Atherosclerosis develops at regions of the arterial tree exposed to disturbed flow. The early stage of atherogenesis involves the adhesion of leukocytes (white blood cells [WBCs]) to and their transmigration across endothelial cells (ECs), which are located in close proximity to smooth muscle cells (SMCs). We investigated the effects of EC/SMC coculture and disturbed flow on the adhesion and transmigration of 3 types of WBCs (neutrophils, peripheral blood lymphocytes [PBLs], and monocytes) using our vertical-step flow (VSF) chamber, in which ECs were cocultured with SMCs in collagen gels. Such coculture significantly increased the adhesion and transmigration of neutrophils, PBLs, and monocytes under VSF, particularly in the reattachment area, where the rolling velocity of WBCs and their transmigration time were decreased, as compared with the other areas. Neutrophils, PBLs, and monocytes showed different subendothelial migration patterns under VSF. Their movements were more random and shorter in distance in the reattachment area. Coculture of ECs and SMCs induced their expressions of adhesion molecules and chemokines, which contributed to the increased WBC adhesion and transmigration. Our findings provide insights into the mechanisms of WBC interaction with the vessel wall (composed of ECs and SMCs) under the complex flow environments found in regions of prevalence for atherogenesis.
Introduction
The preferential development of atherosclerosis at arterial branches and curvatures, where local flow is often disturbed (eg, flow separation, recirculation, flow reattachment, and nonuniform shear stress distribution)1 suggests that hemodynamic factors play a role in atherogenesis. The key cells involved in atherogenesis include endothelial cells (ECs), smooth muscle cells (SMCs), and leukocytes (white blood cells [WBCs]). Adhesion of circulating WBCs (especially monocytes) to and subsequent transmigration across the EC monolayer are early events in atherogenesis.2 Disturbed flow with a low-shear stress and a high-shear stress gradient has been shown to stimulate the expression of EC atherogenic genes and to enhance WBC adhesion.3-5 During the development of atherosclerotic plaques, SMCs change from their physiologic contractile phenotype to the pathophysiologic synthetic phenotype and migrate into the plaque,6 where they interact with ECs under flow and may modulate WBC recruitment into the vessel wall.7,8
There have been several reports on the effects of shear flow on WBC transmigration across the EC monolayer and the underlying mechanisms. Cuvelier and Patel9 reported that the interleukin-4 (IL-4)–induced transmigration of eosinophils across ECs is dependent on shear flow and regulated by extaxin-3. Weber et al10 found that monocyte chemotactic protein-1 (MCP-1) and its receptor CCR2 mediate monocyte transmigration, which occurs under flow but rarely in stasis. Kitayama et al11 showed that shear stress affects the transmigration of neutrophils arrested on ECs and that this is regulated by β1 integrins in the extracellular matrix. Cinamon et al12,13 demonstrated that shear stress is essential for chemokine-induced transmigration of CD3+ peripheral blood lymphocytes (PBLs) and that this involves signaling to lymphocyte-expressed G-protein–coupled receptors.13,14 Although these studies using laminar flow–based migration assays have contributed to the understanding of the effects of shear flow on WBC transmigration, they do not address the complexity of disturbed flow that exists near arterial branches and bends. In addition, EC monolayers as an experimental model may not reflect the in vivo environment of ECs, which exist in close proximity to SMCs that can exert significant influences on WBC transmigration.7,8
In the present study, we constructed a parallel-plate EC/SMC coculture flow system capable of producing disturbed flow and used this system to elucidate the roles of SMCs and disturbed flow in WBC adhesion and transmigration. The results indicate that neutrophils, PBLs, and monocytes exhibit differential behaviors in their adhesion to ECs and subsequent migration across and beneath the EC monolayer. The adhesion, transmigration, and subendothelial migration of these WBCs are modulated by the disturbed flow and by the coculture-induced expressions of various adhesion molecules (ie, intercellular adhesion molecule-1 [ICAM-1], vascular adhesion molecule-1 [VCAM-1], and E-selectin and chemokines [ie, MCP-1, IL-8, interferon-inducible T-cell-α chemoattractant (I-TAC), stromal cell-derived factor (SDF-1), interferon-γ–inducible protein 10 (IP-10), growth related oncogene-α (GRO-α), and regulated-on-activation, normal T cell expressed and secreted chemokine (RANTES)]) in ECs and/or SMCs. Our findings provide insights into mechanisms of interactions between circulating WBCs and vessel wall under complex flow environments found in regions of prevalence of atherosclerosis.
Materials and methods
Cell cultures
ECs were isolated from human umbilical cords by collagenase perfusion15 and grown in Petri dishes in medium 199 (M199; Gibco, Grand Island, NY) supplemented with 20% fetal bovine serum (FBS; Gibco) for 3 days. Secondary cultures were used in all experiments. Third-passage human umbilical cord SMCs were obtained commercially (Clonetics, Palo Alto, CA) and maintained in F12K medium (Gibco) supplemented with 10% FBS. Cells with passages 4 to 6 were used.
WBC isolation
Human neutrophils were isolated from heparinized venous blood by dextran sedimentation and Ficoll-Hypaque density-gradient centrifugation, followed by hypotonic lysis of erythrocytes.16 PBLs and CD14+ monocytes were isolated as described.17,18 In brief, peripheral blood mononuclear cells (PBMCs) were isolated by density-gradient centrifugation over Histopaque 1077 (Sigma Chemicals, St Louis, MO), suspended in medium containing 10% FBS, and incubated on Petri dishes for 45 minutes at 37°C. The nonadherent cells were harvested to yield a PBL-enriched population (> 95% purity). Monocytes were purified from PBMCs by negative selection using the magnetic-activated cell sorting (MACS) monocyte isolation kit (Miltenyi Biotech, Auburn, CA); PBMCs were first treated with FcγR blocking reagent (human IgG), followed by a hapten/antibody mixture (mixture of hapten-conjugated monoclonal anti-CD3, anti-CD7, anti-CD19, anti-CD45RA, anti-CD56, and anti-IgE antibodies). After treatment with MACS antihapten magnetic microbeads conjugated to monoclonal antihapten antibody, the labeled cells were passed over a MACS column, and the effluent was collected as the negative fraction representing enriched monocytes (> 95% purity). PBLs were treated with phorbol myristic acetate (PMA; 10 ng/mL; Sigma Chemicals) for 24 hours before the experiments.
Coculture model
The SMCs were cultured in a lattice made of polymerized collagen as previously described.19 Collagen gels (0.1%) were prepared by mixing 4 mg/mL rat tail collagen I (25%; Fisher Scientific, Pittsburgh, PA), 0.1 M NaOH (5%), 2 × F12K medium (40%), FBS (10%), and complete medium (F12K with 10% FBS; 20%) with or without SMCs (1 × 106 cells/mL). The mixture was allowed to polymerize for at least 1 hour at 37°C, and ECs (1∼2 × 105 cells/cm2) were then seeded on top of the gel for 24 hours before the flow experiments. In some experiments, neutralizing antibodies (20 μg/mL; R&D, Minneapolis, MN) against the designated adhesion molecules and chemokines were added to the coculture for 1 hour before the experiments.
Flow channel
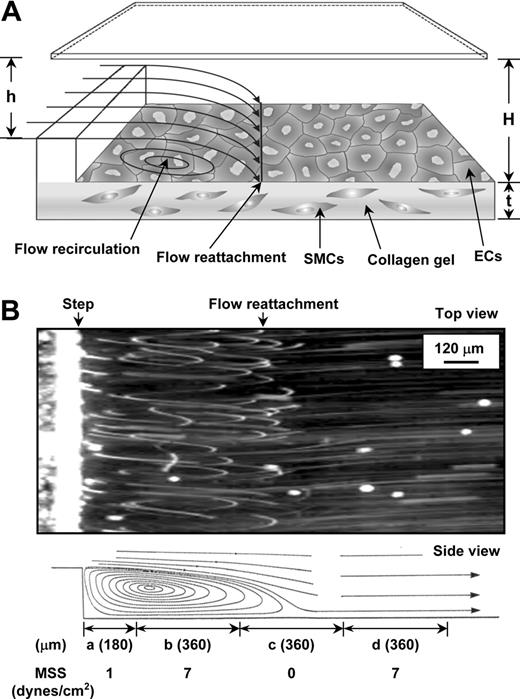
The vertical-step flow (VSF) chamber was previously described.5,20 In the test section of the VSF, the channel width (w) was 10 mm, the entrance height (h) was 0.25 mm, and the main channel height (H) was 0.5 mm (Figure 1A). The total and entrance lengths were 45 and 15 mm, respectively. The Reynolds number used in the experiment was 100, based on the inlet flow rate (26 mL/min) and channel geometry. The flow conditions used in this study provided a well-defined laminar recirculation region downstream to the step without transition to turbulence. The experimental flow patterns were visualized by phase microscopy using marker particles (Figure 1B and Video S1, which is available on the Blood website; see the Supplemental Materials link at the top of the online article). Position “a” is the stagnation flow area; “b” is the area below the center of the recirculation eddy; “c” is the reattachment flow area; and “d” is the area where laminar flow has been redeveloped. The locations of areas a, b, c, and d were measured to be 0.18, 0.54, 0.9, and 1.26 mm from the step, respectively. The mean shear stresses in the areas a, b, c, and d were 1, 7, 0, and 7 dyne/cm2, respectively, as measured previously.5
Schematic configuration and flow pattern in VSF channel. (A) Schematic diagram of the flow channel and test section. ECs were seeded on the collagen gel in the presence of SMCs. The dimensions of the test section are described in “Materials and methods.” The thickness (t) of the gel was measured to be approximately 0.03 mm. (B, top) Phase-contrast photomicrograph (top view) of experimental flow patterns in the VSF channel. (B, bottom) Schematic drawing of the side view of the streamlines in the VSF deduced from the top-view photomicrograph. Flow is from left to right and made visible with marker particles, as described in “Materials and methods.” Flow separation occurs in the region distal to the step, forming 4 specific flow areas: (a) the stagnant flow area, (b) the area of the recirculation eddy, (c) the reattachment flow area, and (d) the area where the flow has developed again to laminar flow, as denoted in the bottom part of the figure. The span of the areas (represented as length) and the mean shear stress (MSS) in each of the areas were measured as indicated. The movie for online microscopic observation of the flow patterns is provided in Video S1.
Schematic configuration and flow pattern in VSF channel. (A) Schematic diagram of the flow channel and test section. ECs were seeded on the collagen gel in the presence of SMCs. The dimensions of the test section are described in “Materials and methods.” The thickness (t) of the gel was measured to be approximately 0.03 mm. (B, top) Phase-contrast photomicrograph (top view) of experimental flow patterns in the VSF channel. (B, bottom) Schematic drawing of the side view of the streamlines in the VSF deduced from the top-view photomicrograph. Flow is from left to right and made visible with marker particles, as described in “Materials and methods.” Flow separation occurs in the region distal to the step, forming 4 specific flow areas: (a) the stagnant flow area, (b) the area of the recirculation eddy, (c) the reattachment flow area, and (d) the area where the flow has developed again to laminar flow, as denoted in the bottom part of the figure. The span of the areas (represented as length) and the mean shear stress (MSS) in each of the areas were measured as indicated. The movie for online microscopic observation of the flow patterns is provided in Video S1.
Adhesion and transmigration assays
The slides with untreated or IL-1β–treated (5 ng/mL, 4 hours) EC/SMC cocultures were mounted in the flow chamber and connected to the perfusion loop system.5 The chamber was placed on the stage of the inverted microscope (Axiovert 200M; Zeiss, Jena, Germany), to which a CCD video camera (CCD-72; Dage-MTI, Michigan City, IN) was attached. The video image was transmitted to a video monitor (HR-1000; Dage-MTI.) and recorder (SR9090U; JVC, Tokyo, Japan), enabling the recording of results in the video fields. The suspension containing neutrophils (5 × 105 cells/mL), PBLs (1 × 106 cells/mL), or monocytes (2 × 105 cells/mL) was perfused over the EC monolayers in the VSF chamber at a flow rate of 26 mL/min (shear stress = 7 dyne/cm2) for 20 minutes. The adhesion and transmigration of WBCs in different areas were determined by image analysis of videotapes.5 Arrested (or adherent) WBCs were defined as cells that did not detach or roll during the 5-second sampling time. The WBCs on the EC monolayer were detected as bright round spots, whereas those transmigrated to the underside of the monolayer were detected as dark images with irregular shapes. The numbers of transmigrated and adherent cells were counted, and their ratio was calculated. To determine WBC transmigration, the video movies were analyzed with the image analysis software NIH Image 1.60b7 (National Institutes of Health, Bethesda, MD) and a MacIntosh computer (MacIntosh, Cupertino, CA). The net migration distance was obtained by comparing the initial and final positions over 20 minutes, and the total distance traversed was obtained for the same 20-minute experiment by summing the individual distances traversed during successive 20-second periods. The ratio of total distance to net distance provides an index of randomness of migration.
Western blot analysis
ECs were harvested from EC/SMC coculture by incubation with a trypsin buffer containing EDTA. SMCs resided in collagen gels were collected by digesting the gels with collagenase and centrifugation. The total proteins from ECs and SMCs were extracted and analyzed by immunoblotting.21 Antibodies against platelet endothelial cell adhesion molecule-1 (PECAM-1; 1:2000; R&D) and smooth muscle α-actin (SMα-actin; 1:1000, Sigma) were used to assess specificities of ECs and SMCs, respectively. The phenotypes of SMCs were determined by using antibodies against SMC contractile marker proteins: SMα-actin, smooth muscle–myosin heavy chain (SM-MHC), h-caldesmon, and calponin (Sigma).
RNA isolation and reverse transcriptase–polymerase chain reaction (RT-PCR)
Total RNAs from ECs or SMCs were isolated by the guanidium isothiocyanate/phenol-chloroform method22 and converted to cDNA using the Superscript II reverse transcriptase system (Life Technologies, Rockville, MD).23 The primer sequences of the genes analyzed and the number of cycles of PCR reaction are summarized in Table S1.
Flow cytometry
The expressions of ICAM-1, VCAM-1, and E-selectin on the surface of ECs cocultured with SMCs were measured by indirect immunofluorescence using flow cytometry and the mouse monoclonal antibody (20 μg/mL; R&D) against ICAM-1, VCAM-1, or E-selectin.24
ELISA
The levels of chemokines (ie, MCP-1, IL-8, I-TAC, SDF-1, IP-10, GRO-α, and RANTES) in the conditioned media and the collagenase-digested collagen gels of the EC/SMC cocultures were determined by using sandwich enzyme-linked immunosorbent assay (ELISA; sensitivity, 4.4 pg/mL; R&D) according to manufacturer's protocols.
Statistical analysis
Results are expressed as mean plus or minus standard error of the mean (SEM). Statistical analysis was performed by analysis of variance (ANOVA) followed by Scheffe test for multiple comparisons. P less than .05 was considered statistically significant.
Results
ECs cocultured with SMCs showed increases in neutrophil, PBL, and monocyte adhesion and transmigration under VSF, especially in the vicinity of flow reattachment
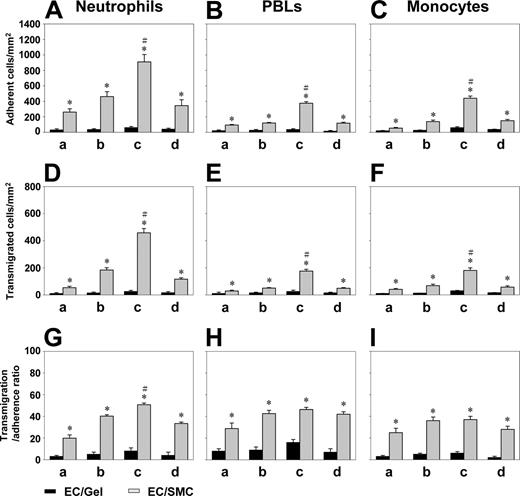
When ECs were cultured alone (EC/Gel), there was virtually no adhesion of neutrophils, PBLs, and monocytes in all areas under VSF (Figure 2A-C). When ECs were cocultured with SMCs (EC/SMC), the adhesion of neutrophils, PBLs, and monocytes to ECs under VSF prominently increased, especially in the flow reattachment area c. Coculture of ECs with SMCs also increased the transmigration of neutrophils, PBLs, and monocytes through the EC monolayers under VSF, as compared with the EC/Gel control (Figure 2D-2F). These increases in WBC transmigration were also particularly remarkable in the reattachment flow area c. SMC coculture increased the transmigration-adhesion ratio for these 3 types of WBCs under the VSF (Figure 2G-I), and this effect was particularly prominent in the flow reattachment area c for neutrophils. As positive controls, IL-1β caused increases in WBC adhesion and transmigration for ECs cultured alone or cocultured with SMCs (data not shown). These results indicate that coculture with SMCs caused ECs to increase the adhesion and transmigration of WBCs and that complex flow patterns had significant influences on WBC recruitment to ECs cocultured with SMCs.
Coculture of ECs with SMCs increased the adhesion of neutrophils, PBLs, and monocytes and their subsequent transmigration under VSF, especially in the vicinity of flow reattachment. ECs were seeded on the collagen gel in the absence (EC/Gel) or presence (EC/SMCs) of SMCs. Purified neutrophils (A,D,G), PBLs (B,E,H), or monocytes (C,F,I) were perfused over the EC monolayers for 20 minutes under the VSF, and their adhesion (A-C) to and transmigration (D-F) across the EC monolayer were counted in each of the areas a, b, c, and d, as described in “Materials and methods.” The transmigration-adherence ratio (G-I) was calculated from the numbers of transmigrated cells relative to adherent cells. As positive controls, EC/Gel and EC/SMC treated with IL-1β (5 ng/mL) for 4 hours caused increases in WBC adhesion and transmigration (data not shown). Data are expressed as mean ± SEM from 3 independent experiments. *P < .05 versus control EC/Gel in the same area. #P < .05 for comparing area c with areas a, b, and d (P < .05 for each comparison).
Coculture of ECs with SMCs increased the adhesion of neutrophils, PBLs, and monocytes and their subsequent transmigration under VSF, especially in the vicinity of flow reattachment. ECs were seeded on the collagen gel in the absence (EC/Gel) or presence (EC/SMCs) of SMCs. Purified neutrophils (A,D,G), PBLs (B,E,H), or monocytes (C,F,I) were perfused over the EC monolayers for 20 minutes under the VSF, and their adhesion (A-C) to and transmigration (D-F) across the EC monolayer were counted in each of the areas a, b, c, and d, as described in “Materials and methods.” The transmigration-adherence ratio (G-I) was calculated from the numbers of transmigrated cells relative to adherent cells. As positive controls, EC/Gel and EC/SMC treated with IL-1β (5 ng/mL) for 4 hours caused increases in WBC adhesion and transmigration (data not shown). Data are expressed as mean ± SEM from 3 independent experiments. *P < .05 versus control EC/Gel in the same area. #P < .05 for comparing area c with areas a, b, and d (P < .05 for each comparison).
Reattachment flow decreases the velocity of rolling and the transmigration time of neutrophils, PBLs, and monocytes adherent to ECs cocultured with SMCs
The velocity of rolling and the transmigration time of neutrophils, PBLs, and monocytes in different areas under VSF were measured by computerized image analysis (examples in areas b and c are provided in Video S2). The velocity of rolling of these 3 different types of WBCs on ECs cocultured with SMCs was slower in flow reattachment area c in comparison to areas a, b, and d (Table 1; P < .05 for each comparison). In each flow area, the 3 types of WBCs showed similar levels of rolling velocity (P > .05 for each comparison). Arrested neutrophils, PBLs, and monocytes transmigrated across the EC layers cocultured with SMCs more rapidly (shorter transmigration time) in the flow reattachment area c than in areas a, b, and d, and static condition (P < .05 for each comparison). In each area, neutrophils transmigrated more rapidly than PBLs and monocytes (P < .05 for each comparison).
Rolling velocities and transmigration times of neutrophils, PBLs, and monocytes on ECs cocultured with SMCs under VSF
. | a . | b . | c . | d . | Static . |
|---|---|---|---|---|---|
| Average rolling velocity, μm/sec | |||||
| Neutrophils | 7.15 ± 0.52 | 11.37 ± 0.46 | 5.72 ± 0.20* | 12.04 ± 0.34 | ND |
| PBLs | 7.03 ± 0.28 | 12.01 ± 0.36 | 5.18 ± 0.27* | 12.54 ± 0.41 | ND |
| Monocytes | 8.41 ± 0.22 | 10.66 ± 0.32 | 4.63 ± 0.34* | 11.57 ± 0.31 | ND |
| Transmigration time, sec | |||||
| Neutrophils | 100.34 ± 4.69 | 95.77 ± 5.79 | 58.27 ± 2.13* | 99.75 ± 4.88 | 103.86 ± 5.10 |
| PBLs | 195.21 ± 7.56 | 173.73 ± 8.52 | 131.21 ± 8.11* | 177.53 ± 7.63 | 183.73 ± 7.31 |
| Monocytes | 313.08 ± 8.49 | 293.83 ± 7.90 | 204.40 ± 7.38* | 291.06 ± 8.10 | 337.92 ± 7.83 |
. | a . | b . | c . | d . | Static . |
|---|---|---|---|---|---|
| Average rolling velocity, μm/sec | |||||
| Neutrophils | 7.15 ± 0.52 | 11.37 ± 0.46 | 5.72 ± 0.20* | 12.04 ± 0.34 | ND |
| PBLs | 7.03 ± 0.28 | 12.01 ± 0.36 | 5.18 ± 0.27* | 12.54 ± 0.41 | ND |
| Monocytes | 8.41 ± 0.22 | 10.66 ± 0.32 | 4.63 ± 0.34* | 11.57 ± 0.31 | ND |
| Transmigration time, sec | |||||
| Neutrophils | 100.34 ± 4.69 | 95.77 ± 5.79 | 58.27 ± 2.13* | 99.75 ± 4.88 | 103.86 ± 5.10 |
| PBLs | 195.21 ± 7.56 | 173.73 ± 8.52 | 131.21 ± 8.11* | 177.53 ± 7.63 | 183.73 ± 7.31 |
| Monocytes | 313.08 ± 8.49 | 293.83 ± 7.90 | 204.40 ± 7.38* | 291.06 ± 8.10 | 337.92 ± 7.83 |
Purified neutrophils, PBLs, and monocytes were perfused over ECs/SMCs under VSF for 20 minutes, and video records were made of the adhesive behaviors in areas a, b, c, and d. The rolling velocity and transmigration time of purified WBCs in different areas were measured by computerized image analysis. Examples for areas b and c are provided in Video S2. The WBC rolling velocity was calculated by dividing the rolling length by the time elapsed every 10 seconds and then averaged. Only WBCs that rolled without stopping during the entire 10-second period were included in this analysis. The transmigration time of WBCs was measured by following individual cells from the time they firmly attached on the EC monolayer (bright appearance on phase microscopy) until they turned phase dark in appearance after passing through the EC monolayer. The results shown are mean ± SEM of 25 cells from 3 independent experiments.
ND indicates no rolling velocity detected.
P < .05 for comparison of area c versus areas a, b, and d
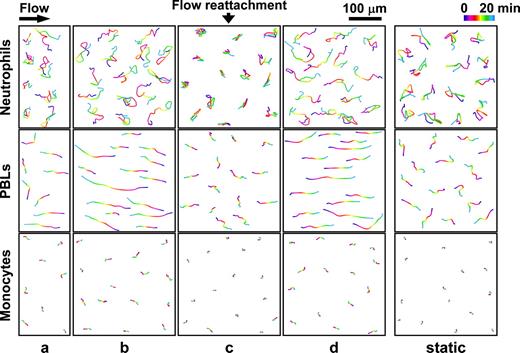
Neutrophils, PBLs, and monocytes show differential patterns of migration beneath the EC monolayers under VSF
The migration of neutrophils, PBLs, and monocytes underneath the EC monolayer was traced for 20 minutes in different areas after their transmigration. The neutrophils showed winding and random migrations beneath the EC monolayer in all areas a, b, c, and d (though the motion was more restricted in c), and also under static condition (Figure 3; Video S3 shows examples for areas c and d). The results of quantitative analyses of the migration tracings are shown in Table 2. Transmigrated neutrophils moved for shorter distances in the flow reattachment area c, as compared with areas a, b, and d, and also the static condition (P < .05, n = 25). The randomness of movement (ratio of the cumulative distance to net distance traversed) was significantly higher in area c than in a, b, and d, as well as in static condition (P < .05). Once transmigrated into the subendothelial space, PBLs stretched their cell bodies dramatically and moved along the flow direction (Figure 3; Video S4 shows examples for areas c and d). These phenomena were particularly pronounced in areas b and d, which have the highest shear stresses. The PBLs in the flow reattachment area c moved for shorter distances (P < .05 versus a, b, and d and static) but did not show significant difference in randomness in comparison to the other areas (P > .05 versus a, b, and d and static) (Table 2). In contrast to neutrophils and PBLs, the transmigrated monocytes showed very low mobility underneath the ECs in each of the areas under VSF and also under static condition (Figure 3; Video S5 shows examples for areas c and d). As in the case of neutrophils, the randomness of movement of monocytes was significantly higher in area c than in a, b, and d, and in static condition (P < .05 for each comparison) (Table 2).
Movements of transmigrated neutrophils, PBLs, and monocytes beneath the EC monolayers under VSF and static condition
. | a . | b . | c . | d . | Static . |
|---|---|---|---|---|---|
| Total distance (T), μm | |||||
| Neutrophils | 252.63 ± 20.06 | 277.52 ± 12.13 | 183.67 ± 11.60* | 256.80 ± 13.75 | 233.66 ± 9.86 |
| PBLs | 89.93 ± 4.40 | 125.07 ± 14.79 | 50.73 ± 3.61* | 116.62 ± 7.84 | 69.77 ± 3.31 |
| Monocytes | 21.82 ± 0.78 | 24.51 ± 1.54 | 10.62 ± 1.08* | 24.28 ± 1.66 | 20.14 ± 1.41 |
| Distance from initial to final position (N), μm | |||||
| Neutrophils | 52.47 ± 6.35 | 60.01 ± 7.10 | 23.27 ± 2.92* | 75.93 ± 6.96 | 43.04 ± 4.45 |
| PBLs | 82.46 ± 4.72 | 121.13 ± 14.32 | 40.07 ± 3.76* | 111.53 ± 7.58 | 50.07 ± 2.49 |
| Monocytes | 14.86 ± 0.55 | 17.74 ± 0.87 | 8.12 ± 0.32* | 16.95 ± 1.14 | 13.76 ± 1.43 |
| Randomness, T/N | |||||
| Neutrophils | 5.27 ± 0.42 | 5.87 ± 0.88 | 10.86 ± 1.92* | 3.98 ± 0.47 | 6.25 ± 0.55 |
| PBLs | 1.12 ± 0.09 | 1.03 ± 0.02 | 1.46 ± 0.25 | 1.05 ± 0.01 | 1.41 ± 0.07 |
| Monocytes | 1.31 ± 0.05 | 1.34 ± 0.07 | 1.12 ± 0.02* | 1.26 ± 0.04 | 1.57 ± 0.11 |
. | a . | b . | c . | d . | Static . |
|---|---|---|---|---|---|
| Total distance (T), μm | |||||
| Neutrophils | 252.63 ± 20.06 | 277.52 ± 12.13 | 183.67 ± 11.60* | 256.80 ± 13.75 | 233.66 ± 9.86 |
| PBLs | 89.93 ± 4.40 | 125.07 ± 14.79 | 50.73 ± 3.61* | 116.62 ± 7.84 | 69.77 ± 3.31 |
| Monocytes | 21.82 ± 0.78 | 24.51 ± 1.54 | 10.62 ± 1.08* | 24.28 ± 1.66 | 20.14 ± 1.41 |
| Distance from initial to final position (N), μm | |||||
| Neutrophils | 52.47 ± 6.35 | 60.01 ± 7.10 | 23.27 ± 2.92* | 75.93 ± 6.96 | 43.04 ± 4.45 |
| PBLs | 82.46 ± 4.72 | 121.13 ± 14.32 | 40.07 ± 3.76* | 111.53 ± 7.58 | 50.07 ± 2.49 |
| Monocytes | 14.86 ± 0.55 | 17.74 ± 0.87 | 8.12 ± 0.32* | 16.95 ± 1.14 | 13.76 ± 1.43 |
| Randomness, T/N | |||||
| Neutrophils | 5.27 ± 0.42 | 5.87 ± 0.88 | 10.86 ± 1.92* | 3.98 ± 0.47 | 6.25 ± 0.55 |
| PBLs | 1.12 ± 0.09 | 1.03 ± 0.02 | 1.46 ± 0.25 | 1.05 ± 0.01 | 1.41 ± 0.07 |
| Monocytes | 1.31 ± 0.05 | 1.34 ± 0.07 | 1.12 ± 0.02* | 1.26 ± 0.04 | 1.57 ± 0.11 |
Purified neutrophils, PBLs, or monocytes were perfused over the EC/SMC cocultures, and the subendothelial migration of arrested WBCs was traced for 20 minutes in areas a, b, c, and d under VSF or under static conditions after their transmigration across the EC monolayer to its underside. The distance traversed by the cell center was measured every 20 seconds and summed for each cell as the total migration distance (T) in 20 minutes. The net distance traversed by the cell center (N) was determined from the displacement between the initial (0 minute) and final (20 minutes) position, and the T/N ratio was calculated as an index of randomness of cell movement. Data shown are mean ± SEM of 25 cells from 3 independent experiments.
P < .05 for comparison of area c versus areas a, b, and d and static
Tracings of the subendothelial migration paths of the transmigrated neutrophils, PBLs, and monocytes in each of the areas under VSF and under static condition. Purified neutrophils, PBLs, and monocytes were perfused over the EC/SMC coculture, and the migration of arrested WBCs was traced for 20 minutes in areas a, b, c, and d under VSF or under static condition after their transmigration across the EC monolayer to the underside. This figure shows the results of a representative experiment containing 8 cells transmigrated in area a and 15 cells transmigrated in areas b, c, and d, as well as under static condition. The positions of the centers of the cells were determined at 20-second intervals from 0 to 20 minutes, and their paths of travel were processed with image analysis software NIH Image 1.60b7. Examples for online microscopic observations of the movements of transmigrated neutrophils, PBLs, and monocytes in areas c and d are provided in Videos S3, S4, and S5, respectively.
Tracings of the subendothelial migration paths of the transmigrated neutrophils, PBLs, and monocytes in each of the areas under VSF and under static condition. Purified neutrophils, PBLs, and monocytes were perfused over the EC/SMC coculture, and the migration of arrested WBCs was traced for 20 minutes in areas a, b, c, and d under VSF or under static condition after their transmigration across the EC monolayer to the underside. This figure shows the results of a representative experiment containing 8 cells transmigrated in area a and 15 cells transmigrated in areas b, c, and d, as well as under static condition. The positions of the centers of the cells were determined at 20-second intervals from 0 to 20 minutes, and their paths of travel were processed with image analysis software NIH Image 1.60b7. Examples for online microscopic observations of the movements of transmigrated neutrophils, PBLs, and monocytes in areas c and d are provided in Videos S3, S4, and S5, respectively.
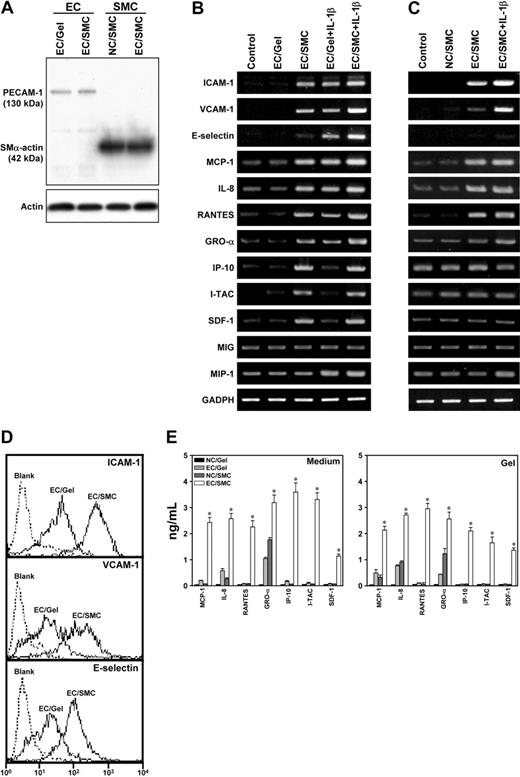
Coculture of ECs and SMCs induces their expression of several adhesion molecules and chemokines relevant to WBC recruitment
Because coculture of ECs and SMCs increased the adhesion of WBCs to ECs and their subsequent transmigration across the ECs under VSF, we investigated whether the coculture of ECs and SMCs would mediate their expression of adhesion molecules and chemokines relevant to WBC recruitment. Three adhesion molecules, including ICAM-1, VCAM-1, and E-selectin, and 9 chemokines, including 3 C-C chemokines (MCP-1, RANTES, and monokine induced by interferon-γ [MIG]) and 6 C-X-C chemokines (IL-8, I-TAC, SDF-1, IP-10, GRO-α, and macrophage-inflammatory protein-1 [MIP]), were examined to assess their potential roles in WBC adhesion to and transmigration across the ECs cocultured with SMCs. ECs and SMCs were purified from their cocultures; immunoblotting of their lysates using the EC marker PECAM-1 and the SMC marker SMα-actin showed minimal contamination with the other cell type (Figure 4A). ECs cocultured with SMCs (EC/SMC) showed markedly higher mRNA levels of ICAM-1, VCAM-1, and E-selectin than ECs cultured on collagen gels without SMCs (EC/Gel) and ECs cultured on Petri dishes (Control) (P < .05 for each comparison) (Figure 4B). Flow cytometric analysis confirmed the increases in ICAM-1, VCAM-1, and E-selectin expressions on ECs induced by coculture with SMCs (Figure 4D). Among the 9 chemokine genes examined, 7 (ie, MCP-1, IL-8, I-TAC, SDF-1, IP-10, GRO-α, and RANTES) showed an increase in EC expression by the coculture (Figure 4B). Coculture of ECs and SMCs increased the mRNA expression in SMCs of ICAM-1, VCAM-1, MCP-1, IL-8, RANTES, and GRO-α, but not E-selectin, IP-10, I-TAC, SDF-1, MIG, and MIP, as compared with SMCs in collagen gels without EC coculture (NC/SMC) and the SMCs cultured on Petri dishes (P < .05 for each comparison) (Figure 4C). Treatments of EC/Gel or EC/SMC with IL-1β led to increased expressions of most of these adhesion molecules and chemokines in ECs and SMCs.
To determine the effects of coculture on secretion of chemokines MCP-1, IL-8, RANTES, GRO-α, IP-10, I-TAC, and SDF-1 by the cells, ELISA was performed to detect these chemokines in both the conditioned media and collagen gels. The concentrations of soluble forms of these chemokines were higher in the conditioned media and gels of the EC/SMC coculture, as compared with the EC/Gel and NC/SMC (P < .05 for each comparison) (Figure 4E). Collagen gel immersed in the medium without cells (Gel) was used as negative control. The results indicate that EC/SMC coculture can induce the production of a number of adhesion molecules and chemokines in both types of cells.
Contributions of adhesion molecules and chemokines to the adhesion of neutrophils, PBLs, and monocytes to ECs and their subsequent migrations across and beneath the ECs cocultured with SMCs under VSF
Because EC/SMC coculture induced their expressions of adhesion molecules ICAM-1, VCAM-1, and E-selectin and chemokines MCP-1, IL-8, RANTES, GRO-α, IP-10, I-TAC, and SDF-1, we further assessed the roles of these adhesion molecules and chemokines in the adhesion of the 3 types of WBCs to ECs and their subsequent migrations across and underneath the ECs cocultured with SMCs under VSF. The effects of various antibodies on EC/SMC coculture were assessed by comparing the results with the controls (including both EC/SMC treated with control IgG and untreated EC/SMC). Incubation of EC/SMC coculture with antibodies against ICAM-1, E-selectin, IL-8, and GRO-α significantly blocked the adhesion and transmigration of neutrophils (Figure 5 shows the results for area c; see Video S1 for complete results for all areas) and increased their transmigration time in each area (P < .05 versus EC/SMC controls) (Table 3 shows the results for area c and Video S2 shows results for all areas; Video S6 shows examples for an increase in transmigration time in area c by the use of antibody to ICAM-1 compared with the control IgG). Only the antibody to ICAM-1 significantly inhibited the total distance and randomness of neutrophil movement underneath the ECs cocultured with SMCs under VSF (Table 3; Table S2). Antibodies to ICAM-1, VCAM-1, MCP-1, RANTES, GRO-α, I-TAC, and SDF-1 caused significant inhibition of PBL adhesion and transmigration in all areas in comparison to the corresponding EC/SMC controls (Figure 5; Figure S1). The antibody against IP-10 had inhibitory effects only on PBL adhesion, but not transmigration, in each of the areas. Although the uses of antibodies to ICAM-1, VCAM-1, MCP-1, and SDF-1 increased the transmigration time of PBLs, only antibody to VCAM-1 inhibited the distance and randomness of PBL movement underneath the ECs cocultured with SMCs under VSF (Table 3 and Table S2; Video S7 shows the example for inhibition of the subendothelial migration of PBLs under VSF by the use of antibody to VCAM-1). The uses of antibodies against ICAM-1, E-selectin, MCP-1, RANTES, GRO-α, IP-10, and SDF-1 significantly inhibited monocyte adhesion and transmigration in each of the areas (P < .05 versus EC/SMC controls) (Figure 5; Figure S1). Antibody against VCAM-1 or IL-8 only blocked the adhesion, but not transmigration, of monocytes in each of the areas. Antibody against ICAM-1 or MCP-1 significantly increased the transmigration time of monocytes in each area (P < .05 versus EC/SMC controls) (Table 3; Table S2).
Effects of neutralizing antibodies against various adhesion molecules and chemokines on the transmigration times of neutrophils, PBLs, and monocytes and their randomness in movements beneath the EC monolayers in the reattachment flow area c under VSF
. | Randomness, T/N . | . | . | Transmigration time, sec . | . | . | ||||
|---|---|---|---|---|---|---|---|---|---|---|
| mAb . | Neutrophils . | PBLs . | Monocytes . | Neutrophils . | PBLs . | Monocytes . | ||||
| NA | 10.88 ± 1.92 | 1.52 ± 0.25 | 1.12 ± 0.02 | 58.27 ± 2.13 | 131.21 ± 8.11 | 204.40 ± 7.44 | ||||
| IgG | 9.73 ± 1.74 | 1.42 ± 0.23 | 1.16 ± 0.02 | 63.28 ± 6.75 | 141.32 ± 6.36 | 195.32 ± 8.22 | ||||
| ICAM-1 | 6.89 ± 1.12* | 1.46 ± 0.17 | 1.12 ± 0.02 | 207.13 ± 9.57* | 189.27 ± 7.22* | 276.28 ± 9.46* | ||||
| VCAM-1 | 11.13 ± 2.17 | 1.04 ± 0.02* | 1.14 ± 0.03 | 65.23 ± 6.42 | 231.39 ± 9.40* | 218.19 ± 8.32 | ||||
| E-selectin | 9.61 ± 1.75 | 1.51 ± 0.31 | 1.11 ± 0.03 | 228.46 ± 11.72* | 125.53 ± 6.31 | 210.64 ± 8.16 | ||||
| MCP-1 | 13.53 ± 2.06 | 1.38 ± 0.17 | 1.10 ± 0.02 | 66.02 ± 8.10 | 204.61 ± 10.06* | 267.24 ± 10.34* | ||||
| IL-8 | 11.72 ± 1.52 | 1.37 ± 0.19 | 1.08 ± 0.03 | 243.50 ± 10.22* | 146.45 ± 8.42 | 214.65 ± 8.75 | ||||
| RANTES | 14.98 ± 1.68 | 1.38 ± 0.14 | 1.13 ± 0.02 | 67.33 ± 7.35 | 148.31 ± 8.20 | 211.51 ± 8.29 | ||||
| GRO-α | 11.14 ± 2.12 | 1.44 ± 0.17 | 1.10 ± 0.03 | 106.43 ± 6.34* | 138.48 ± 7.26 | 217.40 ± 8.40 | ||||
| IP-10 | 11.49 ± 1.66 | 1.42 ± 0.14 | 1.11 ± 0.02 | 65.19 ± 9.61 | 151.32 ± 7.46 | 214.67 ± 9.92 | ||||
| I-TAC | 13.91 ± 2.57 | 1.49 ± 0.11 | 1.12 ± 0.03 | 70.11 ± 8.50 | 144.54 ± 6.17 | 221.74 ± 7.64 | ||||
| SDF-1 | 10.77 ± 1.84 | 1.42 ± 0.12 | 1.11 ± 0.02 | 62.56 ± 6.68 | 168.51 ± 7.74* | 223.71 ± 8.26 | ||||
. | Randomness, T/N . | . | . | Transmigration time, sec . | . | . | ||||
|---|---|---|---|---|---|---|---|---|---|---|
| mAb . | Neutrophils . | PBLs . | Monocytes . | Neutrophils . | PBLs . | Monocytes . | ||||
| NA | 10.88 ± 1.92 | 1.52 ± 0.25 | 1.12 ± 0.02 | 58.27 ± 2.13 | 131.21 ± 8.11 | 204.40 ± 7.44 | ||||
| IgG | 9.73 ± 1.74 | 1.42 ± 0.23 | 1.16 ± 0.02 | 63.28 ± 6.75 | 141.32 ± 6.36 | 195.32 ± 8.22 | ||||
| ICAM-1 | 6.89 ± 1.12* | 1.46 ± 0.17 | 1.12 ± 0.02 | 207.13 ± 9.57* | 189.27 ± 7.22* | 276.28 ± 9.46* | ||||
| VCAM-1 | 11.13 ± 2.17 | 1.04 ± 0.02* | 1.14 ± 0.03 | 65.23 ± 6.42 | 231.39 ± 9.40* | 218.19 ± 8.32 | ||||
| E-selectin | 9.61 ± 1.75 | 1.51 ± 0.31 | 1.11 ± 0.03 | 228.46 ± 11.72* | 125.53 ± 6.31 | 210.64 ± 8.16 | ||||
| MCP-1 | 13.53 ± 2.06 | 1.38 ± 0.17 | 1.10 ± 0.02 | 66.02 ± 8.10 | 204.61 ± 10.06* | 267.24 ± 10.34* | ||||
| IL-8 | 11.72 ± 1.52 | 1.37 ± 0.19 | 1.08 ± 0.03 | 243.50 ± 10.22* | 146.45 ± 8.42 | 214.65 ± 8.75 | ||||
| RANTES | 14.98 ± 1.68 | 1.38 ± 0.14 | 1.13 ± 0.02 | 67.33 ± 7.35 | 148.31 ± 8.20 | 211.51 ± 8.29 | ||||
| GRO-α | 11.14 ± 2.12 | 1.44 ± 0.17 | 1.10 ± 0.03 | 106.43 ± 6.34* | 138.48 ± 7.26 | 217.40 ± 8.40 | ||||
| IP-10 | 11.49 ± 1.66 | 1.42 ± 0.14 | 1.11 ± 0.02 | 65.19 ± 9.61 | 151.32 ± 7.46 | 214.67 ± 9.92 | ||||
| I-TAC | 13.91 ± 2.57 | 1.49 ± 0.11 | 1.12 ± 0.03 | 70.11 ± 8.50 | 144.54 ± 6.17 | 221.74 ± 7.64 | ||||
| SDF-1 | 10.77 ± 1.84 | 1.42 ± 0.12 | 1.11 ± 0.02 | 62.56 ± 6.68 | 168.51 ± 7.74* | 223.71 ± 8.26 | ||||
The EC/SMC cocultures were incubated with neutralizing antibodies (20 μg/mL) against the indicated adhesion molecules and chemokines for 1 hour prior to adhesion and transmigration assays under VSF. The EC/SMC treated with isotype-matched IgG or without antibody treatment (NA) was used as controls. Purified neutrophils, PBLs, or monocytes were perfused over the EC/SMC under VSF for 20 minutes, and video records were made of the behaviors of adhesion and transmigration in the reattachment flow area c. The transmigration times of purified WBCs as well as the total distances (T), net distances (N), and randomness (T/N) of their subendothelial migration were measured by computerized image analysis, as described in “Materials and methods.” An example is provided in Video S6 to show an increase in transmigration time of neutrophils by the use of antibody to ICAM-1 as compared with the control IgG. Data shown in this table are mean ± SEM of 25 cells from three independent experiments. A complete list of the results on all parameters for all areas under VSF and static condition is provided in Table S2.
P < .05 versus control EC/SMC treated with IgG or without antibody treatment
Coculture of ECs and SMCs induced their expressions of adhesion molecules and chemokines relevant to WBC recruitment. ECs were seeded on the collagen gel in the absence (EC/Gel) or presence (EC/SMC) of SMCs. SMCs were also embedded in the collagen gels without coculture of ECs (NC/SMC). The cells cultured on Petri dishes were used as controls (Control). In additional experiments, EC/Gel and EC/SMC were treated with IL-1β for 4 hours. (A) Specificity of ECs and SMCs. ECs and SMCs were purified from their cocultures, and their specificity was examined by Western blot analysis of their lysates using antibodies against PECAM-1 and SMα-actin as markers for ECs and SMCs, respectively. (B-C) Coculture of ECs and SMCs induced their expressions of adhesion molecules and chemokines. The mRNA levels of selected adhesion molecules and chemokines in ECs (B) and SMCs (C) from different experimental conditions were determined by RT-PCR analysis, as described in “Materials and methods.” Amplification of cDNA was performed in parallel samples using human GAPDH primers. Results are representative of triplicate experiments with similar results. (D) Coculture with SMCs increased ICAM-1, VCAM-1, and E-selectin expressions on ECs. ECs isolated from EC/SMC or EC/Gel were analyzed by flow cytometry, as described in “Materials and methods.” The EC/SMC shows a shift of fluorescence intensity profile for ICAM-1, VCAM-1, and E-selectin, as compared with the EC/Gel. ECs incubated with only FITC-conjugated antibody were used as blank controls. Results are representative of duplicate experiments with similar results. (E) Coculture of ECs and SMCs increased the release of chemokines into conditioned media and collagen gels. The soluble forms of the chemokines in both the media and collagen gels of EC/Gel, NC/SMC, and EC/SMC experiments were determined by ELISA assay, as described in “Materials and methods.” The collagen gel incubated with only the media was used as negative controls (NC/Gel). The results shown are mean ± SEM from 3 independent experiments. *P < .05 for EC/SMC versus NC/Gel, EC/Gel, and NC/SMC samples.
Coculture of ECs and SMCs induced their expressions of adhesion molecules and chemokines relevant to WBC recruitment. ECs were seeded on the collagen gel in the absence (EC/Gel) or presence (EC/SMC) of SMCs. SMCs were also embedded in the collagen gels without coculture of ECs (NC/SMC). The cells cultured on Petri dishes were used as controls (Control). In additional experiments, EC/Gel and EC/SMC were treated with IL-1β for 4 hours. (A) Specificity of ECs and SMCs. ECs and SMCs were purified from their cocultures, and their specificity was examined by Western blot analysis of their lysates using antibodies against PECAM-1 and SMα-actin as markers for ECs and SMCs, respectively. (B-C) Coculture of ECs and SMCs induced their expressions of adhesion molecules and chemokines. The mRNA levels of selected adhesion molecules and chemokines in ECs (B) and SMCs (C) from different experimental conditions were determined by RT-PCR analysis, as described in “Materials and methods.” Amplification of cDNA was performed in parallel samples using human GAPDH primers. Results are representative of triplicate experiments with similar results. (D) Coculture with SMCs increased ICAM-1, VCAM-1, and E-selectin expressions on ECs. ECs isolated from EC/SMC or EC/Gel were analyzed by flow cytometry, as described in “Materials and methods.” The EC/SMC shows a shift of fluorescence intensity profile for ICAM-1, VCAM-1, and E-selectin, as compared with the EC/Gel. ECs incubated with only FITC-conjugated antibody were used as blank controls. Results are representative of duplicate experiments with similar results. (E) Coculture of ECs and SMCs increased the release of chemokines into conditioned media and collagen gels. The soluble forms of the chemokines in both the media and collagen gels of EC/Gel, NC/SMC, and EC/SMC experiments were determined by ELISA assay, as described in “Materials and methods.” The collagen gel incubated with only the media was used as negative controls (NC/Gel). The results shown are mean ± SEM from 3 independent experiments. *P < .05 for EC/SMC versus NC/Gel, EC/Gel, and NC/SMC samples.
Discussion
Our present study aims at elucidating the roles of SMCs and disturbed flow in the adhesion of neutrophils, PBLs, and monocytes to and their subsequent migration across and beneath the ECs, and also the molecular mechanisms underlying these processes. Using our newly developed EC/SMC coculture flow system, we demonstrated that coculture of ECs with SMCs significantly increased the adhesion and subsequent transmigration of these 3 types of WBCs under disturbed flow. These changes were particularly pronounced in the flow reattachment area. Several in vitro studies, including ours.5,25,26 have shown increased adhesion of flowing WBCs to activated ECs in the vicinity of the reattachment point. One of the reasons for this regional propensity for adhesion could be a longer residence time of the cells delivered to the area and a higher near-wall concentration.5 We found that neutrophils, PBLs, and monocytes all rolled more slowly and transmigrated more rapidly in regions near the reattachment point in comparison to other areas. These behaviors may contribute, at least in part, to the increased adhesion and transmigration of WBCs in this area. Our findings substantiate the importance of complex flow environment in WBC recruitment to ECs and their neighboring SMCs in the vessel wall.
One of the most striking observations in the present study is the differential migration patterns exhibited by neutrophils, PBLs, and monocytes underneath the ECs in different areas under disturbed flow and under static condition. The transmigrated neutrophils moved without preferred direction underneath the ECs and they displayed a more random course in the reattachment area than in other areas. This observation is comparable with the reports that neutrophils moved more quickly and randomly once they transmigrated into the subendothelial layers.11,27 The migration paths of transmigrated PBLs beneath the EC monolayers under disturbed flow were highly dependent on the flow direction, suggesting that the transmural flow and shear force can modulate PBL migration. The transmigrated PBLs showed the greatest stretch deformation and elongated with the flow direction in areas b and d, where the average shear stress is high (7 dyne/cm2). The monocytes appeared to be virtually at a standstill after their transmigration across the EC monolayers under static and flow conditions, particularly at the regions near the reattachment point. This suggests that monocytes possess the lowest mobility among the WBCs and that they have the greatest likelihood to be deposited in the vessel wall following transmigration into the subendothelial space. This long residence time may contribute to the role of monocytes as the major cellular component in the formation of atherosclerotic plaques.2,28
Inhibitory effects of neutralizing antibodies against various adhesion molecules and chemokines on the adhesion of neutrophils, PBLs, and monocytes to and their transmigration through the ECs cocultured with SMCs in the reattachment flow area c under VSF. The EC/SMC cocultures were incubated with neutralizing antibodies (20 μg/mL) against the indicated adhesion molecules and chemokines for 1 hour prior to adhesion and transmigration assays under VSF. EC/SMC treated with IgG or without antibody treatment were used as controls. Purified neutrophils, PBLs, and monocytes were perfused over the EC/SMC for 20 minutes, and the adhesion and transmigration of the WBCs in the reattachment flow area c were determined, as described in “Materials and methods.” Data represent percentage of inhibition, which was calculated from the number densities (in cells/mm2) of adherent (▪) or transmigrated (▦) WBCs with antibody treatment (CE) and without antibody treatment (CC): % inhibition = 100 [(CC - CE)/CC]. Data are expressed as mean ± SEM, n = 4. *P < .05 versus EC/SMC treated with control IgG or without antibody treatment.
Inhibitory effects of neutralizing antibodies against various adhesion molecules and chemokines on the adhesion of neutrophils, PBLs, and monocytes to and their transmigration through the ECs cocultured with SMCs in the reattachment flow area c under VSF. The EC/SMC cocultures were incubated with neutralizing antibodies (20 μg/mL) against the indicated adhesion molecules and chemokines for 1 hour prior to adhesion and transmigration assays under VSF. EC/SMC treated with IgG or without antibody treatment were used as controls. Purified neutrophils, PBLs, and monocytes were perfused over the EC/SMC for 20 minutes, and the adhesion and transmigration of the WBCs in the reattachment flow area c were determined, as described in “Materials and methods.” Data represent percentage of inhibition, which was calculated from the number densities (in cells/mm2) of adherent (▪) or transmigrated (▦) WBCs with antibody treatment (CE) and without antibody treatment (CC): % inhibition = 100 [(CC - CE)/CC]. Data are expressed as mean ± SEM, n = 4. *P < .05 versus EC/SMC treated with control IgG or without antibody treatment.
To gain insight into the mechanism by which the EC/SMC coculture enhanced WBC adhesion and transmigration, we examined the expression of 3 adhesion molecules (ICAM-1, VCAM-1, and E-selectin), 3 C-C chemokines (MCP-1, RANTES, and MIG), and 6 C-X-C chemokines (IL-8, I-TAC, SDF-1, IP-10, GRO-α, and MIP) in ECs and SMCs in their coculture. These molecules have been shown to be highly expressed in human or experimental atherosclerotic lesions and have strong effects on WBC recruitment.29-32 The expression of some of these genes, such as IP-10, I-TAC, SDF-1, and E-selectin, was induced only in ECs, but not in SMCs, by their coculture. These results suggest that coculture may exert differential effects on EC and SMC gene expressions. The expressions of ICAM-1, VCAM-1, MCP-1, IL-8, RANTES, and GRO-α were induced in both ECs and SMCs by their coculture, suggesting that ECs and SMCs in their coculture may crosstalk with each other and that the effect of the coculture on the gene expression is bidirectional. The mechanisms underlying this bidirectional modulation of gene expression remain to be determined. The phenotype of SMCs may play a role in their interaction with ECs in modulating gene expressions and WBC recruitment. Recent studies suggest that synthetic SMCs migrated to the intima can generate proinflammatory molecules to promote WBC infiltration of the artery wall.6-8 Our recent data showed that the SMCs used in the present experiments had lower levels of protein expression of contractile markers such as SMα-actin, SM-MHC, h-caldesmon, and calponin than those cultured in the medium containing only 0.5% FBS (Figure S2), indicating that the SMCs in our present study may be in a synthetic phenotype. Moreover, several growth factors or cytokines, such as activated transforming growth factor-β1 (TGF-β1) and IL-1β, have been shown to be produced by the ECs and SMCs in coculture and may be involved in some of the effects exerted by the coculture on these cells.33-35 TGF-β1 activation of adhesion molecules has been reported.36 Our present study using IL-1β as positive controls also showed increases in the expression of most of the genes examined in the activated SMCs. It is likely that these growth factors or cytokines produced by ECs or SMCs in their coculture exert autocrine or paracrine effects on themselves or their partners to contribute to the increased expressions of the adhesion molecules and chemokines.
By using neutralizing antibodies against adhesion molecules ICAM-1, VCAM-1, and E-selectin and chemokines MCP-1, IL-8, I-TAC, SDF-1, IP-10, GRO-α, and RANTES, whose expressions were induced in ECs and/or SMCs in their coculture, our present study identified different candidate molecules that may mediate the differences in the adhesion and migration behaviors among neutrophils, PBLs, and monocytes in the EC/SMC coculture under disturbed flow. A summary of the effects of neutralizing antibodies on WBC behaviors is provided in Table 4. Our results on the roles of these molecules in the WBC behaviors showed good agreements with in vivo findings in human or experimental atherosclerotic lesions. For example, as in human atherosclerosis, ICAM-1 and VCAM-1 were shown in our EC/SMC coculture flow system to play significant roles in the adhesion and transmigration of PBLs and monocytes. These results are also consistent with the in vivo findings by Nakashima et al37 showing that up-regulation of ICAM-1 and VCAM-1 was associated with sites of lesion formation in the apolipoprotein E (ApoE)–deficient mice and responsible for focal recruitment of monocytes and lymphocytes. The absence of MCP-1 has been shown to dramatically decrease lesion size in atherosclerosis-susceptible LDL receptor-deficient mice,38 and the absence or decrease in MCP-1 receptor CCR2 also caused a reduction in lesion size in ApoE-deficient mice.39 Our finding on the blockade of adhesion and transmigration of PBLs and monocytes by the MCP-1 antibody supports the importance of MCP-1 in modulating the formation and progression of atherosclerotic lesions. The antibodies to VCAM-1 and MCP-1 did not exert significant effects on neutrophil adhesion and transmigration; this could be due to the lacks of receptors for VCAM-1 (ie, CD49d/CD29) and MCP-1 (ie, CCR2) on neutrophils.29,30 Although PBLs express L-selectin, the receptor of E-selectin, on their surfaces,30 the E-selectin antibody did not show inhibitory effects on PBL adhesion and transmigration for EC/SMC under disturbed flow, suggesting that the E-selectin/L-selectin pair does not contribute to the PBL recruitment to EC/SMC under disturbed flow. The chemokines GRO-α and IL-8 have been shown to mediate the accumulation of monocytes/macrophages in atherosclerotic lesions of LDL receptor-deficient mice.40 In our coculture flow system, GRO-α was shown to mediate adhesion and transmigration of all 3 types of WBCs. The use of IL-8 antibody blocked the recruitment of neutrophils and monocytes, but not PBLs, to the EC/SMC under disturbed flow; this agrees with the findings that neutrophils and monocytes, but not PBLs, express the IL-8 receptors CXCR1 and CXCR2 on their surfaces.29 The results in our coculture flow system, however, showed that antibody to IL-8 did not affect the transmigration of monocytes. This may be explained by the findings by Zernecke et al41 that monocytes exhibit a higher surface expression of CXCR2 than CXCR1 and that CXCR2 plays minor roles in transmigration. RANTES is a central counterligand for CCR1, CCR3, and CCR5 expressed on different types of WBCs.29 The antibody to RANTES had no effect on the behaviors of neutrophils, which express the RANTES receptor CCR1, suggesting that CCR1 is probably not essential for RANTES-induced WBC chemotaxis. This finding is in agreement with the result reported by Ebisawa et al42 that RANTES failed to induce recruitment of neutrophils. The inhibition of adhesion and transmigration of PBLs and monocytes by the RANTES antibody in our coculture flow system is in agreement with the clinical observation in children that RANTES specifically stimulates activation and transmigration of monocytes and lymphocytes.43 The chemokines IP-10 and I-TAC have been shown to be highly expressed in human atherosclerotic lesions and play significant roles in the recruitment of mononuclear cells.44-46 As expected, the uses of IP-10 and I-TAC antibodies did not inhibit the adhesion and transmigration of neutrophils, which lack their counterreceptor CXCR3.29 However, the antibody to IP-10 did inhibit the adhesion and transmigration of monocytes, although they also are lacking CXCR3.29 This result is in agreement with the findings by Taub et al47 and Jinquan et al48 that IP-10 plays important roles in promoting monocyte interaction with ECs. IP-10 antibody had inhibitory effects on only the adhesion, but not transmigration, of PBLs; this is consistent with the results of Roth et al49 that IP-10 was ineffective in their transendothelial assay. Although neutrophils express the SDF-1 receptor CXCR4,29 the SDF-1 antibody had no effects on neutrophil adhesion and transmigration, suggesting a minimal role played by SDF-1/CXCR4 in neutrophil recruitment in our coculture flow system. Interestingly, the uses of ICAM-1 and VCAM-1 antibodies significantly inhibited the movements of neutrophils and PBLs underneath the ECs cocultured with SMCs under disturbed flow, suggesting that ICAM-1 and VCAM-1 may be the major adhesion molecules contributing to subendothelial migration of neutrophils and PBLs under disturbed flow. Taken together, our present findings indicate that various adhesion molecules and chemokines exert differential effects on the recruitment of neutrophils, PBLs, and monocytes in our EC/SMC coculture system under disturbed flow.
Summary of the effects of neutralizing antibodies against various adhesion molecules and chemokines on the adhesion of neutrophils, PBLs, and monocytes and their subsequent transendothelial and subendothelial migration patterns under disturbed flow
. | ICAM-1 . | VCAM-1 . | E-selectin . | MCP-1 . | IL-8 . | RANTES . | GRO-α . | IP-10 . | I-TAC . | SDF-1 . |
|---|---|---|---|---|---|---|---|---|---|---|
| Neutrophil | ||||||||||
| Adhesion | + | – | + | – | + | – | + | – | – | – |
| Transendothelial migration | + | – | + | – | + | – | + | – | – | – |
| Subendothelial migration | + | – | – | – | – | – | – | – | – | – |
| PBL | ||||||||||
| Adhesion | + | + | – | + | – | + | + | + | + | + |
| Transendothelial migration | + | + | – | + | – | + | + | – | + | + |
| Subendothelial migration | – | + | – | – | – | – | – | – | – | – |
| Monocyte | ||||||||||
| Adhesion | + | + | + | + | + | + | + | + | – | + |
| Transendothelial migration | + | + | + | + | – | + | + | + | – | + |
| Subendothelial migration | – | – | – | – | – | – | – | – | – | – |
. | ICAM-1 . | VCAM-1 . | E-selectin . | MCP-1 . | IL-8 . | RANTES . | GRO-α . | IP-10 . | I-TAC . | SDF-1 . |
|---|---|---|---|---|---|---|---|---|---|---|
| Neutrophil | ||||||||||
| Adhesion | + | – | + | – | + | – | + | – | – | – |
| Transendothelial migration | + | – | + | – | + | – | + | – | – | – |
| Subendothelial migration | + | – | – | – | – | – | – | – | – | – |
| PBL | ||||||||||
| Adhesion | + | + | – | + | – | + | + | + | + | + |
| Transendothelial migration | + | + | – | + | – | + | + | – | + | + |
| Subendothelial migration | – | + | – | – | – | – | – | – | – | – |
| Monocyte | ||||||||||
| Adhesion | + | + | + | + | + | + | + | + | – | + |
| Transendothelial migration | + | + | + | + | – | + | + | + | – | + |
| Subendothelial migration | – | – | – | – | – | – | – | – | – | – |
+ for adhesion indicates the neutralizing antibody has a significant effect on the number of adherent WBCs on EC/SMC; + for transendothelial migration, the neutralizing antibody has a significant effect on either the number of transmigrated WBCs or the transmigration time of WBCs; + for subendothelial migration, the neutralizing antibody has a significant effect on the total distance, the net distance, or the randomness of the subendothelial movement of transmigrated WBCs; – for the neutralizing antibody, no significant effect on the indicated behaviors of WBCs.
In summary, our present study demonstrated for the first time that neutrophils, PBLs, and monocytes exhibit diverse behaviors in adhesion, transmigration, and subendothelial migration in the EC/SMC coculture under disturbed flow. The present study also identified different candidate adhesion molecules and chemokines that may mediate the differences in these behaviors of the 3 types of WBCs in EC/SMC coculture under disturbed flow. Our findings provide new insights into the mechanisms of the interactions between WBCs and the vessel wall under the complex flow environments found in regions of prevalence of atherosclerotic lesions.
Prepublished online as Blood First Edition Paper, November 17, 2005; DOI 10.1182/blood-2005-08-3137.
Supported by the National Health Research Institutes (grant ME-094-PP-06) (J.-J.C.), the National Science Council, Taiwan, ROC (94-3112-B-400-005 and 93-2321-B-400-003), and the National Heart, Lung, and Blood Institute(grant HL19454) (S.C.).
The online version of the article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
This work was conducted in part in affiliation with the Center of Tissue Engineering and Stem Cell Research, National Chung-Hsing University, Taichung, Taiwan, ROC.

![Figure 5. Inhibitory effects of neutralizing antibodies against various adhesion molecules and chemokines on the adhesion of neutrophils, PBLs, and monocytes to and their transmigration through the ECs cocultured with SMCs in the reattachment flow area c under VSF. The EC/SMC cocultures were incubated with neutralizing antibodies (20 μg/mL) against the indicated adhesion molecules and chemokines for 1 hour prior to adhesion and transmigration assays under VSF. EC/SMC treated with IgG or without antibody treatment were used as controls. Purified neutrophils, PBLs, and monocytes were perfused over the EC/SMC for 20 minutes, and the adhesion and transmigration of the WBCs in the reattachment flow area c were determined, as described in “Materials and methods.” Data represent percentage of inhibition, which was calculated from the number densities (in cells/mm2) of adherent (▪) or transmigrated (▦) WBCs with antibody treatment (CE) and without antibody treatment (CC): % inhibition = 100 [(CC - CE)/CC]. Data are expressed as mean ± SEM, n = 4. *P < .05 versus EC/SMC treated with control IgG or without antibody treatment.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/107/5/10.1182_blood-2005-08-3137/2/m_zh80050691880005.jpeg?Expires=1769086243&Signature=wNzbUNAStd1uGIBeyRHzj2gQjRFjS-nPUU775UU1TiZywAbtEBVqG5YoZgICsckkLxN4lJeFQ21wkkWbxAbKc9C4LVOT1ItWAMGPjgKHDEWzL4tu8AgHIssnrccqyF0lG8Ev1GLVDC4EvQGj4pPZJYonOk9jG~DoAdzPH0NsK619dqEuNSdHDJ0uhAkiqQtpClHuRlcRpEJGNWYq3Oy8SxmOvBBuEv7eDE-AP9B7KKjPsuOE413HMcozGLCm0LVH5gtc74eKtz6f8QZGn3EdtoFfcYrGnWznu3VNPS-rNruyITgAMaLIYMYOe2xfNRouOKnJj7luVswHOQG2RMyRkg__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
This feature is available to Subscribers Only
Sign In or Create an Account Close Modal