CD4+CD25+ regulatory T cells (Tregs) control immune responses to self- and foreign antigens and play a pivotal role in autoimmune diseases, infectious and noninfectious inflammation, and graft rejection. Since recent experimental studies have indicated that Tregs were able to ameliorate graft-versus-host disease (GvHD), we analyzed the number of infiltrating Tregs in the intestinal mucosa as one site of GvH reactivity using immunoenzymatic labeling to enumerate FOXP3+ T cells in 95 intestinal biopsies from 49 allografted patients in comparison with healthy controls and patients with infectious inflammation. While patients with cytomegalovirus (CMV)-colitis or diverticulitis showed a concomitant increase of CD8+ effectors and Tregs, acute and chronic GvHD were characterized by the complete lack of a counter-regulation indicated by a FOXP3+/CD8+ T-cell ratio identical to healthy controls. In contrast, specimens without histologic signs of GvHD demonstrated increased numbers of FOXP3+ per CD8+ T cells, indicating that the potential for Treg expansion is principally maintained in allografted patients. Our findings provide evidence that GvHD is associated with an insufficient up-regulation of Tregs in intestinal GvHD lesions. The determination of FOXP3+/CD8+ ratio can be a helpful tool to discriminate GvHD from infectious inflammation after allogeneic stem cell transplantation.
Introduction
Despite the prophylactic use of potent immunosuppressants, severe graft-versus-host disease (GvHD) is—besides infections—the most relevant complication after allogeneic stem cell transplantation. Immunologically, acute GvHD is characterized by an expansion of donor lymphocytes with cytotoxic reactivity against recipient histocompatibility antigens. The resulting clinical picture includes life-threatening destruction of skin, gut, and liver tissue.
In acute GvHD, the transferred immune system lacks the capability to gain control over alloreactive T-cell clones. Therefore, the understanding of mechanisms by which an organism controls allo- or autoimmune reactivity is crucial for the development of successful strategies to prevent and/or control GvHD. Recently, these mechanisms have been further elucidated by the description of a distinct CD4+ T-cell population with the capability to confer nonresponsiveness to T cells against autologous and allogeneic antigens.1-3
These suppressor or regulatory T cells were originally described as a lymphocyte subset that prevents autoimmunity caused by neonatal thymectomy in mice1 and are characterized by the expression of the interleukin-2 (IL-2) receptor α-chain (CD25). More recently, FOXP3, which encodes for a forkhead/winged helix transcription factor called Scurfin, has been identified to be a key regulatory gene required for the development and functional activity of regulatory T cells.4-7 Tregs are able to suppress CD4+ and CD8+ T-cell responses to auto- and alloantigens in a contact-dependent fashion.8 In animal models, they can prevent graft rejection2,3,9 and autoimmune diseases,8,10 and there is also evidence that inappropriate numbers of Tregs may contribute to the development of chronic inflammatory diseases.11
Consequently, the question has been raised whether Tregs are also capable of suppressing GvHD. Indeed, it has been shown in different murine models that freshly isolated or ex vivo-expanded donor-type CD4+CD25+ Tregs are able to delay or even prevent GvH reactivity.12-15 Consistent with this, the selective depletion of Tregs leads to an increased severity of acute GvHD in vivo.16
In humans, however, available data are ambiguous. Whereas Clark et al found elevated numbers of CD4+CD25high cells in the peripheral blood of patients with chronic GvHD,17 Miura et al observed a significantly decreased FOXP3 mRNA expression in the peripheral blood of patients suffering from allogeneic or autologous GvHD.18 Since the presence of Tregs in the peripheral blood does not necessarily reflect their presence in the target organs of GvHD, the conclusions that can be drawn from these findings are limited. So far, methodologic restrictions have made it difficult to quantify the number of Tregs at the major sites of GvH manifestation (ie, the liver and gastrointestinal tract). To address this issue, we applied a recently described immunohistochemical technique11 to identify Tregs at the single-cell level by their expression of FOXP3 in paraffin-embedded tissue samples.
With the help of this new method, we aimed to investigate if a reduction of Tregs in the gastrointestinal mucosa would be associated with the development and progression of acute intestinal GvHD. Our data indicate that the frequency of immunoregulatory cells and their quantitative relation to CD8+ effector T cells differ significantly between allografted patients with histologically proven GvHD and symptomatic allografted patients without histologic signs of GvHD. Our findings may contribute to a better understanding of the pathophysiology of GvHD and could allow for better discrimination between GvHD- and non-GvHD-associated intestinal inflammation after allogeneic stem cell transplantation.
Patients, materials, and methods
Patients and samples
A retrospective analysis of 95 paraffin-embedded tissue samples obtained from intestinal biopsies (duodenum and colon) during diagnostic endoscopic examination derived from 49 patients undergoing allogeneic stem cell transplantation was performed. Biopsies of allografted patients were taken when GvHD was suspected due to gastrointestinal symptoms such as nausea, vomiting, diarrhea, and abdominal pain, or in the follow-up while on immunosuppressive therapy. In all cases, microbiologic standard evaluation of feces including screening for Clostridium difficile was performed. Additionally, cytomegalovirus (CMV) infections were excluded by immunohistology and/or pp65 staining in peripheral blood samples according to routine procedures.
Histopathologic signs of intestinal GvHD were present in 62 biopsies from 38 patients (GvHD grade 1: n = 28, GvHD grade 2: n = 14, GvHD grade 3: n = 16, GvHD grade 4: n = 4), whereas no GvHD was detectable in 33 biopsies based on published histologic criteria.19 Biopsies were taken in patients without systemic immunosuppression (n = 6), with systemic steroids alone (n = 12), with cyclosporine therapy alone (n = 18), with additional systemic steroids (n = 36), with previous treatment with anti-CD25 antibodies (basiliximab or daclizumab; n = 9), with systemic steroids and mycophenolate mofetil (MMF) (n = 6), with cyclosporine and MMF (n = 4), and with a combination therapy (n = 4). Sixty-three biopsies were taken before day 100, and 32 biopsies after day 100 after transplantation.
Twenty-five patients received a conventional myeloablative conditioning regimen (8 patients high-dose chemotherapy alone, 17 patients chemotherapy combined with radiotherapy), and 24 patients received reduced-intensity conditioning regimen. All patients were treated with standard prophylaxis for GvHD using cyclosporine and low-dose methotrexate or MMF (n = 22) and in the case of nonsibling transplantation additional antithymocyte globulin (ATG; n = 25). Three patients had alemtuzumab-containing prophylaxis. The majority (46 patients) received full-matched transplants (21 of related donors, 25 of unrelated donors); 3 patients received a mismatch transplant of unrelated donors. Clinical GvHD was graded according to standard criteria.20,21 As controls, intestinal biopsies were obtained from the normal mucosa of 13 patients during screening endoscopy for colorectal cancer, from inflamed mucosa of 6 patients with diverticulitis, and from 6 HIV-negative patients with CMV-colitis who were under immunosuppressive therapy due to rheumatic diseases.
In addition, paired samples of peripheral blood and lamina propria mononuclear cells from 24 patients with suspected acute intestinal GvHD were analyzed by flow cytometry for lymphocyte subsets with a special focus on the presence of CD4+CD25high cells. All samples were collected after patients provided written informed consent on this study approved by the institutional review board of the Charité-University Medicine Berlin, consistent with the principles outlined in the Declaration of Helsinki.
Double immunoenzymatic labeling
For immunostaining, 4-μm thick serial sections were cut, deparaffinized, and subjected to a heat-induced epitope retrieval step before incubation with antibodies. Sections were immersed in sodium citrate buffer solutions at pH 6.0 and heated in a high-pressure cooker. The slides were rinsed in cool running water, washed in Tris-buffered saline (pH 7.4), and incubated with primary antibodies. The primary antibodies included monoclonal antibodies against CD4 obtained from Novocastra (clone 1F6, dilution 1:25; Newcastle, United Kingdom) and CD8 purchased from DakoCytomation (C8/144B, 1:100; Glostrup, Denmark). For detection, the alkaline-phosphatase-anti-alkaline phosphatase complex (APAAP) method was used. Alkaline phosphatase was revealed by Fast Red as chromogen. For double immunoenzymatic labeling of FOXP3/CD3, slides were blocked using a commercial peroxidase-blocking reagent (DakoCytomation) and incubated for 30 minutes with goat polyclonal antibody against the C terminus of the FOXP3 protein (ab2481, dilution 1:50; Abcam Limited, Cambridge, United Kingdom), followed by biotin-conjugated rabbit anti-goat and the EnVision peroxidase kit (DakoCytomation). Sections were then incubated for 30 minutes with the second antibody against CD3 (clone UCHT1, 1:25; DakoCytomation) and visualized by the APAAP method. Tonsilar tissue with follicular hyperplasia served as positive control displaying scattered T cells in the interfollicular area with nuclear expression of FOXP3. Negative controls were performed by omitting primary antibodies. To exclude nonspecific staining of the FOXP3 antibody, we double labeled FOXP3 and CD25 in all biopsies, and confirmed coexpression of CD25 on all FOXP3-positive cells. Only a few weakly CD25-stained cells were negative for FOXP3 staining, indicating a preferential staining of the CD25high cell population. For double labeling of FOXP3/CD25, the anti-CD25 antibody (clone 4C9, 1:100; Novocastra) was incubated for 30 minutes as second primary antibody and detected using a biotinylated donkey antimouse antibody (1:200; Dianova, Hamburg, Germany) and streptavidin-AP (DakoCytomation).
Isolation of lamina propria mononuclear cells
Lamina propria mononuclear cells were isolated from 6 intestinal biopsies derived from small or large intestine by a modified method for the isolation of lymphocytes from resected specimens.22
Biopsies were washed once in 20 mL NaCl 0.9% to remove adherent blood and mucus and cut into small fragments. Fragments were preincubated overnight at 4°C in 50 mL RPMI 1640 medium (Gibco, Karlsruhe, Germany) containing 10% fetal calf serum (FCS), 100 U/mL penicillin, 100 μg/mL streptomycin, 50 μg/mL gentamycin, 2.5 μg/mL amphotericin, 25 mM HEPES buffer (all from Seromed Biochrom, Berlin, Germany), 0.05 M 2-mercaptoethanol (Gibco), 0.01% collagenase (CLS III; Seromed Biochrom), 0.01% trypsin inhibitor (type I-S; Sigma-Aldrich, Steinheim, Germany), and 0.01% DNAse (DNAse I from bovine pancreas; Hoffmann-La Roche, Basel, Switzerland) and then incubated for 4 hours (37°C) in a humid chamber (5% CO2). Cells were processed through a 60-μm nylon mesh to obtain a single cell suspension. After washing in RPMI 1640 containing 10% FCS, cells were resuspended in 30% isotonic Percoll (Seromed Biochrom), underlayered with 70% isotonic Percoll, and centrifuged at 1200g for 25 minutes. Lymphocytes were harvested from the interphase, washed, and resuspended in RPMI 1640 containing 10% FCS. The number of mononuclear cells derived from 6 intestinal biopsies varied from 0.8 to 1.6 × 106. Viability of lymphocytes, as determined by trypan blue staining exclusion, was always more than 80%. Viability was not different in samples of patients with versus without GvHD.
Fluorescence-activated cell sorter (FACS) analysis of lamina propria lymphocytes
Two-color staining of intestinal lymphocytes was performed using fluorescein isothiocyanate (FITC)- or phycoerythrin (PE)-conjugated monoclonal antibodies (MoAbs) against CD3 (Becton Dickinson, Heidelberg, Germany), CD4, CD8 (Beckman Coulter, Krefeld, Germany), and CD25 (clone B1.49.9; Beckman Coulter) in saturating concentrations as determined in previous experiments.
Lymphocytes were gated by forward and sideward scatter properties and an additional gate was set to include only T cells as determined by the detection of CD3-FITC and CD3-PE and quantified using CellQuest software (BD Biosciences, Mountain View, CA). Each fluorescence analysis included a double-negative isotype control immunoglobulin. Results are given as percentage of positive cells per CD3+ T cells, since the absolute number of positive cells per microliter cannot be calculated in intestinal biopsies.
FACS analysis of peripheral blood lymphocytes
Whole blood samples were costained for 2-color FACS: anti-CD3-FITC and anti-CD4-PE, anti-CD3-FITC and anti-CD8-PE, anti-CD4-PE and anti-CD25-FITC (details are listed in “FACS analysis of lamina propria lymphocytes”). CD4+CD25high Tregs appeared as a subpopulation to the right from the major population of CD4+CD25- and CD4+CD25low cells with a lower CD4 expression than the CD4+CD25low or CD4+CD25- population as described by Baecher-Allan et al.23
For the staining of surface antigens, 200 μL peripheral blood was incubated with the MoAbs at 4°C for 30 minutes. After separating the cell pellet from red cells by red cell lysis (red cell lysis buffer), cells were resuspended in 200 μL FACS buffer (PBS with 0.5% bovine serum albumin and 0.01% NaN3), followed by analysis with a FACScalibur (BD Biosciences). Each fluorescence analysis included a double-negative isotype control immunoglobulin.. Lymphocytes were gated by forward and sideward scatter properties and quantified using CellQuest software (BD Biosciences).
Statistical analysis
Results are expressed as mean ± SEM. To evaluate comparative statistical significance, the nonparametric 2-tailed Mann-Whitney U-test was used with P values less than .05 considered significant.
Results
Increased T-cell infiltration in the mucosa of patients with gastrointestinal symptoms after allogeneic stem cell transplantation
Using standard immunohistologic techniques, number and phenotype of mucosa-infiltrating lymphocytes were determined in 95 intestinal biopsies of 49 allografted patients who demonstrated gastrointestinal symptoms suggesting the presence of GvHD. The histologic evaluation revealed no signs of GvHD in 33 of these biopsies. Forty-four biopsies were classified as acute GvHD and 18 as chronic GvHD depending on the onset of GvHD (acute GvHD beginning before day 100 after transplantation, chronic GvHD beginning after day 100).
Whereas healthy controls demonstrated a mean of only 130 mucosa-infiltrating CD3+ T cells per 10 high-power fields (1 hpf = 0.237 mm2), their number was extensively increased in biopsies taken after allogeneic transplantation (approximately 400 CD3+ cells per 10 hpf's). Surprisingly, samples without histologic evidence of GvHD also showed a significant increase. No significant difference was observed between patients with acute or chronic GvHD (Table 1). The highest T-cell numbers were present in the mucosa of patients with CMV-colitis under immunosuppressive therapy (836.7 ± 146.9) or diverticulitis (1346.0 ± 134.8).
Phenotype of the lymphocyte infiltrate in small and large intestine biopsies measured by immunoenzymatic labeling
. | . | No GvHD . | . | Acute GvHD . | . | . | Chronic GvHD, cells/10 hpf's . | |||
|---|---|---|---|---|---|---|---|---|---|---|
| Cell type . | Healthy ctrls, cells/10 hpf's . | Cells/10 hpf's . | P vs healthy ctrls . | Cells/10 hpf's . | P vs healthy ctrls . | P vs no GvHD . | . | |||
| CD3+ | 130.3 ± 16.5 | 341.4 ± 70.4 | NS | 482.7 ± 56.3 | < .001 | < .01 | 501.7 ± 53.7 | |||
| CD4+ | 86.0 ± 10.2 | 208.5 ± 46.0 | NS | 199.3 ± 25.2 | < .01 | NS | 240.6 ± 33.2 | |||
| CD8+ | 44.3 ± 6.4 | 132.8 ± 26.7 | NS | 283.4 ± 33.6 | < .001 | < .001 | 260.6 ± 34.0 | |||
| FOXP3+ | 1.0 ± 0.3 | 18.6 ± 5.9 | < .001 | 5.3 ± 1.4 | < .01 | < .001 | 5.6 ± 1.5 | |||
. | . | No GvHD . | . | Acute GvHD . | . | . | Chronic GvHD, cells/10 hpf's . | |||
|---|---|---|---|---|---|---|---|---|---|---|
| Cell type . | Healthy ctrls, cells/10 hpf's . | Cells/10 hpf's . | P vs healthy ctrls . | Cells/10 hpf's . | P vs healthy ctrls . | P vs no GvHD . | . | |||
| CD3+ | 130.3 ± 16.5 | 341.4 ± 70.4 | NS | 482.7 ± 56.3 | < .001 | < .01 | 501.7 ± 53.7 | |||
| CD4+ | 86.0 ± 10.2 | 208.5 ± 46.0 | NS | 199.3 ± 25.2 | < .01 | NS | 240.6 ± 33.2 | |||
| CD8+ | 44.3 ± 6.4 | 132.8 ± 26.7 | NS | 283.4 ± 33.6 | < .001 | < .001 | 260.6 ± 34.0 | |||
| FOXP3+ | 1.0 ± 0.3 | 18.6 ± 5.9 | < .001 | 5.3 ± 1.4 | < .01 | < .001 | 5.6 ± 1.5 | |||
Number of CD3+, CD4+, CD8+, and FOXP3+ lymphocytes per 10 high-power fields (hpf's) in biopsies from healthy controls (n = 13), as well as allografted symptomatic patients without GvHD (n = 33), with acute GvHD (n = 44), and with chronic GvHD (n = 18). Results are expressed as mean ± SEM. Acute and chronic GvHD showed no significant differences.
NS indicates not significant.
Lower CD4+/CD8+ ratio in patients with histologic signs of GvHD
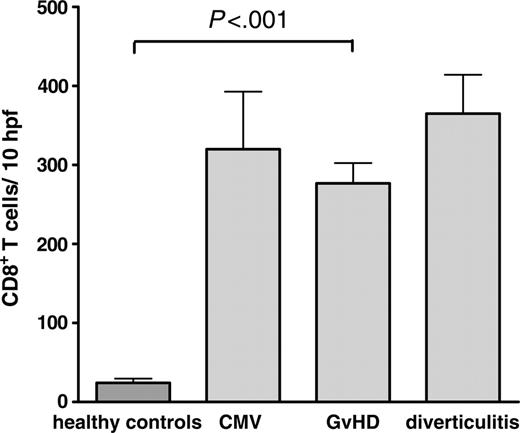
Despite similar T-cell numbers, the composition of the mucosa-infiltrating T lymphocytes differed remarkably between patients with and without GvHD. Specimens of patients with the histologic diagnosis of GvHD showed increased numbers of CD8+ T cells, resulting in a CD4+/CD8+ ratio of 0.8 (± 0.1) in this group compared with 1.9 (± 0.2; P < .001) in samples without GvHD and 2.0 (± 0.1; P < .001) in healthy controls. Similar findings have been previously reported.24-26 However, a comparable increase in the number of infiltrating CD8+ cells was also observed in patients with other inflammatory conditions such as CMV-colitis (Figure 1). Therefore, this finding alone is of limited value for the differential diagnosis of GvHD.
The immunohistologic results were confirmed by flow cytometry analysis of isolated lymphocytes derived from mucosal biopsies of 24 patients. While CD8+ lymphocytes were the predominant population found in samples of patients with GvHD (60.1% ± 4.9%), specimens of patients without GvHD demonstrated no such shift toward a predominance of CD8+ T cells (45.5% ± 6.2%).
Reduced numbers of FOXP3+ regulatory T cells in the mucosa of patients with acute and chronic GvHD
Using double immunoenzymatic labeling for FOXP3 and CD3, the number of Tregs in the mucosa of patients with acute and chronic GvHD was quantified and related to the number of CD8+ and CD4+ T cells.
Comparison of the number of CD8+ T cells per 10 hpf's in intestinal biopsies. Paraffin-embedded colon biopsies of healthy controls (n = 13), and patients after allogeneic transplantation with GvHD (n = 62; chronic GvHD, n = 18; acute GvHD, n = 44), CMV-colitis (n = 6), and diverticulitis (n = 6) were stained for CD8 by immunohistochemistry. The number of CD8+ cells was counted per 10 high-power fields. Results are expressed as mean ± SEM. The differences of CD8+ T cells in GvHD, CMV, and diverticulitis were not statistically significant.
Comparison of the number of CD8+ T cells per 10 hpf's in intestinal biopsies. Paraffin-embedded colon biopsies of healthy controls (n = 13), and patients after allogeneic transplantation with GvHD (n = 62; chronic GvHD, n = 18; acute GvHD, n = 44), CMV-colitis (n = 6), and diverticulitis (n = 6) were stained for CD8 by immunohistochemistry. The number of CD8+ cells was counted per 10 high-power fields. Results are expressed as mean ± SEM. The differences of CD8+ T cells in GvHD, CMV, and diverticulitis were not statistically significant.
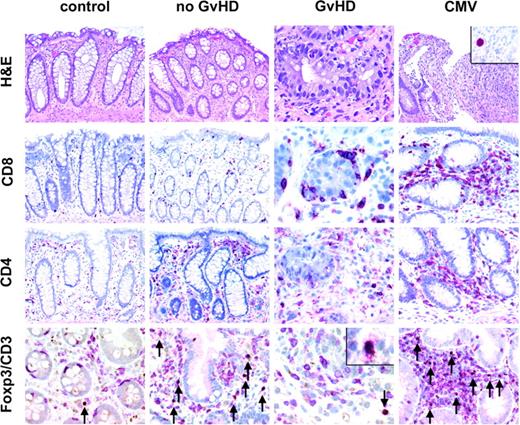
Histology and immunohistochemistry for CD4+, CD8+, and CD3+FOXP3+ T cells of representative colonic biopsies from healthy controls and patients with no GvHD, with GvHD after bone marrow transplantation, and with CMV infection. A normal amount of CD3+ T cells (red, membranous) with very few regulatory T cells coexpressing FOXP3 (brown, nuclear) is found in healthy controls, whereas mucosal biopsies of patients without GvHD display a high number of CD3+FOXP3+ T cells in the lamina propria (arrows, bottom row) as opposed to a low number of regulatory T cells in patients with GvHD. All FOXP3+ cells in addition expressed CD25 (inset/bottom row). The number of CD8+ T cells is increased in biopsies from patients with GvHD especially in and around crypts displaying many characteristic apoptotic bodies. In CMV infection (inset/upper panel: nuclear staining for CMV antigen), the number of CD3+FOXP3+ regulatory T cells is also elevated compared with patients with GvHD. Original magnifications, × 200; and × 600 (inset). Microscope: Olympus AX70 (Olympus, Melville, NY); numeric aperture of objective lenses: × 20, 0.70 mm; × 60, 1.40. Stains: hematoxylin and eosin (H&E; top row); APAAP and immunoperoxide. Camera: JVC KY-F70 (JVC, Yokohama, Japan); acquisition software: DISKUS (Koenigswinter, Germany); and software used for image processing: Adobe Photoshop 7.0 (Adobe Systems, San Jose, CA).
Histology and immunohistochemistry for CD4+, CD8+, and CD3+FOXP3+ T cells of representative colonic biopsies from healthy controls and patients with no GvHD, with GvHD after bone marrow transplantation, and with CMV infection. A normal amount of CD3+ T cells (red, membranous) with very few regulatory T cells coexpressing FOXP3 (brown, nuclear) is found in healthy controls, whereas mucosal biopsies of patients without GvHD display a high number of CD3+FOXP3+ T cells in the lamina propria (arrows, bottom row) as opposed to a low number of regulatory T cells in patients with GvHD. All FOXP3+ cells in addition expressed CD25 (inset/bottom row). The number of CD8+ T cells is increased in biopsies from patients with GvHD especially in and around crypts displaying many characteristic apoptotic bodies. In CMV infection (inset/upper panel: nuclear staining for CMV antigen), the number of CD3+FOXP3+ regulatory T cells is also elevated compared with patients with GvHD. Original magnifications, × 200; and × 600 (inset). Microscope: Olympus AX70 (Olympus, Melville, NY); numeric aperture of objective lenses: × 20, 0.70 mm; × 60, 1.40. Stains: hematoxylin and eosin (H&E; top row); APAAP and immunoperoxide. Camera: JVC KY-F70 (JVC, Yokohama, Japan); acquisition software: DISKUS (Koenigswinter, Germany); and software used for image processing: Adobe Photoshop 7.0 (Adobe Systems, San Jose, CA).
As described previously,11 infectious and inflammatory conditions usually demonstrate an increase of Tregs that accompanies the primary infiltration by effector T cells. While the expected increase could be found in the mucosa of patients with CMV-colitis (where the number of Tregs per 10 hpf's rose to 85.3 ± 17.9) and diverticulitis (raising to 202.0 ± 31.9 Tregs per 10 hpf's; data previously published11 ), only a slight increase (5.4 ± 1.1 vs 1.0 ± 0.3 FOXP3+ cells per 10 hpf's) was observed in patients with acute and chronic GvHD (Table 1), with the small number of FOXP3+ cells concentrated around and in between apoptotic crypts (Figure 2). In contrast, allografted patients without GvHD showed a remarkable increase in the number of FOXP3+ cells, resulting in a mean of 18.6 FOXP3+ cells per 10 hpf's (Table 1).
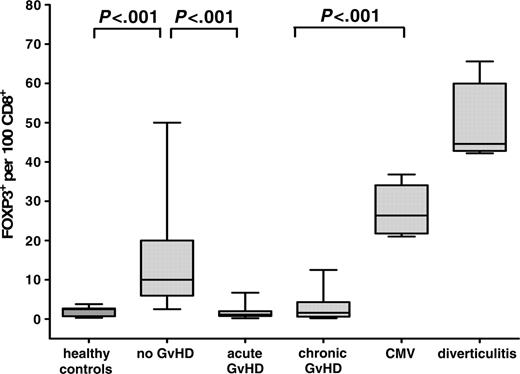
Since it can be expected that the overall effect of Tregs depends on their number in relation to CD8+ effector T cells, we determined the corresponding ratio for each patient in comparison with inflammatory controls (Figure 3). This analysis revealed an even more pronounced difference between the groups and showed that biopsies of patients with acute and chronic GvHD are characterized by a complete lack of a counterregulatory reaction as indicated by values identical to those of healthy controls (2.0 ± 0.3 FOXP3+ cells per 100 CD8+ cells) (Figure 3). In contrast, specimens without histologic signs of GvHD demonstrated significantly increased numbers of FOXP3+ per CD8+ T cells (14.1 ± 2.0; P < .001), though usually not as high as those seen in CMV-colitis (27.4 ± 2.5) or diverticulitis (50.0 ± 4.4).
Number of FOXP3+ Tregs per 100 CD8+ lymphocytes in healthy controls, and patients without GvHD, with acute and chronic GvHD, with CMV-colitis, and with diverticulitis. Paraffin-embedded intestinal biopsies of healthy controls (n = 13), and patients after allogeneic transplantation without GvHD (n = 33), with acute GvHD (n = 44), with chronic GvHD (n = 18), with CMV-colitis (n = 6), and with diverticulitis (n = 6) were immunohistochemically stained for CD8 and costained with anti-CD3/anti-FOXP3. The number of CD8+ cells and CD3+/FOXP3+ cells was counted per 10 high-power fields. Results are expressed as mean ± SEM. The ratio of FOXP3+ Tregs per 100 CD8+ lymphocytes was significantly increased in samples without GvHD versus acute and chronic GvHD.
Number of FOXP3+ Tregs per 100 CD8+ lymphocytes in healthy controls, and patients without GvHD, with acute and chronic GvHD, with CMV-colitis, and with diverticulitis. Paraffin-embedded intestinal biopsies of healthy controls (n = 13), and patients after allogeneic transplantation without GvHD (n = 33), with acute GvHD (n = 44), with chronic GvHD (n = 18), with CMV-colitis (n = 6), and with diverticulitis (n = 6) were immunohistochemically stained for CD8 and costained with anti-CD3/anti-FOXP3. The number of CD8+ cells and CD3+/FOXP3+ cells was counted per 10 high-power fields. Results are expressed as mean ± SEM. The ratio of FOXP3+ Tregs per 100 CD8+ lymphocytes was significantly increased in samples without GvHD versus acute and chronic GvHD.
To exclude that these findings can be explained by a general reduction in the number of CD4+ cells in patients with GvHD, the ratio of FOXP3+ per CD4+ cells was also determined. Samples without GvHD showed significantly (P < .001) higher numbers of FOXP3+ T cells per 100 CD4+ lymphocytes (10.5 ± 3.2) compared with GvHD samples (2.3 ± 0.3). This finding underlines that the pool of Tregs among all CD4+ cells is reduced in the mucosa of patients with intestinal GvHD.
Fourteen patients were analyzed sequentially over time: In 3 patients without intestinal GvHD who developed GvHD in the follow up, the absolute number of FOXP3+ cells as well as the number of FOXP3+/CD8+ cells decreased, while in 3 patients who initially had intestinal GvHD that responded to treatment, a final biopsy without GvHD signs showed that the absolute number of FOXP3+ as well as the number of FOXP3+/CD8+ cells had increased. Eight patients with acute GvHD who did not respond to treatment were also analyzed sequentially. During ongoing intestinal GvHD, the number of Tregs remained stable at a very low level without any increase over time.
In an univariate analysis of additional factors potentially influencing the number of mucosa-infiltrating Tregs, neither the conditioning regimen (myeloablative vs dose-reduced) nor the time point of the biopsy or the immunosuppressive treatment turned out to be statistically relevant. The number of Tregs was not correlated to the histologic or clinical GvHD grade.
Reduced numbers of CD4+CD25high regulatory T cells in the peripheral blood of patients with acute GvHD
We additionally analyzed the peripheral blood lymphocytes of 24 patients who underwent biopsy by flow cytometry and determined the percentage of CD4+CD25high T cells as previously described.23 While 14 of these patients had histologic signs of intestinal GvHD (grade 1-3), 10 showed no evidence of GvHD in the gut, skin, or liver.
The frequency of CD4+CD25high T cells in peripheral blood lymphocytes of patients with GvHD (0.8% ± 0.2%) was nearly identical to that of healthy individuals (1.0% ± 0.1%) (Table 2),23,27-28 again suggesting the lack of a counterregulatory response in patients with GvHD. Compared with allografted patients without GvHD (2.8% ± 0.6%), GvHD specimens demonstrated significantly (P < .001) lower CD4+CD25high cell numbers. However, the difference of Tregs in the peripheral blood compartment of patients with and without GvHD was not as pronounced as in the intestinal mucosa.
Percentage of T-cell subsets in peripheral blood from healthy controls, patients without GvHD, and patients with acute intestinal GvHD
. | . | No GvHD . | . | Acute GvHD . | . | . | |||
|---|---|---|---|---|---|---|---|---|---|
| Cell type . | Healthy ctrls, % of total lymphocytes . | % of total lymphocytes . | P vs healthy ctrls . | % of total lymphocytes . | P vs healthy ctrls . | P vs no GvHD . | |||
| CD4+/CD3+ | 57.4 ± 4.6 | 49.3 ± 6.8 | NS | 42.9 ± 5.5 | NS | NS | |||
| CD8+/CD3+ | 37.2 ± 2.7 | 38.9 ± 5.3 | NS | 55.4 ± 4.2 | <.01 | <.05 | |||
| CD4+CD25high/CD3+ | 1.0 ± 0.1 | 2.8 ± 0.6 | <.01 | 0.8 ± 0.2 | NS | <.001 | |||
| CD4+CD25high/CD4+ | 2.3 ± 0.2 | 8.5 ± 1.0 | <.001 | 4.2 ± 1.1 | NS | <.01 | |||
| CD4+CD25high/CD8+ | 6.4 ± 0.7 | 16.6 ± 5.4 | NS | 3.9 ± 1.2 | NS | <.01 | |||
. | . | No GvHD . | . | Acute GvHD . | . | . | |||
|---|---|---|---|---|---|---|---|---|---|
| Cell type . | Healthy ctrls, % of total lymphocytes . | % of total lymphocytes . | P vs healthy ctrls . | % of total lymphocytes . | P vs healthy ctrls . | P vs no GvHD . | |||
| CD4+/CD3+ | 57.4 ± 4.6 | 49.3 ± 6.8 | NS | 42.9 ± 5.5 | NS | NS | |||
| CD8+/CD3+ | 37.2 ± 2.7 | 38.9 ± 5.3 | NS | 55.4 ± 4.2 | <.01 | <.05 | |||
| CD4+CD25high/CD3+ | 1.0 ± 0.1 | 2.8 ± 0.6 | <.01 | 0.8 ± 0.2 | NS | <.001 | |||
| CD4+CD25high/CD4+ | 2.3 ± 0.2 | 8.5 ± 1.0 | <.001 | 4.2 ± 1.1 | NS | <.01 | |||
| CD4+CD25high/CD8+ | 6.4 ± 0.7 | 16.6 ± 5.4 | NS | 3.9 ± 1.2 | NS | <.01 | |||
Analyses were performed by flow cytometry of whole blood samples from healthy controls (n = 5), as well as allografted symptomatic patients without GvHD (n = 10) and with acute GvHD (n = 16). Results are expressed as mean ± SEM.
NS indicates not significant.
To exclude that the effects were based on a general decrease in the number of CD4+ cells in patients with GvHD, the ratio of CD4+CD25high cells per CD4+ was determined as an indicator for the expansion or contraction of the regulatory T-cell pool. The results confirmed that CD4+CD25high cells were enriched among the CD4+ T cells in the group of allografted patients without GvHD (Table 2). Unfortunately, insufficient T-cell numbers prevented the measurement of CD4+CD25high cells by flow cytometry in mucosal biopsies, emphasizing the value of the immunohistochemical evaluation of the FOXP3+ Tregs.
Discussion
After allogeneic transplantation, GvHD is characterized by donor leukocyte infiltrates in the gut, skin, and liver.29 Recruitment, activation, and expansion of mature donor T cells at the site of inflammation represent the key processes during the initiation phase of GvHD that leads to tissue damage, transmission of infectious agents, consecutive multiorgan failure, and, in worst case, death of the patient.
In mice, experimental data have revealed that the activity of alloreactive T cells can be controlled by CD4+CD25+ immunoregulatory T cells. So far, the examination of these cells in humans was hampered by the lack of a marker that specifically identifies Tregs. The identification and enumeration of Tregs was rather based on the staining of CD4+CD25+ lymphocytes by FACS analysis with additional in vitro confirmation of their regulatory capacity, as described by Baecher-Allen et al.23 The recent identification of FOXP3 as a key regulator gene required for the development and functional activity of Tregs offered an additional and more specific marker, which has been applied for the molecular characterization and quantification of T cells with regulatory capacity in a number of studies.4-6,11
Using a polyclonal antibody against FOXP3, we have established a method for immunohistochemical staining in combination with the surface markers CD3 and CD25. In contrast to previous studies, which primarily investigated the Treg population in peripheral blood, this new approach enabled us to evaluate the number of Tregs directly at the site of GvH reactivity and compare it with specimens with CMV infection or diverticulitis.
Our results revealed striking differences of FOXP3+ Tregs in relation to CD8+ effector T cells in the mucosa of patients with GvHD versus intestinal infections. While the number of CD8+ cells in GvHD was similar to that found in CMV colitis and diverticulitis, the number of FOXP3+ Tregs per CD8+ T cells was significantly lower in the mucosa of patients with GvHD compared with patients under immunosuppressive therapy with CMV infection or with patients with diverticulitis (Figure 3).
This finding suggests an insufficient increase or a loss of the Treg pool possibly due to diminished generation, impaired migration, increased apoptosis, or a change in phenotype, and a disproportional increase of CD8+ effector T cells in intestinal GvHD. Of interest, the potential for Treg expansion was principally maintained in allografted patients as indicated by a significant increase of the FOXP3+ Treg/CD8+ ratio in stem cell recipients without GvHD compared with healthy controls.
Our data confirm previous studies demonstrating that GvHD is characterized by a CD3+-dominated lymphocyte infiltration. Unfortunately, this finding alone is of limited value for the diagnosis of GvHD since significantly increased T-cell numbers are also present in specimens without histologic signs of GvHD, in CMV colitis and bacterial infections such as diverticulitis. Although no causative infectious agents could be identified despite extensive diagnostic maneuvers in the majority of these cases, we suggest that these infiltrates are caused by an inflammation due to viral or bacterial infections, which led to gastrointestinal symptoms, such as persisting nausea and vomiting, intestinal pain, or diarrhea, which were the indication for endoscopic examination.
Although 33 patients with gastrointestinal symptoms had no histologic signs of GvHD, only 3 had pp65-positive cells in peripheral blood or immunohistochemical signs indicating a CMV infection, and only 2 others had evidence of Clostridium difficile infection. Differentiation between infection and GvHD is a common obstacle after allogeneic stem cell transplantation and is often impossible by available routine methods. Cox et al analyzed 126 patients with diarrhea after allogeneic stem cell transplantation and found gastrointestinal GvHD in 48% and intestinal infection in 13% of episodes (viruses in 8%, nosocomially acquired bacterial infection including Clostridium difficile in 5%). However, in 39% no underlying etiology could be identified for self-limited diarrhea.30
Our results contribute to a series of recent publications indicating a crucial role of Tregs in GvHD pathophysiology and, most interestingly, in their potential to ameliorate GvHD. In murine stem cell transplantation models, Taylor et al demonstrated that the depletion of CD25+ T cells accelerated the development of GvHD,13 while the infusion of isolated and/or ex vivo-expanded donor-type CD4+CD25+ Tregs was able to delay or even prevent GvHD.12-16,31-34
Inconclusive data obtained in human studies of Tregs could be related to different methodologic approaches in the studies published so far. Whereas the present study and Miura et al18 have determined the expression of FOXP3, either by immunohistologic staining or mRNA expression, flow cytometry was used in the 2 other studies17,35 to estimate the number of CD4+CD25high cells. An increased number of Tregs in the peripheral blood17,35 or in stem cell grafts36 of patients with GvHD was found only in studies that used overexpression of CD25 to characterize the Treg population. It is not unlikely that the classification of Tregs by an arbitrary cut-off point for the definition of CD25high cells could result in diverse findings, since CD25 expression is not restricted to regulatory CD4+ cells.22,36 Moreover, low CD4+ T-cell numbers can make it extremely difficult to set the cut-off for the CD25high cell population.
While the quantification of Tregs by FACS analysis is restricted to high cell numbers, immunoenzymatic staining of nuclear FOXP3 in combination with CD3 allows straightforward identification and quantification even of very low numbers of Tregs. Through the herewith described quantification of lymphoid infiltrates in the target tissues of GvHD (ie, liver, gut, and skin), it will be possible to study retrospectively and prospectively the influence of regulatory T cells on the disease development and to monitor the efficacy of transferred Tregs in future therapeutic studies.
In conclusion, our findings provide strong evidence that acute and chronic GvHD are characterized by an insufficient increase or a loss of Tregs in GvHD target organs, while increased numbers of Tregs are found in non-GvHD-associated inflammation after transplantation and in inflammatory controls. This finding may indicate that the suppressive potency of Tregs is impaired due to numeric deficiency during GvHD, especially with regard to the high number of effector T lymphocytes as indicated by a very low FOXP3/CD8+ ratio. We suggest that the determination of FOXP3+ T cells could help to discriminate GvHD from infectious inflammation after allogeneic stem cell transplantation and thus may be of value in clinical practice.
Prepublished online as Blood First Edition Paper, November 8, 2005; DOI 10.1182/blood-2005-06-2529.
Supported by the Deutsche Forschungsgemeinschaft Deutschland (DFG) grants KFO 104/1 and Z1SFB633.
K.R. and C.L. contributed equally to this work.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
We would like to thank Simone Spieckermann for excellent technical assistance.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal