Donor lymphocyte infusions (DLIs) induce potent graft versus tumor (GVT) effects for relapsed chronic myelogenous leukemia (CML) after allogeneic stem cell transplantation (SCT) but are disappointing for other diseases. Disease resistance can occur if donor T cells are not appropriately activated in vivo. Ex vivo T-cell activation might overcome disease-induced anergy and augment GVT activity. We performed a phase 1 trial of ex vivo–activated DLI (aDLI) for 18 patients with relapse after SCT. Activated donor T cells are produced through costimulation with anti-CD3– and anti-CD28–coated beads. Patients with aggressive malignancies received induction chemotherapy, and all patients received conventional DLI (median, 1.5 × 108 mononuclear cells/kg) followed 12 days later by aDLI. Activated DLI was dose escalated from 1 × 106 to 1 × 108 CD3+ cells per kilogram in 5 levels. Seven patients developed acute graft versus host disease (GVHD) (5 grade I-II, 2 grade III), and 4 developed chronic GVHD. Eight patients achieved complete remission, including 4 of 7 with acute lymphocytic leukemia (ALL), 2 of 4 with acute myelogenous leukemia (AML), 1 with chronic lymphocytic leukemia (CLL), and 1 of 2 with non-Hodgkin lymphoma (NHL). Four complete responders relapsed while 4 remain alive in remission a median 23 months after aDLI. Overall, 10 of 18 remain alive 11 to 53 months after aDLI. Adoptive transfer of costimulated activated allogeneic T cells is feasible, does not result in excessive GVHD, and may contribute to durable remissions in diseases where conventional DLI has been disappointing.
Introduction
The success of allogeneic stem cell transplantation (SCT) is dependent not only on the conditioning therapy but also on the graft versus tumor (GVT) properties of the donor graft. Unfortunately, many patients will relapse after SCT and have limited treatment options. Second SCT may cure some relapsed patients but at the expense of extensive morbidity and mortality.1-3 Donor lymphocyte infusions (DLIs) can induce a direct and potent GVT effect for some patients who relapse after allogeneic SCT and are particularly effective for relapsed chronic-phase chronic myelogenous leukemia (CML).4-7 Response rates to DLI for patients with relapsed acute leukemia (acute myelogenous leukemia [AML] or acute lymphocytic leukemia [ALL]) or advanced-phase CML have been disappointing to date. For patients with AML, response rates to DLI vary from 15% to 30%,5,6 and many of these remissions are transient.8 The high tumor burden and rapid proliferation of leukemic blasts often limit the response to DLI; because GVT effects may be delayed, there is significant disease-related mortality after DLI but before the antileukemia effect is manifested. Recently, a prospective trial of DLI was performed using pre-DLI induction chemotherapy to limit the leukemia burden in patients with relapsed AML after allogeneic SCT.9 Although 42% of patients in this trial achieved a complete remission (CR), only 14% were in continuous CR at a median of 29 months after DLI. These results suggested that pretreatment with chemotherapy increased the CR rate and that AML could be sensitive to GVT induction but that more effective therapies are clearly needed. Similarly, patients with ALL respond poorly to conventional DLI, with reported remission rates between 0% and 18%.5,6,10 In one large retrospective analysis, the 3-year probability of survival for 44 recipients of DLI for ALL was 13%, and only 2 of 44 patients remained in CR 2 years after DLI.10 Notably, however, while response rates are low, durable remissions are possible, and one of the earliest recipients of DLI was treated for relapsed ALL with a sustained remission for more than 8 years at the time of last report.11 There are limited data regarding efficacy of DLI for patients with other hematologic malignancies such as myeloma, non-Hodgkin and Hodgkin lymphoma, and myelodysplastic syndrome (MDS), but overall response rates have been disappointing, ranging from 0% to 50%.12 Clearly, more effective approaches to relapsed disease (other than early-phase CML) are needed.
While GVT induction seems to be disease specific, the actual mechanisms for disease resistance are not known. It is possible that donor T cells are not appropriately activated in vivo to induce an antitumor response. Activation of T cells requires 2 signals: engagement of the T-cell receptor (TCR) and a second, costimulatory signal. This second signal, when combined with primary antigen-dependent stimulation of the TCR, is required for the T cell to maximally synthesize and secrete cytokine and divide in response to antigen. The major positive costimulatory receptor on T cells is CD28, and its ligands are the B7 family of molecules CD80 and CD86, which are abundantly expressed on activated antigen-presenting cells (APCs). T-cell costimulation is critical for induction of full T-cell effector function and therefore represents an attractive immunotherapeutic approach for treatment of cancer and may maximize GVT effects of allogeneic donor T cells. Inadequate T-cell activation could occur for many reasons, including lack of costimulatory ligands on tumor cells, failure to present antigens to T cells, direct suppression of cytotoxic effector cells by suppressor T cells or cytokines, failure to stimulate CD4+ cells, or quantitative lack of sufficient cytotoxic effector cells.
We hypothesized that ex vivo costimulation of T cells via CD3 and CD28 can produce activated T cells that can overcome disease-induced anergy, preserve and augment CD4 function, and enhance GVT activity. Activated donor T cells are produced by costimulation and expansion following exposure to magnetic beads coated with anti-CD3 (OKT3) and anti-CD28.13 In the setting of autologous SCT, administration of ex vivo–costimulated T cells can reverse both in vivo and in vitro functional T-cell defects in patients with lymphoma.14 To explore the feasibility and toxicity of adoptive immunotherapy with expanded activated allogeneic T cells, we performed a phase 1 trial of DLI followed by escalating doses of ex vivo–costimulated donor T cells (activated DLI [aDLI]) for patients with relapse of diseases other than chronic-phase CML after allogeneic SCT. Activated DLI in this trial has been well tolerated without excessive toxicity, and response rates are impressive for diseases that historically have not responded well to conventional DLI.
Patients, materials, and methods
Eligibility criteria
Patients with relapsed disease (except for chronic-phase CML) after allogeneic SCT from an HLA-matched sibling were eligible for this study. Patients with relapsed CML were eligible only if they had advanced disease (accelerated or blast phase) at relapse. Prior to DLI, patients could not have active acute graft versus host disease (GVHD) above grade I, active chronic GVHD, and could not require active immunosuppression to control GVHD. The study was approved by the institutional review boards of the Hospital of the University of Pennsylvania and Children's Hospital of Philadelphia and was conducted under a Food and Drug Administration (FDA)–approved Investigational New Drug Application. Voluntary written informed consent was obtained from all patients and donors or from an appropriate guardian in the case of a minor.
Study design
Patients receiving immunosuppression at the time of relapse had therapy rapidly tapered or discontinued with the intent to observe for GVHD and GVT for approximately 4 weeks. However, 2 patients with acute leukemia began induction chemotherapy within 2 weeks of stopping immunosuppression due to rapid progression of disease. Patients who experienced above grade I acute GVHD or a GVT response after discontinuing immunosuppression would not receive DLI.
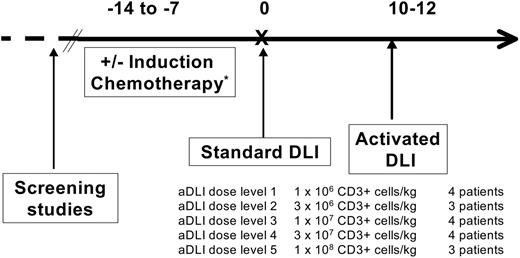
This pilot study was designed as a phase 1 dose escalation trial of aDLI focusing on feasibility and safety as the primary end points. The treatment regimen is shown in Figure 1. Patients with “aggressive malignancies,” defined as relapsed AML, ALL, lymphoblastic lymphoma, or blast-phase CML (more than 30% blasts in the blood and/or marrow), were treated with conventional induction chemotherapy 7 to 14 days before DLI9 ; any standard induction regimen for leukemia was acceptable and was determined by the patient's past therapy and clinical condition.
Donor leukocyte infusions
All patients received conventional, unstimulated DLI prior to receiving aDLI. The original stem-cell donor underwent large-volume (10 to 15 L) leukapheresis for mononuclear-cell collection on 1 or 2 sequential days. A target goal of 1 × 108 to 2 × 108 mononuclear cells per kilogram of recipient body weight was administered on the day of collection to all patients (day 0) as conventional DLI; mononuclear-cell and CD3+ cell doses are shown in Tables 1, 2. The median actual dose of unstimulated DLI administered was 1.5 × 108 CD3+ cells per kilogram (range, 0.9 × 108 to 3.5 × 108 cells/kg). For patients who received induction chemotherapy, donor leukocytes were collected and administered at the hematologic nadir, 10 to 12 days after chemotherapy (Figure 1). The DLI product served in all cases as the source of cells for CD3+/CD28+ T-cell expansion.
Patient characteristics
Characteristic . | Value . |
|---|---|
| Diagnosis, no. of patients | |
| ALL | 7 |
| AML | 4 |
| CML-BC | 1 |
| CLL | 1 |
| NHL | 2 |
| HD | 1 |
| Myeloma | 1 |
| LL | 1 |
| Median age, y (range) | 46 (12-57) |
| Sex, male/female | 12/6 |
| Median donor age, y (range) | 42 (10-62) |
| Sex-mismatched donors, no. | 6 |
| Graft source of original BMT, no. of patients | |
| Bone marrow | 13 |
| Peripheral blood stem cells | 5 |
| Conditioning regimen intensity of original BMT, no. of patients | |
| Myeloablative | 15 |
| Nonmyeloablative | 3 |
| Conditioning regimen of original BMT, no. of patients | |
| TBI based* | 12 |
| Bu/Cy | 3 |
| Flu/Cy | 3 |
| Induction chemotherapy used prior to DLI†, no. of patients | |
| Ida/AraC | 3 |
| CVAD | 3 |
| Mito/AraC | 2 |
| Flu/AraC | 2 |
| DVP, Asp | 1 |
| Ida | 1 |
| ICE | 1 |
| Mylotarg | 1 |
| Time from BMT to relapse, mo (range) | 11.5 (2-90) |
| Time from relapse to DLI, wk (range) | 6 (2-30) |
| Median DLI-unstimulated mononuclear cells, × 108 (range) | 1.5 (0.9-3.5) |
Characteristic . | Value . |
|---|---|
| Diagnosis, no. of patients | |
| ALL | 7 |
| AML | 4 |
| CML-BC | 1 |
| CLL | 1 |
| NHL | 2 |
| HD | 1 |
| Myeloma | 1 |
| LL | 1 |
| Median age, y (range) | 46 (12-57) |
| Sex, male/female | 12/6 |
| Median donor age, y (range) | 42 (10-62) |
| Sex-mismatched donors, no. | 6 |
| Graft source of original BMT, no. of patients | |
| Bone marrow | 13 |
| Peripheral blood stem cells | 5 |
| Conditioning regimen intensity of original BMT, no. of patients | |
| Myeloablative | 15 |
| Nonmyeloablative | 3 |
| Conditioning regimen of original BMT, no. of patients | |
| TBI based* | 12 |
| Bu/Cy | 3 |
| Flu/Cy | 3 |
| Induction chemotherapy used prior to DLI†, no. of patients | |
| Ida/AraC | 3 |
| CVAD | 3 |
| Mito/AraC | 2 |
| Flu/AraC | 2 |
| DVP, Asp | 1 |
| Ida | 1 |
| ICE | 1 |
| Mylotarg | 1 |
| Time from BMT to relapse, mo (range) | 11.5 (2-90) |
| Time from relapse to DLI, wk (range) | 6 (2-30) |
| Median DLI-unstimulated mononuclear cells, × 108 (range) | 1.5 (0.9-3.5) |
HD indicates Hodgkin disease; LL, lymphoblastic lymphoma; TBI, total body irradiation; Bu, busulfan; Cy, cyclophosphamide; Ida, idarubicin; AraC, cytosine arabinoside; CVAD, cyclophosphamide, vincristine, adriamycin, dexamethosone; Mito, mitoxantrone; Flu, fludarabine; DVP, daunarubicin, vincristine, prednisone; Asp, asparaginase; and ICE, ifosphamide, carboplatinum, VP16 (etoposide).
With either Cy, VP16, thiotepa/Cy, or thiotepa/Cy/VP16
For patients with AML, ALL, CML-BC, and LL; n = 14
Patient outcomes
UPN . | Age at DLI, y . | Diagnosis . | Time from BMT to relapse, mo . | Time from relapse to DLI, wk . | DLI dose, MNCs × 108/kg . | aDLI dose, CD3+ cells × 106/kg . | Maximal grade of aGVHD . | Maximal response and duration . | Current status . | Time from DLI to last follow-up, mo . |
|---|---|---|---|---|---|---|---|---|---|---|
| 4799-03 | 54 | CLL | 45 | 6 | 3.5 | 1.0 | II | CR, 53+ mo | Alive in CR | 53+ |
| 649-02 | 19 | ALL | 2 | 3 | 1.0 | 1.0 | NE | NE | Died, disease | 0.8 |
| 4799-05 | 57 | AML | 90 | 2 | 1.5 | 1.0 | I | CR, 40 mo | Relapse after CR, alive with disease | 49+ |
| 649-03 | 16 | ALL (Ph+) | 24 | 24 | 1.0 | 1.0 | 0 | NR | Died, disease | 2.6 |
| 4799-07 | 44 | ALL | 5 | 5 | 0.9 | 3.0 | II | CR, 7 mo | Relapse after CR, died, disease | 8.2 |
| 4799-08 | 52 | NHL | 20 | 6 | 1.0 | 3.0 | 0 | CR, 35+ mo | Alive in CR | 35+ |
| 4799-11 | 45 | Myeloma | 5 | 5 | 1.6 | 3.0 | I | NR | Died, disease | 13.8 |
| 4799-09 | 53 | AML | 2 | 3 | 2.4 | 10.0 | III | PR, 6 mo | Died, disease | 6.7 |
| 4799-13 | 32 | ALL | 10 | 9 | 1.5 | 10 | 0 | CR, 22 mo | Relapse after CR, alive with disease | 24+ |
| 4799-14 | 28 | LL | 3 | 2 | 1.8 | 10 | III | NR | Died, disease | 3.0 |
| 4799-15 | 36 | CML-BC | 13 | 2 | 1.6 | 10 | 0 | NR | Died, disease | 3.6 |
| 649-04 | 12 | ALL (Ph+) | 8 | 6 | 1.1 | 30 | 0 | CR, 16 mo | Relapse after CR, alive with disease | 16+ |
| 4799-16 | 54 | HD | 38 | 6 | 1.5 | 30 | 0 | NR | Alive with disease | 11+ |
| 4799-18 | 49 | NHL | 29 | 9 | 1.5 | 30 | 0 | NR | Alive with disease | 13+ |
| 4799-20 | 36 | AML | 83 | 8 | 1.0 | 30 | 0 | NR | Died, disease | 4 |
| 4799-21 | 43 | ALL | 4 | 31 | 1.3 | 100 | 0 | SD, 13 mo | Alive with disease | 13+ |
| 4799-23 | 46 | ALL | 19 | 10 | 2.0 | 100 | 0 | CR, 11+ mo | Alive in CR (PCR-) | 11+ |
| 4799-24 | 46 | APML | 5 | 3 | 2.2 | 100 | I | CR, 12+ mo | Alive in CR (PCR-) | 12+ |
UPN . | Age at DLI, y . | Diagnosis . | Time from BMT to relapse, mo . | Time from relapse to DLI, wk . | DLI dose, MNCs × 108/kg . | aDLI dose, CD3+ cells × 106/kg . | Maximal grade of aGVHD . | Maximal response and duration . | Current status . | Time from DLI to last follow-up, mo . |
|---|---|---|---|---|---|---|---|---|---|---|
| 4799-03 | 54 | CLL | 45 | 6 | 3.5 | 1.0 | II | CR, 53+ mo | Alive in CR | 53+ |
| 649-02 | 19 | ALL | 2 | 3 | 1.0 | 1.0 | NE | NE | Died, disease | 0.8 |
| 4799-05 | 57 | AML | 90 | 2 | 1.5 | 1.0 | I | CR, 40 mo | Relapse after CR, alive with disease | 49+ |
| 649-03 | 16 | ALL (Ph+) | 24 | 24 | 1.0 | 1.0 | 0 | NR | Died, disease | 2.6 |
| 4799-07 | 44 | ALL | 5 | 5 | 0.9 | 3.0 | II | CR, 7 mo | Relapse after CR, died, disease | 8.2 |
| 4799-08 | 52 | NHL | 20 | 6 | 1.0 | 3.0 | 0 | CR, 35+ mo | Alive in CR | 35+ |
| 4799-11 | 45 | Myeloma | 5 | 5 | 1.6 | 3.0 | I | NR | Died, disease | 13.8 |
| 4799-09 | 53 | AML | 2 | 3 | 2.4 | 10.0 | III | PR, 6 mo | Died, disease | 6.7 |
| 4799-13 | 32 | ALL | 10 | 9 | 1.5 | 10 | 0 | CR, 22 mo | Relapse after CR, alive with disease | 24+ |
| 4799-14 | 28 | LL | 3 | 2 | 1.8 | 10 | III | NR | Died, disease | 3.0 |
| 4799-15 | 36 | CML-BC | 13 | 2 | 1.6 | 10 | 0 | NR | Died, disease | 3.6 |
| 649-04 | 12 | ALL (Ph+) | 8 | 6 | 1.1 | 30 | 0 | CR, 16 mo | Relapse after CR, alive with disease | 16+ |
| 4799-16 | 54 | HD | 38 | 6 | 1.5 | 30 | 0 | NR | Alive with disease | 11+ |
| 4799-18 | 49 | NHL | 29 | 9 | 1.5 | 30 | 0 | NR | Alive with disease | 13+ |
| 4799-20 | 36 | AML | 83 | 8 | 1.0 | 30 | 0 | NR | Died, disease | 4 |
| 4799-21 | 43 | ALL | 4 | 31 | 1.3 | 100 | 0 | SD, 13 mo | Alive with disease | 13+ |
| 4799-23 | 46 | ALL | 19 | 10 | 2.0 | 100 | 0 | CR, 11+ mo | Alive in CR (PCR-) | 11+ |
| 4799-24 | 46 | APML | 5 | 3 | 2.2 | 100 | I | CR, 12+ mo | Alive in CR (PCR-) | 12+ |
UPN indicates unique patient number; MNCs, mononuclear cells; aGVHD, acute GVHD; CR, complete remission; NE, not evaluable; NR, no response; PR, partial response; LL, lymphoblastic lymphoma; CML-BC, chronic myelogenous leukemia-blast crisis; HD, Hodgkin disease; SD, stable disease; APML, acute promyelocytic leukemia; and PCR, polymerase chain reaction.
Ex vivo costimulation and expansion of donor T cells
An aliquot of cells from the donor leukocyte product collected on the first or second day of leukapheresis was removed prior to DLI for ex vivo expansion. The washed apheresis product was enriched for lymphocytes using magnetic bead depletion of monocytes in a closed system if monocytes constituted more than 20% of white blood cells (WBCs) as gated on a Coulter Multisizer3 (Beckman Coulter, Fullerton, CA). T cells were processed in a manner consistent with appropriate FDA guidelines and regulations on Good Manufacturing Practices as previously described, with the exception that CD8+ T cells and CD20+ B cells were not removed from the starting culture.14,15
The cells were seeded into gas-permeable flasks (Baxter Oncology, Deerfield, IL) containing X VIVO 15 (Cambrex, Walkersville, MD) supplemented with 5% normal human AB serum (Valley Biomedical, Winchester, VA), 2 mM l-glutamine (Cambrex), and 20 mM HEPES (Cambrex). Magnetic beads (Dynal, Brown Deer, WI) with conjugated anti-CD3 (OKT3; Ortho Biotech, Bridgewater, NJ) and anti-CD28 (clone 9.3) monoclonal antibodies were added at a 3:1 bead/CD3+ cell ratio, and the cultures were maintained for up to 12 days prior to harvest and preparation for infusion. After completion of cell culture, the magnetic beads were removed using a Baxter Fenwal Maxsep magnetic cell separation system, and the cells were washed, concentrated, and resuspended in 100 to 250 mL PlasmaLyte A (Baxter Oncology)/5% dextrose 0.45% NaCl containing 1% human serum albumin (Baxter Oncology). All infused T-cell products were required to meet release criteria specified for T-cell phenotype, cell viability, pyrogenicity, sterility, and freedom from bead contamination.
The ex vivo–expanded and –activated cells were infused approximately 12 days after standard DLI as aDLI (Figure 1). Activated DLI was dose escalated based on CD3+ cell number between sequential groups of patients as shown in Figure 1. Five dose levels of activated cells were tested ranging from 1 × 106 CD3+ cells per kilogram to 1 × 108 CD3+ cells per kilogram.
The third patient on each dose level was observed for a minimum of 4 weeks before enrolling subsequent patients to ensure severe GVHD did not develop. In some cases 4 patients were treated at a given dose level. Dose-limiting toxicity was defined as any grade 4 nonhematologic toxicity or any grade 4 acute GVHD. The maximum tolerated dose was defined as the dose level resulting in 2 of 6 (33%) patients experiencing dose-limiting toxicity.
Statistical analyses
This phase 1 study was designed to determine the feasibility and safety of administering ex vivo–costimulated and expanded donor lymphocyte infusions to patients with relapsed disease after allogeneic SCT. Characteristics and outcomes are described with summary statistics including median and mean values when appropriate. The probability of survival, progression-free survival, and disease-free survival (for patients achieving CR) were estimated using the Kaplan-Meier method. These analyses were performed with the Statview statistical software package (Abacus Concepts, Berkeley, CA). Data were analyzed as of May 1, 2005.
Results
Patient characteristics
The characteristics of the 18 patients are shown in Table 1. There were 11 male and 7 female patients with a median age of 46 years (range, 12 to 57 years). The median donor age was 42 years (range, 10 to 62 years), and donors were sex matched in 12 cases and mismatched in 6 cases. All but 1 patient received DLI and aDLI (preceded by induction chemotherapy for patients with aggressive leukemia and lymphoma) for overt clinical relapse without treatment other than hydroxyurea to control blood counts prior to study entry. One patient with ALL (patient no. 649-04) was treated with induction chemotherapy and received aDLI 4 weeks after DLI at the time of minimal residual disease rather than at the hematologic nadir.
The median time from transplantation to relapse was 11.5 months (range, 2 to 90 months) and from relapse to DLI was 6 weeks (range, 2 to 30 weeks). Three patients (1 each with non-Hodgkin lymphoma [NHL], Hodgkin disease, and myeloma) received aDLI for relapse after a nonmyeloablative-conditioned transplantation, and all other patients were treated for relapse after a conventional myeloablative allogeneic SCT. The indications for DLI were ALL (n = 7), AML (n = 4), CML–blast phase (n = 1), chronic lymphocytic leukemia (CLL) (n = 1), NHL (n = 2), Hodgkin disease (n = 1), myeloma (n = 1), and lymphoblastic lymphoma (n = 1). Individual patient characteristics and outcomes are shown in Table 2. The 13 patients with ALL, AML, lymphoblastic lymphoma, and CML–blast crisis (CML-BC) received chemotherapy prior to DLI and aDLI.
Ex vivo expansion and administration of donor T cells
Ex vivo cultures of donor lymphocytes were initiated with 50 × 106 to 700 × 106 cells depending on the target dose level. After approximately 10 to 12 days in culture, CD3+ cells were 94.1% ± 1.0% viable by trypan blue dye exclusion. T cells in the cultures expanded a median of 113-fold and consisted of 60.7% CD3+CD4+ cells and 30.4% CD3+CD8+ cells (Table 3). The CD4/CD8 ratio of the starting cell population (3.26 ± 0.67) was maintained in the expanded cells (3.33 ± 0.82). We have previously demonstrated that healthy donor cells expanded in this fashion demonstrate a Th1 cytokine profile, maintain the T-cell Vβ repertoire for at least 60 days, and express high levels of telomerase.13,16,17
Ex vivo expansion of allogeneic T cells
UPN . | Initial CD3, % . | Final CD3, % . | Final CD3+ CD4+, % . | Final CD3+ CD8+, % . | Total cell fold expansion . | CD3+ fold expansion . |
|---|---|---|---|---|---|---|
| 4799-03D | 65.2 | 90.7 | 44.0 | 41.3 | 27.4 | 38.1 |
| 4799-05D | 65.5 | 96.3 | 83.9 | 14.7 | 121.0 | 177.9 |
| 649-02D | 56.2 | 90.3 | 46.0 | 34.8 | 59.2 | 95.1 |
| 649-03D | 56.7 | 98.5 | 29.5 | 70.6 | 82.6 | 143.5 |
| 4799-07D | 50.1 | 93.5 | 66.4 | 25.7 | 49.4 | 92.2 |
| 4799-08D | 61.1 | 95.1 | 84.3 | 10.8 | 30.0 | 46.7 |
| 4799-11D | 54.6 | 95.8 | 50.6 | 37.4 | 28.2 | 49.5 |
| 4799-09D | 51.7 | 95.0 | 72.4 | 26.5 | 69.4 | 127.5 |
| 4799-13D | 50.8 | 84.5 | 49.5 | 28.5 | 43.0 | 71.5 |
| 4799-14D | 39.5 | 98.3 | 45.4 | 51.0 | 40.6 | 101.0 |
| 4799-15D | 68.5 | 85.3 | 52.2 | 31.6 | 422.0 | 525.5 |
| 649-04D | 84.4 | 96.3 | 45.7 | 46.3 | 77.0 | 87.9 |
| 4799-16D | 69.9 | 96.4 | 71.5 | 23.3 | 91.2 | 125.8 |
| 4799-20D | 71.6 | 94.1 | 52.5 | 39.5 | 24.2 | 31.8 |
| 4799-18D | 64.9 | 97.4 | 67.9 | 28.6 | 100.5 | 150.8 |
| 4799-21D | 35.8 | 89.7 | 74.1 | 5.0 | 32.7 | 81.8 |
| 4799-23D | 74.0 | 95.6 | 78.8 | 16.4 | 26.3 | 34.0 |
| 4799-24D | 62.6 | 98.3 | 78.7 | 16.0 | 34.3 | 53.8 |
| Average | 58.9 | 94.0 | 60.7 | 30.4 | 75.5 | 113.0 |
| SE of the mean | 2.9 | 1.0 | 3.9 | 3.8 | 21.5 | 26.3 |
UPN . | Initial CD3, % . | Final CD3, % . | Final CD3+ CD4+, % . | Final CD3+ CD8+, % . | Total cell fold expansion . | CD3+ fold expansion . |
|---|---|---|---|---|---|---|
| 4799-03D | 65.2 | 90.7 | 44.0 | 41.3 | 27.4 | 38.1 |
| 4799-05D | 65.5 | 96.3 | 83.9 | 14.7 | 121.0 | 177.9 |
| 649-02D | 56.2 | 90.3 | 46.0 | 34.8 | 59.2 | 95.1 |
| 649-03D | 56.7 | 98.5 | 29.5 | 70.6 | 82.6 | 143.5 |
| 4799-07D | 50.1 | 93.5 | 66.4 | 25.7 | 49.4 | 92.2 |
| 4799-08D | 61.1 | 95.1 | 84.3 | 10.8 | 30.0 | 46.7 |
| 4799-11D | 54.6 | 95.8 | 50.6 | 37.4 | 28.2 | 49.5 |
| 4799-09D | 51.7 | 95.0 | 72.4 | 26.5 | 69.4 | 127.5 |
| 4799-13D | 50.8 | 84.5 | 49.5 | 28.5 | 43.0 | 71.5 |
| 4799-14D | 39.5 | 98.3 | 45.4 | 51.0 | 40.6 | 101.0 |
| 4799-15D | 68.5 | 85.3 | 52.2 | 31.6 | 422.0 | 525.5 |
| 649-04D | 84.4 | 96.3 | 45.7 | 46.3 | 77.0 | 87.9 |
| 4799-16D | 69.9 | 96.4 | 71.5 | 23.3 | 91.2 | 125.8 |
| 4799-20D | 71.6 | 94.1 | 52.5 | 39.5 | 24.2 | 31.8 |
| 4799-18D | 64.9 | 97.4 | 67.9 | 28.6 | 100.5 | 150.8 |
| 4799-21D | 35.8 | 89.7 | 74.1 | 5.0 | 32.7 | 81.8 |
| 4799-23D | 74.0 | 95.6 | 78.8 | 16.4 | 26.3 | 34.0 |
| 4799-24D | 62.6 | 98.3 | 78.7 | 16.0 | 34.3 | 53.8 |
| Average | 58.9 | 94.0 | 60.7 | 30.4 | 75.5 | 113.0 |
| SE of the mean | 2.9 | 1.0 | 3.9 | 3.8 | 21.5 | 26.3 |
UPN indicates unique patient number.
No severe toxicity was associated with administration of aDLI. Mild infusional toxicity of fevers and chills (grade II) developed in 4 patients at the highest dose levels, but otherwise aDLI was well tolerated. Dose-limiting toxicity was not reached at the tested dose levels.
Response
Individual responses to therapy are shown in Table 2. Eight patients achieved a complete response. This includes 4 of 7 patients with ALL, 2 of 4 patients with AML, 1 patient with CLL, and 1 of 2 patients with NHL (mantle-cell lymphoma). One patient with AML manifested by recurrent extramedullary chloromas had a partial response, and 1 patient with ALL has had stable disease for 13 months after aDLI.
Three patients were treated for relapse after nonmyeloablative allogeneic SCT (patients 4799-11, 4799-16, and 4799-18) for myeloma, NHL, or Hodgkin disease. None of these patients had a complete response to DLI plus aDLI (Table 2).
Of the 17 patients evaluable for response, 11 had relapsed more than 6 months after the original bone marrow transplantation (BMT) and 7 had relapsed within 6 months of transplantation. Six of 11 aDLI recipients who had relapsed more than 6 months after SCT and 2 of 6 who relapsed within 6 months of SCT achieved CR; this difference was not statistically significant (P = .4), though the number of patients studied is quite small. When only the 10 patients evaluable for response with ALL or AML are considered, 2 of 4 patients who relapsed within 6 months of transplantation achieved CR and 4 of 6 recipients of aDLI who relapsed more than 6 months from transplantation achieved CR (P = .6).
Disease-free and overall survival
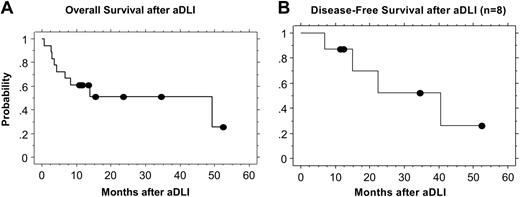
The estimated 2-year overall survival after aDLI for all patients is 51% (Figure 2A). Ten of 18 patients remain alive a median of 16 months after aDLI (range, 11 to 53 months), and 8 have died. Four of the 8 patients who achieved CR remain alive in CR 10 to 52 months after aDLI (median, 23 months), including 1 patient each with NHL, AML, CLL, and ALL. Four of the complete responders subsequently relapsed. Three of these 4 patients had ALL and relapsed after 7-, 16-, and 22-month remissions. One of these patients died of recurrent disease, and 2 remain alive and are undergoing alternate therapy. One patient with AML achieved CR and recently relapsed after a 40-month remission and is alive undergoing alternate therapy.
Treatment scheme for the phase 1 trial of DLI plus activated DLI.*Patients with acute leukemia (AML, ALL) or lymphoblastic lymphoma were pretreated with induction chemotherapy.
Treatment scheme for the phase 1 trial of DLI plus activated DLI.*Patients with acute leukemia (AML, ALL) or lymphoblastic lymphoma were pretreated with induction chemotherapy.
Progression-free survival at 1 and 2 years for the entire cohort was 44% and 23%, respectively. For the subset of 11 patients with AML and ALL, progression-free survival at 1 and 2 years was 45% and 15%, respectively. The estimated 2-year disease-free survival after DLI for the 8 patients who achieved CR is 52% (Figure 2B). Of these 8 patients, 6 received chemotherapy prior to DLI for AML or ALL. The median follow-up in these 6 patients is 13 months (range, 7 to 40 months). Two of these 6 patients remain in remission 11 and 12 months after aDLI. Five patients received DLI and aDLI without induction chemotherapy. One of these 5 patients with CLL remains in CR 53 months after aDLI, and 1 with mantle-cell lymphoma is in CR 35 months after aDLI.
Nine patients had no response (or stable disease) after therapy (Table 2); the diagnoses in these 9 patients were AML (n = 2), NHL (n = 1), ALL (n = 2), CML-BC (n = 1), myeloma (n = 1), lymphoblastic lymphoma (n = 1), and Hodgkin disease (n = 1). Six of these patients have died of progressive disease, and 3 remain alive with disease on alternate therapies. One patient with ALL was not evaluable for response due to early death from progressive disease.
Eight patients have died of persistent or recurrent disease 1 to 14 months after aDLI (median, 4 months) and include 2 patients with AML, 3 with ALL, and 1 each with CML-BC, myeloma, and lymphoblastic lymphoma.
Graft versus host disease
After the original SCT, 14 of the 18 patients experienced no acute GVHD, 2 developed grade I, and 2 patients developed grade III acute GVHD. Six patients experienced chronic GVHD. At the time of DLI no patient had active GVHD and all patients were off immunosuppression for a minimum of 2 weeks (median, 16 weeks; range, 2 to 162 weeks).
After DLI and aDLI, 7 patients developed grade I (n = 3), grade II (skin only, n = 2), and grade III (n = 2) acute GVHD (Table 2). GVHD was not evaluable in 1 patient due to early death. Acute GVHD occurred a median of 28 days after aDLI (range, 16 to 38 days) or 40 days after conventional DLI. The actuarial probability of developing grade II-IV or grade III-IV acute GVHD by day 100 was 33% (standard error, 11%) and 22% (standard error, 10%), respectively. No patient died from complications related to GVHD. Of the 7 patients who developed acute GVHD, 3 remain alive in remission and 4 have died from recurrent or progressive disease. In this small series of patients there was no association with acute GVHD and survival (P = .35).
Chronic GVHD developed in 4 patients and was limited stage in 2 patients and extensive in 2 patients. Three of the 4 patients with chronic GVHD remain alive in remission, and 2 require ongoing immunosuppression for mucosal or skin involvement. The fourth patient died of progressive myeloma 14 months after aDLI. In this limited number of patients there was no association with chronic GVHD and survival (P = .51). Therefore, even at the highest dose level of aDLI, GVHD was not a dose-limiting toxicity.
T-cell recovery after DLI and aDLI
The administration of DLI and aDLI resulted in either no or only a slight increase in CD3 and CD4 cell counts. The median fold increase in CD3+ cell numbers at 1, 2, and 3 months after aDLI was 1.5 (range, 0.33 to 8.3), 2.1 (range, 0.24 to 18.8), and 1.8 (range, 0.26 to 25.0), respectively. The median fold increase in CD4+ cell numbers at 1, 2, and 3 months was 1.7 (range, 0.21 to 10.1), 1.2 (range, 0.30 to 6.4), and 1.2 (range, 0.33 to 19.5), respectively. The change in CD3+ or CD4+ numbers was not significantly different in responders compared with nonresponders (data not shown).
Kaplan-Meier estimates of overall and disease-free survival after aDLI. (A) The estimated overall survival at 2 years for all patients is 51%. (B) The estimated disease-free survival at 2 years for the 8 patients who achieved a CR is 52%.
Kaplan-Meier estimates of overall and disease-free survival after aDLI. (A) The estimated overall survival at 2 years for all patients is 51%. (B) The estimated disease-free survival at 2 years for the 8 patients who achieved a CR is 52%.
Discussion
GVI induction with DLI is dramatically successful for patients with chronic-phase CML who relapse after allogeneic SCT but is disappointing for patients with other hematologic malignancies. The mechanisms for disease specificity of DLI are not known. In some instances, such as in patients with AML and ALL, rapid tumor progression may occur before GVT effects from DLI develop. Induction chemotherapy given before DLI may limit the tumor burden and increase response rates but is of limited long-term benefit.9,10 Poor responses to DLI also would occur if donor T cells were anergic or suppressed in vivo. In this case, ex vivo activation could overcome immune system unresponsiveness or disease resistance to DLI. Several lines of evidence support the potential for adoptive immunotherapy with ex vivo–activated donor T cells, including the following: (1) Some patients resistant to DLI achieve CR after administration of IL-2 or after infusions of donor T cells activated ex vivo with IL-211 (notably, IL-2 results in preferential activation of cytotoxic CD8+ T cells; several reports in humans suggest that CD8+ cells are primary mediators of GVHD while CD4+ cells are thought to provide the greater contribution to GVT activity18-20 ). (2) Tumors that lack costimulatory ligands are poor stimulators of immune effector cells and can induce peripheral tolerance of tumor reactive T cells.21,22 (3) Tolerance induction generated in vivo by down-regulatory signals such as CTLA4 can be avoided by ex vivo costimulation.23 (4) Some tumors may produce factors that interfere with T-cell or APC intracellular signaling, resulting in suboptimal costimulation.24,25 (5) Methods are available to stimulate and expand CD4+ cells,26 which may have a primary role in GVT induction.18-20 (6) Ex vivo costimulation of autologous T cells by CD3 and CD28 can reverse both in vivo and in vitro functional T-cell defects in patients with lymphoma.14
We hypothesized that ex vivo costimulation of T cells via CD3 and CD28 might overcome disease-induced anergy, preserve and augment CD4 function, and enhance GVT activity and that the activated and expanded donor T cells would induce GVT effects in patients who do not otherwise respond well to DLI. All patients first received unstimulated DLI because this was felt to be the most effective established treatment option available. To improve the poor response rates seen in earlier studies of conventional DLI, a small aliquot of donor T cells was removed from the DLI product for ex vivo costimulation and expansion and administered as aDLI after 10 to 12 days of culture. To limit the risk of rapid progression before GVT effects could develop and to induce a minimal disease state prior to DLI, patients with AML, ALL, advanced-phase CML, and lymphoblastic lymphoma were pretreated with induction chemotherapy and DLI was administered during the hematologic nadir.9,10
Seventeen patients were evaluable for a response, and 8 achieved a CR, including 4 of 6 patients with ALL, 2 of 4 with AML, and 1 each with CLL and NHL. Unfortunately, 4 of these 8 patients have relapsed; these are 4 of the 6 patients with acute leukemia who had achieved CR. This is similar to other trials of DLI showing that remissions may not be durable for many patients with acute leukemia in CR after DLI.9,10 However, duration of remission in these patients with acute leukemia varied between 8 and 40 months (median, 18.5 months). This is significant given that the median time to relapse after chemotherapy plus standard DLI was reported as approximately 6 months (range, 1.5 to 25 months) for patients with advanced myeloid malignancies9 and 1.5 to 6 months for patients with ALL treated with chemotherapy and DLI.10 The number of patients treated in our phase 1 trial was too small to determine if time from transplantation to relapse influenced overall response and outcome after aDLI and was too small to definitively determine if outcomes are improved compared with historical controls.
There are several reasons this therapy may not result in durable remissions. It is likely that in many cases the GVT effect (with or without induction chemotherapy) is unable to eradicate minimal residual disease. It is also possible that the number of T cells was insufficient to induce GVT activity in patients with significant tumor burdens. For instance, in patients with CML, evidence suggests there may be a dose-response effect; lower DLI doses are effective for patients with minimal disease while higher doses of T cells are required for remission induction in patients with greater disease burdens.27 While some studies of DLI have not shown a benefit to higher doses of DLI,5 the range of T cells administered has generally been narrow. The ability to expand donor T cells ex vivo through costimulation will permit the therapeutic use of T-cell doses that cannot be achieved through steady-state pheresis. Future dose escalation of aDLI is practical and may be able to identify a possible dose-response effect. However, it is also possible that regardless of the initial T-cell dose, tolerance to tumor cells develops. In this case, repetitive dosing of DLI and aDLI may be useful to enhance long-term GVT activity and minimize relapse risk.
Acute GHVD occurs in up to 40% to 60% of patients after conventional DLI and, excluding patients with early-phase relapse of CML, most patients who respond to DLI develop acute GVHD.5,6,12 Expansion through costimulation results in polyclonal activation and theoretically could result in excessive toxicity from GVHD; therefore, aDLI was dose escalated from 1 × 106 to 1 × 108 activated T cells per kilogram. A maximum tolerated dose was not identified within this range. The incidence of acute and chronic GVHD was acceptable and compared favorably with other conventional DLI studies.5,6,9,10,12 Although acute GVHD developed in 7 of 17 (41%) evaluable patients, grade III acute GVHD developed in only 2 patients (12%) (actuarial probability of grade III-IV acute GVHD, 33%; standard error, 11%) and no patient died of complications related to GVHD.
There was no obvious correlation of aDLI dose and acute GVHD, and in this limited number of patients acute GVHD did not correlate with response or survival. Chronic GVHD developed in 4 patients but was extensive in only 2 patients and was not associated with response or survival. Although all 4 patients with chronic GVHD achieved CR, patients who did not respond were likely to die from progressive disease before they were at risk for chronic GVHD.
Because all patients also received conventional DLI and patients with acute leukemia were pretreated with chemotherapy, it is not possible to separate the unique contribution of aDLI to the high response rates in this pilot dose-escalation trial. However, the high response rate is of interest given that remission rates after DLI in similar clinical settings have been uniformly disappointing.5,9,10,12 Of particular note, 2 of 4 patients (1 each with CLL and mantle-cell lymphoma) who received DLI and aDLI without induction chemotherapy remain in remission 35 and 53 months after aDLI. Three patients with relapse of Hodgkin lymphoma, NHL, and myeloma did not respond to therapy. Interestingly, these were the 3 patients treated for relapse after nonmyeloablative allogeneic SCT (NST). Because NST depends on GVT activity for maximal response, it is possible that recipients who relapse after NST are less susceptible to GVT induction by any means.
This phase 1 trial demonstrates that allogeneic T cells can be expanded effectively through ex vivo costimulation and administered with acceptable toxicity from GVHD and other complications. Response rates and remission durations were impressive for a group of diseases where conventional DLI has been disappointing. The ability for effective ex vivo expansion will permit even further dose escalation of aDLI to levels of donor T cells not practical with conventional apheresis. In addition, because late relapses remain a concern after DLI and aDLI and may imply that tolerance to tumor cells develops, repetitive dosing over time with aDLI will be feasible and practical. Ultimately, studies to determine the mechanism of tumor resistance to allogeneic T cells will best define the role of aDLI, but based on these data future studies of adoptive immunotherapy with aDLI are warranted.
Prepublished online as Blood First Edition Paper, November 3, 2005; DOI 10.1182/blood-2005-08-3373.
Supported in part by a grant from The Leukemia & Lymphoma Society (7000-02).
Two of the authors (C.H.J., B.L.L.) have declared a financial interest in a company (Xcyte) that holds license to the technology studied in this work.
All authors have contributed substantially to this work. D.L.P. was responsible for conception, design, and execution of research, data review and analysis, and primary authorship; B.L.L., conception and design, performance or supervision of ex vivo costimulation, biologic assays, data review, and manuscript preparation; N.B., conception, design, and execution of research, data review, and assistance with manuscript preparation; E.A.S., S.M.L., S.G., A.L., J.P., S.N., A.P., S.S., and D.T., execution of research, data review, and assistance with manuscript preparation; A.S., data collection, quality control, data review, and assistance with manuscript preparation; E.V., data collection, execution of research, quality control, and assistance with manuscript preparation; S.E., conception and design of research, data review, and assistance with manuscript preparation; and C.H.J., conception, design, and execution of research, data review and analysis, and manuscript preparation.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
The authors acknowledge outstanding technical support from Julio Cotte, Zhaohui Zheng, Rebecca Mair, Andrea Cannon, Kathryn Grandfield, Nina Chi, Christa Eisenman, Robert Sachs, Gene DeLeo, and Mark Wahl.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal