In allogeneic hematopoietic cell transplantation (allo-HCT), the immune recognition of host antigens by donor T lymphocytes leads to a beneficial graft-versus-leukemia (GvL) effect as well as to life-threatening graft-versus-host disease (GvHD). Genetic modification of T lymphocytes with a retroviral vector (RV) expressing the herpes simplex virus-thymidine kinase (TK) suicide gene confers selective sensitivity to the prodrug ganciclovir (GCV). In patients, the infusion of TK+ lymphocytes and the subsequent administration of GCV resulted in a time-wise modulation of antihost reactivity for a GvL effect, while controlling GvHD. Because activation required for genetic modification with RV may reduce antihost reactivity, we investigated the requirements for maximizing the potency of human TK+ lymphocytes. Whereas T-cell receptor triggering alone led to effector memory (EM) TK+ lymphocytes, the addition of CD28 costimulation through cell-sized beads resulted in the generation of central memory (CM) TK+ lymphocytes. In a quantitative model for GvHD using nonobese diabetic/severely combined immunodeficient mice, CM TK+ lymphocytes were more potent than EM TK+ lymphocytes. GCV administration efficiently controlled GvHD induced by CM TK+ lymphocytes. These results warrant the clinical investigation of CM suicide gene-modified human T lymphocytes for safe and effective allo-HCT.
Introduction
Allogeneic hematopoietic cell transplantation (allo-HCT) is the curative option for many hematologic malignancies. Moreover, it is being investigated to treat solid tumors. In allo-HCT, the recognition of host antigens by donor T lymphocytes evokes a graft-versus-leukemia (GvL) effect.1 This is associated with the risk of graft-versus-host disease (GvHD). T-cell depletion prevents GvHD but increases the probability of leukemia relapse. Suicide gene therapy offers a pragmatic solution to the “T-cell dilemma” of allo-HCT.2 A suicide gene codifies for a protein able to convert, at a cellular level, a nontoxic prodrug into a toxic product. Suicide gene modification of donor lymphocytes aims at exploiting allo-HCT for a GvL effect, while providing a selective “switch” to GvHD.3 The thymidine kinase of herpes simplex virus (TK) is a cell cycle-dependent suicide gene, that is, it catalyzes the generation of triphosphate ganciclovir (GCV), which is toxic to proliferating cells.4 In HLA-identical allo-HCT, delayed infusions of TK+ lymphocytes were effective against relapsing leukemia, lymphoma, multiple myeloma, and Epstein-Barr virus (EBV)–related lymphoproliferative disorders.5 When administered at the time of transplantation, TK+ lymphocytes facilitated the engraftment of hematopoietic cells.6,7 In patients developing GvHD, GCV administration eliminated circulating TK+ cells and controlled the disease.5-7
Despite these encouraging results, dissemination of the TK technology has been limited by the difficulty in defining optimal conditions for cell manipulation. Current protocols for genetic modification of T lymphocytes with retroviral vectors (RVs) may reduce antigen responsiveness in vitro8 and antihost reactivity in vivo.9,10 Given the strict clinical association between GvL and GvHD, it may be postulated that the therapeutic efficacy of TK+ lymphocytes will be improved by protocols designed to maximize antihost reactivity in vivo.
Genetic modification of T lymphocytes with RVs relies on cell proliferation and leads to a memory phenotype.11,12 Different models have been proposed to describe mature T-cell differentiation. The linear model, described first by Sallusto et al in 1999,13 dictates that antigen exposure control the transition from naive, to central memory (CM) and, eventually, to effector memory (EM) cells. Lymphocytes belonging to the different stages can be identified by the coexpression of CD45RA and the chemokine receptor 7 (CCR7): CD45RA+CCR7+ naive cells home to lymphoid organs and, on antigen encounter, proliferate extensively but lack immediate effector functions, CD45RA-CCR7+ CM cells retain homing to lymphoid organs and display intermediate proliferative and effector capacities, and finally, CD45RA-CCR7- effector memory cells travel to inflamed tissues, where they poorly proliferate but are endowed with potent effector functions.14 Recently, it has been proposed that the ability to cause GvHD may be confined to specific stages of T-cell differentiation.15-18 Although confirmed in animal models, this hypothesis has not been documented in humans so far.
Clinical protocols to generate TK+ lymphocytes with RV comprise polyclonal activation through anti-CD3 monoclonal antibodies (aCD3 mAbs) and selection for a marker gene.3 Each of these steps may affect T-cell physiology and is currently being optimized. The addition of anti-CD28 mAb (aCD28) generates higher numbers of gene-modified lymphocytes with maintained T-cell receptor hypervariable region β (TCR-Vβ) and homing receptors repertoires19,20 that resist apoptosis.21 The benefit of mAb conjugation with cell-sized beads remains to be elucidated.22 On the other hand, immunomagnetic selection for a surface-marker gene such as the truncated form of the low-affinity nerve growth factor receptor (ΔLNGFR) avoids the toxicity of drug-resistance systems and spares anti-EBV reactivity.8,23
In this study, we hypothesized that targeting early stages of mature T-cell differentiation would maximize antihost reactivity of suicide gene-modified human lymphocytes in vivo. Activation through anti-CD3 and anti-CD28 mAbs conjugated with cell-sized beads (baCD3/CD28) enriched for CM TK+ lymphocytes, whereas activation with aCD3 mainly resulted in EM cells. In a quantitative model for GvHD using nonobese diabetic/severely combined immunodeficient (NOD/scid) mice, CM TK+ lymphocytes were more potent than EM cells. GvHD caused by CM TK+ lymphocytes could be effectively controlled with GCV. These results warrant the clinical investigation of CM suicide gene-modified human T lymphocytes for safe and effective allo-HCT.
Materials and methods
Small-scale generation of TK+ human lymphocytes
Approval for these studies was obtained from the S. Raffaele Scientific Institutional Review Board. Peripheral blood mononuclear cells (PBMCs) were isolated by Lymphoprep (Fresenius, Oslo, Norway) gradient separation from buffy coats of healthy donors obtained after informed consent according to the Declaration of Helsinki was obtained. PBMCs were cultured in RPMI 1640 medium (Gibco-BRL, Gaithersburg, MD) supplemented with antibiotics, glutamine, and 10% heat-inactivated FBS (BioWhittaker-Italia, Milan, Italy). PBMCs were seeded in 6-well plates (1 × 106/mL) and activated with aCD3 (OKT3 30 ng/mL; OrthoBiotech, Raritan, NJ), aCD3 and aCD28 (28.1 1 μg/mL, PharMingen, San Diego, CA), or paramagnetic baCD3/CD28 (3:1 beads/T cell, Xcyte Therapies, Seattle, WA). T cells were enriched by baCD3/CD28 before culture.24 Human recombinant interleukin 2 (IL-2; Chiron, Emeryville, CA) was added at 600 IU/mL in aCD3 cultures or at 200 IU/mL in aCD3/CD28 and baCD3/CD28 cultures. At days 2 and 3, cells were transduced with the SFCMM3 retroviral supernatant (Molmed, Milan, Italy) by spinoculation at 1200 g for 2 hours at 37°C with 8 μg/mL Polybrene (Sigma, St Louis, MO). The SFCMM3 RV encodes for TK under the long terminal repeat and the ΔLNGFR under the simian virus 40 promoter.25,26 After spinoculation, cells were cultured in medium containing IL-2 for 14 days. At days 6, 10, and 14 growth curves were calculated by multiplying the percentages of LNGFR+ cells determined by flow cytometry with the trypan blue counts.
Flow cytometry
Flow cytometry was used for analysis of surface phenotype, transduction efficiency, cell cycle, cytokine production, and expression of lytic granules. The following mAbs were purchased from PharMingen: fluorescein isothiocyanate (FITC)–conjugated mAb to human CD4, CD8, CD25, CD45RA, CD27, IFN-γ, and granzyme A; phycoerythrin (PE)–conjugated mAb to human CD4, CD8, LNGFR, CCR7, CD28, IL-4, IL-2, CD40L, and perforin B; peridinin chlorophyll-a protein (PerCP)–conjugated mAb to mouse CD45 (Ly5.1); and allophycocyanin (APC)–conjugated mAb to human CD3 and CD8. In some experiments, CCR7 or LNGFR expression was revealed by PerCP-conjugated streptavidin after staining with purified anti–human CCR7 mAb and biotinylated anti–mouse IgM or after staining with biotinylated anti–human LNGFR mAb, respectively. Before cell cycle analysis, cells were incubated in a buffer containing 50 μg/mL propidium iodide (Sigma), 0.00015% NP40 (Sigma), and 100 μg/mL RNAse (Boehringer Mannheim, Mannheim, Germany) for 1 hour at 37°C. Samples were run through a FACSCalibur flow cytometer (Becton Dickinson, Mountain View, CA) after isotype-matched fluorochrome-conjugated irrelevant mAb-stained control and data were analyzed using CellQuest software (Becton Dickinson).
Cytokine production, CD40L up-regulation, and expression of lytic granules
For determination of cytokine production, cells were seeded in 24-well plates (1 × 106/mL) and stimulated with 50 ng/mL phorbol myristate acetate (PMA; Sigma) and 1 μg/mL ionomycin (Sigma). After 4 hours, brefeldin A (Sigma) was added for additional 2 hours (10 μg/mL). Cells were then stained with the appropriate fluorochrome-conjugated anti–surface marker antibodies and fixed with 1% paraformaldehyde at 4°C for 10 minutes. Intracellular staining was performed with the appropriate fluorochrome-conjugated anticytokine antibodies after incubation for 20 minutes at room temperature (RT) in PBS 2% FBS containing 0.05% saponin (Sigma). For CD40L up-regulation, cells were stimulated with PMA and ionomycin. At different time points, cells were stained with fluorochrome-conjugated anti-CD40L antibodies. For expression of lytic granules, cells were stained with the appropriate fluorochrome-conjugated antilytic granule antibodies after incubation with saponin. Samples were run through a FACSCalibur flow cytometer after mock-stained unstimulated control.
Up-scale production and selection of TK+ human lymphocytes
PBMCs were seeded in VueLife bags (American Fluoroseal, Gaithersburg, MD) in X-VIVO15 (Cambrex BioScience, Verviers, Belgium) supplemented with 3% autologous human plasma and activated either with aCD3 and 600 IU/mL IL-2 or with baCD3/CD28 and 200 IU/mL IL-2. After 2 days, cells were transduced with the SFCMM3 vector by overnight incubation (1 × 106/mL) in bags precoated with the CH-296 fibronectin fragment (Retronectin, Takara, Kyoto, Japan). Cells were then propagated at 0.2 to 0.5 × 106/mL for 10 days. At day 6, baCD3/CD28 cells were magnetically removed and gene-modified cells were sorted using anti-LNGFR mAb 20.4 (1 μg/20 × 106) and Dynabeads M-450 sheep anti–mouse IgG (Nexell Therapeutics, Irvine, CA; 5 × 106 beads/106 positive).
GvHD model and GCV administration
Six- to 8-week-old female NOD/scid mice were obtained from Charles-River Italia (Calco, Italy). The experimental protocol was approved by the internal committee for animal studies of our institution (Institutional Animal Care and Use Committee [IACUC]). One week before infusion, mice were transferred from laminar-flow isolators to normal cages and kept under specific pathogen-free conditions receiving sterile water and irradiated pellets ad libitum. The day before the experiment, mice were given 1 mg blocking ant-mouse IL-2Rβ mAb intraperitoneally to neutralize residual natural killer (NK) activity.27 The antibody was produced as described28 from the TMβ-1 hybridoma kindly provided by Prof Tanaka (Osaka University, Japan). At day 0, mice received total body irradiation with a single dose of 350 cGy (γ irradiation from a linear accelerator) and were immediately infused with unmodified peripheral blood lymphocytes (PBLs) or TK+ human lymphocytes. Unmodified PBLs were obtained from PBMCs after the depletion of contaminating monocytes, B cells, and NK cells with Pan T-cell isolation kit (Miltenyi Biotec, Bergisch Gladbach, Germany). Cells were resuspended in 500 μL X-VIVO15 medium and infused intraperitoneally. Mice were then monitored for clinical GvHD 3 times per week as described.29 The following signs were included in the clinical index: weight loss (0 for weight loss < 10%, 1 for 10%-20%, 2 for > 20%), hunching (0-2), activity (0-2), fur texture (0-2), and skin integrity (0-2, maximum index = 10). Weight loss was also estimated as an independent variable. Moribund mice were humanely killed for ethical reasons. Human chimerism was determined weekly by flow cytometry after bleeding from the tail vein. Human chimerism was calculated as follows: human chimerism (%) = [huCD3+/(huCD3+ + mCD45+)] × 100.
At the time of severe GvHD, defined as the concomitant presence of human chimerism greater than 10% and weight loss greater than 5%, mice were treated with GCV by means of mini-osmotic Alzet pumps (Durect, Cupertino, CA). Briefly, pumps were filled with 200 μL GCV (25 mg/mL) and implanted subcutaneously after general anesthesia with 3,3,3 tribromoethanol (Avertin, Sigma). This protocol ensures the release of the prodrug at a constant rate over 7 days. After 7 days, pumps were surgically removed. During treatment mice were followed daily for weight loss and clinical index.
Histology and immunohistochemistry
Postmortem collection of organs included spleen, bone marrow, liver, heart, lungs, kidney, skin, and gut. Formalin-fixed, paraffin-embedded organs were cut in 4-μm thick sections and stained with hematoxylin and eosin for morphologic evaluation. Immunohistochemical assessment for the presence of human T lymphocytes was carried out with monoclonal anti–human CD3 antibody (Dako, Glostrup, Denmark) at 1:100 dilution, by way of the avidin/biotin peroxidase complex method using an automized Dako immunostainer. Staining reaction was revealed by the tetrahydrochloride chromogen method and sections were counterstained with hematoxylin. A pathologic score based on semiquantitative evaluation of the degree of infiltration of spleen, liver, and gut was designed. Each organ was scored (0-2, normal to increasing severity) after a double-blinded analysis by 2 independent pathologists (M.P. and F.S.) of at least 3 sections per organ. A global pathologic score was calculated for each group of treated animals as follows: global pathologic score (%) = [(spleen + liver + gut scores of all animals in the group)/6 × number of animals in the group] × 100. All images were acquired with a Zeiss Axioskope Plus microscope (Zeiss, Heidelberg, Germany) with Plan Neofluar 40×/0.75 NA objective lenses and no imaging medium. Pictures were taken with a Zeiss Axiocam HRC.
Statistical analysis
Statistical analysis for comparison of the distribution of cells in phenotypic/functional subsets was performed with a 2-tailed Student t test for paired samples using Excel software (Microsoft, Seattle, WA). Severe GvHD-free survival of mice infused with PBLs or TK+ lymphocytes was analyzed with a Fisher exact test using Prism Software (GraphPad, San Diego, CA). Statistical comparison between global pathologic scores was analyzed with a χ2 test (Prism).
Costimulation through baCD3/CD28 efficiently generates TK+ human lymphocytes with a preserved CD4/CD8 ratio. PBMCs (5 × 106) were stimulated either with aCD3, aCD3/CD28, or baCD3/CD28. (A) After 48 hours, CD4+ (top row) or CD8+ cells (bottom row) were analyzed by flow cytometry for DNA content. The horizontal lines overlie cells in the S/M phase of the cell cycle with the relative percentages. Results with cells from one representative donor of 2 are shown. (B) At 48 and 72 hours after stimulation, cells were transduced with the RV. At day 6, TK+ cells were quantified by flow cytometry as LNGFR-expressing cells, along with staining for CD8. Quadrants were set according to isotype-control staining. The inserts report the percentages of cells for each quadrant. Results with cells from one representative of 5 different donors are shown. (C) At days 6, 10, and 14, cells were counted by trypan blue exclusion and analyzed by flow cytometry. Cell counts for CD4+ (○) or for CD8+ (•) TK+ lymphocytes are reported over time. Results with cells from one representative donor of 4 are shown.
Costimulation through baCD3/CD28 efficiently generates TK+ human lymphocytes with a preserved CD4/CD8 ratio. PBMCs (5 × 106) were stimulated either with aCD3, aCD3/CD28, or baCD3/CD28. (A) After 48 hours, CD4+ (top row) or CD8+ cells (bottom row) were analyzed by flow cytometry for DNA content. The horizontal lines overlie cells in the S/M phase of the cell cycle with the relative percentages. Results with cells from one representative donor of 2 are shown. (B) At 48 and 72 hours after stimulation, cells were transduced with the RV. At day 6, TK+ cells were quantified by flow cytometry as LNGFR-expressing cells, along with staining for CD8. Quadrants were set according to isotype-control staining. The inserts report the percentages of cells for each quadrant. Results with cells from one representative of 5 different donors are shown. (C) At days 6, 10, and 14, cells were counted by trypan blue exclusion and analyzed by flow cytometry. Cell counts for CD4+ (○) or for CD8+ (•) TK+ lymphocytes are reported over time. Results with cells from one representative donor of 4 are shown.
Results
CD28 costimulation through cell-sized beads generates CM suicide gene-modified human lymphocytes
Because cell proliferation is required for retroviral transduction, we compared cell cycle analyses of lymphocytes 2 days after stimulation with aCD3, aCD3/CD28, or baCD3/CD28. The addition of CD28 costimulation significantly increased T-cell recruitment in the cell cycle (Figure 1A). The results were more striking with baCD3/CD28 and were due to a preferential recruitment of CD4+ cells. Accordingly, transduction efficiency was significantly higher after stimulation with baCD3/CD28 (LNGFR+ cells, 52.4% ± 16.8%) than with aCD3 (19.6%± 5.7%; P < .001) or aCD3/CD28 (24.6% ± 7.9%; P < .001). The CD4/CD8 ratio was also higher after stimulation with baCD3/CD28 (1.84 ± 0.76) than with aCD3 (0.41 ± 0.11; P < .01) or aCD3/CD28 (1.26 ± 0.45; P < .05; Figure 1B). Moreover, stimulation through baCD3/CD28 led to greater expansion of TK+ lymphocytes (fold growth at day 10, 6.3 ± 2.1) than stimulation with aCD3 (2.4 ± 0.7; P < .01) or aCD3/CD28 (3.6 ± 1.2; P < .05; Figure 1C). Due to the clear benefits of CD28 costimulation through cell-sized beads, further experiments were performed with TK+ lymphocytes generated with baCD3/CD28 (baCD3/CD28-TK+) or with aCD3 (aCD3-TK+).
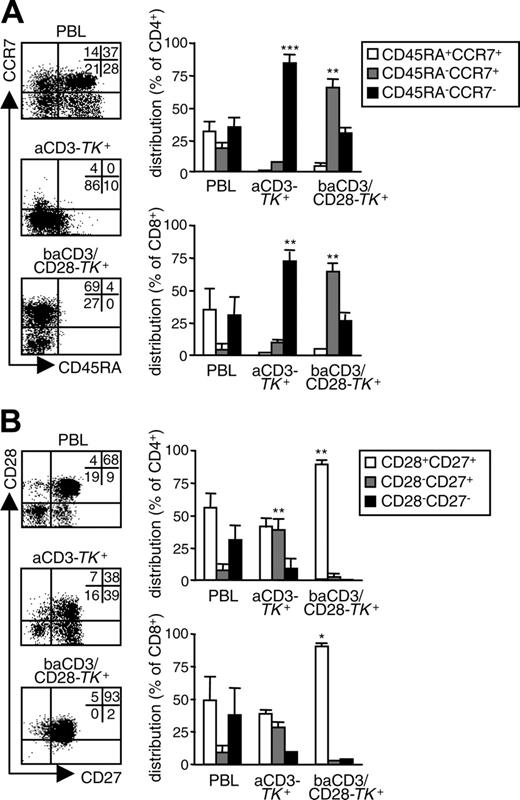
Polyclonal activation required for RV transduction of T lymphocytes enriches for memory cells.11,12 To determine the relative distribution of memory subsets in human TK+ lymphocytes, we analyzed CD45RA/CCR7 coexpression. At day 10, aCD3-TK+ lymphocytes were mainly CD45RA-CCR7- EM cells. On the contrary, baCD3/CD28-TK+ lymphocytes were highly enriched for CD45RA-CCR7+ CM cells (Figure 2A). This phenomenon was observed in both CD4+ and CD8+ cells. To better define the memory phenotype, we also analyzed CD28/CD27 coexpression. Whereas aCD3-TK+ lymphocytes showed a mixed population with predominance of CD28+CD27+ and CD28+CD27- cells, baCD3/CD28-TK+ lymphocytes were homogenously CD28+CD27+ (Figure 2B).
CM suicide gene-modified human lymphocytes are unpolarized cells that produce IL-2 and up-regulate CD40L
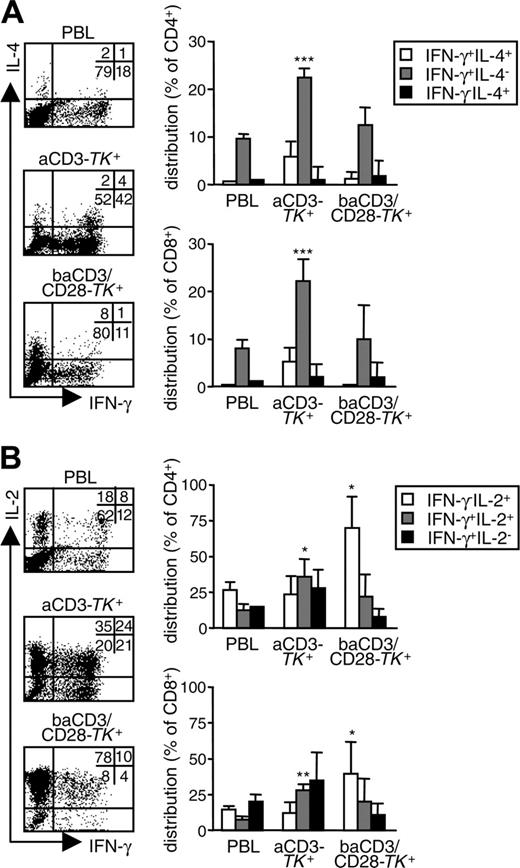
To challenge the phenotypic definition of CM and EM TK+ human lymphocytes, 10 days after initial stimulation, we restimulated the different TK+ populations and performed functional assays. First, we analyzed cytokine production. Compared with unmodified PBLs, a higher proportion of aCD3-TK+ lymphocytes produced IFN-γ in the absence of IL-4, indicating a preferential T-helper 1/T-cytotoxic 1 polarization (Figure 3A). A fraction of IFN-γ–producing aCD3-TK+ lymphocytes also produced IL-2 (Figure 3B). In sharp contrast, the majority of baCD3/CD28-TK+ lymphocytes were unpolarized cells that produced IL-2 in the absence of IFN-γ. TK+ cells did not produce IL-10 (data not shown).
Costimulation through baCD3/CD28 generates TK+ human lymphocytes with a CM phenotype. At day 10, aCD3- or baCD3/CD28-TK+ lymphocytes were compared with the corresponding PBLs for memory phenotype. (A) After gating for CD3 in the case of PBLs and for LNGFR in the case of TK+ cells, cells were analyzed by flow cytometry for CD45RA and CCR7 expression (dot plots). Quadrants were set according to isotype-control staining. The inserts report the percentages of cells for each quadrant. Results with cells from one representative donor of 5 are shown. Averages ± SD of the relative distribution of CD45RA+CCR7+, CD45RA-CCR7+, or CD45RA-CCR7- cells are reported for CD4+ (top histograms) or for CD8+ cells (lbottom histograms) in PBLs, aCD3- or baCD3/CD28-TK+ lymphocytes. (B) Cells were also analyzed by flow cytometry for CD27 and CD28 expression (dot plots). Quadrants were set according to isotype-control staining. The inserts report the percentages of cells for each quadrant. Results with cells from one representative donor of 5 are shown. Averages ± SD of the relative distribution of CD28+CD27+, CD28-CD27+, or CD28-CD27- cells are reported for CD4+ (top histograms) or CD8+ cells (bottom histograms) in PBLs or saCD3- or baCD3/CD28-TK+ lymphocytes. Symbols overlying saCD3- or baCD3/CD28-TK+–related bars are for statistical comparison with the corresponding PBL-related bars (*P < .05; **P < .01; ***P < .005).
Costimulation through baCD3/CD28 generates TK+ human lymphocytes with a CM phenotype. At day 10, aCD3- or baCD3/CD28-TK+ lymphocytes were compared with the corresponding PBLs for memory phenotype. (A) After gating for CD3 in the case of PBLs and for LNGFR in the case of TK+ cells, cells were analyzed by flow cytometry for CD45RA and CCR7 expression (dot plots). Quadrants were set according to isotype-control staining. The inserts report the percentages of cells for each quadrant. Results with cells from one representative donor of 5 are shown. Averages ± SD of the relative distribution of CD45RA+CCR7+, CD45RA-CCR7+, or CD45RA-CCR7- cells are reported for CD4+ (top histograms) or for CD8+ cells (lbottom histograms) in PBLs, aCD3- or baCD3/CD28-TK+ lymphocytes. (B) Cells were also analyzed by flow cytometry for CD27 and CD28 expression (dot plots). Quadrants were set according to isotype-control staining. The inserts report the percentages of cells for each quadrant. Results with cells from one representative donor of 5 are shown. Averages ± SD of the relative distribution of CD28+CD27+, CD28-CD27+, or CD28-CD27- cells are reported for CD4+ (top histograms) or CD8+ cells (bottom histograms) in PBLs or saCD3- or baCD3/CD28-TK+ lymphocytes. Symbols overlying saCD3- or baCD3/CD28-TK+–related bars are for statistical comparison with the corresponding PBL-related bars (*P < .05; **P < .01; ***P < .005).
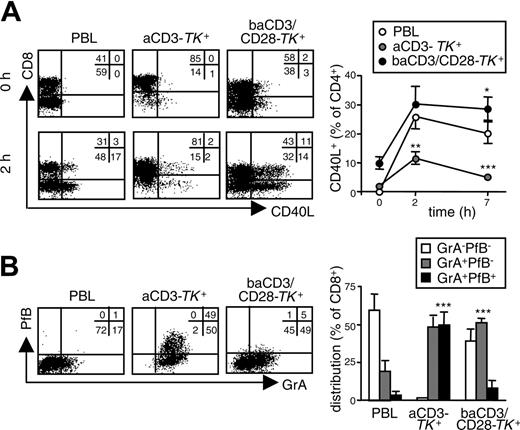
On antigen encounter, CM CD4+ lymphocytes up-regulate CD40L by which they instruct lymph node-residing cells, such as B lymphocytes and interdigitating dendritic cells.14 After restimulation, we analyzed the kinetics of CD40L expression on the different TK+ cells. Differently from CD4+ aCD3-TK+ lymphocytes, CD4+ baCD3/CD28-TK+ cells promptly up-regulated CD40L, which remained expressed up to 7 hours (Figure 4A). EM CD8+ lymphocytes continuously patrol peripheral tissues where, in case of recall infection, they kill virus-infected cells by targeted exocytosis of lytic granules (Figure 4A). A substantial fraction of CD8+ aCD3-TK+ lymphocytes was fully equipped with granzyme A and perforin B (Figure 4B). In contrast, only a proportion of CD8+ baCD3/CD28-TK+ lymphocytes expressed granzyme A in the absence of perforin B.
Scale-up production of CM suicide gene-modified human lymphocytes is feasible
Suicide gene therapy applied to allo-HCT requires the production of high numbers of suicide gene-modified lymphocytes.3 To test the feasibility of the strategy at a clinical level, we scaled up the production of CM TK+ human lymphocytes using culture bags precoated with the CH296 fibronectin fragment. Starting with PBMCs from 4 different donors, we confirmed higher transduction efficiency after activation through baCD3/CD28 (LNGFR+ cells, 41.6% ± 5.7%) compared with the activation with aCD3 (27.4% ± 6.5%; P < .001). TK+ lymphocytes were then sorted to purity with anti-LNGFR antibodies and magnetic beads. TK+ lymphocyte recovery and purity were not significantly different in the 2 populations (35.7% ± 19.1% and 90.6% ± 7.1% for aCD3; 37.1% ± 11.6% and 95.1% ± 5.7% for baCD3/CD28).
baCD3/CD28-TK+ human lymphocytes are unpolarized cells that produce IL-2. At day 10, aCD3-TK+ or baCD3/CD28-TK+ lymphocytes were compared with the corresponding PBLs for cytokine production at a single cell level. (A) After gating for CD3 in the case of PBLs, and for LNGFR in the case of TK+ cells, cells were analyzed by flow cytometry for IFN-γ and IL-4 production (dot plots). Quadrants were set according to isotype-control staining. The inserts report the percentages of cells for each quadrant. Results with cells from one representative donor of 4 are shown. Averages ± SD of the relative distribution of IFN-γ+IL-4+, IFN-γ-IL-4+, or IFN-γ-IL-4- are reported for CD4+ (top histograms) or CD8+ cells (lower histograms) in PBLs or aCD3- or baCD3/CD28-TK+ lymphocytes. (B) Cells were also analyzed by flow cytometry for IFN-γ and IL-2 production (dot plots). Quadrants and percentages were set according to isotype-control staining. The inserts report the percentages of cells for each quadrant. Results with cells from one representative donor of 4 are shown. Averages ± SD of the relative distribution of IFN-γ+IL-2+, IFN-γ-IL-2+, or IFN-γ-IL-2- cells are reported for CD4+ (top histograms) or CD8+ cells (bottom histograms) in PBLs or aCD3- or baCD3/CD28-TK+ lymphocytes. Symbols overlying aCD3- or baCD3/CD28-TK+-related bars are for statistical comparison with the corresponding PBL-related bars (*P < .05; **P < .01; ***P < .005).
baCD3/CD28-TK+ human lymphocytes are unpolarized cells that produce IL-2. At day 10, aCD3-TK+ or baCD3/CD28-TK+ lymphocytes were compared with the corresponding PBLs for cytokine production at a single cell level. (A) After gating for CD3 in the case of PBLs, and for LNGFR in the case of TK+ cells, cells were analyzed by flow cytometry for IFN-γ and IL-4 production (dot plots). Quadrants were set according to isotype-control staining. The inserts report the percentages of cells for each quadrant. Results with cells from one representative donor of 4 are shown. Averages ± SD of the relative distribution of IFN-γ+IL-4+, IFN-γ-IL-4+, or IFN-γ-IL-4- are reported for CD4+ (top histograms) or CD8+ cells (lower histograms) in PBLs or aCD3- or baCD3/CD28-TK+ lymphocytes. (B) Cells were also analyzed by flow cytometry for IFN-γ and IL-2 production (dot plots). Quadrants and percentages were set according to isotype-control staining. The inserts report the percentages of cells for each quadrant. Results with cells from one representative donor of 4 are shown. Averages ± SD of the relative distribution of IFN-γ+IL-2+, IFN-γ-IL-2+, or IFN-γ-IL-2- cells are reported for CD4+ (top histograms) or CD8+ cells (bottom histograms) in PBLs or aCD3- or baCD3/CD28-TK+ lymphocytes. Symbols overlying aCD3- or baCD3/CD28-TK+-related bars are for statistical comparison with the corresponding PBL-related bars (*P < .05; **P < .01; ***P < .005).
baCD3/CD28-TK+ human lymphocytes lack lytic granules but strongly up-regulate CD40L on restimulation. At day 10, aCD3- or baCD3/CD28-TK+ lymphocytes were compared with the corresponding PBLs for CD40L up-regulation and for expression of lytic granules. (A) Before (top dot plots) and 2 hours after stimulation with PMA and ionomycin (bottom dot plots), cells were analyzed by flow cytometry for CD40L and CD8 expression after gating for CD3 in the case of PBLs and for LNGFR in the case of TK+ cells. Quadrants and percentages were set according to isotype control staining. The inserts report the percentages of cells for each quadrant. Results with cells from one representative donor of 6 are shown. Curves depict averages ± SD of the proportion CD4+ cells in PBLs or aCD3- or baCD3/CD28-TK+ lymphocytes that also express CD40L over time. (B) Cells were also analyzed by flow cytometry for the expression of granzyme A (Gr-A) and perforin B (Pf-B). Quadrants and percentages were set according to isotype control staining. The inserts report the percentages of cells for each quadrant. Results with cells from one representative donor of 3 are shown. Averages ± SD of the relative distribution of GrA-PfB-, GrA+PfB-, or GrA+PfB+ in CD8+ cells from PBLs or aCD3- or baCD3/CD28-TK+ lymphocytes are reported (histograms). Symbols overlying aCD3- or baCD3/CD8-TK+–related bars are set for statistical comparison with the corresponding PBL-related symbols or bars (*P < .05; **P < .01; ***P < .005).
baCD3/CD28-TK+ human lymphocytes lack lytic granules but strongly up-regulate CD40L on restimulation. At day 10, aCD3- or baCD3/CD28-TK+ lymphocytes were compared with the corresponding PBLs for CD40L up-regulation and for expression of lytic granules. (A) Before (top dot plots) and 2 hours after stimulation with PMA and ionomycin (bottom dot plots), cells were analyzed by flow cytometry for CD40L and CD8 expression after gating for CD3 in the case of PBLs and for LNGFR in the case of TK+ cells. Quadrants and percentages were set according to isotype control staining. The inserts report the percentages of cells for each quadrant. Results with cells from one representative donor of 6 are shown. Curves depict averages ± SD of the proportion CD4+ cells in PBLs or aCD3- or baCD3/CD28-TK+ lymphocytes that also express CD40L over time. (B) Cells were also analyzed by flow cytometry for the expression of granzyme A (Gr-A) and perforin B (Pf-B). Quadrants and percentages were set according to isotype control staining. The inserts report the percentages of cells for each quadrant. Results with cells from one representative donor of 3 are shown. Averages ± SD of the relative distribution of GrA-PfB-, GrA+PfB-, or GrA+PfB+ in CD8+ cells from PBLs or aCD3- or baCD3/CD28-TK+ lymphocytes are reported (histograms). Symbols overlying aCD3- or baCD3/CD8-TK+–related bars are set for statistical comparison with the corresponding PBL-related symbols or bars (*P < .05; **P < .01; ***P < .005).
CM suicide gene-modified human lymphocytes induce GvHD
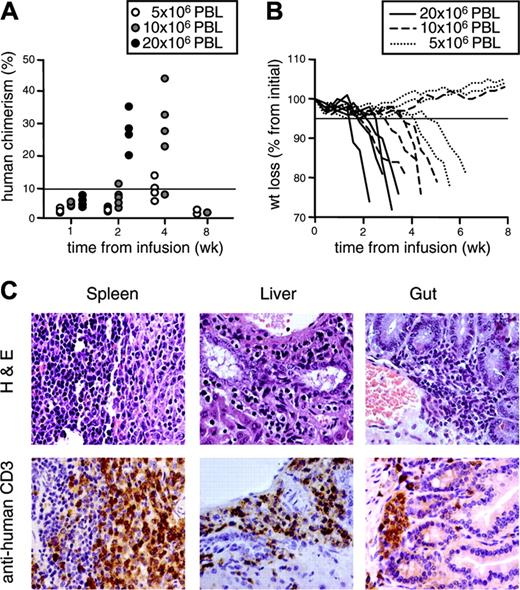
To compare the antihost reactivity of the differently generated TK+ cells in vivo, we infused suicide gene-modified lymphocytes in NOD/scid mice conditioned with nonlethal irradiation and anti-NK antibodies. In preliminary experiments, we infused NOD/scid mice with increasing doses of human purified CD3+ PBLs obtained from healthy donors. Engraftment and GvHD incidence were dose-dependent (Figure 5A-B). GvHD was characterized by ruffled fur, hunchback, reduced activity, weight loss and, eventually, death of the animals. Incidence of lethal GvHD was 100% at a dose of 20 × 106 CD3+ cells/mouse, 87.5% at 10 × 106, and 62.5% at 5 × 106. A severe form of the disease, defined as weight loss greater than 5% concomitant with human chimerism greater than 10%, was invariantly lethal. Postmortem detailed histologic analysis showed heavy mononuclear infiltration by medium-to-large cells with somewhat irregular nuclei, especially in the spleen, where the pattern was diffuse, the liver and the gut, where the pattern was focal, with perivascular and subepithelial predominance, respectively (Figure 5C). Immunohistochemical analysis confirmed these cells as CD3+ human lymphocytes. None of the conditioned mice infused with vehicle alone show clinical or histologic signs of GvHD.
We infused cohorts of NOD/scid mice either with CM baCD3/CD28-TK+ or with EM aCD3-TK+ human lymphocytes at a dose of 20 × 106 CD3+/mouse. As controls, mice were infused with human purified PBLs obtained from the same donor. CM baCD3/CD28-TK+ lymphocytes were significantly more efficient at engrafting than EM aCD3-TK+ cells (human chimerism at week 1: average, 5.8% and range, 0.1%-15.3% versus average, 1.8% and range, 0.1%-4.6%; P < .05; Figure 6A; Table 1). The CD4/CD8 ratio of engrafting CM baCD3/CD28-TK+ lymphocytes was higher than that of EM aCD3-TK+ cells, albeit the difference was not statistically significant (Figure 6B; Table 1). Engrafting cells retained their memory phenotype in vivo, with predominance of the CD45RA-CCR7+ subset for baCD3/CD28-TK+ lymphocytes and of the CD45RA-CCR7- subset for aCD3-TK+ cells. This was not observed for CD28/CD27 expression, with the majority of engrafting cells being CD28+CD27+, independently of the original phenotype. In almost all mice infused with EM aCD3-TK+ lymphocytes, human chimerism decreased after week 1 and mice did not develop GvHD (Figure 6A,C; Table 1). In contrast, persistent human chimerism was observed in the majority of mice infused with CM baCD3/CD28-TK+ lymphocytes and 14 mice of 27 (55%) developed severe GvHD. In the absence of severe GvHD, human chimerism steadily declined and survival was more than 120 days. If compared with PBLs, CM baCD3/CD28-TK+ lymphocytes caused GvHD with a subacute course (latency, 30.2 ± 8.2 days versus 15.9 ± 4.1 days; P < .001).
Engraftment and severe GvHD incidence
. | PBLs . | aCD3-TK+ . | baCD3/CD28-TK+ . |
|---|---|---|---|
| Engraftment | |||
| Average human chimerism, % (range) | 6.7 (2.0-21.1) | 1.8 (0.1-4.6) | 5.8 (0.1-15.3)‡ |
| CD4+/CD8+ | 2.0 ± 0.7 | 0.7 ± 0.2 | 1.0 ± 0.5 |
| CD28+CD27+, % | 79.2 ± 7.6 | 70.1 ± 11.5 | 74.6 ± 9.8 |
| Average CD28–CD27+, % | 15.8 ± 2.1 | 18.6 ± 2.7 | 20.2 ± 3.2 |
| Average CD45RA–CCR7+, % | 67.5 ± 11.7 | 1.1 ± 0.7 | 58.9 ± 7.3‡ |
| Average CD45RA–CCR7–, % | 15.4 ± 4.8 | 87.0 ± 9.2 | 25.2 ± 5.4‡ |
| Severe GvHD | |||
| Incidence* | 25/25 | 3/27 | 15/27‡ |
| Latency, d† | 15.9 ± 4.1 | 33.5 ± 6.4 | 30.2 ± 8.2 |
| Average human chimerism, % (range) | 33.1 (16.5-59.9) | 19.7 (10.3-29.9) | 26.9 (17.5-35.2) |
| Average clinical index, arbitrary units | 5.5 ± 0.9 | 4.3 ± 0.6 | 4.8 ± 1.0 |
. | PBLs . | aCD3-TK+ . | baCD3/CD28-TK+ . |
|---|---|---|---|
| Engraftment | |||
| Average human chimerism, % (range) | 6.7 (2.0-21.1) | 1.8 (0.1-4.6) | 5.8 (0.1-15.3)‡ |
| CD4+/CD8+ | 2.0 ± 0.7 | 0.7 ± 0.2 | 1.0 ± 0.5 |
| CD28+CD27+, % | 79.2 ± 7.6 | 70.1 ± 11.5 | 74.6 ± 9.8 |
| Average CD28–CD27+, % | 15.8 ± 2.1 | 18.6 ± 2.7 | 20.2 ± 3.2 |
| Average CD45RA–CCR7+, % | 67.5 ± 11.7 | 1.1 ± 0.7 | 58.9 ± 7.3‡ |
| Average CD45RA–CCR7–, % | 15.4 ± 4.8 | 87.0 ± 9.2 | 25.2 ± 5.4‡ |
| Severe GvHD | |||
| Incidence* | 25/25 | 3/27 | 15/27‡ |
| Latency, d† | 15.9 ± 4.1 | 33.5 ± 6.4 | 30.2 ± 8.2 |
| Average human chimerism, % (range) | 33.1 (16.5-59.9) | 19.7 (10.3-29.9) | 26.9 (17.5-35.2) |
| Average clinical index, arbitrary units | 5.5 ± 0.9 | 4.3 ± 0.6 | 4.8 ± 1.0 |
Engraftment was determined 1 week after infusion. Where applicable, values are ± SD.
Defined as weight loss greater than 5% and human chimerism greater than 10%
Calculated as time from infusion to severe GvHD
P < .01 for comparison with mice infused with aCD3-TK+ lymphocytes
Human purified PBLs cause lethal GvHD in NOD/scid mice in a dose-dependent fashion. NOD/scid mice were conditioned with anti-NK antibodies and sublethally irradiated prior to the intraperitoneal transfer of increasing numbers of human purified PBLs. (A) After infusion, mice were followed for human chimerism in peripheral blood over time. Each symbol represents a single mouse infused with 5 × 106, 10 × 106, or 20 × 106 PBLs. (B) Mice were also followed for weight loss over time. Each line represents a single mouse infused with 5 × 106, 10 × 106, or 20 × 106 PBLs. Results from mice infused with cells from one representative donor of 2 are shown. All mice with severe GvHD, defined weight loss greater than 5% (solid horizontal line in panel B) concomitant with human chimerism greater than 10% (solid horizontal line in panel A) died before week 8. (C) Spleen, liver, and gut were excised from dying animals and analyzed by histopathology after staining with hematoxylin and eosin (top row). Tissue samples were simultaneously analyzed by immunohistochemistry after counterstaining with human monoclonal anti-CD3 antibodies and peroxidase-conjugated second-step reagent (bottom row). Sections from one representative of 18 analyzed animals are shown.
Human purified PBLs cause lethal GvHD in NOD/scid mice in a dose-dependent fashion. NOD/scid mice were conditioned with anti-NK antibodies and sublethally irradiated prior to the intraperitoneal transfer of increasing numbers of human purified PBLs. (A) After infusion, mice were followed for human chimerism in peripheral blood over time. Each symbol represents a single mouse infused with 5 × 106, 10 × 106, or 20 × 106 PBLs. (B) Mice were also followed for weight loss over time. Each line represents a single mouse infused with 5 × 106, 10 × 106, or 20 × 106 PBLs. Results from mice infused with cells from one representative donor of 2 are shown. All mice with severe GvHD, defined weight loss greater than 5% (solid horizontal line in panel B) concomitant with human chimerism greater than 10% (solid horizontal line in panel A) died before week 8. (C) Spleen, liver, and gut were excised from dying animals and analyzed by histopathology after staining with hematoxylin and eosin (top row). Tissue samples were simultaneously analyzed by immunohistochemistry after counterstaining with human monoclonal anti-CD3 antibodies and peroxidase-conjugated second-step reagent (bottom row). Sections from one representative of 18 analyzed animals are shown.
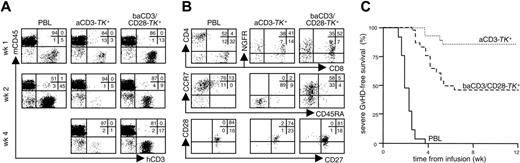
baCD3/CD28-TK+ human lymphocytes induce severe GvHD in NOD/scid mice. NOD/scid mice were conditioned, infused intraperitoneally with 20 × 106 PBLs or aCD3- or baCD3/CD28-TK+ lymphocytes and followed for human chimerism and incidence of severe GvHD. (A) Human chimerism was assessed weekly by flow cytometry after staining for human CD3 and mouse CD45. Quadrants and percentages were set according to isotype control staining. The inserts report the percentages of cells for each quadrant. Results from a single representative mouse per condition are shown over weeks 1, 2, and 4. (B) At week 1, engrafting cells were analyzed by flow cytometry for CD8 and CD4 or LNGFR expression (top dot plots). After gating for CD3, cells were also analyzed for CD45RA and CCR7 expression (middle dot plots) or for CD27 and CD28 expression (bottom dot plots). Quadrants and percentages were set according to isotype-control staining. The inserts report the percentages of cells for each quadrant. Results from a single representative mouse per condition are shown. (C) Mice were followed for severe GvHD-free survival over time. Severe GvHD was defined as weight loss greater than 5% concomitant to human chimerism greater than 10%. In the study 25 mice were infused with PBLs, 27 with aCD3/CD28-TK+, and 27 with baCD3/CD28-TK+ lymphocytes. Cells were derived from 7 human donors. For overall results on engraftment and incidence of severe GvHD, see Table 1.
baCD3/CD28-TK+ human lymphocytes induce severe GvHD in NOD/scid mice. NOD/scid mice were conditioned, infused intraperitoneally with 20 × 106 PBLs or aCD3- or baCD3/CD28-TK+ lymphocytes and followed for human chimerism and incidence of severe GvHD. (A) Human chimerism was assessed weekly by flow cytometry after staining for human CD3 and mouse CD45. Quadrants and percentages were set according to isotype control staining. The inserts report the percentages of cells for each quadrant. Results from a single representative mouse per condition are shown over weeks 1, 2, and 4. (B) At week 1, engrafting cells were analyzed by flow cytometry for CD8 and CD4 or LNGFR expression (top dot plots). After gating for CD3, cells were also analyzed for CD45RA and CCR7 expression (middle dot plots) or for CD27 and CD28 expression (bottom dot plots). Quadrants and percentages were set according to isotype-control staining. The inserts report the percentages of cells for each quadrant. Results from a single representative mouse per condition are shown. (C) Mice were followed for severe GvHD-free survival over time. Severe GvHD was defined as weight loss greater than 5% concomitant to human chimerism greater than 10%. In the study 25 mice were infused with PBLs, 27 with aCD3/CD28-TK+, and 27 with baCD3/CD28-TK+ lymphocytes. Cells were derived from 7 human donors. For overall results on engraftment and incidence of severe GvHD, see Table 1.
Prodrug administration controls GvHD induced by CM suicide gene-modified human lymphocytes
In NOD/scid mice, the different TK+ populations expressed the transgene at similar levels (Figure 6B). At the time of severe GvHD, mice infused with CM baCD3/CD28-TK+ human lymphocytes were given subcutaneous implants with a GCV-releasing osmotic pump or with a pump filled with saline. After 7 days of GCV treatment, human chimerism dropped by more than one-log (Figure 7A; Table 2). After 14 days, 87.5% of GCV recipients were alive with a near normal weight and favorable clinical scores. Surviving mice were followed up to 120 days without signs of recurrent disease. All mice given saline or infused with unmodified PBLs died. As for unmodified PBLs, CM baCD3/CD28-TK+ lymphocytes caused mononuclear infiltration in the spleen, liver, and gut (Figure 7B-C). At the end of the study, GCV-rescued mice had significantly lower mononuclear infiltration in target organs than animals dying from GvHD (global pathologic score, 23.3% versus 66.6%; P < .005). Conditioned mice injected with vehicle alone only showed background pathologic findings (global pathologic score, 6.7%).
Suicide gene therapy of severe GvHD
Cells, treatment . | Average human chimerism, % (range) . | Average clinical index, arbitrary units . | Survival . |
|---|---|---|---|
| PBLs | |||
| Saline | |||
| Day 0 | 29.8 (16.5-53.2) | 5.2 ± 1.2 | 6/6 |
| Day +7 | 78.5 (73.1-83.9)* | 8.5 ± 0.7† | 2/6 |
| Day +14 | — | — | 0/6 |
| GCV | |||
| Day 0 | 35.8 (18.9-83.9) | 5.4 ± 0.9 | 8/8 |
| Day +7 | 85.8 (69.9-94.2)† | 8.3 ± 0.6* | 3/8 |
| Day +14 | — | — | 0/8 |
| baCD3/CD28-TK+ | |||
| Saline | |||
| Day 0 | 24.3 (10.3-34.1) | 4.3 ± 1.5 | 4/4 |
| Day +7 | 43.4 (33.1-58.3)† | 7.7 ± 0.6† | 3/4 |
| Day +14 | — | — | 0/4 |
| GCV | |||
| Day 0 | 27.8 (19.5-35.2) | 4.7 ± 0.8 | 6/6 |
| Day +7 | 1.8 (0.9-3.1)* | 6.0 ± 0.7† | 5/6 |
| Day +14 | 0.7 (0.4-1.7)‡ | 4.4 ± 1.1 | 5/6§ |
Cells, treatment . | Average human chimerism, % (range) . | Average clinical index, arbitrary units . | Survival . |
|---|---|---|---|
| PBLs | |||
| Saline | |||
| Day 0 | 29.8 (16.5-53.2) | 5.2 ± 1.2 | 6/6 |
| Day +7 | 78.5 (73.1-83.9)* | 8.5 ± 0.7† | 2/6 |
| Day +14 | — | — | 0/6 |
| GCV | |||
| Day 0 | 35.8 (18.9-83.9) | 5.4 ± 0.9 | 8/8 |
| Day +7 | 85.8 (69.9-94.2)† | 8.3 ± 0.6* | 3/8 |
| Day +14 | — | — | 0/8 |
| baCD3/CD28-TK+ | |||
| Saline | |||
| Day 0 | 24.3 (10.3-34.1) | 4.3 ± 1.5 | 4/4 |
| Day +7 | 43.4 (33.1-58.3)† | 7.7 ± 0.6† | 3/4 |
| Day +14 | — | — | 0/4 |
| GCV | |||
| Day 0 | 27.8 (19.5-35.2) | 4.7 ± 0.8 | 6/6 |
| Day +7 | 1.8 (0.9-3.1)* | 6.0 ± 0.7† | 5/6 |
| Day +14 | 0.7 (0.4-1.7)‡ | 4.4 ± 1.1 | 5/6§ |
Values for average clinical index are ± SD.
— indicates not applicable.
P < .01 for comparison with day 0
P < .05 for comparison with day 0
P < .01 for comparison with day 0
P < .001 for comparison with mice infused with baCD3/CD28-TK+ lymphocytes and treated with saline
Discussion
In allo-HCT, several biotechnologic approaches are currently being proposed to increase the therapeutic index of donor T lymphocytes. Anti-inflammatory therapy aims at interfering with the “cytokine storm” induced by bacterial translocation secondary to the epithelial toxicity of conditioning regimens.29 Cell therapy approaches are planning the use of effectors specific for leukemia,30 lineage markers,21 oncogenic viruses,31 or hematopoietic tissue32,33 or of alloreactive NK cells.34 Alternative options comprise the depletion of alloreactive T cells35 or the use of recipient-specific regulatory T lymphocytes.36,37 Clinical and experimental observations, however, suggest that the GvL effect is at least in part mediated by the same effectors responsible for GvHD. Therefore, a major challenge for successful allo-HCT is to control rather than avoid antihost reactivity. Suicide gene therapy is actualizing this concept. With a cell cycle-dependent suicide gene, such as TK, it is possible, by a time-wise administration of GCV, to exploit antihost reactivity to the point of GvL,38,39 while controlling GvHD.40,41 Recently, a non–cell cycle-dependent suicide gene based on a modified caspase-9 fused with a prodrug-binding site has also been described.42
Ganciclovir administration rescues NOD/scid mice from severe GvHD induced by baCD3/CD28-TK+ human lymphocytes. At the time of severe GvHD, NOD/scid mice were treated with GCV or with saline. (A) Individual recipients of PBL (left graphs) or baCD3/CD28-TK+ lymphocytes (right graphs) were implanted subcutaneously either with a saline-filled or a GCV-releasing osmotic pump. At days 0, 7, and 14 from the implantation of the pumps mice were assessed for human chimerism by flow cytometry (▪) and scored for GvHD by a clinical index (□, top histograms). Mice were also followed for weight loss and mortality over time (bottom curves). Open arrows indicate saline; dashed arrows, GCV administration. The broken horizontal line is set at 95%. Results from one representative mouse per condition are shown. (B) Available mice infused with PBLs (left graphs) or with baCD3/CD28-TK+ lymphocytes (right graphs) and either treated with saline or with GCV were analyzed by histopathology and scored. GCV-rescued mice were humanely killed at the end of the study, 120 days after the infusion of baCD3/CD28-TK+ cells. Each symbol represents the spleen, gut, or liver score of individual animals. (C) Histologic sections from the spleen, liver, and gut of representative animals infused with baCD3/CD28-TK+ lymphocytes and treated either with saline (top row) or with GCV (bottom row) are reported.
Ganciclovir administration rescues NOD/scid mice from severe GvHD induced by baCD3/CD28-TK+ human lymphocytes. At the time of severe GvHD, NOD/scid mice were treated with GCV or with saline. (A) Individual recipients of PBL (left graphs) or baCD3/CD28-TK+ lymphocytes (right graphs) were implanted subcutaneously either with a saline-filled or a GCV-releasing osmotic pump. At days 0, 7, and 14 from the implantation of the pumps mice were assessed for human chimerism by flow cytometry (▪) and scored for GvHD by a clinical index (□, top histograms). Mice were also followed for weight loss and mortality over time (bottom curves). Open arrows indicate saline; dashed arrows, GCV administration. The broken horizontal line is set at 95%. Results from one representative mouse per condition are shown. (B) Available mice infused with PBLs (left graphs) or with baCD3/CD28-TK+ lymphocytes (right graphs) and either treated with saline or with GCV were analyzed by histopathology and scored. GCV-rescued mice were humanely killed at the end of the study, 120 days after the infusion of baCD3/CD28-TK+ cells. Each symbol represents the spleen, gut, or liver score of individual animals. (C) Histologic sections from the spleen, liver, and gut of representative animals infused with baCD3/CD28-TK+ lymphocytes and treated either with saline (top row) or with GCV (bottom row) are reported.
Suicide gene therapy in the context of allo-HCT has been one of the first successful attempts of human gene therapy.5,6,43 In this study we investigated the requirements for maximizing antihost reactivity of human TK+ lymphocytes to increase the therapeutic efficacy of suicide gene therapy in allo-HCT.
The mAbs conjugated with cell-sized beads deliver a stronger signal than soluble or plate-bound mAbs,44 probably because, by mimicking antigen-presenting cells, they facilitate polarized interactions required for the formation of the immunologic synapse.45-47 Herein, we demonstrate that CD28 costimulation through cell-sized beads fosters the generation of CM TK+ lymphocytes, whereas stimulation with aCD3 alone ends up mainly with EM cells. A possible explanation is that a tridimensional signal is actually recruiting a wider repertoire of lymphocytes into the cell cycle and in particular naive cells, which have a higher threshold for activation.48 Accordingly, if compared with aCD3-TK+, baCD3/CD28-TK+ lymphocytes have been shown to display a highly polyclonal TCR-Vβ repertoire, similar to that of naive cells.20 Genetic modification of naive precursors and further differentiation into CM cells is also suggested by the fact that the majority of TK+ lymphocytes generated with baCD3/CD28 are unpolarized cells (IL-4-, IFN-γ-) that produce IL-2 and strongly up-regulate CD40L, all hallmarks of recently antigen-experienced lymphocytes.49
Mouse naive lymphocytes are significantly more efficient than memory cells at causing GvHD.15,16 Nevertheless, GvHD persistence in vivo is associated with a memory phenotype.18 In vitro, human CD62L+ naive and CM lymphocytes proliferate equally in response to alloantigens.50 In a set of experiments we found that cells generated with baCD3/CD28 proliferate significantly more than cells generated with aCD3 in response to human allogeneic, as well as to mouse xenogeneic, dendritic cells (A.B. and C. Bonini, unpublished observations, January 2005). To test the relative antihost reactivity of CM baCD3/CD28-TK+ and EM aCD3-TK+ human lymphocytes in vivo, we infused the cells in NOD/scid mice conditioned with irradiation and with anti-NK antibodies and used the incidence and severity of GvHD across a xenogeneic barrier as a read-out. Mice with immunologic defects in the adaptive (scid, recombination-activating genes-/-) as well as in the innate compartment (NOD, common γ chain-/-) are commonly used to study human lymphocyte biology in vivo and, in particular, to model GvHD.28,51,52 Human TK+ lymphocytes have been already shown to engraft in NOD/scid mice.53,54 In the previous studies, however, engraftment was poor and lethal GvHD was not observed unless a high number of cells were given (> 100 × 106 cells/mouse). Because residual NK cell activity limits the engraftment of human cells in NOD/scid mice,55 we functionally inactivated NK cells with anti–mouse IL-2Rβ antibodies prior to TK+ lymphocytes' transfer.27 Following NK cell inactivation, we reproducibly observed engraftment of human TK+ lymphocytes at a dose as low as 20 × 106/mouse. The extent and the kinetics of engraftment, however, greatly differed between the TK+ populations. Whereas EM TK+ lymphocytes induced a slight and transient human chimerism, in the case of CM TK+ cells this was robust and sustained. To better characterize engrafting cells, we analyzed CD28/CD27 coexpression. It has been proposed that, as memory cells differentiate from CD28+CD27+ to CD28-CD27+ and finally to CD28-CD27- cells, they progressively lose proliferative potential and acquire effector functions.56 Despite the fact that CM TK+ lymphocytes were homogenously CD28+CD27+, whereas EM TK+ cells were a mixed population in regard to CD28/CD27 coexpression, in both cases engrafting cells were almost invariantly CD28+CD27+. This observation suggests that the CD28+CD27+ phenotype provides a survival advantage in vivo, as recently proposed in a clinical trial of adoptive immunotherapy, in which, following the infusion of melanoma-specific effectors enriched for CD28-CD27- cells, a selective persistence of CD28+CD27+ cells was reported.57 Intrinsic characteristics of CM TK+ lymphocytes, such as high proliferative potential, resistance to apoptosis, or ability to respond to homeostatic cytokines, may be at the basis of their superior ability to persist in vivo.
In conditioned NOD/scid mice infused with human lymphocytes, persistent human chimerism was associated with GvHD. In accordance, the incidence of severe GvHD was higher with CM TK+ lymphocytes than with EM TK+ cells, confirming our hypothesis that targeting early stages of T-cell maturation maximizes antihost reactivity of suicide gene-modified lymphocytes in vivo. Albeit more potent than EM TK+ lymphocytes, CM TK+ cells clearly caused less GvHD than syngeneic PBLs. Because in TK+ lymphocytes we did not observe a selective enrichment of cells with a putative regulatory activity, such as CD4+CD25+ or IL-10–producing cells (data not shown), the most likely explanation is that the highest potential of PBLs to cause GvHD relies on the fact that they contain naive cells.
In the clinic, GCV administration to patients suffering from GvHD due to aCD3-TK+ lymphocytes led to complete disease control.5-7 With the aim of using baCD3/CD28-TK+ cells in patients, it is crucial to demonstrate that it is possible to control GvHD even when induced by cells with maximized antihost reactivity. To this purpose, at the time of severe GvHD, we treated the recipients of baCD3/CD28-TK+ human lymphocytes with GCV. Although in control mice the disease was invariantly lethal, GCV administration to mice infused with CM TK+ cells resulted in a dramatic decrease in human chimerism with complete resolution of all signs and symptoms of GvHD.
In conclusion, we established that tridimensional CD28 costimulation generates CM suicide gene-modified human lymphocytes that display high persistence and high antihost reactivity in vivo. Moreover, we demonstrated that prodrug administration efficiently controls GvHD induced by suicide gene-modified CM human lymphocytes. Clinical studies are warranted to demonstrate the clinical benefit of suicide gene therapy with CM lymphocytes, in particular in high-risk or relapsing patients, which critically demand for a strong and safe GvL effect.
Prepublished online as Blood First Edition Paper, November 17, 2005; DOI 10.1182/blood-2005-09-3716.
This work was supported by the European Community (QLK3-CT-2001-01265), the Italian Ministry of Health, and the Italian Association for Cancer Research (AIRC).
M.B. is an employee and has stock in a company (Xcyte Therapies) whose (potential) product was studied in the present work. C.T., S.T., M.R., and S.L.S.-C. are employees of a company (Molmed) whose (potential) product was studied in the present work. C. Bordignon is a consultant of a company (Molmed) whose (potential) product was studied in the present work.
A.B., F.C., C. Bordignon, and C. Bonini designed the research; A.B., V.V., Z.M., M.P., and S.L.S.-C. performed the research; M.B., S.T., and M.R. provided vital new reagents; A.B., M.P., K.F., C.T., F.S., and C. Bonini analyzed the data; and A.B. and C. Bonini wrote the paper.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
We thank Prof Tanaka (Osaka University, Japan) for kindly providing the TMβ-1 hybridoma, Dr Paolo Dellabona for stimulating discussions, and Dr Patricia Vera Cruz Lorena for help with English.



This feature is available to Subscribers Only
Sign In or Create an Account Close Modal