Iron deficiency is a known complication of achlorhydria and may precede the development of pernicious anemia. Among 160 patients with autoimmune gastritis identified by hypergastrinemia and strongly positive antiparietal antibodies, we explored the overlap between 83 subjects presenting with iron deficiency anemia (IDA), 48 with normocytic indices, and 29 with macrocytic anemia. Compared with macrocytic patients, patients with IDA were 21 years younger (41 ± 15 years versus 62 ± 15 years) and mostly women. All groups had a high prevalence of thyroid disease (20%) and diabetes (8%) suggestive of the autoimmune polyendocrine syndrome. Stratification by age cohorts from younger than 20 years to older than 60 years showed a regular and progressive increase in mean corpuscular volume (MCV) from 68 ± 9 to 95 ± 16 fl, serum ferritin levels from 4 ± 2 to 37 ± 41 μg/L, gastrin level from 166 ± 118 to 382 ± 299 pM/L (349 ± 247 to 800 ± 627 pg/mL), and a decrease in cobalamin level from 392 ± 179 to 108 ± 65 pg/mL. The prevalence of Helicobacter pylori infection was 87.5% at age younger than 20 years, 47% at age 20 to 40 years, 37.5% at 41 to 60 years, and 12.5% at age older than 60 years. These findings challenge the common notion that pernicious anemia is a disease of the elderly and imply a disease starting many years before the establishment of clinical cobalamin deficiency, by an autoimmune process likely triggered by H pylori.
Introduction
Pernicious anemia (PA) in Western countries is the most common cause of megaloblastic anemia resulting from cobalamin (Cbl) deficiency. In PA the portion of gastric mucosa containing parietal cells is destroyed by an autoimmune mechanism. The resulting failure of intrinsic factor production prevents normal absorption of Cbl, which over the years will lead to Cbl depletion and the well-known hematologic and neurologic consequences of Cbl deficiency.1,2 However, damage to parietal cells also abolishes acid secretion resulting in achlorhydria, which is regularly associated with PA. Gastric acid secretion, in turn, is critical for the solubilization and reduction of food iron permitting normal iron absorption. Consequently, iron deficiency is a known complication of PA at presentation or following Cbl treatment.3,4
In a previous prospective study conducted among 150 ambulatory patients with iron deficiency anemia (IDA) without apparent gastrointestinal disease, we have found autoimmune gastritis indicated by hypergastrinemia in combination with strongly positive antiparietal antibody titers in a very high (27%) proportion of subjects.5 In about one half of these subjects the serum Cbl level was subnormal. Unlike classic PA, most of our patients with autoimmune gastritis presenting with IDA were young women and many of them had coexistent Helicobacter pylori infection. This combination of findings has been described previously as “atrophic body gastritis” in subjects with refractory IDA without gastrointestinal symptoms.6-11
The objectives of the present study were to explore the degree of overlap between IDA with coexistent hypergastrinemia and serum antiparietal antibodies on the one hand and Cbl deficiency associated with autoimmune gastritis on the other. To this end, a comparison was made between patients referred to us for evaluation of low serum Cbl levels and all patients investigated for causes of IDA in whom autoimmune atrophic gastritis has been diagnosed by a systematic screening using serum gastrin and antiparietal-cell antibody (APCA) determinations.
Patients, materials, and methods
Target population
The study included all patients diagnosed with hypergastrinemia in combination with antiparietal antibodies identified in a prospective study over a 4-year period starting September 1, 2001. Approval was obtained from the Shaare Zedek Medical Center Institutional Review Board for these studies. Informed consent was provided according to the Declaration of Helsinki. The tests were performed in all patients presenting with IDA or those referred for investigation of low serum Cbl levels. In addition to 127 patients with hypergastrinemia and antiparietal antibodies identified in the prospective study, 33 patients diagnosed and followed-up with hypergastrinemia and antiparietal antibodies at our clinic between the years 1995 and 2000 were also included, resulting in a total of 160 patients. Causes of referral were refractory IDA in 41, combined IDA and Cbl deficiency in 48, de novo finding of macrocytosis in 26, de novo finding of low Cbl level in 28, known PA in relapse or referred for follow-up in 13, anemia referred for investigation in 2, and unexplained hypergastrinemia or atrophic gastritis discovered on endoscopy in 1 patient each.
Anemia was defined as a hemoglobin level of 115 g/L (11.5 g/dL) or less in women and 135 g/L (13.5 g/dL) or less in men. Iron deficiency was defined by the coexistence of transferrin saturation less than 15% and a serum ferritin level less than 15 μg/L. IDA was defined as microcytic (mean corpuscular volume [MCV] < 80 fl) anemia and the described criteria for iron deficiency. Detailed information on the initial workup of IDA, gastrointestinal studies, and method of treatment is presented in a previous report.5
The diagnosis of PA rested on the combined presence of (1) low (< 181 ng/L) fasting serum Cbl level; (2) abnormal Schilling test or positive intrinsic factor type I blocking antibodies; (3) increased fasting serum gastrin concentration; (4) increased (2+ to 4+) titers of serum antiparietal-cell antibody (APCA); (5) endoscopy demonstrating atrophic gastritis; and (6) response of macrocytic anemia to B12 (hydroxocobalamin) therapy by achieving normal hemoglobin (as defined) and MCV (< 100 fl) levels. Definite diagnosis of PA required an abnormal Schilling test or anti-intrinsic factor positivity in combination with 3 additional abnormal parameters. When a Schilling test or anti-intrinsic factor result was not available, probable PA was defined as the combination of 4 abnormal parameters. In patients failing to fulfill the criteria, evidence for PA was considered insufficient.
Healthy control subjects
The control group of 60 nonanemic subjects (9 men and 51 women) aged 36 ± 15 years was recruited from among asymptomatic patients referred for evaluation of suspected thrombophilia (44), no disease (10), or borderline white-cell counts (6).
Laboratory workup
All patients had a complete blood count, serum iron, total iron-binding capacity (TIBC), serum ferritin, serum B12 and folate, sedimentation rate, fibrinogen, and CRP studies performed. Routine biochemistry included serum albumin and cholesterol determinations. Celiac serology included antiendomysial and antigliadin antibodies. H pylori IgG antibodies were determined along with APCAs, anti-intrinsic factor antibodies, and serum gastrin concentration.
Laboratory methods
Complete blood counts (CBCs) were performed by a Sysmex SE-9000 automated analyzer (Kobe, Japan) standardized daily with standards provided by the manufacturer. Routine biochemical measurements were performed by a Hitachi 747 automated analyzer. Both instruments were standardized daily with commercial controls provided by the manufacturers. Quality control assurance was directed to both instruments by UKNEQAS (Birmingham, United Kingdom). Fasting serum B12 levels were determined by the ADVIA Centaur VB12 assay, which is an automated competitive immunoassay using direct chemiluminescent technology. The lower limit of normal was defined as less than 181 ng/L. Cbl absorption was measured by the phase #1 Schilling test (available until 2002) using 1 μg radioactive cyanocobalamin in water ingested in the fasting state, followed by the intramuscular injection of 1 mg unlabeled cyanocobalamin at 2 hours. The lower limit of normal 24-hour urinary excretion of radioactivity was 8% or higher. Fasting serum gastrin levels were determined by GammaDab Gastrin 125I RIA Kit (DiaSorin, Stillwater, MN) and expressed as picomoles per liter (pg/mL), with normal limits less than 51 pM (107 pg/mL). For the detection of IgG antibodies to H pylori in human serum, the IMMULITE 2000 H pylori IgG, a solid-phase chemiluminescent immunoimetric assay (DPC Diagnostics, Los Angeles, CA) was used; it has an analytic sensitivity limit of 0.4 U/mL, with less than 0.9 U/mL considered to be negative. Anti–endomysial IgG and IgA antibodies and APCAs were determined by an indirect immunofluorescence antibody test for semiquantitative detection of antibodies using the ImmuGlo Anti-Endomysial Antibody Test System (IMMCO Diagnostics, Buffalo, NY). Results were considered positive if equal to or more than 1:40 dilution. Intrinsic factor antibodies were assayed by enzyme-linked immunoassay (ALPHADIA, Wavre, Belgium) detecting type I blocking antibodies. Results exceeding 1.1 were considered positive. Antigliadin antibodies were measured by an indirect assay using peroxidase-labeled rabbit anti–human IgG and IgA antibodies using the BINDAZYME Human Anti-Gliadin IgG and IgA kits (The Binding Site, Birmingham, United Kingdom). Results were considered positive if equal to or more than 10 U/mL for IgG and 5 U/mL for IgA antibodies.
The 13C-urease breath test was performed as described by Gal et al.12 Briefly, patients were given 75 mg urea labeled with 13C in 200 mL orange juice and breath samples were collected at times 0 before, and 30 minutes after 13C intake. Results were expressed as the difference between the 2 scores (delta over baseline). The cut-off 13C/12C of T30′ minus T0′ was Δ3.5%. Patients were instructed to discontinue treatment with H2 antagonists, proton pump inhibitors, or any antibiotics 1 week before the test.
Triple therapy for H pylori eradication consisted of omeprazole 20 mg twice a day, amoxicillin 1 g twice a day, and clarithromycin 500 mg twice a day for 7 days. Repeat tests for validation of H pylori eradication were performed after at least 1 month of completing triple therapy.
Statistical analysis
We used the Student t test for continuous variables and the χ2 test for categorical variables. For continuous variables that had measurements before and after interventions (eg, hemoglobin levels) we used the paired t test. All tests were 2-tailed. For all the analyses we used the SPSS 10.0 for Windows (SPSS Software, Chicago, IL).
Results
The presenting features of the 160 patients studied are described in Table 1.
Autoimmune gastritis: mode of presentation, associated findings, and results of endoscopy
. | Macrocytic, n = 29 . | Normocytic, n = 48 . | Microcytic, n = 83 . |
|---|---|---|---|
| Age, y, ± SD (M/F) | 62 ± 15 (17/12) | 58 ± 17 (18/30) | 41 ± 15 (18/65) |
| Anemic, no. (%) | 18 (62) | 19 (40) | 83 (100) |
| Low B12 level, no. (%) | 29 (100) | 44 (92) | 38 (46) |
| Iron deficiency, no. (%) | 3 (10) | 24 (50) | 83 (100) |
| Thyroid disease, no. (%) | 3 (10) | 14 (29) | 15 (18) |
| Hypothyroid, no. | 3 | 12 | 12 |
| Graves disease, no. | 0 | 1 | 2 |
| Hashimoto thyroiditis, no. | 0 | 1 | 1 |
| Vitiligo, no. (%) | 2 (7) | 0 (0) | 0 (0) |
| Diabetes mellitus, no. (%) | 1 (3) | 4 (8) | 7 (8) |
| Neurologic complications, no. (%) | 5 (17) | 2 (4) | 0 (0) |
| Gastric histology, total no. | 13 | 24 | 32 |
| Atrophic gastritis, no. (%) | 9 (69) | 13 (54) | 13 (41) |
| Chronic gastritis, no. (%) | 2 (15) | 9 (38) | 18 (56) |
| MALT lymphoma, no. (%) | 1 (8) | 1 (4) | 0 (0) |
| Neoplasia, no., type (%) | 1, adenocarcinoma (8) | 1, polyp (4) | 1, polyp (3) |
. | Macrocytic, n = 29 . | Normocytic, n = 48 . | Microcytic, n = 83 . |
|---|---|---|---|
| Age, y, ± SD (M/F) | 62 ± 15 (17/12) | 58 ± 17 (18/30) | 41 ± 15 (18/65) |
| Anemic, no. (%) | 18 (62) | 19 (40) | 83 (100) |
| Low B12 level, no. (%) | 29 (100) | 44 (92) | 38 (46) |
| Iron deficiency, no. (%) | 3 (10) | 24 (50) | 83 (100) |
| Thyroid disease, no. (%) | 3 (10) | 14 (29) | 15 (18) |
| Hypothyroid, no. | 3 | 12 | 12 |
| Graves disease, no. | 0 | 1 | 2 |
| Hashimoto thyroiditis, no. | 0 | 1 | 1 |
| Vitiligo, no. (%) | 2 (7) | 0 (0) | 0 (0) |
| Diabetes mellitus, no. (%) | 1 (3) | 4 (8) | 7 (8) |
| Neurologic complications, no. (%) | 5 (17) | 2 (4) | 0 (0) |
| Gastric histology, total no. | 13 | 24 | 32 |
| Atrophic gastritis, no. (%) | 9 (69) | 13 (54) | 13 (41) |
| Chronic gastritis, no. (%) | 2 (15) | 9 (38) | 18 (56) |
| MALT lymphoma, no. (%) | 1 (8) | 1 (4) | 0 (0) |
| Neoplasia, no., type (%) | 1, adenocarcinoma (8) | 1, polyp (4) | 1, polyp (3) |
As defined by MCV, 29 patients had macrocytic (MCV > 100 fl), 83 had microcytic (MVC < 80 fl), and 48 had normocytic (MCV 80-100 fl) indices at presentation. The mean age of patients with microcytic anemia was 41 ± 15 years, 18 years younger than the mean age of 59 ± 16 years for all other groups combined (P < .001). Of the 83 subjects with microcytic anemia, only 18 (22%) were men compared with 35 of 77 (46%) for all other groups combined (χ2 = 0.0001). Although all microcytic patients were anemic, anemia was present in only 62% of the macrocytic and 40% of the normocytic subjects. Low (defined as < 181 ng/L) serum vitamin B12 (Cbl) levels were found in 100% of the macrocytic, 92% of the normocytic, and 46% of the microcytic groups. Iron deficiency (as defined in “Patients, materials, and methods”) was found in all patients with microcytic anemia, but also in 50% of the normocytic and 10% of the macrocytic patients. Thus, a considerable proportion of patients had combined iron and Cbl deficiency. Additional diseases known to be associated with the autoimmune polyendocrine syndromes were present in the following proportion of patients: thyroid disease including hypothyroidism (27), Graves disease (3), and Hashimoto thyroiditis (2) in 20% of patients, diabetes mellitus (12) in 8%, and vitiligo (2) in 1.3%. The 18% prevalence of thyroid disease in the microcytic group was not significantly different from its 22% prevalence in the other 2 groups (χ2 = 0.272).
Neurologic complications that may be attributed to Cbl deficiency including paresthesia (4), confusion (1), impaired memory (1), and blurred vision (1) were encountered in 9% of the macrocytic and normocytic but in none of the microcytic subjects. Improvement in neurologic symptoms was observed in all patients within several months of starting Cbl therapy.
Results of endoscopic studies were available in 87 patients and gastric mucosal histology in 69. Histology was defined as atrophic gastritis in 50% and chronic gastritis (chronic inflammation) in 42%, mucosa-associated lymphoid tissue (MALT) lymphoma in 3%, gastric polyp in 3%, and adenocarcinoma of the stomach in 1 patient (1%). In an additional 18 cases the gastric mucosa was described as normal macroscopically, but no biopsies were taken. The proportion of patients with atrophic gastritis was 9 (69%) of 13 in patients with macrocytic anemia and 13 (41%) of 32 in those with microcytic anemia. Conversely, the proportion of patients with chronic inflammation in the macrocytic group was 2 (15%) of 13 compared with 18 (56%) of 32 in microcytic anemia.
Some of the patients with microcytic anemia had evidence of increased blood loss including 10 with a history of menorrhagia, 4 with positive occult blood tests, and 3 with bleeding gastrointestinal lesions including colon cancer, gastric polyp, and gastric erosions. Two patients were referred with anemia of pregnancy and one was known to have celiac disease. Altogether, 20 of the 83 patients with IDA had common and well-established causes of IDA and in the rest, the high proportion of fertile women indicated an increased likelihood of menstrual blood loss as the underlying cause of IDA. In addition, the presence of H pylori infection in 42% of patients with IDA may have been a significant contributing factor.
The presenting laboratory findings in the various groups defined by the absence, presence, and type of anemia are described in Table 2.
Laboratory findings in autoimmune gastritis by type of anemia
Presenting features . | No. . | Hemoglobin level, g/L . | MCV, fl . | Serum iron level, μM . | TIBC, μM . | Ferritin level, μg/L . | B12 concentration, pM . | Gastrin level, pM . | H pylori, no. positive/total (%) . |
|---|---|---|---|---|---|---|---|---|---|
| Macrocytic anemia | 18 | 88 ± 26 (8.8 ± 2.6 g/dL) | 119.3 ± 7.8 | 14.7 ± 8.1 μM (82.2 ± 45.1 μg/dL) | 48.7 ± 11.5 (272.3 ± 64.5 μg/dL) | 51.5 ± 57.8 | 60.8 ± 25.3 (82.4 ± 34.3 pg/mL) | 403.4 ± 259.0 (845.7 ± 551.0 pg/mL) | 2/7 (28.6) |
| Macrocytic nonanemic | 11 | 139 ± 10 (13.9 ± 1.0 g/dL) | 109.4 ± 5.7 | 19.9 ± 7.7 (110.9 ± 43.1 μg/dL) | 48.3 ± 8.1 (269.6 ± 45.2 μg/dL) | 73.4 ± 63.6 | 64.9 ± 35.3 (88.0 ± 47.9 pg/mL) | 303.3 ± 110.1 (635.8 ± 230.8 pg/mL) | 1/7 (14.3) |
| Normocytic nonanemic | 29 | 137 ± 11 (13.7 ± 1.1 g/dL) | 87.2 ± 4.4 | 13.1 ± 6.7 (73.0 ± 37.6 μg/dL) | 57.9 ± 9.2 (323.3 ± 51.6 μg/dL) | 31.0 ± 30.6 | 92.8 ± 53.0 (128.5 ± 71.8 pg/mL) | 347.4 ± 335.1 (728.4 ± 702.5 pg/mL) | 9/24 (37.5) |
| Normocytic anemia | 19 | 10.6 ± 1.7 | 87.2 ± 5.2 | 10.1 ± 3.5 (56.7 ± 19.6 μg/dL) | 59.4 ± 9.7 (331.9 ± 54.1 μg/dL) | 15.4 ± 16.6 | 82.4 ± 69.0 (111.8 ± 93.4 pg/mL) | 364.0 ± 286.1 (763.0 ± 599.8 pg/mL) | 2/9 (22.2) |
| Microcytic anemia | 83 | 97 ± 14 (9.7 ± 1.4 g/dL) | 71.7 ± 7.8 | 5.4 ± 2.8 (30.4 ± 16.2 μg/dL) | 60.1 ± 7.3 (340.5 ± 40.8 μg/dL) | 6.3 ± 8.8 | 159.0 ± 87.6 (215.4 ± 118.7 pg/mL) | 254.6 ± 190.0 (541.6 ± 397.6 pg/mL) | 31/75 (41.3) |
| Healthy control subjects | 60 | 126 ± 21 (12.6 ± 2.1 g/dL) | 86.7 ± 4.5 | 17.1 ± 7.2 (95.8 ± 40.0 μg/dL) | 50.7 ± 10.1 (283.0 ± 56.5 μg/dL) | 51.0 ± 63.3 | 212.1 ± 96.2 (287.5 ± 130.4 pg/mL) | 29.2 ± 8.3 (61.3 ± 17.4 pg/mL) | 31/60 (51.7) |
Presenting features . | No. . | Hemoglobin level, g/L . | MCV, fl . | Serum iron level, μM . | TIBC, μM . | Ferritin level, μg/L . | B12 concentration, pM . | Gastrin level, pM . | H pylori, no. positive/total (%) . |
|---|---|---|---|---|---|---|---|---|---|
| Macrocytic anemia | 18 | 88 ± 26 (8.8 ± 2.6 g/dL) | 119.3 ± 7.8 | 14.7 ± 8.1 μM (82.2 ± 45.1 μg/dL) | 48.7 ± 11.5 (272.3 ± 64.5 μg/dL) | 51.5 ± 57.8 | 60.8 ± 25.3 (82.4 ± 34.3 pg/mL) | 403.4 ± 259.0 (845.7 ± 551.0 pg/mL) | 2/7 (28.6) |
| Macrocytic nonanemic | 11 | 139 ± 10 (13.9 ± 1.0 g/dL) | 109.4 ± 5.7 | 19.9 ± 7.7 (110.9 ± 43.1 μg/dL) | 48.3 ± 8.1 (269.6 ± 45.2 μg/dL) | 73.4 ± 63.6 | 64.9 ± 35.3 (88.0 ± 47.9 pg/mL) | 303.3 ± 110.1 (635.8 ± 230.8 pg/mL) | 1/7 (14.3) |
| Normocytic nonanemic | 29 | 137 ± 11 (13.7 ± 1.1 g/dL) | 87.2 ± 4.4 | 13.1 ± 6.7 (73.0 ± 37.6 μg/dL) | 57.9 ± 9.2 (323.3 ± 51.6 μg/dL) | 31.0 ± 30.6 | 92.8 ± 53.0 (128.5 ± 71.8 pg/mL) | 347.4 ± 335.1 (728.4 ± 702.5 pg/mL) | 9/24 (37.5) |
| Normocytic anemia | 19 | 10.6 ± 1.7 | 87.2 ± 5.2 | 10.1 ± 3.5 (56.7 ± 19.6 μg/dL) | 59.4 ± 9.7 (331.9 ± 54.1 μg/dL) | 15.4 ± 16.6 | 82.4 ± 69.0 (111.8 ± 93.4 pg/mL) | 364.0 ± 286.1 (763.0 ± 599.8 pg/mL) | 2/9 (22.2) |
| Microcytic anemia | 83 | 97 ± 14 (9.7 ± 1.4 g/dL) | 71.7 ± 7.8 | 5.4 ± 2.8 (30.4 ± 16.2 μg/dL) | 60.1 ± 7.3 (340.5 ± 40.8 μg/dL) | 6.3 ± 8.8 | 159.0 ± 87.6 (215.4 ± 118.7 pg/mL) | 254.6 ± 190.0 (541.6 ± 397.6 pg/mL) | 31/75 (41.3) |
| Healthy control subjects | 60 | 126 ± 21 (12.6 ± 2.1 g/dL) | 86.7 ± 4.5 | 17.1 ± 7.2 (95.8 ± 40.0 μg/dL) | 50.7 ± 10.1 (283.0 ± 56.5 μg/dL) | 51.0 ± 63.3 | 212.1 ± 96.2 (287.5 ± 130.4 pg/mL) | 29.2 ± 8.3 (61.3 ± 17.4 pg/mL) | 31/60 (51.7) |
Data are mean ± 1 SD.
Comparing macrocytic anemia in the top row of Table 2 with microcytic anemia at the bottom, there was a stepwise and regular decrease in MCV from 119 ± 8 to 72 ± 8 fl (P < .001); serum iron level from 15 ± 8 to 5 ± 3 μM (82 ± 45 to 30 ± 16 μg/dL; P < .001); serum ferritin concentration from 52 ± 58 to 6 ± 9 μg/L (P < .001); and serum gastrin level from 403 ± 263 to 255 ± 187 pM (846 ± 551 to 542 ± 398 pg/mL; P = .011). Conversely, in the same order of groups, serum Cbl increased from 82 ± 34 to 215 ± 19 pg/mL (P < .001) and TIBC from 49 ± 12 to 61 ± 7 μM (272 ± 65 to 341 ± 41 μg/dL; P < .001). Among subjects with normocytic indices, the only feature distinguishing anemic from nonanemic patients was the lower value of serum iron (P = .029) and serum ferritin (P = .018) in anemic subjects, implying that the apparently normocytic anemia was in fact dimorphic anemia resulting from joint iron and Cbl deficiency. Compared with the 60 healthy control subjects (gastrin 29 ± 8 pM [61 ± 17 pg/mL], APCA negative), the serum gastrin level was 9- to 14-fold higher, and the serum Cbl level was significantly lower in all groups (P < .001), including microcytic anemia (P = .004). The Schilling test (available only before 2002) was performed in 34 patients only. It was abnormal in 11 of 11 patients with macrocytic, 11 of 13 patients with normocytic, and 3 of 10 patients with microcytic indices. Using the criteria for the diagnosis of PA defined in “Patients, materials, and methods,” 100% (29 of 29) of macrocytic patients had definite or probable PA compared with 54% (26 of 48) of normocytic and 27% (22 of 83) of microcytic patients. Comparison by sex has shown slightly higher hemoglobin levels in men than in women within the various subgroups: 140 ± 7 versus 126 ± 15 g/L (14.0 ± 0.7 versus 12.6 ± 1.5 g/dL; mean ± 1 SD) in healthy control subjects, 99 ± 25 versus 71 ± 18 g/L (9.9 ± 2.5 versus 7.1 ± 1.8 g/dL) in macrocytic anemia, 148 ± 7 versus 131 ± 10 g/L (14.8 ± 0.7 versus 13.1 ± 1.0 g/dL) in macrocytic nonanemic, 148 ± 9 versus 127 ± 6 g/L (14.8 ± 0.9 versus 12.7 ± 0.6 g/dL) in normocytic nonanemic, 110 ± 19 versus 103 ± 16 g/L (11.0 ± 1.9 versus 10.3 ± 1.6 g/dL) in normocytic anemic, and 109 ± 15 verus 94 ± 13 g/L (10.9 ± 1.5 versus 9.4 ± 1.3 g/dL) in microcytic anemic subjects (P = .001 to P = .02). However, there were no significant sex-related differences in all other laboratory findings shown in Table 2.
The rate of seropositivity for H pylori in the healthy control subjects was 52% (31 of 60). Active H pylori infection confirmed by positive urease breath test was encountered in 41% (31 of 75) of those with microcytic anemia and 30% (14 of 47) of all other groups combined. Intrinsic factor blocking antibodies, available in 56 patients, were positive in 20% (2 of 10) of the macrocytic, 40% (8 of 20) normocytic, and 38% (10 of 26) of the microcytic subjects.
Because the age of patients with microcytic anemia was significantly younger, and their seropositivity to H pylori higher than of all other groups, we wished to explore the effect of age on the presenting features of autoimmune gastritis as expressed in various laboratory measurements (Table 3).
Effect of age on presenting features of autoimmune gastritis
Age, y . | No. . | Sex, M/F . | Hemoglobin level, g/L . | MCV, fl . | Serum iron level, μM . | TIBC, μM . | Ferritin level, μg/L . | B12 level, pM . | Gastrin level, pM . | H pylori, no. positive/total (%) . |
|---|---|---|---|---|---|---|---|---|---|---|
| Younger than 20 | 8 | 2/6 | 96 ± 18 (9.6 ± 1.8 g/dL) | 67.6 ± 9.0 | 6.1 ± 3.2 (33.8 ± 18.1 μg/dL) | 56.7 ± 3.62 (317.0 ± 20.2 μg/dL) | 3.5 ± 2.2 | 289.1 ± 132.0 (391.9 ± 178.9 pg/mL) | 166.6 ± 118 (349.3 ± 247.4 pg/mL) | 7/8 (87.5) |
| 20-40 | 40 | 7/33 | 101 ± 21 (10.1 ± 2.1 g/dL) | 78.1 ± 15.2 | 6.9 ± 6.3 (38.6 ± 35.2 μg/dL) | 60.3 ± 8.5 (337.0 ± 47.7 μg/dL) | 9.9 ± 16.6 | 140.0 ± 87.4 (189.6 ± 118.4 pg/mL) | 222.8 ± 156.2 (467.1 ± 327.5 pg/mL) | 16/34 (47.1) |
| 41-60 | 58 | 15/43 | 106 ± 24 (10.6 ± 2.4 g/dL) | 81.8 ± 16.9 | 8.7 ± 6.5 (48.4 ± 36.3 μg/dL) | 60.0 ± 9.1 (333.8 ± 50.9 μg/dL) | 17.2 ± 22.3 | 116.8 ± 58.4 (158.3 ± 79.2 pg/mL) | 310.4 ± 224.3 (650.7 ± 470.2 pg/mL) | 18/48 (37.5) |
| Older than 60 | 54 | 28/26 | 115 ± 25 (11.5 ± 2.5 g/dL) | 95.0 ± 16.0 | 12.5 ± 6.3 (69.8 ± 35.1 μg/dL) | 54.3 ± 10.6 (303.6 ± 59.0 μg/dL) | 36.8 ± 40.5 | 79.9 ± 48.3 (108.3 ± 65.4 pg/mL) | 381.6 ± 299.2 (800.0 ± 627.3 pg/mL) | 4/32 (12.5) |
Age, y . | No. . | Sex, M/F . | Hemoglobin level, g/L . | MCV, fl . | Serum iron level, μM . | TIBC, μM . | Ferritin level, μg/L . | B12 level, pM . | Gastrin level, pM . | H pylori, no. positive/total (%) . |
|---|---|---|---|---|---|---|---|---|---|---|
| Younger than 20 | 8 | 2/6 | 96 ± 18 (9.6 ± 1.8 g/dL) | 67.6 ± 9.0 | 6.1 ± 3.2 (33.8 ± 18.1 μg/dL) | 56.7 ± 3.62 (317.0 ± 20.2 μg/dL) | 3.5 ± 2.2 | 289.1 ± 132.0 (391.9 ± 178.9 pg/mL) | 166.6 ± 118 (349.3 ± 247.4 pg/mL) | 7/8 (87.5) |
| 20-40 | 40 | 7/33 | 101 ± 21 (10.1 ± 2.1 g/dL) | 78.1 ± 15.2 | 6.9 ± 6.3 (38.6 ± 35.2 μg/dL) | 60.3 ± 8.5 (337.0 ± 47.7 μg/dL) | 9.9 ± 16.6 | 140.0 ± 87.4 (189.6 ± 118.4 pg/mL) | 222.8 ± 156.2 (467.1 ± 327.5 pg/mL) | 16/34 (47.1) |
| 41-60 | 58 | 15/43 | 106 ± 24 (10.6 ± 2.4 g/dL) | 81.8 ± 16.9 | 8.7 ± 6.5 (48.4 ± 36.3 μg/dL) | 60.0 ± 9.1 (333.8 ± 50.9 μg/dL) | 17.2 ± 22.3 | 116.8 ± 58.4 (158.3 ± 79.2 pg/mL) | 310.4 ± 224.3 (650.7 ± 470.2 pg/mL) | 18/48 (37.5) |
| Older than 60 | 54 | 28/26 | 115 ± 25 (11.5 ± 2.5 g/dL) | 95.0 ± 16.0 | 12.5 ± 6.3 (69.8 ± 35.1 μg/dL) | 54.3 ± 10.6 (303.6 ± 59.0 μg/dL) | 36.8 ± 40.5 | 79.9 ± 48.3 (108.3 ± 65.4 pg/mL) | 381.6 ± 299.2 (800.0 ± 627.3 pg/mL) | 4/32 (12.5) |
Data are mean ± 1 SD.
Stratifying our patients according to age groups ranging from younger than 20 years to over 60 years of age revealed a strong and steadily progressive effect regarding all laboratory parameters. Starting with the youngest age group of less than 20 years and ending with the oldest, there was a decrease with increasing age in serum Cbl level from 392 ± 179 to 108 ± 65 pg/mL (P < .001), a decrease in H pylori positivity from 87% to 12.5% (χ2 < 0.0001), and increase in serum gastrin concentration from 166 ± 118 to 376 ± 299 pM (349 ± 247 to 800.0 ± 627 pg/mL; P = .028). Conversely, there was a progressive decrease with decreasing age in levels of serum iron (P = .006), serum ferritin (P < .001), and MCV (P < .001) and in the proportion of men, which was 52% in the oldest age group and 19% in patients younger than 40 years of age (χ2 < 0.0001).
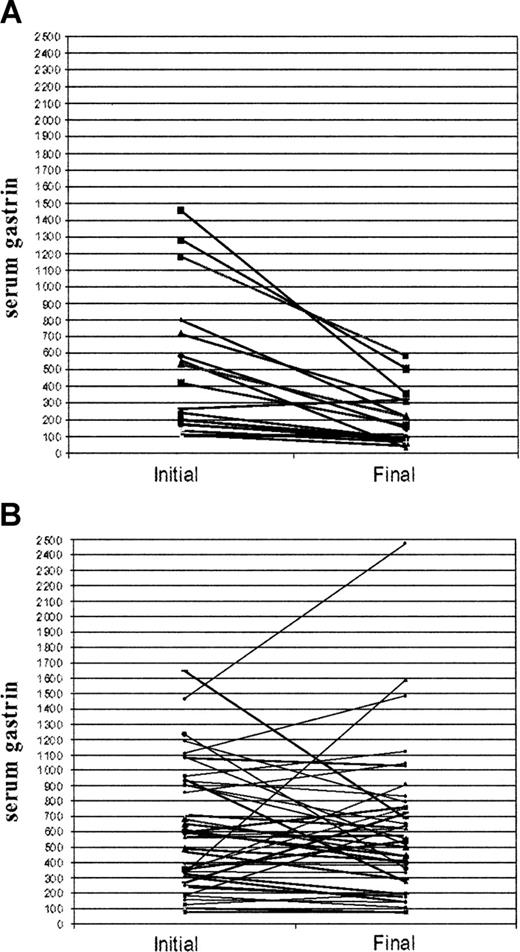
Of the 45 patients positive for H pylori infection (positive serology confirmed by urease breath test) 33 completed triple therapy for H pylori eradication. Of these, 24 had successful eradication documented by negative repeat urease test, 3 were failures, and in 6 results are unavailable. The results of H pylori eradication in the 24 patients responding to triple therapy are shown in Figure 1A-B.
Treatment resulted in a decrease of serum gastrin level from 224 ± 187 to 104 ± 105 pM (476 ± 391 to 218 ± 220 pg/mL; paired t test, P = .001) and a parallel decrease in serum H pylori IgG antibodies from 4.1 ± 3.0 to 1.8 ± 1.5 U/mL (paired t test, P = .004) after a follow-up period of 19 ± 12 months. By contrast, no spontaneous change in serum gastrin concentration was encountered in 36 matched untreated patients seronegative for H pylori after a follow-up period of 17 ± 15 months: 293 ± 198 pM (615 ± 397 pg/mL) initial compared with 287 ± 221 pM (602 ± 470 pg/mL) final (paired t test, P = .843). As a rule, the effect of H pylori eradication on the intensity of APCA titers was unimpressive. However, in 2 of our patients, in addition to a sharp decrease in serum gastrin, APCA titers decreased from 1:640 to 1:20 and from 1:1280 to 1:40 28 and 23 months, respectively, following H pylori eradication. Likewise, complete clinical remission has been achieved following H pylori eradication in the 2 patients presenting with gastric MALT lymphoma.
Serum gastrin levels related to H pylori eradication. Serum gastrin (pg/mL) in 24 H pylori–positive patients before H pylori eradication and after a follow-up period of 19 ± 12 months (A) and in 36 untreated H pylori–negative patients after a follow-up period of 17 ± 15 months (B).
Serum gastrin levels related to H pylori eradication. Serum gastrin (pg/mL) in 24 H pylori–positive patients before H pylori eradication and after a follow-up period of 19 ± 12 months (A) and in 36 untreated H pylori–negative patients after a follow-up period of 17 ± 15 months (B).
Discussion
Our data indicate that iron deficiency of various degrees is a common finding at presentation of autoimmune gastritis. Of the 160 patients diagnosed as having autoimmune gastritis by the combined presence of hypergastrinemia and strongly positive APCAs, 83 (52%) presented with IDA manifested in low serum ferritin levels, low transferrin saturations, and microcytic anemia. Normocytic red blood cell indices were found at presentation in 48 (30%) patients of whom 24 (50%) had coexistent iron deficiency indicated by abnormal transferrin saturation and serum ferritin concentration. By contrast, among the 29 patients presenting with macrocytosis, iron deficiency was encountered in only 3. Using the definitions for the diagnosis of PA (see “Patients, materials, and methods”), 100% of the subjects presenting with macrocytosis and a low serum vitamin B12 level in combination with hypergastrinemia and APCAs had definite or probable PA, compared with 54% of the normocytic, and 27% of the microcytic groups.
In a previous prospective study of refractory IDA we have shown that autoimmune atrophic gastritis manifested in hypergastrinemia and strongly positive anti–parietal serum antibodies existed in 40 or 27% of the 150 patients with IDA studied.5 In the present study including the previous 40 patients but extended to 160 patients with autoimmune atrophic gastritis, IDA was the presenting feature in 52% of all patients, implying that it is the most common hematologic presentation of autoimmune gastritis. This is a conservative estimate because prior to 2001 we were unaware of IDA as a possible presenting feature of atrophic gastritis. Consequently, among the 33 patients with Cbl deficiency and atrophic gastritis studied before 2001 and included in the present study, only 3 were identified as having IDA.
Despite the common feature of autoimmune gastritis in all 160 subjects, patients presenting with IDA are distinguished from those presenting with classic PA characterized by Cbl deficiency and macrocytosis, by a number of features. They are about 20 years younger, they are predominantly women (Table 1), and they are more likely to have active H pylori infection as evidenced by positive serology and results of the urease breath test. Histologically, although they do have a high proportion with atrophic gastritis, active chronic inflammation is nearly 4 times more common in patients with IDA than in classic PA patients presenting with macrocytosis, likely reflecting their higher rate of active H pylori infection.
On the other hand, patients with autoimmune gastritis presenting with IDA have many features overlapping or identical with those of classic PA. They have an 18% prevalence of thyroid disease and 8% of diabetes mellitus, diseases well known for their association with PA as part of the autoimmune polyendocrine syndromes.13 Patients with IDA and autoimmune gastritis have not only a 46% prevalence of abnormal serum Cbl levels, but also a 41% prevalence of atrophic gastritis documented by endoscopic biopsy. Finally, seropositivity in patients with IDA for intrinsic factor blocking antibodies, a low sensitivity but high specificity test for PA was 38%, similar to the other 2 subgroups in the present study. The apparently lower rate of anti–intrinsic factor seropositivity in macrocytic anemia is in all likelihood a sampling error caused by the limited number of observations.
In view of these characteristics of IDA associated with atrophic gastritis, the question should be raised whether indeed we may be witnessing an earlier phase in the evolution of one and the same disease. A definitive answer to this question would require a 20-year follow up of the IDA cohort with autoimmune gastritis. An alternative, although less specific, approach would be to stratify the entire population of 160 patients by age (Table 3). Such stratification reveals a remarkable, regular correlation between age and the presenting hematologic complications of autoimmune gastritis. With advancing age of presentation, there was a progressive increase in MCV and increase in the severity of hypergastrinemia and the severity of Cbl deficiency. Iron deficiency, and active H pylori infection, both of which were very common in the youngest age group were gradually replaced by Cbl deficiency and H pylori seronegativity, most pronounced in the oldest age group. The concept of IDA progressing to classic PA was first introduced in 1966 by Dagg et al,14 who demonstrated a 19% prevalence of histamine-fast achlorhydria and APCAs in patients with IDA, coined the term “latent pernicious anemia,” and predicted that 32% of such patients will develop PA.
The high prevalence of H pylori positivity in young patients with autoimmune gastritis and its almost total absence in elderly patients with PA raises the question whether H pylori gastritis may represent an early phase of disease in which an infectious process is gradually replaced by an autoimmune disease terminating in burned-out infection and the irreversible destruction of gastric body mucosa. Although this question has long intrigued investigators, the relation between H pylori and the pathogenesis of PA is still unsettled.15 H pylori–infected subjects have circulating IgG antibodies directed against epitopes on gastric mucosal cells. Of these, the H+K+-ATPase protein, the most common autoantigen in PA, is the most likely target of an autoimmune mechanism triggered by H pylori and directed against gastric parietal cells by means of antigenic mimicry.16-22 Conversely, H pylori eradication in patients with autoimmune atrophic gastritis is followed by improved gastric acid and ascorbate secretion in many, and complete remission of atrophic gastritis in a variable proportion of patients.9,23,24 In the present study, H pylori eradication was followed by a significant decrease in serum gastrin levels in almost all patients (Figure 1), likely reflecting improved acid secretion. In our previous study of IDA5 we have also shown that H pylori eradication results in improved therapeutic response in all patients previously refractory to oral iron. However, complete serologic remission manifested in disappearance of circulating APCA was only observed in 2 of 24 patients. Failure to achieve complete remission by H pylori eradication in the majority of patients does not necessarily argue against the role of H pylori in the pathogenesis of autoimmune gastritis but, more likely indicates that a point of no return may be reached beyond which the autoimmune process may no longer require the continued presence of the inducing pathogen.
The dual importance of normal gastric secretion for both iron and Cbl absorption has been an integral part of PA research from its earliest days. The landmark studies of Minot and Murphy on the cure of PA by a meat and liver diet were based on a fortuitous misinterpretation of Whipple's report on the efficiency of such diet promoting hemoglobin regeneration in dogs made anemic by venesection,25 most likely representing iron deficiency and not Cbl depletion. Likewise, the seminal observations of Faber and Bloch26 on gastric atrophy involving the proximal two thirds of the stomach are recognized not only as a major insight into the pathogenesis of PA, but also as a characteristic feature of achylia gastrica, a syndrome of chronic IDA associated with gastric atrophy first described by the same author in 190927 and recognized subsequently as a major cause of IDA.28,29 Achylia gastrica was largely forgotten and completely ignored in subsequent major surveys of gastrointestinal causes of IDA.30,31 More recently, achlorhydric gastric atrophy has been rediscovered by Dickey et al32 and implicated in 20% of patients with IDA with no evidence of gastrointestinal blood loss. This observation was confirmed and greatly extended in a series of important studies by Annibale et al7-10 who found 27% of patients with refractory IDA without gastrointestinal symptoms to have atrophic body gastritis, a percentage identical with the proportion of subjects with autoimmune gastritis found in our previous studies.5 Impaired iron absorption in PA is corrected by normal, but not by neutralized, gastric juice—indicating that lack of gastric acidity is the key factor in abnormal iron absorption.33 Other studies have also shown that iron absorption is heavily dependent on normal gastric secretion and acidity for solubilizing and reducing dietary iron.34,35
If autoimmune atrophic gastritis impairs both food iron and food Cbl absorption, what are the factors determining its clinical presentation in the form of microcytic IDA or macrocytic megaloblastic anemia? Age, sex, and duration and severity of disease may all be important in this respect. In addition, coexistent H pylori gastritis per se may have been a factor contributing to the development of IDA.6,9,10 It is generally believed that achlorhydric gastric atrophy may precede by many years the clinical onset of pernicious anemia. As shown in the present study and in the study of Marignani et al,11 compared with macrocytic anemia, patients with atrophic body gastritis presenting with microcytic anemia are about 20 years younger and almost exclusively women. These features imply that menstrual blood loss may have been an important primary factor in the development of iron deficiency, aggravated by the inability to compensate by improving food iron absorption. Conversely, depletion of Cbl stores may take many years longer, implies a more severe impairment of intrinsic factor secretion manifested in an abnormal Schilling test, and equally involves men and women. Thus, although atrophic gastritis may impair both Cbl and iron absorption simultaneously, in fertile women in whom menstruation represents an added strain on iron requirements, iron deficiency will develop many years before the depletion of vitamin B12 stores. It is, however, the crucial loss of the remaining gastric intrinsic factor that may only develop in a proportion of patients with autoimmune gastritis that determines the prevalence of PA.
Prepublished online as Blood First Edition Paper, October 20, 2005; DOI 10.1182/blood-2005-09-3534.
An Inside Blood analysis of this article appears at the front of this issue.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
The outstanding skills and devotion of Edna Assis and Nicole Adar in the processing and recording of data are gratefully acknowledged.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal