The metalloproteinase ADAMTS13 regulates the size of released von Willebrand factor (VWF) multimers bound to endothelial cells, but it is unknown whether it can cleave plasma VWF during thrombogenesis. To address this issue, we perfused blood over immobilized VWF and used videomicroscopy to visualize an activation-independent platelet aggregation process mediated by soluble VWF at shear rates greater than 10 000 s-1. At normal Ca2+ concentration, platelets formed rolling as well as surface-attached clusters that grew larger during the first 5 minutes but then lost more than 70% of their mass by 10 minutes. In contrast, platelet clusters were stable in size when metal ions were chelated, anti-ADAMTS13 IgG were added, or washed blood cells were perfused with purified VWF but no plasma. In the latter case, addition of recombinant ADAMTS13 reduced platelet cluster size by more than 70%. Incubating ADAMTS13 with VWF before perfusion did not prevent the initial platelet clustering, indicating that the enzyme may act on platelet-bound VWF under shear stress. At the concentrations tested, ADAMTS13 had no effect on platelet aggregates formed upon blood perfusion over collagen fibrils. ADAMTS13, therefore, may regulate thrombus size preferentially when the cohesion between platelets depends on VWF binding induced by pathologically elevated shear stress.
Introduction
The cleavage of von Willebrand factor (VWF) multimers by ADAMTS13,1 a metalloproteinase, may prevent abnormal platelet aggregation and thrombus formation. This concept is based on the evidence that genetic defects or autoantibodies that alter ADAMTS13 function are associated with thrombotic microangiopathies in which platelet-rich thrombi occlude the microcirculation, causing severe organ damage.2,3 In many patients with thrombotic thrombocytopenic purpura (TTP), unusually large VWF multimers can be detected in plasma4 and may become a concurrent cause of acute thrombotic episodes triggered by still-unknown factors. “Unusually large” refers to the size of VWF multimers that are present within endothelial-cell Weibel-Palade bodies5,6 but not normally in circulating plasma, where they appear after release induced by agonists.7,8 Unusually large VWF multimers are also contained in platelet α-granules,9 from which they are released upon platelet activation.10 ADAMTS13 is thought to cleave VWF still bound to the endothelial cell membrane at the time of release,11 and a similar process may occur during thrombogenesis when stimulated platelets release VWF, thus explaining why the largest multimers are not circulating under normal resting conditions. That VWF undergoes a physiologic proteolytic processing is shown by the fact that subunit fragments are found in the plasma of all healthy individuals.12 The isolation and sequence characterization of these fragments revealed their origin from a single peptide bond cleavage at Tyr842-Met843,13 later identified as the specific ADAMTS13 cleavage site.14-17
The information on how ADAMTS13 cleaves newly released VWF11,18 is paralleled by a lack of knowledge as to whether the protease may further process circulating VWF. Platelet thrombus formation requires VWF function in areas of the circulation with rapid blood flow and shear rate above a threshold limit of approximately 1000 s-1 conditions that occur in the microcirculation.19 Plasma VWF multimers support platelet adhesion at sites of vascular injury after becoming immobilized onto extracellular matrix components,20 and contribute to platelet aggregation after binding to membrane receptors.21 The largest multimers, such as those newly released from endothelial cells and platelets, may be particularly efficient in performing these functions because of their ability to form strong adhesive bonds with cell receptors.22 Normal circulating multimers, however, display adequate thrombogenic function in ex vivo systems21 and may contribute significantly to platelet adhesion and aggregation during hemostasis and thrombosis in vivo. It should also be noted that plasma VWF multimers may enhance their adhesive properties by self-aggregating onto surfaces,23 such that their cleavage could represent an additional mechanism for the control of platelet aggregate size by ADAMTS13 during thrombogenesis. To investigate this possibility, we have established a model of activation-independent platelet cohesion mediated by plasma VWF under conditions of extremely elevated shear stress, and evaluated whether ADAMTS13 had any influence on the size of aggregates formed. Our findings delineate a mechanism for the modulation of plasma VWF-mediated platelet responses to thrombogenic stimuli.
Patients, materials, and methods
Patients and control samples
Blood for perfusion studies was drawn from the antecubital veins of healthy and medication-free human volunteers and collected into syringes containing PPACK (d-phenylalanyl-l-prolyl-l-arginine chloromethyl ketone dihydrochloride, final concentration 93 μM; Bachem Bioscience, King of Prussia, PA) to prevent clotting. Plasma for the preparation of anti-ADAMTS13 IgG was obtained from patients with acquired TTP during therapeutic exchange procedures. Platelet-poor plasma was prepared by centrifuging blood containing 0.011 M trisodium citrate as the anticoagulant for 20 minutes at 3000g and approximately 22°C (room temperature). All studies involving human subjects were conducted in accordance with the Declaration of Helsinki and were approved by institutional review boards of The Scripps Research Institute and Mario Negri Institute. Informed consent to participate in the studies was obtained from all volunteers.
Construction of a recombinant plasmid expressing ADAMTS13
Total RNA (2 μg) from human liver (Stratagene, La Jolla, CA) was heated for 5 minutes at 65°C and reverse transcribed for 50 minutes at 42°C with the SuperScript First-Strand synthesis system (Invitrogen, Carlsbad, CA) using random primers according to the manufacturer's instructions. The resulting cDNA was subjected to polymerase chain reaction (PCR) using a 5′ end BglII tail primer (5′-CTAGCCAGATCTGCTGCAGGCGGCATCCTACACCTGGAG) and a 3′ end AgeI tail primer (5′-TATGCCACCGGTTGCGGTTCCTTCCTTTCCCTTCC; underlining identifies the nucleotide sequences recognized by the indicated restriction enzymes). The amplified ADAMTS13 coding region started with the codon for the first Ala residue known to be the amino terminus of the plasma enzyme. PCR was performed using a high-fidelity DNA polymerase (Platinum Pfx; Invitrogen) with the following cycle profile: 15 seconds at 94°C, 30 seconds at 55°C, and 280 seconds at 68°C (1-minute extension for approximately 1 kb), repeated for 30 cycles. The resulting amplified product was blunt-end ligated into an intermediate cloning vector, PCR-Blunt (Invitrogen). Individual clones were then screened to verify that no spontaneous mutations had arisen during the PCR. The ADAMTS13 cDNA was subsequently removed from the PCR-Blunt construct by digestion with BglII and AgeI, and cloned into the corresponding restriction sites of the expression vector pMTBip/V5-HisA (Invitrogen) in frame with a sequence coding for 6 His residues. The final expression vector, which contained the coding sequence for the BiP secretion signal, directed the synthesis of a soluble carboxyl terminal His-tagged ADAMTS13 fusion protein under the control of a copper sulfate–inducible metallothionein promoter.
Expression of recombinant ADAMTS13
Plasmid DNA was purified through a CsCl2 gradient prior to transfection into Drosophila melanogaster S2 cells (Invitrogen). The cells were maintained at 24°C in Schneider Drosophila medium supplemented with 10% heat-inactivated fetal bovine serum and transfected (2-3 × 106/mL, > 95% viability, 3 mL/well in 6-well plates) using the Calcium Phosphate Transfection Kit (Invitrogen). To obtain stable cell lines expressing human ADAMTS13, 19 μg purified pMT/Bip/ADAMTS13 vector was cotransfected with 1 μg pCoHYGRO, a vector carrying a hygromycin B resistance gene. Cells resistant to hygromycin (used at a final concentration of 300 μg/mL) were selected after 4 weeks as a stable polyclonal population and induced with 0.5 mM copper sulfate in serum-free expression medium. Cell-free conditioned medium was collected 3 days after induction, clarified by centrifugation, and examined for ADAMTS13 expression.
ADAMTS13 Western blot analysis
Proteins in solution were separated by 6% polyacrylamide gel electrophoresis (PAGE) in the presence of 2% sodium dodecyl sulfate (SDS) and after disulfide bond reduction with 5 mM dithiothreitol, and then transferred to nitrocellulose membranes (Invitrogen). Membranes were blocked for 1 hour with TBS-T (20 mM Tris [pH 7.6], 150 mM NaCl, 0.1% Tween 20) containing 5% nonfat dry milk (Blotto solution). Expression of ADAMTS13 was monitored by detection with an antihexahistidine monoclonal antibody diluted 1: 2500 in Blotto solution. After incubation with the antibody, the membranes were washed 3 times with TBS-T and then incubated with a polyclonal horseradish peroxidase–conjugated rabbit anti–mouse IgG anti-serum (1:2000 dilution). Reacting protein bands were identified using an enhanced chemiluminescence detection system (Pierce Super Signal; Pierce Biotechnology, Rockford, IL).
Purification of ADAMTS13 from culture medium
The first step in the purification of recombinant (r) ADAMTS13 was performed using immobilized metal ion affinity chromatography (IMAC) for the direct capture of the His-tagged protein from conditioned medium. Filtered conditioned medium, 1.8 L, was incubated with 120 mL 75% slurry of Chelating Sepharose Fast Flow (Pfizer, New York, NY). The mixture was kept in agitation overnight at 4°C, and then washed 3 times with 5 volumes of a buffer composed of 20 mM HEPES, 300 mM NaCl, and 5 mM imidazole (pH 7.4). Elution was carried out with 2 gel volumes of elution buffer (20 mM HEPES, 300 mM NaCl, and 300 mM imidazole [pH 7.4]), and this step was repeated a total of 3 times. The 3 eluted fractions were subsequently pooled and dialyzed against 20 mM HEPES (pH 8) for further purification on a heparin-Sepharose (Pfizer) column prepared with 45 mL gel and equilibrated with the same HEPES buffer. The IMAC-eluted sample was loaded onto the column at a flow rate of 3 mL/min, and the column was then washed with 20 mM HEPES (pH 7.4) until the absorbance at 280 nm was at baseline. Heparin-bound proteins were eluted with a stepwise NaCl gradient from 0 to 150 mM, 150 to 300 mM, 300 to 450 mM, and 450 to 600 mM. Absorbance was monitored at 280 nm, and peak fractions obtained with 0 to 150 mM and 150 to 300 mM NaCl were collected as 2 pools; they displayed the same functional activity, as evaluated by cleavage of a recombinant VWF A1-A2-A3 fragment, and were eventually mixed. The protein concentration was measured with the micro-BCA assay (Pierce) according to the manufacturer's instructions, and the degree of purification was evaluated by densitometric analysis of the bands corresponding to the proteins in solution separated by SDS-PAGE (see above) and stained with Coomassie blue. The VWF-cleaving activity of purified ADAMTS13 was assessed by measuring the residual collagen binding activity of VWF in a plasma substrate incubated with the test sample under conditions previously described.24 A pool of normal plasma samples obtained from 25 healthy donors was used as a reference for the assays and arbitrarily defined to contain 100% of the protease activity. The values of ADAMTS13 activity were calculated from a dose-response curve obtained by testing serial dilutions (from 1:5 to 1:320) of the reference plasma.
Cleavage of a recombinant VWF A1-A2-A3 fragment by rADAMTS13
A fragment of VWF spanning residues 508 to 1111 of the mature subunit and comprising the 3 type-A domains in the molecule was expressed in Drosophila melanogaster S2 cells, using a combination of the methods described for ADAMTS13 and previously for obtaining domain A1 in Chinese hamster ovary cells.25 The expressed fragment was purified by IMAC and heparin-Sepharose chromatography followed by gel-permeation chromatography. A mixture of VWF fragment (1 μg/mL) and rADAMTS13 (12% of normal plasma activity) was incubated for 2 hours at 37°C in 5 mM Tris buffer (pH 8) containing 1.5 M urea and 3 mM BaCl2. The proteins in solution were then separated by SDS-PAGE (10% polyacrylamide) followed by blotting onto a nitrocellulose membrane. Fragments of the VWF subunit were detected by reaction with a mouse monoclonal antibody directed against an epitope located within the A3 domain, thus carboxyl terminal with respect to the target 842-843 bond.12,13,26,27 Under these conditions, the intact rVWF fragment of approximately 80 kDa and the carboxyl terminal fragment of approximately 30 kDa derived from cleavage at 842-843 could be easily differentiated. Proteins that reacted with the antibody were revealed by incubation with a rabbit anti–mouse IgG coupled to horseradish peroxidase followed by a chemiluminescence reaction. For inhibition of ADAMTS13 activity, rADAMTS13 was incubated for 10 minutes at 37°C with purified IgG antibodies (2 mg/mL) isolated by protein A chromatography from the plasma of a patient with acquired TTP.
Isolation of plasma IgG from a patient with acquired TTP
Plasma from a patient with high-titer anti-ADAMTS13 antibodies causing acquired TTP was obtained from therapeutic plasma exchange and collected in standard citrate-phosphate-dextrose (CPD) anticoagulant. After recalcification, the resulting serum was applied to a Sepharose–Protein A column (Sigma Chemical, St Louis, MO), and IgG was eluted with 0.1 M citric acid at pH 6, 4.5, and 3. All fractions were tested with respect to the ability of inhibiting the ADAMTS13-dependent cleavage of VWF multimers reflected in a collagen-binding test.24 The IgG fraction eluted at pH 3 displayed the highest inhibitory activity and, at the concentration of 2 mg/mL, completely blocked ADAMTS13 function. The same concentration of commercially available IgG (Sigma) served as a control.
Measurement of VWF-mediated and activation-independent platelet cohesion
The detailed description of this assay will be reported elsewhere (Z.M.R., J.N.O., Rolf Habermann, Augusto B. Federici, Armin J. Reininger, manuscript submitted). In brief, we have established a videomicroscopy method to demonstrate that platelets adhere to one another in an activation-independent manner when exposed to a surface presenting immobilized VWF and in the presence of soluble VWF multimers, but only when the wall shear rate is above a threshold of approximately 10 000 s-1. The assay was performed with PPACK-containing human blood. When indicated, prostaglandin (PG) E1 (10 μM; Sigma Chemical) was added to inhibit platelet activation and EDTA (5 mM; Sigma) to chelate divalent cations, respectively. To remove plasma VWF, in some cases blood cells were washed in modified Tyrode buffer (pH 7.4) as reported previously.20,21 The platelet count and hematocrit were adjusted to the original values in whole blood. Perfusion experiments were conducted at 37°C using as adhesive substrate human multimeric VWF, purified from plasma as previously described,28 or fibrillar type I collagen from bovine tendon (acid insoluble; Sigma) immobilized onto glass coverslips that were subsequently assembled into a parallel-plate rectangular flow chamber.20 The perfusion chamber was mounted on the stage of an inverted microscope (Axiovert 135M; Carl Zeiss, Thornwood, NY) for real-time visualization of platelet interactions with the immobilized substrates and with one another. Platelets were rendered fluorescent by the addition of 10 μM mepacrine (quinacrine dihydrochloride; Sigma).29 Images were obtained with a Fluar 20 ×/0.75 numeric aperture (NA) (Figures 1 and 4) or a Plan-Neofluar 40 ×/0.75 NA (Figures 2, 3) objective (Carl Zeiss). Blood flow through the chamber was maintained with a peristaltic pump and adjusted to obtain selected wall shear rates. All experiments were recorded on S-VHS videotape using a silicon-intensified high sensitivity camera (Hamamatsu, Bridgewater, NJ) and a VCR (SVO-9500MD; Sony, Tokyo, Japan) at the acquisition rate of 30 frames/second. Image analysis was performed offline using the Metamorph software package (Universal Imaging, West Chester, PA).29 The number and size (surface area and length) of each individual object detected on and interacting with the surface, as defined by a slow translocation or longer-lasting adhesion, was determined on images obtained at different positions in the flow path of the chamber. Platelet clusters were identified by the computerized program as continuous single objects larger than individual platelets and moving or stationary over a 5-second time period. Quantitative information was typically measured in 5 different positions for each experimental point. The size of these clusters was taken to indicate the cohesive function of larger VWF multimers. A threshold was applied to the images to distinguish platelets and platelet clusters from the background, and the images were binarized before object measurement. The movies on the Blood website (see the Supplemental Videos link at the top of the online article) were prepared by digitizing and editing the recorded analog tapes with Adobe Premiere (Adobe Systems, San Jose, CA).
Measurement of platelet thrombus formation on immobilized collagen fibrils
These experiments were performed essentially as previously described20 by perfusing blood containing 93 μM PPAK as anticoagulant over immobilized acid-insoluble type I collagen fibrils from bovine tendon (Sigma Chemical). The volume of the platelet thrombi formed onto the collagen fibrils was measured from confocal sections as previously described.20,21
Statistical analyses
The statistical significance of differences between measured parameters was evaluated with the Student t test for unpaired groups of values. A P value less than .05 was considered significant.
Results
In relation to the terminology used here, note that traditionally, “aggregation” refers to platelet-platelet cohesion dependent on activation and mediated by adhesive ligands bound to the integrin αIIbβ3, while “agglutination” indicates an activation-independent process of platelet cohesion that in most instances is induced by exogenous modulators, such as ristocetin in the case of the typical VWF/GP Ibα–mediated platelet agglutination. In this study, the term “aggregation” is used in a broad sense to describe the formation of platelet clusters even independently of activation or αIIbβ3 function, as seen in blood perfused at high shear rate over immobilized VWF without addition of exogenous components.
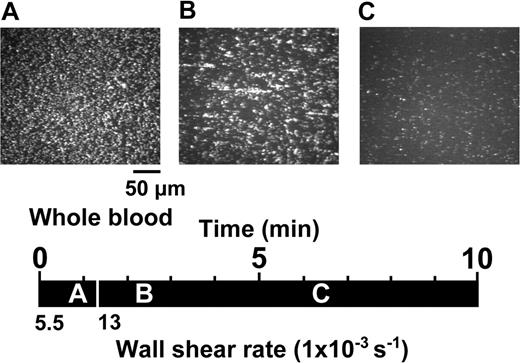
Activation-independent and shear rate-dependent aggregation and disaggregation of platelets perfused over immobilized VWF. Whole blood containing 93 μM PPACK as the anticoagulant, 10 μM PG E1 to block platelet activation, and 10 μM mepacrine to render platelets fluorescent for visualization, was perfused over a surface coated with immobilized VWF. The images shown are single frames from a real-time recording (Video S1). A timeline (black horizontal bar) indicates at what moment during the perfusion experiment a given image was taken, as shown by the position of the corresponding letter (A, 55 seconds; B, 130 seconds; and C, 380 seconds).Avertical white bar separates the timeline in segments corresponding to periods of perfusion at different wall shear rates (5500 or 13 000 s-1). The images are representative of 3 separate experiments performed with blood from different donors.
Activation-independent and shear rate-dependent aggregation and disaggregation of platelets perfused over immobilized VWF. Whole blood containing 93 μM PPACK as the anticoagulant, 10 μM PG E1 to block platelet activation, and 10 μM mepacrine to render platelets fluorescent for visualization, was perfused over a surface coated with immobilized VWF. The images shown are single frames from a real-time recording (Video S1). A timeline (black horizontal bar) indicates at what moment during the perfusion experiment a given image was taken, as shown by the position of the corresponding letter (A, 55 seconds; B, 130 seconds; and C, 380 seconds).Avertical white bar separates the timeline in segments corresponding to periods of perfusion at different wall shear rates (5500 or 13 000 s-1). The images are representative of 3 separate experiments performed with blood from different donors.
The perfusion of PG E1–containing blood over immobilized VWF exposed to a wall shear rate of 5500 s-1 resulted in the adhesion of single platelets exhibiting continuous translocation in the direction of flow (Figure 1A; Video S1). PG E1, as previously demonstrated,29 has no effect on the initial VWF interaction with GP Ibα but, by inhibiting platelet activation, impairs the subsequent stable adhesion and aggregation mediated by ligand binding to activated αIIbβ3. In contrast, a new modality of platelet adhesion and aggregation emerged when the shear rate was increased to 13 000 s-1. After approximately 1 minute of perfusion at this higher shear rate, the number of individual platelets interacting with immobilized VWF decreased and clusters of several platelets linked to one another appeared, either rolling on the surface with variable shape or forming chainlike structures aligned in the direction of flow and stationary for variable periods of time (Figure 1B; Video S1). Translocating platelet clusters tended to arrest transiently onto the stationary ones, often detaching them from immobilized VWF and incorporating them into rolling aggregates that increased in size as they progressed along the surface. The number and size of the aggregates formed under these experimental conditions initially increased up to a maximum at approximately 3 minutes, but then started to decrease progressively and, after approximately 5 minutes of perfusion, mostly single platelets were seen interacting with the surface (Figure 1C; Video S1).
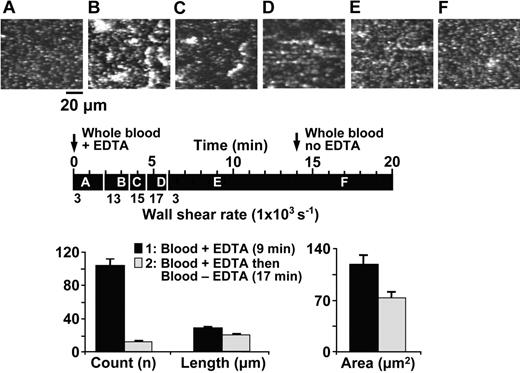
Effect of EDTA on the activation-independent and shear rate–dependent aggregation and disaggregation of platelets perfused over immobilized VWF. Whole blood containing 93 μM PPACK, 10 μM PG E1, 10 μM mepacrine, and the addition of EDTA (5 mM) from the beginning of flow, was perfused over immobilized VWF at varying wall shear rates. The images shown are single frames from a real-time recording (Video S2). The timeline (black horizontal bar) shows at what moment during the experiment a given image was taken as well as the wall shear rate during different periods of perfusion, as explained in the legend to Figure 1 (A, 50 seconds, 3000 s-1; B, 180 seconds, 13 000 s-1; C, 240 seconds, 15 000 s-1; D, 340 seconds, 17 000 s-1; E, 540 seconds, 3000 s-1; and F, 1020 seconds, 3000 s-1). An arrow at 14 minutes on the timeline indicates when blood without EDTA replaced the blood with EDTA (from the same donor) perfused from the beginning of the experiment. The bar graph shows the average count, length, and area of all the platelet aggregates present in 5 different positions on the surface after 540 seconds (▪) and 1020 seconds (▦) of perfusion under the conditions described. All values are reported as mean ± SEM and differences are statistically significant (count: P < .001; length: P < .001; area: P < .006).
Effect of EDTA on the activation-independent and shear rate–dependent aggregation and disaggregation of platelets perfused over immobilized VWF. Whole blood containing 93 μM PPACK, 10 μM PG E1, 10 μM mepacrine, and the addition of EDTA (5 mM) from the beginning of flow, was perfused over immobilized VWF at varying wall shear rates. The images shown are single frames from a real-time recording (Video S2). The timeline (black horizontal bar) shows at what moment during the experiment a given image was taken as well as the wall shear rate during different periods of perfusion, as explained in the legend to Figure 1 (A, 50 seconds, 3000 s-1; B, 180 seconds, 13 000 s-1; C, 240 seconds, 15 000 s-1; D, 340 seconds, 17 000 s-1; E, 540 seconds, 3000 s-1; and F, 1020 seconds, 3000 s-1). An arrow at 14 minutes on the timeline indicates when blood without EDTA replaced the blood with EDTA (from the same donor) perfused from the beginning of the experiment. The bar graph shows the average count, length, and area of all the platelet aggregates present in 5 different positions on the surface after 540 seconds (▪) and 1020 seconds (▦) of perfusion under the conditions described. All values are reported as mean ± SEM and differences are statistically significant (count: P < .001; length: P < .001; area: P < .006).
To evaluate the hypothesis that the progressive disappearance of activation-independent platelet aggregates formed under high shear stress resulted from changes in VWF, possibly caused by the metal ion–dependent protease ADAMTS13, we performed a set of experiments using blood containing the metal ion chelator EDTA (5 mM) from the beginning of flow. The wall shear rate was kept at 3000 s-1 for approximately 2 minutes to allow the adhesion of single translocating platelets (Figure 2A), then between approximately 2 and 6 minutes it was increased progressively to 17 000 s-1 to induce activation-independent aggregation (Figure 2B-D), and finally it was decreased to 3000 s-1 to monitor aggregate stability over a total period of approximately 15 minutes (Figure 2E; Video S2). At this point, the EDTA-containing blood was replaced with the same blood but without EDTA and the perfusion continued for about 5 minutes. Platelet clusters that formed under high shear stress in the presence of EDTA were stable for over 10 minutes, while they typically lasted only 2 to 3 minutes when forming in the absence of EDTA (compare Figures 1 and 2 and Videos S1 and S2).
Accordingly, platelet aggregates that had been stable for several minutes in the presence (+) of EDTA (5 mM) were markedly and rapidly reduced in number when the latter was removed (–) from the perfusing blood (+EDTA, 104 ± 6.70; -EDTA, 12 ± 1.43; Figure 2). The few aggregates that remained after 3 minutes of perfusion without EDTA were significantly reduced in size, in both average length and surface area (Figure 2). The effect of removing EDTA was even more apparent when the dimensions of the single largest aggregate rather than the average dimensions of all the aggregates present in a field of view were evaluated (Table 1). Note that activation-independent platelet clustering required a shear rate above 10 000 s-1 to occur effectively, but afterward persisted even at the lower shear rate of 3000 s-1 (Video S2). ADAMTS13 could efficiently reduce aggregate size even at the lower shear rate, suggesting that the protease does not require extremely high shear stress to cleave VWF.
Change in activation-independent platelet aggregate size in the presence or absence of EDTA
. | EDTA present . | EDTA absent . |
|---|---|---|
| Area minimum, μm2 | 23.39 ± 0.60 | 23.73 ± 0.65 |
| Area maximum, μm2 | 1187.29 ± 187.58 | 329.00 ± 43.92 |
| Length minimum, μm | 6.06 ± 0.32 | 8.64 ± 1.00 |
| Length maximum, μm | 210.35 ± 36.81 | 61.31 ± 10.55 |
. | EDTA present . | EDTA absent . |
|---|---|---|
| Area minimum, μm2 | 23.39 ± 0.60 | 23.73 ± 0.65 |
| Area maximum, μm2 | 1187.29 ± 187.58 | 329.00 ± 43.92 |
| Length minimum, μm | 6.06 ± 0.32 | 8.64 ± 1.00 |
| Length maximum, μm | 210.35 ± 36.81 | 61.31 ± 10.55 |
Experimental details are given in the legend to Figure 2. Blood containing PPACK and PG E1 was perfused over immobilized VWF at varying shear rates. The blood contained 5 mM EDTA for the initial 14 minutes, and then blood from the same donor but without EDTA was perfused for an additional 6 minutes. The values (mean ± SEM of 5 separate fields of view; Figure 2) represent the area of the single smallest and largest aggregate and the length of the single shortest and longest aggregate detected on the surface at 9 minutes (blood with EDTA) or 17 minutes (blood without EDTA) from the beginning of perfusion. The differences in maximal area (P < .001) and length (P < .001) are significant.
To confirm the identity of the plasma component responsible for the disappearance of activation-independent platelet aggregates formed under high shear stress, we carried out perfusion experiments with blood cells washed free of plasma and resuspended in a buffer containing 20 μg/mL purified VWF multimers. Preliminary experiments (not shown) demonstrated that no platelet clusters formed in the absence of added soluble VWF even above the threshold shear rate of 10 000 s-1. In the presence of soluble VWF, large platelet aggregates could be seen attached to the immobilized VWF surface over an observation period of approximately 6 minutes, but their stability was greatly reduced when recombinant ADAMTS13 was added to the blood cell suspension together with the VWF multimers before the beginning of perfusion (Figure 3; Video S3). The protease used in these experiments could cleave a recombinant VWF fragment comprising domains A1-A2-A3 under denaturing conditions and in the absence of flow (not shown).
Effect of recombinant ADAMTS13 on VWF-mediated and activation-independent platelet thrombus formation under flow. Plasma-free washed blood cells were suspended in a HEPES/Tyrode buffer (10 mM HEPES, 135 mM NaCl, 2.7 mM KCl, 0.4 mM NaH2PO4, and 2.8 mM dextrose), pH 7.4, containing 10 μM PG E1, 10 μM mepacrine, 20 μg/mL purified human VWF multimers, 2 mM Ca2+, and 0.5 mM Mg2+. The blood cell suspension (platelet count, 180-390 × 109/L; hematocrit, 0.38-0.43) was perfused over a surface coated with immobilized VWF without or with the addition of recombinant ADAMTS13, as indicated. The protease activity added corresponded to 17.5% of that present in normal blood. The images shown are single frames from a real time recording (Video S3). The timeline (black horizontal bar) shows at what moment during the experiment a given image was taken, as well as the wall shear rate during different periods of perfusion, as explained in the Figure 1 legend (A, 30 seconds, 5500 s-1; B, 170 seconds, 13 000 s-1; and C, 370 seconds, 13 000 s-1). The bar graph shows the average count, length, and area (mean ± SEM) of all the platelet aggregates measured in 5 separate fields of view after 10 minutes of perfusion in the absence or presence of recombinant ADAMTS13 during each of 3 experiments performed with blood cells from different donors. Differences are statistically significant for count (P < .009) and area (P < .04), but not for length (P > .05).
Effect of recombinant ADAMTS13 on VWF-mediated and activation-independent platelet thrombus formation under flow. Plasma-free washed blood cells were suspended in a HEPES/Tyrode buffer (10 mM HEPES, 135 mM NaCl, 2.7 mM KCl, 0.4 mM NaH2PO4, and 2.8 mM dextrose), pH 7.4, containing 10 μM PG E1, 10 μM mepacrine, 20 μg/mL purified human VWF multimers, 2 mM Ca2+, and 0.5 mM Mg2+. The blood cell suspension (platelet count, 180-390 × 109/L; hematocrit, 0.38-0.43) was perfused over a surface coated with immobilized VWF without or with the addition of recombinant ADAMTS13, as indicated. The protease activity added corresponded to 17.5% of that present in normal blood. The images shown are single frames from a real time recording (Video S3). The timeline (black horizontal bar) shows at what moment during the experiment a given image was taken, as well as the wall shear rate during different periods of perfusion, as explained in the Figure 1 legend (A, 30 seconds, 5500 s-1; B, 170 seconds, 13 000 s-1; and C, 370 seconds, 13 000 s-1). The bar graph shows the average count, length, and area (mean ± SEM) of all the platelet aggregates measured in 5 separate fields of view after 10 minutes of perfusion in the absence or presence of recombinant ADAMTS13 during each of 3 experiments performed with blood cells from different donors. Differences are statistically significant for count (P < .009) and area (P < .04), but not for length (P > .05).
By densitometric analysis of Coomassie blue–stained protein bands separated by SDS-PAGE, we estimated that ADAMTS13 represented 25% of the protein present in the preparation. The corresponding functional activity, measured as cleavage of the larger VWF multimers resulting in reduced binding to collagen, was 231% of that present in a normal reference plasma. Thus, based on the amounts added, the ADAMTS13 activity in the washed blood cell suspension used for the experiment shown in Figure 3 was 17.5% of that in normal blood. Yet, at least in the absence of other plasma proteins, this level of activity was sufficient to regulate VWF-mediated platelet cohesion. In agreement with the results obtained in the presence or absence of EDTA, the effect of ADAMTS13 on activation-independent platelet aggregates was particularly evident in the reduction of their number (-ADAMTS13, 86.3 ± 17.6; +ADAMTS13, 4.0 ± 1.5; Figure 3) as well as of the maximal size and length that they could attain (Table 2). Of note, the addition of ADAMTS13 did not prevent the initial formation of activation-independent platelet aggregates, which in fact were as numerous and large as in the absence of the protease within approximately 3 minutes, but caused their disappearance as opposed to persistence over the subsequent 3 minutes (Figure 3; Video S3). Preincubation of ADAMTS13 with the blood cell/VWF suspension for over 1 hour before perfusion did not affect the initial formation of activation-independent platelet aggregates or accelerate their disappearance, indicating that the enzyme likely acts under flow conditions after VWF multimers are bound to platelet GP Ibα and exposed to shear stress.
Change in activation-independent platelet aggregate size in the presence or absence of recombinant ADAMTS13
. | ADAMTS13 absent . | ADAMTS13 present . |
|---|---|---|
| Area minimum, μm2 | 91.96 ± 0.59 | 84.25 ± 25.41 |
| Area maximum, μm2 | 2400.00 ± 10.41 | 320.54 ± 132.04 |
| Length minimum, μm | 13.53 ± 0.91 | 20.00 ± 2.57 |
| Length maximum, μm | 238.82 ± 22.36 | 53.83 ± 14.24 |
. | ADAMTS13 absent . | ADAMTS13 present . |
|---|---|---|
| Area minimum, μm2 | 91.96 ± 0.59 | 84.25 ± 25.41 |
| Area maximum, μm2 | 2400.00 ± 10.41 | 320.54 ± 132.04 |
| Length minimum, μm | 13.53 ± 0.91 | 20.00 ± 2.57 |
| Length maximum, μm | 238.82 ± 22.36 | 53.83 ± 14.24 |
Experimental details are given in the legend to Figure 3. A suspension of washed blood cells with added soluble VWF and without or with added ADAMTS13 (protease activity added before perfusion equals 17.5% of that present in normal blood) was perfused over immobilized VWF at varying shear rates. The values (mean ± SEM of 5 different fields of view in 3 separate experiments; Figure 3) represent the area of the single smallest and largest aggregate and the length of the single shortest and longest aggregate detected on the surface. The differences in maximal area (P < .001) and length (P < .002) are significant.
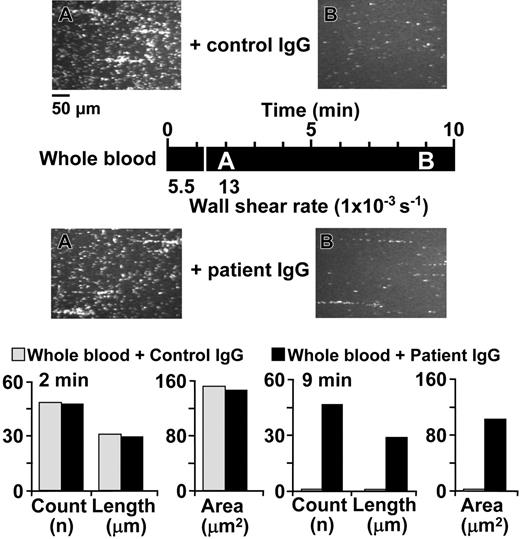
Additional experiments confirmed the role of ADAMTS13 in controlling the number and size of activation-independent platelet aggregates formed under high shear stress. First, we used the IgG fraction derived from the plasma of a patient with recurrent TTP who had developed function-blocking anti-ADAMTS13 antibodies. When this IgG was added to normal blood, the subsequent perfusion over immobilized VWF resulted in the formation of activation-independent aggregates that were more numerous, were larger in size and persisted for longer periods of time than after addition of control IgG prepared from the plasma of a healthy volunteer (Figure 4; Table 3).
Change in activation-independent platelet aggregate size in the presence or absence of IgG from a patient with neutralizing antibodies against ADAMTS13
. | Control IgG . | . | Patient IgG . | . | ||
|---|---|---|---|---|---|---|
. | 2 minutes . | 9 minutes . | 2 minutes . | 9 minutes . | ||
| Area minimum, μm2 | 22.60 | 0 | 22.60 | 22.60 | ||
| Area maximum, μm2 | 806.82 | 0 | 971.80 | 553.70 | ||
| Length minimum, μm | 6.21 | 0 | 6.37 | 5.45 | ||
| Length maximum, μm | 155.66 | 0 | 143.16 | 122.67 | ||
. | Control IgG . | . | Patient IgG . | . | ||
|---|---|---|---|---|---|---|
. | 2 minutes . | 9 minutes . | 2 minutes . | 9 minutes . | ||
| Area minimum, μm2 | 22.60 | 0 | 22.60 | 22.60 | ||
| Area maximum, μm2 | 806.82 | 0 | 971.80 | 553.70 | ||
| Length minimum, μm | 6.21 | 0 | 6.37 | 5.45 | ||
| Length maximum, μm | 155.66 | 0 | 143.16 | 122.67 | ||
Experimental details are given in the legend to Figure 4. Normal blood with added IgG isolated from normal plasma or the plasma of a patient with neutralizing antibodies against ADAMTS13 was perfused over immobilized VWF at varying shear rates. The values represent the average area of the single smallest and largest aggregate and the average length of the single shortest and longest aggregate detected in a field of view of the surface at different times of perfusion, as indicated.
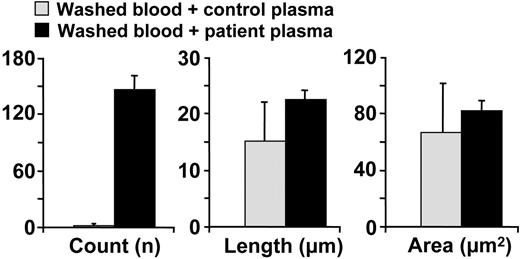
In another experiment, we prepared washed normal blood cells and resuspended them either in normal plasma or in the plasma of a TTP patient with a congenital deficiency of ADAMTS13. In the latter case, the activation-independent platelet aggregates formed upon perfusion over immobilized VWF were more numerous and larger in size after 10 minutes of perfusion than seen with normal plasma (Figure 5; Table 4).
Change in activation-independent platelet aggregate size in the presence of normal plasma or plasma from a patient with undetectable ADAMTS13 activity
. | Control plasma . | Patient plasma . |
|---|---|---|
| Area minimum, μm2 | 45.20 ± 28.62 | 22.62 ± 0.23 |
| Area maximum, μm2 | 109.15 ± 61.71 | 652.91 ± 136.62 |
| Length minimum, μm | 11.44 ± 6.28 | 5.50 ± 0.13 |
| Length maximum, μm | 22.32 ± 9.62 | 142.65 ± 30.00 |
. | Control plasma . | Patient plasma . |
|---|---|---|
| Area minimum, μm2 | 45.20 ± 28.62 | 22.62 ± 0.23 |
| Area maximum, μm2 | 109.15 ± 61.71 | 652.91 ± 136.62 |
| Length minimum, μm | 11.44 ± 6.28 | 5.50 ± 0.13 |
| Length maximum, μm | 22.32 ± 9.62 | 142.65 ± 30.00 |
Experimental details are given in the legend to Figure 5. Washed blood cells were resuspended in plasma either from a healthy individual or from a patient with unmeasurable ADAMTS13 activity and then perfused over immobilized VWF at varying shear rates. The values represent the area of the single smallest and largest aggregate and the length of the single shortest and longest aggregate detected on the surface (mean ± SEM of 5 separate fields of view; Figure 5). The differences in maximal area (P < .007) and length (P < .005) are significant.
Effect of IgG from a patient with TTP on VWF-mediated and activation-independent platelet thrombus formation under flow. Whole blood containing 93 μM PPACK as anticoagulant and 10 μM PG E1 to inhibit platelet activation was supplemented with 2 mg/mL IgG purified from the plasma of a healthy individual (control IgG) or 2 mg/mL IgG purified from the plasma of a patient with function-blocking antibodies against ADAMTS13 (patient IgG) and then perfused over immobilized VWF. The images shown are single frames from a real-time recording. The timeline (black horizontal bar) shows at what moment during the experiment a given image was taken as well as the wall shear rate during different periods of perfusion, as explained in the Figure 1 legend (A, 120 seconds, 13 000 s-1; B, 540 seconds, 13 000 s-1). The bar graph shows the average count, length, and area of all the platelet aggregates present in a field of view after 2 and 9 minutes of perfusion in the presence of control or patient IgG, as indicated.
Effect of IgG from a patient with TTP on VWF-mediated and activation-independent platelet thrombus formation under flow. Whole blood containing 93 μM PPACK as anticoagulant and 10 μM PG E1 to inhibit platelet activation was supplemented with 2 mg/mL IgG purified from the plasma of a healthy individual (control IgG) or 2 mg/mL IgG purified from the plasma of a patient with function-blocking antibodies against ADAMTS13 (patient IgG) and then perfused over immobilized VWF. The images shown are single frames from a real-time recording. The timeline (black horizontal bar) shows at what moment during the experiment a given image was taken as well as the wall shear rate during different periods of perfusion, as explained in the Figure 1 legend (A, 120 seconds, 13 000 s-1; B, 540 seconds, 13 000 s-1). The bar graph shows the average count, length, and area of all the platelet aggregates present in a field of view after 2 and 9 minutes of perfusion in the presence of control or patient IgG, as indicated.
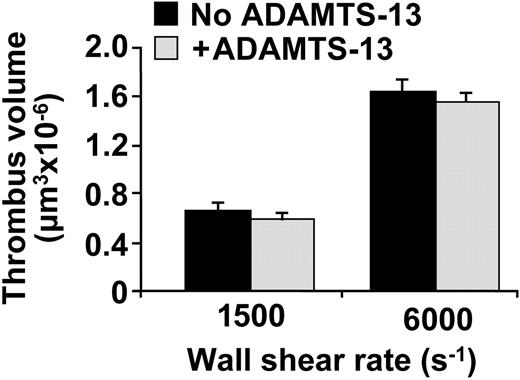
For comparison, we then evaluated whether the addition of ADAMTS13 could influence the stability of platelet aggregates formed upon perfusion of blood over immobilized collagen type I fibrils. In this case, inhibitors of platelet activation were not included in the perfusion. Recombinant ADAMTS13, added to enhance the endogenous activity already present in normal blood, had no significant effect on the size of platelet thrombi formed at shear rates as high as 6000 s-1 (Figure 6).
The supplementary protease activity was 17.5% of that present in normal blood, the same concentration that by itself was sufficient to reduce considerably the size of activation-independent and VWF-mediated platelet clusters (see Figure 3). Whether higher concentrations of ADAMTS13 could yield an effect on collagen-induced platelet thrombi remains to be established, but it is clear that, in any case, this would require concentrations of the protease well above physiologic levels.
Effect of plasma from a congenital TTP patient on VWF-mediated and activation-independent platelet thrombus formation under flow. Normal washed blood cells were resuspended either in the plasma of a healthy individual (control plasma) or that of a patient with TTP obtained from therapeutic plasmapheresis (patient plasma). The bar graph shows the average count, length, and area (mean ± SEM) of all the platelet aggregates present in 5 separate fields of view measured after a 10-minute perfusion. Only the difference in aggregate count was significant (P < .001).
Effect of plasma from a congenital TTP patient on VWF-mediated and activation-independent platelet thrombus formation under flow. Normal washed blood cells were resuspended either in the plasma of a healthy individual (control plasma) or that of a patient with TTP obtained from therapeutic plasmapheresis (patient plasma). The bar graph shows the average count, length, and area (mean ± SEM) of all the platelet aggregates present in 5 separate fields of view measured after a 10-minute perfusion. Only the difference in aggregate count was significant (P < .001).
Discussion
Our findings indicate that ADAMTS13 can cleave plasma VWF multimers bound to platelets, as judged from the reduction in the number and size of platelet clusters formed under conditions of elevated shear stress and in the absence of activation. This unique form of platelet cohesion depends on a mechanism that we will report in detail elsewhere (Z.M.R., J.N.O., Rolf Habermann, Augusto B. Federici, Armin J. Reininger, manuscript submitted). It is known that individual platelets interact with and roll onto immobilized VWF when exposed to the shear rates found in the normal arteriolar circulation.29 As shown here, however, clustered rather than individual platelets roll onto immobilized VWF when the shear rate is above a critical threshold of approximately 10 000 s-1. This phenomenon is strictly dependent on the presence of immobilized VWF to tether platelets onto the surface as well as soluble VWF to mediate interplatelet cohesion, and occurs in whole blood or washed blood cells suspended in a buffer with purified VWF multimers. The resulting clumps, which may consist of hundreds of platelets, attach more firmly to immobilized VWF and acquire an elongated stringlike shape when the shear rate is above 20 000 s-1. In the latter situation, the length of such strings can exceed 200 μm. Platelet clusters do not form if soluble VWF is not present or is preincubated with a monoclonal antibody blocking A1 domain function. Formation of these VWF-dependent clusters does not require platelet activation or integrin function, as it may occur in the presence of PG E1 and EDTA. In this respect, therefore, the process of platelet cohesion visualized onto an immobilized VWF surface in the presence of soluble VWF is different from the previously described shear-induced aggregation detected with platelets in suspension in the absence of surface interactions.5,30-33 Common to both processes is the dependence on soluble VWF multimers and elevated shear stress, but the clusters visualized here onto immobilized VWF are clearly distinct in that they do not require platelet activation or αIIbβ3 function. The activation-independent and VWF/GP Ibα–mediated platelet cohesion shown here may represent in an amplified manner the step preceding activation during the previously described shear-induced aggregation, and the ability to detect it may depend exclusively on the real-time visualization of the interaction with immobilized VWF. Of note, activation-independent and VWF/GP Ibα–mediated platelet cohesion involves only physiologic blood components and thus has the potential to occur in vivo under conditions of pathologically elevated shear stress. This process may become relevant in thrombotic microangiopathies when induced in the context of an altered vessel wall that promotes platelet aggregate stability.
Effect of recombinant ADAMTS13 on platelet thrombus formation on fibrillar collagen type I under flow conditions. The bar graph shows the volume of thrombi formed after perfusion of whole blood containing 93 μM PPACK over a surface of 62 500 μm2 coated with collagen type I fibrils. The blood was supplemented or not with recombinant ADAMTS13, as indicated, which was added at least 15 minutes before perfusion. The protease activity added corresponded to 17.5% of that present in normal blood. The wall shear rate was 1500 s-1 for 7 minutes or 6000 s-1 for 4.5 minutes, as indicated. The values represent the mean ± SEM of 2 experiments performed with blood from different donors. The results obtained with or without addition of ADAMTS13 were not significantly different.
Effect of recombinant ADAMTS13 on platelet thrombus formation on fibrillar collagen type I under flow conditions. The bar graph shows the volume of thrombi formed after perfusion of whole blood containing 93 μM PPACK over a surface of 62 500 μm2 coated with collagen type I fibrils. The blood was supplemented or not with recombinant ADAMTS13, as indicated, which was added at least 15 minutes before perfusion. The protease activity added corresponded to 17.5% of that present in normal blood. The wall shear rate was 1500 s-1 for 7 minutes or 6000 s-1 for 4.5 minutes, as indicated. The values represent the mean ± SEM of 2 experiments performed with blood from different donors. The results obtained with or without addition of ADAMTS13 were not significantly different.
The function of ADAMTS13 has been demonstrated previously on nascent ultralarge VWF multimers newly released from and still bound to the surface of endothelial cells,11,34 but several unique characteristics distinguish the process described here in the presence of plasma VWF. The platelets appearing as “beads on a string,” as reported by Dong and colleagues,11 clearly represent single platelets each adhering individually to long VWF multimers, and could only assemble on the surface of stimulated endothelial cells but not on unstimulated cells in the presence of plasma VWF. Moreover, this type of adhesion to ultralarge multimers occurred in a range of shear stress typical of both venous and arterial blood flow, reportedly11 between 2.5 and 50 dyn/cm2 (with washed platelets resuspended in buffer, these values correspond to shear rates between 250 and 5000 s-1). Even the highest value is well below the threshold we found to be necessary for inducing VWF-mediated and activation-independent platelet cohesion (with platelets in whole blood, the threshold of 10 000 s-1 corresponds to a shear stress of 400 dyn/cm2). It is noteworthy that platelet adhesion to ultralarge endothelial VWF multimers was reported to decrease progressively with increasing shear stress,11 a behavior opposite to the enhanced VWF-mediated platelet cohesion reported here with increasing shear rates. These differences are likely to reflect distinct mechanisms involved in the 2 processes. Adhesion to endothelial VWF appears to be a special case of platelet interaction with immobilized VWF, which is known to occur independently of elevated shear stress,29 and is characterized by the much greater stability of the bonds formed. Shear-induced platelet cohesion, on the other hand, may require the induction of conformational changes in soluble plasma VWF multimers to allow the initiation of the interaction with platelet GP Ibα. In either case, the conclusion that endothelial or plasma VWF multimers are cleaved by ADAMTS13 was based on the demonstrated breakdown of platelet adhesion or cohesion clearly dependent exclusively on VWF. Albeit indirect, the evidence presented here was obtained with different types of experiments in which the prevention or acceleration of the breakdown of platelet cohesion was correlated to the inhibition or enhancement, respectively, of ADAMTS13 function. Our findings, therefore, add to the concept of ADAMTS13-mediated regulation of VWF multimer size at the time of secretion from stimulated endothelial cells, as it is now apparent that plasma VWF multimers can be further cleaved as they mediate platelet-to-platelet cohesion under elevated shear stress. This observation does not exclude the possibility that ADAMTS13 may cleave plasma VWF exposed to shear stress even when not bound to platelets. In any case, cleavage of VWF multimers is a time-dependent process, as suggested by the observation that even supplementation of normal blood, which already contains ADAMTS13, with additional amounts of recombinant protease could not prevent the initial formation of VWF-mediated platelet aggregates.
It is difficult to ascribe a direct pathophysiologic significance to the observations presented here beyond the evidence that ADAMTS13 can effectively regulate the size and persistence in time of plasma VWF-mediated platelet aggregates. In contrast, there was apparently no similar effect on the size of thrombi formed upon blood perfusion over collagen type I fibrils, a situation in which platelets are fully activated and platelet-to-platelet cohesion is mediated by αIIbβ3 through the binding of different ligands.20;21 It is possible that under the latter conditions the role of VWF interacting with GP Ibα is temporally limited to supporting the very initial platelet-surface and platelet-platelet contacts,20,21 while stable bonds are then created by other ligands, such as fibrinogen and fibronectin,35,36 which cannot be cleaved by ADAMTS13. In agreement with these observations, the functional absence of ADAMTS13 is a known risk factor for the development of platelet-rich thrombi in the microarteriolar circulation, where shear rates are the highest, but not for the thrombotic occlusion of larger arteries. Our results suggest that VWF may be the main adhesive protein bridging platelets to one another and precipitating microarteriolar thrombosis in patients with acute episodes of TTP. The presence of an abnormal endothelial cell surface altered by inflammatory processes and the local release of unusually large VWF multimers may be necessary to initiate such episodes, but it is apparent from our findings that plasma VWF may also contribute to platelet aggregation if not regulated by ADAMTS13.
Prepublished online as Blood First Edition Paper, November 17, 2005; DOI 10.1182/blood-2005-07-2972.
Supported by grants HL31950, HL42846, and HL78784 from the National Institutes of Health. C.C. is a recipient of a grant from Fondazione Ainti per la Ricerca sulle Malattie Rare (ARMR) through a donation in memory of Maria Zanetti.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
We thank Dr Miriam Galbusera for her help in the study of patients with TTP, and Mr Rolf Haberman for help in preparing the supplementary videos.


This feature is available to Subscribers Only
Sign In or Create an Account Close Modal