Comment on Ge et al, page 1570
Children with Down syndrome (DS) show a remarkable predisposition to develop acute megakaryocytic leukemia. In this issue of Blood, Ge and colleagues identify a set of genes that are differentially expressed in DS versus non-DS megakaryocytic leukemias. These findings may help explain many of the unique features of DS malignancies.
It is well established that children with Down syndrome are at an elevated risk of developing leukemia.1 In particular, the risk of acute megakaryocytic leukemia (AMKL) in children with DS is increased 500-fold over that of children without DS. It has become clear in the last 10 years that DS children with acute myeloid leukemia (AML) have a significantly better outcome than non-DS children with AML, with event-free survival (EFS) approximating 70% to 80%.2 This improved outcome has been attributed to the increased sensitivity of DS megakaryoblasts to cytosine arabinoside (ara-C) therapy.3
In addition, AMKL patients with DS, but not those without DS, harbor somatic mutations in the X-linked hematopoietic transcription factor GATA-1.4 In all patients, the mutations lead to loss of full-length GATA-1, but persistent expression of a shortened isoform, GATA-1s, that lacks the N-terminal transactivation domain. Thus, it is likely that these mutations contribute to leukemia by leading to altered expression of GATA-1 target genes. The identification of these genes that are misexpressed in DS-AMKL blasts is an essential step in understanding the mechanism of leukemogenesis in DS.
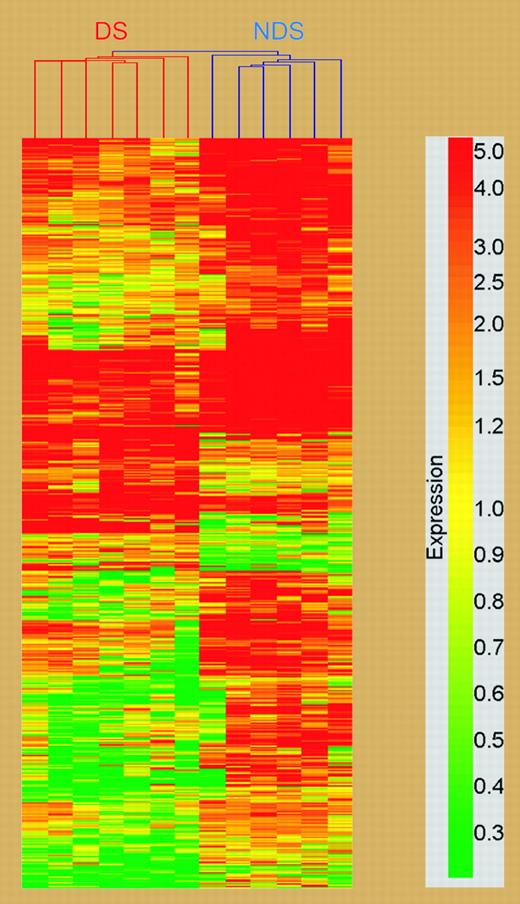
In order to determine the effect of GATA1 mutations and trisomy 21 on gene expression in AMKL, Ge and colleagues performed gene expression profiling of leukemia samples from DS and non-DS children with AMKL. Using Affymetrix U133A microarray chips, they identified 551 differentially expressed genes, consisting of 105 genes overexpressed in the DS group and 446 genes in the non-DS group (see figure). Of note, these genes were widely localized to different chromosomes, with only 7 genes located on chromosome 21. One of these genes, bone marrow stromal cell antigen (BST2) was chosen for further study because previous reports have suggested that bone marrow stromal cells exert a protective effect on leukemic cells. BST2 expression was reduced 8.5-fold in the DS-AMKL group. Using multiple approaches, Ge et al confirmed that BST2 is a bona fide GATA-1 target gene and showed that full-length GATA-1, but not GATA-1s, could efficiently activate BST2 transcription in vivo. Next, they demonstrated that ectopic expression of BST2 in a DS-AMKL cell line, which does not endogenously express this gene, resulted in a significant reduction in ara-C-induced apoptosis when cells were cocultured with bone marrow stromal cells. Thus, restoration of BST2 expression likely facilitated an association with the stromal cells, leading to protection from ara-C-induced cytotoxicity. Decreased expression of BST2 in DS-AMKL may act in conjunction with alterations in expression of genes that govern the metabolism of ara-C, such as cytidine deaminase,3 to elicit the remarkable hypersensitivity of DS megakaryoblasts to therapy.
Cluster analysis of differentially expressed genes between DS and non-DS megakaryoblasts; chromosomal localization of genes in cluster analysis. See the complete figure in the article beginning on page 1570.
Cluster analysis of differentially expressed genes between DS and non-DS megakaryoblasts; chromosomal localization of genes in cluster analysis. See the complete figure in the article beginning on page 1570.
DS-AMKL blasts are distinct from those in non-DS AMKL in that they harbor both trisomy 21 and a GATA1 mutation. This new study by Ge and colleagues suggests that the GATA1 mutations themselves may lead to the differential regulation of target genes that contribute to the unique features of DS-AMKL, such as the increased susceptibility to ara-C and high EFS rates. Surprisingly, their data provide few insights into the causative role of trisomy 21 in the disease. Future studies to determine the contributions of trisomy 21 to the initiation and/or progression of AMKL will be necessary to further advance our understanding of this malignancy. ▪

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal