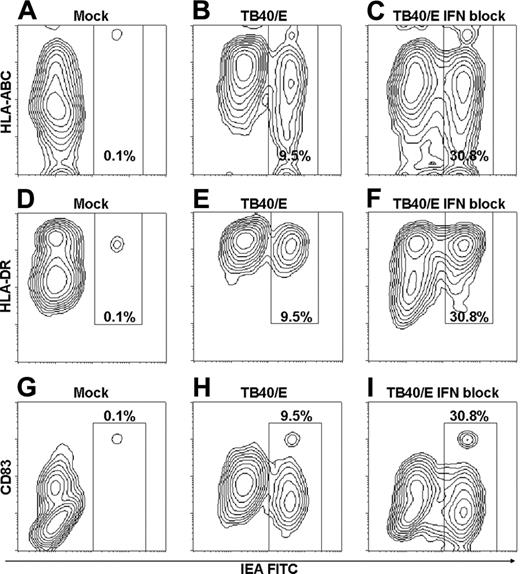
HCMV exposure increases levels of MHC class I, MHC class II, and CD83 on CD11c+ DCs through IFN type 1-dependent and -independent mechanisms
Studies have shown that MHC and costimulatory molecules are down-regulated on moDCs during HCMV infection, resulting in impaired function.2-7 In contrast, levels of MHC class I, MHC class II, and CD83 increased during the incubation of CD11c+ DCs with HCMV (Figures 5, 6). The increase relative to mock was observed for IEA-positive and IEA-negative cells. Expression of MHC class I and CD83, however, was lower on IEA-positive than on IEA-negative cells (Figures 5, 6). An increase in MHC class II (mean, 213%; P < .01; n = 7; separate donors) was also observed on HCMV-exposed PDCs. Levels of CD83 were low on PDCs and remained unaffected by HCMV exposure (not shown).
We examined whether the increased expression of MHC class I, MHC class II, and CD83 on CD11c+ DCs was the result of HCMV-induced autocrine production of IFN-α. Amplitudes were reduced in the presence of the IFN-blocking cocktail but always remained higher than mock levels (Figures 5, 6B). Collectively, these results suggest that HCMV induces maturation of CD11c+ DCs by IFN type 1-dependent and -independent mechanisms.
PDCs and CD11c+ DCs produce substantial amounts of IFN-α on incubation with TB40/E, whereas imoDCs do not. PDCs (A), CD11c+ DCs (B), and imoDCs (C) were incubated with mock or TB40/E (MOI, 10-20) and were cultured for 84 hours. IFN-α production in the supernatants was measured by ELISA (B; P < .001). Secretion of inflammatory cytokines by PDCs and CD11c+ DCs on viral challenge was measured in supernatants by CBA using flow cytometry (D-E). All mean ± SEM are indicated (A, n = 3; B, n = 13; C, n = 5; D, n = 8; E, n = 9; separate donors).
PDCs and CD11c+ DCs produce substantial amounts of IFN-α on incubation with TB40/E, whereas imoDCs do not. PDCs (A), CD11c+ DCs (B), and imoDCs (C) were incubated with mock or TB40/E (MOI, 10-20) and were cultured for 84 hours. IFN-α production in the supernatants was measured by ELISA (B; P < .001). Secretion of inflammatory cytokines by PDCs and CD11c+ DCs on viral challenge was measured in supernatants by CBA using flow cytometry (D-E). All mean ± SEM are indicated (A, n = 3; B, n = 13; C, n = 5; D, n = 8; E, n = 9; separate donors).
CD11c+ DCs are protected against HCMV infection by autocrine production of IFN-α, whereas PDCs might use other mechanisms. PDCs (A-C) or CD11c+ DCs (D-G) were incubated with mock or TB40/E (MOI, 20) in the absence or presence of IFN-blocking cocktail, as indicated. Infection was assessed by flow cytometric analysis of anti-IEA in 7 experiments with separate donors (G; P < .001). CD11c+ DCs were incubated with HCMV2006 in the absence (H) or presence (I) of exogenous IFN-α at a concentration of 10 ng/mL. Results of 1 of 3 representative experiments are shown (separate donors).
CD11c+ DCs are protected against HCMV infection by autocrine production of IFN-α, whereas PDCs might use other mechanisms. PDCs (A-C) or CD11c+ DCs (D-G) were incubated with mock or TB40/E (MOI, 20) in the absence or presence of IFN-blocking cocktail, as indicated. Infection was assessed by flow cytometric analysis of anti-IEA in 7 experiments with separate donors (G; P < .001). CD11c+ DCs were incubated with HCMV2006 in the absence (H) or presence (I) of exogenous IFN-α at a concentration of 10 ng/mL. Results of 1 of 3 representative experiments are shown (separate donors).
Reproducing the data of others,3-8 we found lower expression of MHC class II on IEA-negative (90%) and IEA-positive (74%) moDCs compared with mock. Similarly, CD83 expression was considerably lower on noninfected (62%) and infected (48%) mmoDCs. In accordance with the results of Senechal et al,7 levels of MHC class I on mmoDCs were relatively unchanged by viral exposure (not shown).
HCMV-infected CD11c+ DCs retain T cell-stimulatory capacity
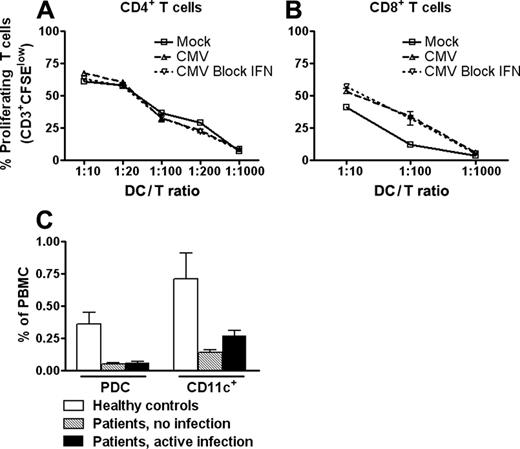
We next examined the T cell-stimulatory capability of virally exposed CD11c+ DCs. To test DCs with a high degree of infection, cells exposed to TB40/E in the presence of IFN-blocking cocktail were included. CD11c+ DCs exposed to virus in the absence or presence of IFN-blocking cocktail contained, on average, 9% and 31% IEA-positive cells, respectively (n = 3). Interestingly, the allostimulatory capacity of CD11c+ DCs under either of these culture conditions was not significantly changed compared with mock control, regardless of whether CD4+ or CD8+ T cells were used as responder cells (Figure 7A-B). Thus, our data show that infection does not functionally paralyze the CD11c+ DCs either in the presence or the absence of IFN type 1.
PDC and CD11c+ DC frequencies are lower in renal transplant recipients than in healthy controls
Previous studies have demonstrated that patients receiving therapy have low numbers of DCs in peripheral blood.29,30 We investigated the relative frequencies of CD11c+ DCs and PDCs in peripheral blood from patients who had undergone renal transplantation and followed immunosuppressive drug regimens with or without HCMV infection and compared them with those of healthy controls. In patients, the percentages of PDCs in PBMCs were 1:6 of those in healthy controls (Figure 7C). Similarly, the relative frequency of CD11c+ DCs was 1:5 or 1:3 for patients without infection and with active infection, respectively, compared with healthy controls (Figure 7C). No significant differences were observed between the 2 patient groups for either DC subset. These data indicate that the reduced DC numbers are a consequence of the immunosuppressive therapy rather than active HCMV infection.
Discussion
The present study shows that HCMV activates the 2 major DC subsets in human blood. Exposure of PDCs and CD11c+ DCs to the virus enhanced survival and induced the production of cytokines and the maturation of CD11c+ DCs in vitro. Importantly, CD11c+ DCs, which were infectable by the virus, retained full T cell-stimulatory capacity even when infected at high frequencies. These results were in sharp contrast to the suppressive effects reported for HCMV on moDCs and showed that HCMV did not induce a global reduction in APC function in humans. The activation of innate immunity by HCMV was consistent with the observation that healthy controls experience HCMV infection and reactivation asymptomatically.
Our results are in agreement with those showing that MCMV activates naturally occurring DCs in mice.13 The functional paralysis of HCMV-exposed human DCs reported in previous studies may, therefore, be specific for DCs generated in vitro from monocytes and CD34+ bone marrow cells. We show here that human PDCs produce extensive amounts of IFN-α when exposed to HCMV, similar to their murine counterparts.13 This cytokine is a key component of innate immunity to HCMV. Disruption of IFN type 1 signaling in IFNR-αβ knockout mice renders them highly susceptible to MCMV infection,31 and prophylactic administration of IFN-α prevents HCMV reactivation in patients who have undergone transplantation.32,33 Yet the resistance of human PDCs to HCMV infection appeared to be independent of secreted IFN type 1 because frequencies of IEA-positive cells were lower than 4%, even in the presence of antibodies blocking IFN type 1 signals. A possible explanation could be that PDCs are continuously stimulated by autocrine low-level constitutive secretion of IFN-α and, hence, are already primed for HCMV resistance. Our data demonstrated the production of low amounts of IFN-α by PDCs in the absence of viral stimulation. High levels of IFN-α-inducible proteins, such as MxA34 and IRF-7,35-37 in freshly isolated PDCs also support this view. Furthermore, IFN-α promotes the survival of PDCs,38,39 which may explain the improved survival observed on HCMV exposure. PDCs seem, however, to be more resistant to HCMV than to other viruses. Their susceptibility to infection with influenza virus and HIV-1 is considerably higher despite the induction of similar levels of IFN-α.40,41 Regardless of the mechanism, PDCs are clearly resistant to HCMV and are likely to represent a cornerstone in the innate defense against this virus in humans.
HCMV exposure increases levels of MHC class I, MHC class II, and CD83 on CD11c+ DCs through IFN-α-dependent and -independent mechanisms. (A-I) CD11c+ DCs were incubated with mock or TB40/E (MOI, 20) in the absence or presence of IFN-blocking cocktail, as indicated, and were cultured for 84 hours. Cells were then surface labeled with antibodies to MHC class I (HLA-ABC), MHC class II (HLA-DR), or CD83, respectively, followed by intracellular staining of IEA. Results of 1 of 3 to 8 representative experiments are shown (separate donors).
HCMV exposure increases levels of MHC class I, MHC class II, and CD83 on CD11c+ DCs through IFN-α-dependent and -independent mechanisms. (A-I) CD11c+ DCs were incubated with mock or TB40/E (MOI, 20) in the absence or presence of IFN-blocking cocktail, as indicated, and were cultured for 84 hours. Cells were then surface labeled with antibodies to MHC class I (HLA-ABC), MHC class II (HLA-DR), or CD83, respectively, followed by intracellular staining of IEA. Results of 1 of 3 to 8 representative experiments are shown (separate donors).
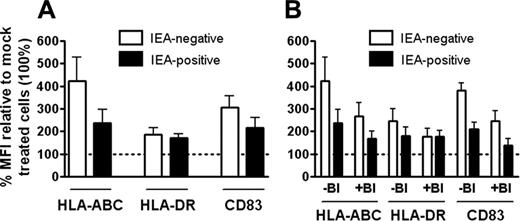
MFI values for antibodies to MHC class I, MHC class II, and CD83 on IEA-positive and -negative CD11c+ DCs. Mean fluorescence intensity (MFI) of antibodies to MHC class I (HLA-ABC), MHC class II (HLA-DR), and CD83 on virally exposed cells staining positively and negatively for IEA were calculated in the percentages of MFIs of mock-infected cells, as measured by flow cytometry (A-B). Results are shown as mean ± SEM. (A) n = 3 for HLA-ABC, n = 8 for HLA-DR (P < .05 and P < .01 for IEA-positive and -negative cells, respectively), and n = 8 for CD83 (P < .005 and P < .05 for IEA-positive and -negative cells, respectively). (B) n = 3. Numbers of experiments correspond to numbers of separate donors. IEA-positive and -negative cells showed higher expression of all 3 markers compared with mock (= 100) regardless of absence (-BI) or presence (+BI) of IFN-blocking.
MFI values for antibodies to MHC class I, MHC class II, and CD83 on IEA-positive and -negative CD11c+ DCs. Mean fluorescence intensity (MFI) of antibodies to MHC class I (HLA-ABC), MHC class II (HLA-DR), and CD83 on virally exposed cells staining positively and negatively for IEA were calculated in the percentages of MFIs of mock-infected cells, as measured by flow cytometry (A-B). Results are shown as mean ± SEM. (A) n = 3 for HLA-ABC, n = 8 for HLA-DR (P < .05 and P < .01 for IEA-positive and -negative cells, respectively), and n = 8 for CD83 (P < .005 and P < .05 for IEA-positive and -negative cells, respectively). (B) n = 3. Numbers of experiments correspond to numbers of separate donors. IEA-positive and -negative cells showed higher expression of all 3 markers compared with mock (= 100) regardless of absence (-BI) or presence (+BI) of IFN-blocking.
CD11c+ DCs were more susceptible to HCMV infection than PDCs. Still, frequencies of infected cells were much lower than those previously reported for moDCs. Interestingly, we found that CD11c+ DCs were protected by autocrine production of IFN-α but that moDCs were not. Inhibition of IFN type 1 in the cultures increased the frequency of infected CD11c+ DCs 4-fold, to levels comparable with those seen for moDCs. However, in contrast to the lytic changes induced by HCMV in imoDCs, as shown in this study and in previous studies,7 viral exposure moderately enhanced the survival of CD11c+ DCs. Viability was independent of the IFN-α produced by the cells. IFN-α secretion by CD11c+ DCs in response to HCMV was an unexpected finding. In previous studies, CD11c+ DCs produced negligible amounts of IFN-α when stimulated by herpes simplex virus-1, HIV-1, or influenza virus.40-42 Furthermore, PDCs were the only murine DCs that secreted IFN-α in response to MCMV.13 Based on the extremely high purity of CD11c+ DCs in the cultures (Figure 1B), we were able to exclude contaminating PDCs as the source of IFN-α. CD11c+ DCs are approximately 2-fold more frequent than PDCs in peripheral blood and secreted 13% of the amount of IFN-α produced by PDCs in response to HCMV. This suggests that CD11c+ DCs represent a significant source of IFN-α in HCMV defense. Recruitment of both of these DC types to sites of viral replication may protect other cells, including other antigen-presenting cells, from infection and functional paralysis.43
The stimulatory effects of HCMV on CD11c+ DCs were not limited to induction of IFN-α production. In sharp contrast to what has been shown for moDCs, HCMV exposure led to a 2- to 4-fold increase in expression of MHC class I and MHC class II and the maturation marker CD83 (Figure 6A). This maturation effect was observed even in IEA-positive cells, though to a lesser degree. Thus, the expression of MHC class I, in particular, was lower on IEA-positive than on IEA-negative HCMV-exposed cells. These observations are best explained as the results of counteracting mechanisms—the well-characterized down-regulating effect induced by virally encoded proteins in cells actually infected by HCMV44 combined with a more powerful up-regulation of maturation molecules on all the cells exposed to HCMV (Figure 6A).
Up-regulation was only partially reduced by inhibition of IFN type 1. The striking increase in infection frequency induced by the IFN-blocking antibodies demonstrated that IFN type 1 activity was effectively inhibited. HCMV exposure, therefore, triggers the maturation of CD11c+ DCs by IFN-dependent and -independent mechanisms. The up-regulation of surface CD83 expression suggests that CD11c+ DCs do not shed this molecule. This may have important implications in view of its recently identified role as immunosuppressant of T-cell responses.7 Interestingly, our results on maturation markers for CD11c+ DCs were similar to those reported for murine CD11b+ DCs.13 MCMV-induced maturation of CD11b+ DCs was relatively unaffected in infected IFNR-αβ knockout mice, whereas maturation of CD8α+ DCs was IFN dependent. This effect is likely to be virus specific because HIV-1 is unable to mature human CD11c+ DCs.41 Collectively, the results suggest that specific subsets of human and murine DCs have the ability to recognize CMV components and to respond with cellular activation.
HCMV-infected CD11c+ DCs retain full T cell-stimulatory capacity, and frequencies of PDCs and CD11c+ DCs are lower in recipients of renal transplants than in healthy controls. CD11c+ DCs were incubated with mock or TB40/E (MOI, 20) in the absence or presence of IFN-blocking cocktail. Equal numbers of viable DCs were cocultured at different ratios with 105 allogeneic CD4+ (A) or CD8+ (B) T cells from an HCMV-negative control. T cells were stained with the cell-tracking dye CFSE, and proliferation was measured by flow cytometry on day 5 as the percentage of CFSElow cells among CD3+CD11c- cells. Results of 1 of 3 representative experiments are shown (separate donors). Differences among the various types of allocultures were nonsignificant. Numbers of PDCs and CD11c+ DCs in the percentage of PBMCs were determined by flow cytometry in peripheral blood from recipients of renal transplants with or without HCMV infection and from healthy controls (C). Data are shown as mean ± SEM (n = 10 and n = 12 healthy controls; n = 21 and n = 26 patients with active infection; n = 10 and n = 10 patients with no infection) for PDCs and CD11c+ DCs, respectively). Relative numbers of both DCs were significantly lower in patients than in healthy controls (P < .005 for PDCs; P < .05 for CD11c+ DCs).
HCMV-infected CD11c+ DCs retain full T cell-stimulatory capacity, and frequencies of PDCs and CD11c+ DCs are lower in recipients of renal transplants than in healthy controls. CD11c+ DCs were incubated with mock or TB40/E (MOI, 20) in the absence or presence of IFN-blocking cocktail. Equal numbers of viable DCs were cocultured at different ratios with 105 allogeneic CD4+ (A) or CD8+ (B) T cells from an HCMV-negative control. T cells were stained with the cell-tracking dye CFSE, and proliferation was measured by flow cytometry on day 5 as the percentage of CFSElow cells among CD3+CD11c- cells. Results of 1 of 3 representative experiments are shown (separate donors). Differences among the various types of allocultures were nonsignificant. Numbers of PDCs and CD11c+ DCs in the percentage of PBMCs were determined by flow cytometry in peripheral blood from recipients of renal transplants with or without HCMV infection and from healthy controls (C). Data are shown as mean ± SEM (n = 10 and n = 12 healthy controls; n = 21 and n = 26 patients with active infection; n = 10 and n = 10 patients with no infection) for PDCs and CD11c+ DCs, respectively). Relative numbers of both DCs were significantly lower in patients than in healthy controls (P < .005 for PDCs; P < .05 for CD11c+ DCs).
Although DC-mediated IFN-α secretion may limit viral replication in the early phases of CMV infection or reactivation, T cells are essential for containing HCMV infections.1 Thus, the ability to activate cytotoxic T cells is fundamental for a successful immune response. Here we show that CD11c+ DCs retain their full capacity to stimulate the proliferation of allogeneic CD4+ and CD8+ T cells on HCMV exposure. The mechanisms responsible for HCMV-induced immunosuppression of moDC-T-cell interactions are clearly not operable in CD11c+ DCs. Thus, potent proliferation of CD4+ and CD8+ T cells was observed even in cell preparations containing high frequencies of infected DCs resulting from the effect of IFN-blocking antibodies. We did not observe any increase in allostimulatory activity of HCMV-exposed CD11c+ DCs. This might have been expected in view of the maturation effect of the virus. However, unlike PDCs and monocytes, CD11c+ DCs are highly potent antigen-presenting cells even in the absence of maturational stimuli.14 Our data show that this function is not lost as a result of HCMV infection.
The present study may indicate that the 2 major subsets of DCs in blood represent a limiting factor in HCMV immunity. It was, therefore, of interest to observe that in recipients of renal transplants, frequencies of CD11c+ DCs and PDCs in blood were more reduced than were those of other leukocytes. This decrease was independent of active HCMV infection (Figure 7C). The results are consistent with those of previous studies showing a reduction in frequencies of PDCs and CD11c+ DCs in patients treated with corticosteroids and a lower production of IFN-α per PDC.29,30,45 An attractive hypothesis is that immunosuppressive drugs render patients susceptible to HCMV infection and reactivation by simultaneously inhibiting T-cell activation and DC generation. This hypothesis may be tested by measuring effects of various pharmacologic immunosuppressants in patients. Furthermore, the influence of these drugs on the generation of CD11c+ DCs and PDCs from CD34+ progenitors can be studied in vitro. Such studies might provide a means to tailor drug regimens to those with less suppressive effects on HCMV immunity for patients with posttransplantation HCMV infection.
The present study raises another question that may be equally important for future therapy: what is the mechanism for HCMV-induced activation of PDCs and CD11c+ DCs? PDCs may be activated through the engagement of toll-like receptor 9 (TLR-9) by HCMV DNA, as has recently been shown in the mouse.46 CD11c+ DCs do not express TLR-9 but can produce IFN-α in response to the TLR-3 ligand poly I:C.47 IFN-α levels reported for poly I:C stimulation were, however, lower than those found in this study in response to HCMV. Furthermore, double-stranded RNA, which is the known ligand for TLR-3, is not produced during replication of DNA viruses such as CMV.48 An intriguing possibility is that CD11c+ DCs recognize other HCMV components, such as envelope glycoprotein B. A soluble form of this protein induced the production of IFN type 1 in human fibroblasts.48 A search for receptors and signaling pathways engaged in CD11c+ DCs on HCMV exposure may provide novel ways to enhance HCMV immune responses.
In conclusion, the present study shows that HCMV activates innate immunity by interaction with CD11c+ DCs and PDCs. The cells secrete high levels of IFN-α and retain full T cell-stimulatory capacity when exposed to virus. Given that CMV has been shown to paralyze other antigen-presenting cells, CD11c+ DCs and PDCs may represent a limiting factor for successful immunity. Our results may explain why healthy persons experience HCMV infection and reactivation asymptomatically, and they may be used to explore novel ways of improving HCMV immunity in immunosuppressed patients.
Prepublished online as Blood First Edition Paper, November 3, 2005; DOI 10.1182/blood-2005-05-2016.
Supported by grants from the University of Oslo, the Research Council of Norway, and Medinnova.
E.Ø.K. and J.D. contributed equally to this study.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
We thank Solveig Beck for providing excellent technical assistance, and Dr Christian Sinzger for kindly providing TB40/E.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal