Abstract
Tissue factor (TF) initiates blood coagulation, but its expression in the vascular space requires a finite period of time. We hypothesized that targeting exogenous tissue factor to sites of vascular injury could lead to accelerated hemostasis. Since phosphatidylserine (PS) is exposed on activated cells at sites of vascular injury, we cloned the cDNA for a chimeric protein consisting of the extracellular domain of TF (called soluble TF or sTF) and annexin V, a human PS-binding protein. Both the sTF and annexin V domains had ligand-binding activities consistent with their native counterparts, and the chimera accelerated factor X activation by factor VIIa. The chimera exhibited biphasic effects upon blood coagulation. At low concentrations it accelerated blood coagulation, while at higher concentrations it acted as an anticoagulant. The chimera accelerated coagulation in the presence of either unfractionated or low-molecular-weight heparins more potently than factor VIIa and shortened the bleeding time of mice treated with enoxaparin. The sTF-annexin V chimera is a targeted procoagulant protein that may be useful in accelerating thrombin generation where PS is exposed to the vasculature, such as may occur at sites of vascular injury or within the vasculature of tumors.
Introduction
The generation of thrombin is crucial for normal hemostasis. Thrombin generation is initiated by the interaction of the plasma serine protease, factor VIIa, with its protein cofactor, tissue factor (TF). TF is a membrane-bound protein not expressed on the surface of cells in contact with the bloodstream until they become activated.1 Upon its expression, TF binds either factor VII (promoting its activation to factor VIIa) or factor VIIa, increasing its catalytic efficiency in converting factor X to factor Xa. Expression of TF's extracellular domain (amino acids 1-219) of TF in E coli generates an approximately 26-kDa polypeptide that retains the ability to bind to factor VIIa and to allosterically activate it. The truncated TF (called soluble TF or sTF) does not bind to cellular membranes and is therefore much less efficient than native TF in promoting factor VII autoactivation or activation of factor X by factor VIIa.2,3 Engineering of the cDNA encoding sTF so that it was expressed on the surface of mammalian cells as a glycosylphosphatidylinositol-anchored protein resulted in a protein with the same specific procoagulant activity as native TF, underscoring the importance of membrane attachment for this protein.4
It has long been recognized that congenital factor VIII deficiency is characterized by abnormal thrombin generation when blood coagulation is triggered by low concentrations of TF.5 More recently recognized is the fact that disorders of platelet function are also associated with decreased thrombin generation.6-9 The vitamin K-dependent blood coagulation proteins, which are required for thrombin generation, assemble on the surface of activated platelets, endothelial cells, and/or monocytes by binding to anionic phospholipids, especially phosphatidylserine (PS). Thrombin, a potent platelet agonist, amplifies the activation of platelets initiated by contact with subendothelial collagen exposure. Delayed thrombin generation may therefore underlie or amplify the bleeding tendency accompanying disorders of plasma coagulation factors or blood platelets.
Others have reported that the intravenous injection of thrombo-plastin (membrane-bound TF) into animals results in generalized activation of the coagulation system,10,11 as does injection of both sTF and factor VIIa. The latter results in beneficial effects upon bleeding in experimental animals, suggesting that sTF might serve as the basis of a therapy designed to reduce bleeding.12
We hypothesized that targeting sTF to sites of vascular injury could maximize its ability to function as a hemostatic agent while minimizing the chance of inducing disseminated intravascular coagulation (DIC). Although targeting might be undertaken by using an antibody specific for activated platelets, endothelial cells, or monocytes, a targeting moiety capable of binding to all 3 cell types would be preferable, insofar as it would be less sensitive to alterations in the number of targeted cells that might be present in any given individual. Since each of the cell types of interest expresses PS on its surface when activated, we considered targeting sTF to PS-containing membranes by coupling it to annexin V, a human PS-binding protein that has been shown to bind to activated platelets, endothelial cells, and monocytes.13-15 Arguing against the use of annexin V was the fact that it is an anticoagulant protein, due to its ability to compete with vitamin K-dependent proteins for binding to PS-containing membranes.16
We report here that a chimeric protein (sTF-annV) consisting of amino acid residues 1 to 218 of TF (sTF), a linker region, and the entire coding sequence for annexin V has both pro- and anticoagulant properties. By using a chimera (called sTFAA-annV) whose sTF domain was functionally impaired by alanine substitution, we have found that the predominance of one or the other of these opposing domains varies with the local concentration of PS, in a predictable manner.
Materials and methods
Materials
Recombinant membrane-anchored TF, recombinant sTF (TF1-219), and monoclonal antibodies to factor VII and TF were prepared as described.17-19 A monoclonal antibody reacting with the His6 tag of recombinant proteins was from Cell Signaling Technology (Beverly, MA). Chromozym t-PA (N-methysulfonyl-d-phenylalanylglycyl-l-arginine-4-nitroanilide acetate) and Chromozym X (N-methoxycarbonyl-d-norleucyl-glycyl-l-arginine-4-nitranilide acetate) were from Roche Diagnostics (Indianapolis, IN). Purified human factors X, Xa, VII, and VIIa were from Enzyme Research Laboratories (South Bend, IN). Unless otherwise specified, all other reagents were from Sigma Chemical (St Louis, MO).
Phospholipids were obtained from Avanti Polar Lipid (Alabaster, AL) as stock solutions in chloroform and used to prepare unilamellar phospholipid vesicles.20 Aliquots were mixed at a molar ratio of phosphatidylcholine (PC) to PS of 4:1, and the chloroform was removed by evaporation under argon. Phospholipids were resuspended in 0.1 M NaCl, 0.05 M Tris-HCl, pH 7.5, and sonicated until the suspension was almost clear, yielding PC/PS vesicles. The phospholipid concentration was determined by phosphate analysis.21
Relipidation of TF into phospholipid vesicles was performed using the octyl-beta-d-glucopyranoside method,22,23 typically at a TF-phospholipid molar ratio of 1:8700. The effective TF concentration was determined by titrating with increasing concentrations of factor VIIa in solutions containing Chromozym t-PA and comparing the resulting amidolytic activity to that obtained by incubating factor VIIa with known concentrations of sTF. The phospholipid concentration of the TF preparation was determined by phosphate analysis21 so that comparable amounts of PS were present when TF was compared directly to sTF-annV.
Fluorescein isothiocyanate (FITC)-annexin V was prepared by incubating FITC with annexin V at a 2:1 molar ratio for 1 hour in the dark at room temperature in 0.5 M carbonate buffer, pH 9.5. Unbound FITC was removed by gel filtration in 150 mM NaCl, 0.01 M Tris-HCl, pH 8.2, followed by dialysis against the same buffer for an additional 48 hours.
Construction of expression vectors
Annexin V cDNA (in the pET-22b(+) vector) was a generous gift from Dr Antony Rosen. The sTF-annV construct was generated by ligating the cDNA encoding amino acids 1 to 218 of TF to the 5′ end of the annexin V cDNA. The sTF cDNA was cloned by polymerase chain reaction (PCR) using the forward primer 5′-CATGCCATGGCAGGCGCTTCAGGCACTACAAATAC-3′ and the reverse primer 5′-CCCAAGTCTTGGGTTCTCTGAATTCCCCTTTCTC-3′. A linker sequence encoding (GGGGS)3 was inserted between the sTF and annexin V cDNA using QuikChange Multi Site-Directed Mutagenesis Kit (Stratagene, La Jolla, CA) with the following primer: 5-GGGGAATTCAGAGAAGGTGGCGGTTCAGGCGGTGGAGGTTCAGGAGGTGGCGGATCAATGGCACAGGTTCTC-3′. The sTF-annV gene was cloned into the NcoI and HindIII sites of the pET-22b(+) vector. This vector codes for a protein with a His6-tag at the carboxy terminus.
A chimera composed of a mutated form of sTF24 linked to annexin V (sTFAA-annV) was created by site-directed mutagenesis. Residues 165 and 166 of sTF were mutated with the QuikChange Multi Site-Directed Mutagenesis Kit (Stratagene) from lysine to alanine using the primer 5′-CTTTATTATTGGAAATCTTCAAGTTCAGGAGCCGCAACAGCCAAAACAAACACTAATGAGTTTTTG-3′. The sTFAA-annV gene was cloned into the NcoI and HindIII sites of pET-22b(+). DNA sequencing confirmed the composition of all 3 constructs.
Expression and purification of sTF-annV, sTFAA-annV, and annexin V
The pET-22b(+) vectors with the sTF-annV, sTFAA-annV, or annV genes were inserted into E coli strain BL-21/DE3 (Novagen, Madison, WI). This vector provides a signal peptide that directs the recombinant protein to the periplasmic space. Expression was induced when the A600 of the bacterial suspension reached 0.8 by adding 0.3 mM isopropyl-beta-D-thiogalactopyranoside (IPTG) for 15 hours at 25°C. Cells were harvested and subjected to osmotic shock (5 mM MgSO4), and the periplasmic fraction was collected. Recombinant proteins were purified by immobilized metal affinity chromatography using a His-Select column (Sigma, St Louis, MO) equilibrated with 0.3 M NaCl, 0.05 M NaH2PO4, pH 8.0. The column was washed with the same solution in the presence of 10 and 20 mM imidazole and then eluted with 250 mM imidazole. The eluate was dialyzed against 0.1 M NaCl, 50 mM Tris-HCl, pH 7.4. The purity and identity of recombinant proteins were examined by sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) and Western blotting.
Phospholipid binding assay
The affinity of chimeras for phospholipid vesicles containing PS was determined by modifications of published assays25,26 using a commercially available aPTT coagulation reagent (Dade Actin FSL; Dade Behring, Newark, DE) as the source of PS. The reagent was reconstituted according to the manufacturer's instructions and diluted 1:10 with 140 mM NaCl, 0.01 M HEPES, pH 7.5 (HBS) plus 2.5 mM Ca++ (HBS-Ca++). The diluted phospholipid suspension (10 μL) was incubated with 50 nM FITC-annexin V (a concentration resulting in saturation of the PS present in the assay, as determined experimentally) in HBS-Ca++ in coated microcentrifuge tubes (Slickseal; National Scientific, Claremont, CA). After incubation at 37°C for 15 minutes, the mixtures were centrifuged at 16 000g for 20 minutes. The pellets were washed in HBS-Ca++ and then resuspended in HBS-Ca++ containing various amounts of each of the unlabeled competing proteins (annexin V, sTF-annV, or sTFAA-annV). After an additional 12 hours of incubation at 37°C, the mixtures were centrifuged and the pellets washed in HBS-Ca++. The pellets were then resuspended in HBS plus 10 mM EDTA and incubated for 16 hours at room temperature to elute bound FITC-annexin V. The tubes were centrifuged and the supernatant fluid was removed. The amount of FITC-annexin in the supernatant was determined by measuring its fluorescence (excitation wavelength, 485 nm; emission wavelength, 535 nm) in a fluorescent microplate reader (Victor2, Wallac; PerkinElmer, Boston, MA).
Factor VII autoactivation assay
The ability of the chimeric proteins to promote the autoactivation of factor VII was assessed by adding each (final concentration, 80 nM) to factor VII (final concentration, 80 nM) in 0.1 M NaCl, 50 mM Tris-HCl, 0.1% bovine serum albumin, pH 7.4 (TBS) with 5 mM CaCl2 (TBS/Ca++). For experiments using sTF-annV or sTF, PC/PS vesicles were added to the incubation mixtures at a composition and concentration identical to that present in the native TF preparation (total phospholipid concentration, 810 μM; PC/PS molar ratio = 4:1). At selected intervals, 10-μL aliquots were removed from each incubation mixture and transferred to polystyrene tubes containing 40 μL TBS plus 5 mM EDTA to stop the reaction. The amount of factor VIIa generated was assayed by adding 150 μL TBS/Ca++, excess sTF, and Chromozym t-PA (final concentrations, 5 mM, 124 nM, and 5 mM, respectively) and measuring the change in A405.
Factor VIIa binding assay
The sTF/TF-induced increase in factor VIIa amidolytic activity was used to quantify the binding of these proteins to factor VIIa. Increasing concentrations of sTF-annV, sTFAA-annV, or sTF were incubated with factor VIIa (5 nM) in TBS/Ca++ in 96-well assay plates. After 10 minutes at room temperature, Chromozym t-PA was added (1 mM final) and the initial rate of substrate hydrolysis was measured at 405 nm using a microplate reader (Molecular Devices, Menlo Park, CA). The background activity of factor VIIa in the absence of sTF, sTF-annV, or sTFAA-annV was subtracted from the measured values. Kinetic parameters were calculated using Prism 4 (GraphPad Software, San Diego, CA).
Factor X activation
The activation of factor X by factor VIIa in the presence of sTF, sTF-annV, sTFAA-annV, or native relipidated TF was monitored in a continuous one-stage assay. sTF, sTF-annV, sTFAA-annV, or native TF was added to 1 nM factor VIIa, factor X, PC/PS (molar ratio, 4:1), and 0.5 mM Chromozym X in TBS/Ca++. The rate of chromogenic substrate hydrolysis (change in A405) was monitored over 20 minutes and converted to factor Xa concentrations by reference to a standard curve prepared with purified human factor Xa. The derivative of the resulting parabolic progress curve was taken (Softmax Pro; Molecular Devices) to determine the rate of factor Xa generation, and kinetic parameters were calculated using Prism 4 (GraphPad Software).
Plasma coagulation assays
Coagulation times. Clotting times of normal human citrated plasma following the addition of CaCl2 in the presence of various concentrations of recombinant proteins and PC/PS (molar ratio, 4:1) were measured with a mechanical coagulometer (ST-4; Diagnostica Stago, Parsippany, NJ), which had an upper limit of measurement of 999 seconds.
Dilute activated partial thromboplastin time. A commercially available aPTT reagent (Dade Actin; Dade Behring) was diluted 1:50 in TBS and used to measure the coagulation time of citrated human plasma in the presence of various concentrations of sTF, annexin V, or sTF-annV. Plasma was incubated with dilute aPTT reagent and recombinant proteins for 3 minutes at 37°C, followed by the addition of 25 mM CaCl2. The time required for a clot to form was measured with a mechanical coagulometer.
Activated partial thromboplastin time (aPTT). The aPTT of plasma containing either 1 unit/mL heparin sodium (American Pharmaceutical Partners, Los Angeles, CA) or enoxaparin sodium (Aventis Pharmaceuticals, Bridgewater, NJ) and various concentrations of sTF-annV or factor VIIa was measured with a commercially available aPTT reagent (Actin FSL; Dade Behring) according to the manufacturer's instructions.
Mouse tail-bleeding times
Mice were housed in accordance with and studied using a protocol approved by the University of Oklahoma Health Sciences Center Institutional Animal Care and Use Committee. They were injected subcutaneously with enoxaparin sodium and 2 hours later were anesthetized with pentobarbital (60 mg/kg, given intraperitoneally). The tail was warmed in normal saline at 37°C, transected with a scalpel blade at a point where it was 2 mm in diameter, and then placed in a tube of normal saline maintained at 37°C. The time required for bleeding to stop was recorded. In preliminary dose-finding experiments, increasing doses of enoxaparin sodium were administered. Once a suitable dose was identified (20 mg/kg), a second bleeding time was performed 5 minutes after the first. The additional bleeding time was performed by transecting the tail at a point 5 mm proximal to the first. The second bleeding times were not significantly different from the first (P = .3, 2-tailed, paired t test, Prism 4; Graphpad Software). Untested animals were then treated in a similar manner, but received an intravenous injection of sTF-annV (90 μg/kg) immediately after the first bleeding time determination. A second bleeding time was performed 5 minutes after the injection. A 2-tailed, paired t test was used to compare the 2 bleeding times. An additional group of animals were then studied. Two hours after the subcutaneous injection of saline or enoxaparin (20 mg/kg), mice were given intravenous injections of sTF-annV (90 μg/kg), saline, sTF, annexin V, or a combination of sTF and annexin V. All proteins were injected in amounts equimolar with sTF-annV. Tail-bleeding times were measured 10 minutes later.
Results
Expression and purification of annexin V and annexin V chimeras
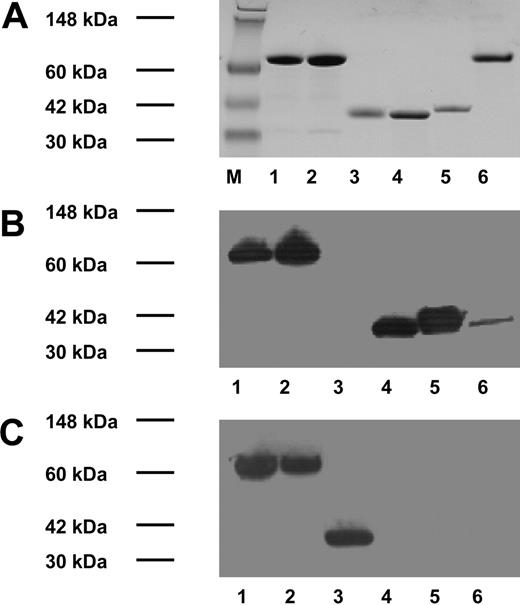
cDNA constructs encoding 3 proteins (annexin V, sTF-annV, and sTFAA-annV) were expressed in the periplasmic space of E coli and the proteins were purified by immobilized metal affinity chromatography (see Figure 1 for analyses of the purified proteins by SDS-PAGE and Western blotting).
sTF-annV accelerates plasma coagulation
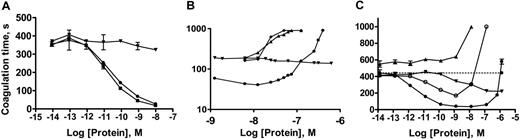
In initial studies, the abilities of sTF, native TF, and sTF-annV to accelerate plasma coagulation in the presence of identical concentrations of added phospholipid and CaCl2 were compared. As shown in Figure 2A, sTF-annV and native TF shortened the plasma coagulation time to a much greater extent than did sTF. A dilute aPTT assay was then used to determine whether the effects of sTF-annV could be duplicated by adding sTF to annexin V. Plasma was recalcified in the presence of dilute aPTT reagent (used as a source of phospholipid) and sTF, annexin V, the combination of sTF and annexin V, or sTF-annV. As shown in Figure 2B, the simultaneous addition of sTF and annexin V did not reproduce the procoagulant effect of sTF-annV. Under these conditions, sTF-annV demonstrated a biphasic effect on plasma coagulation, prolonging coagulation at higher concentrations.
These results suggested that at lower concentrations the procoagulant effect was due to the sTF domain, while at higher concentrations the anticoagulant effect of annexin V predominated. To test this hypothesis, a construct was prepared in which the lysines at positions 165 and 166 of the TF/sTF domain were mutated to alanine, a change known to impair factor X activation.24,27 The resulting chimera (sTFAA-annV) also exhibited biphasic effects on plasma coagulation (Figure 2C), but had less procoagulant activity than sTF-annV and exhibited its anticoagulant activity at lower concentrations. Given the evidence that sTF-annV and sTFAA-annV were able to alter plasma coagulation, detailed functional characterization of their constituent domains was undertaken.
Protein purification and Western blotting of recombinant proteins. Top: SDS-PAGE and Coomassie blue staining. Middle: Western blot using a mouse monoclonal antibody to human TF. Bottom: Western blot using mouse monoclonal antibody to His6. M indicates molecular weight marker. Lanes 1 to 5 are identical in panels A, B, and C: lane 1, sTF-annexin V; lane 2, sTFAA-annexin V; lane 3, annexin V; lane 4, sTF; and lane 5, recombinant TF.18 Lane 6, panel A: bovine serum albumin; panels B and C: recombinant TF (Innovin; Dade Behring).
Protein purification and Western blotting of recombinant proteins. Top: SDS-PAGE and Coomassie blue staining. Middle: Western blot using a mouse monoclonal antibody to human TF. Bottom: Western blot using mouse monoclonal antibody to His6. M indicates molecular weight marker. Lanes 1 to 5 are identical in panels A, B, and C: lane 1, sTF-annexin V; lane 2, sTFAA-annexin V; lane 3, annexin V; lane 4, sTF; and lane 5, recombinant TF.18 Lane 6, panel A: bovine serum albumin; panels B and C: recombinant TF (Innovin; Dade Behring).
Influence of recombinant proteins on plasma coagulation. (A) The coagulation time of citrated human plasma with different concentrations of native TF (▪), sTF (▾), or sTF-annV (•). The total amount of PC/PS added to each sample was 10 μM (PC/PS molar ratio, 4:1). Shown are means ± SEM. (B) Plasma coagulation times with sTF (▾), annexin V (▴), both sTF and annexin V (♦), or sTF-annV (•) measured using a dilute partial thromboplastin time protocol, as described in “Materials and methods.” (C) Plasma coagulation times, in the absence of added phospholipids, with different concentrations of sTF (▾), annexin V (▴), sTF-annV (•), or sTFAA-annV (○). Shown are means ± SEM.
Influence of recombinant proteins on plasma coagulation. (A) The coagulation time of citrated human plasma with different concentrations of native TF (▪), sTF (▾), or sTF-annV (•). The total amount of PC/PS added to each sample was 10 μM (PC/PS molar ratio, 4:1). Shown are means ± SEM. (B) Plasma coagulation times with sTF (▾), annexin V (▴), both sTF and annexin V (♦), or sTF-annV (•) measured using a dilute partial thromboplastin time protocol, as described in “Materials and methods.” (C) Plasma coagulation times, in the absence of added phospholipids, with different concentrations of sTF (▾), annexin V (▴), sTF-annV (•), or sTFAA-annV (○). Shown are means ± SEM.
Phospholipid binding activity
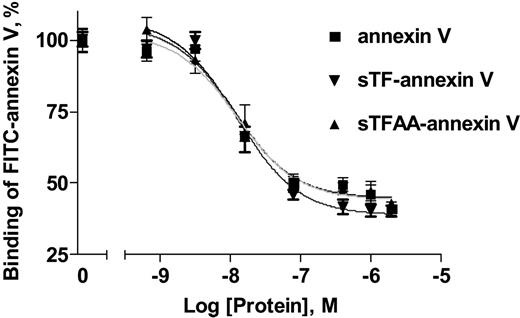
The ability of sTF-annV and sTFAA-annV to bind PS was assessed with a modification of a published PS-binding assay26 in which annexin V, sTF-annV, or sTFAA-annV competed with FITC-annexin V for binding to phospholipid vesicles. As shown in Figure 3, both chimeras exhibited PS-binding activity comparable with annexin V. The Kd for annexin V was 12 nM; for sTF-annV, 13 nM; and for sTFAA-annV, 11 nM.
Factor VII autoactivation activity
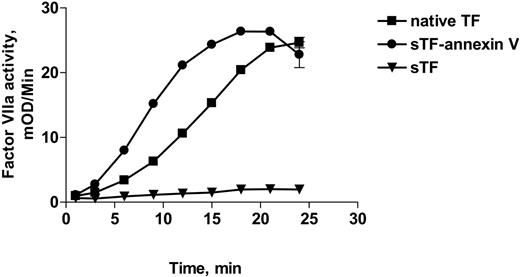
It has been shown previously that TF, but not sTF, promotes autoactivation of factor VII in the presence of PS and Ca++.3,28 The failure of sTF to accelerate factor VII autoactivation has been attributed to the low affinity of sTF:VIIa complexes for PS-containing membranes.29 Since the annexin V domain of the chimera exhibited high affinity for PS-containing liposomes, we postulated that sTF-annV would promote factor VII autoactivation in a manner comparable to TF, rather than sTF. As shown in Figure 4, sTF-annV promoted factor VII autoactivation at a rate comparable to native TF, rather than sTF (which was inactive). These results also suggest that sTF-annV is able to bind factor VII as well as native TF.
Factor VIIa binding activity
To measure the binding of factor VIIa to the chimeras, we quantified the increase in factor VIIa's amidolytic activity when it binds to TF/sTF.30 Because sTF does not bind membranes well, we performed the analysis in the absence of PS. Both sTF-annV and sTFAA-annV increased the amidolytic activity of factor VIIa to the same extent as sTF, indicating that the annexin V domain did not interfere with the ability of the sTF domain to bind factor VIIa (data not shown). The Kd for binding of factor VIIa to sTF was 8.17 nM; for sTF-annV, 7.05 nM; and for sTFAA-annV, 3.47 nM, all in the absence of phospholipid vesicles.
Phospholipid binding of sTF-annV and sTFAA-annV. The ability of sTF-annV (▾) and sTFAA-annV (▴) to bind PC/PS vesicles was compared with that of annexin V (▪) by assessing the ability of each protein to displace FITC-annexin V from a phospholipid suspension, as described in “Materials and methods.” The curves were computer-generated using nonlinear regression and a one-site ligand-binding equation. Data are expressed as the percent (mean ± SEM) of EDTA-elutable fluorescence.
Phospholipid binding of sTF-annV and sTFAA-annV. The ability of sTF-annV (▾) and sTFAA-annV (▴) to bind PC/PS vesicles was compared with that of annexin V (▪) by assessing the ability of each protein to displace FITC-annexin V from a phospholipid suspension, as described in “Materials and methods.” The curves were computer-generated using nonlinear regression and a one-site ligand-binding equation. Data are expressed as the percent (mean ± SEM) of EDTA-elutable fluorescence.
Factor X activation
The effect of sTF-annV and sTFAA-annV on the rate of factor X activation by factor VIIa was assessed. As shown in Figure 5A, the rate of factor X activation in the presence of either sTF-annV or sTFAA-annV was greater than that seen in the presence of sTF. As expected from prior studies of sTFAA,24,27 sTF-annV was more potent than sTFAA-annV in supporting factor X activation. As shown in Table 1, the catalytic efficiency (kcat/Km) of factor VIIa in the presence of sTF-annV was approximately 67% of that found in the presence of native TF, but approximately 5-fold greater than that of sTFAA-annV.
Effect of sTF, sTF-annexin V, and sTFAA-annV on the rate of factor X activation by factor VIIa
Cofactor . | Vmax, nM × min–1 . | Km, nM . | kcat, s–1 . | kcat/Km, μM–1s–1 . |
|---|---|---|---|---|
| TF | 0.71 ± 0.008 | 46 ± 1 | 2.37 | 51.5 |
| sTF-annV | 0.66 ± 0.03 | 64 ± 7 | 2.2 | 34.4 |
| sTFAA-annV | 0.71 ± 0.06 | 389 ± 58 | 2.37 | 6.1 |
Cofactor . | Vmax, nM × min–1 . | Km, nM . | kcat, s–1 . | kcat/Km, μM–1s–1 . |
|---|---|---|---|---|
| TF | 0.71 ± 0.008 | 46 ± 1 | 2.37 | 51.5 |
| sTF-annV | 0.66 ± 0.03 | 64 ± 7 | 2.2 | 34.4 |
| sTFAA-annV | 0.71 ± 0.06 | 389 ± 58 | 2.37 | 6.1 |
Since high concentrations of sTF-annV were associated with prolongation of the plasma coagulation times, we studied the effect of increasing concentrations of sTF-annV on the rate of factor X activation by factor VIIa. As shown in Figure 5B, sTF-annV exhibited a biphasic effect on factor X activation, paralleling its effects on plasma coagulation.
Autoactivation of factor VIIa in the presence of native TF, sTF-annV, or sTF. Factor VII (80 nM) was added to equimolar concentrations of native TF (▪), sTF (▾), or sTF-annV (•) in the presence of PC/PS (810 μM, PC/PS molar ratio = 4:1) and CaCl2. At the indicated times, aliquots were removed and the reaction was stopped by dilution in EDTA-containing solutions. The factor VIIa content of each sample was then determined by measuring the rate of hydrolysis of Chromozym t-PA in the presence of added sTF and CaCl2. Shown are means ± SEM.
Autoactivation of factor VIIa in the presence of native TF, sTF-annV, or sTF. Factor VII (80 nM) was added to equimolar concentrations of native TF (▪), sTF (▾), or sTF-annV (•) in the presence of PC/PS (810 μM, PC/PS molar ratio = 4:1) and CaCl2. At the indicated times, aliquots were removed and the reaction was stopped by dilution in EDTA-containing solutions. The factor VIIa content of each sample was then determined by measuring the rate of hydrolysis of Chromozym t-PA in the presence of added sTF and CaCl2. Shown are means ± SEM.
Generation of factor Xa by factor VIIa in the presence of recombinant proteins. (A) Various concentrations of factor X were added to 1 nM factor VIIa, 5 μM PC/PS (molar ratio, 4:1), 5 mM CaCl2, and Chromozym X substrate in the presence of 5 pM native TF (▪), sTF (▾), sTF-annV (•), or sTFAA-annV (○). Initial rates of substrate hydrolysis were measured as described in “Materials and methods,” to which the Michaelis-Menten equation was fitted. (B) Initial rates of factor Xa generation were measured in solutions containing 28 nM factor X, 5 mM CaCl2, 1 nM factor VIIa, 0.347 μM PC/PS, and various concentrations of sTF (▾) or sTF-annV (•). Shown are the means ± SEM. (Most error bars are too small to be seen.)
Generation of factor Xa by factor VIIa in the presence of recombinant proteins. (A) Various concentrations of factor X were added to 1 nM factor VIIa, 5 μM PC/PS (molar ratio, 4:1), 5 mM CaCl2, and Chromozym X substrate in the presence of 5 pM native TF (▪), sTF (▾), sTF-annV (•), or sTFAA-annV (○). Initial rates of substrate hydrolysis were measured as described in “Materials and methods,” to which the Michaelis-Menten equation was fitted. (B) Initial rates of factor Xa generation were measured in solutions containing 28 nM factor X, 5 mM CaCl2, 1 nM factor VIIa, 0.347 μM PC/PS, and various concentrations of sTF (▾) or sTF-annV (•). Shown are the means ± SEM. (Most error bars are too small to be seen.)
Effect of sTF-annexin on coagulation in the presence of heparin
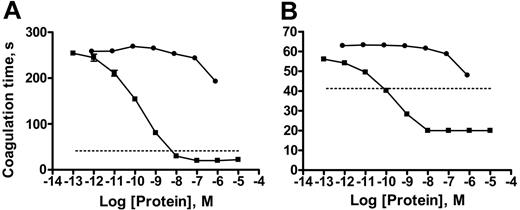
Heparin is a commonly used anticoagulant that works by enhancing the ability of the serpin, antithrombin, to inhibit (primarily) factor Xa and thrombin.31 We added unfractionated heparin sodium or enoxaparin, a low-molecular-weight heparin, to citrated plasma along with various concentrations of sTF-annV and then determined the plasma aPTT. sTF-annV shortened the aPTT of plasma containing either 1 unit/mL unfractionated heparin (Figure 6A) or 1 U/mL enoxaparin (Figure 6B). Factor VIIa, used as a therapeutic agent to treat bleeding in numerous clinical conditions associated with decreased thrombin generation,32 also decreased the aPTT of heparin-treated plasma, although less potently than sTF-annV (Figure 6A-B).
Effect of sTF-annV on mouse tail-bleeding times
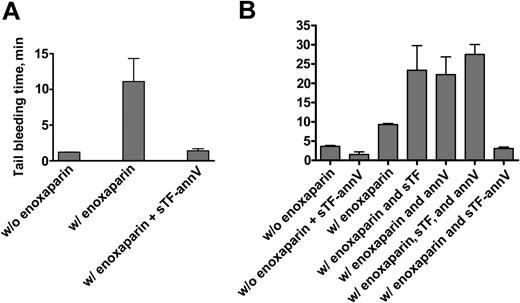
Although the template bleeding time assay in humans is thought to predominantly reflect platelet function, defects in thrombin generation in mice are reflected by prolonged bleeding times.33-36 We therefore administered enoxaparin to normal mice to determine whether sTF-annV would affect an in vivo measure of thrombin generation. Mice were injected subcutaneously with enoxaparin sodium (20 mg/kg), a drug known to inhibit thrombin generation,31 and the bleeding time was measured 2 hours later. After the bleeding from the bleeding time wound stopped, the animals were given an intravenous injection of sTF-annV (90 μg/kg) and the bleeding time was repeated 5 minutes later. (Preliminary experiments showed that a second bleeding time in enoxaparin-treated animals was not significantly different from the first, P = .3, paired 2-tailed t test.) As shown in Figure 7A, sTF-annV significantly shortened the bleeding time of enoxaparin-treated mice (P = .01, paired 2-tailed t test). Enoxaparin treatment resulted in a mean bleeding time of 10.7 minutes (median, 6.1 minutes). The mean bleeding time after sTF-annV treatment was 1.4 minutes (mean, 1.1 minutes). The mean bleeding time of untreated animals was 1.2 minutes.
The effect of sTF-annV or factor VIIa on the aPTT of heparin-treated plasma. (A) aPTT assays of plasma containing heparin sodium (1 unit/mL) were measured in the presence of various concentrations of factor VIIa (•) or sTF-annV (▪). (B) The aPTT of plasma containing enoxaparin sodium (1 unit/mL) was measured in the presence of various concentrations of factor VIIa (•) or sTF-annV (▪). The dotted lines show the mean clotting time for the aPTT assay in the absence of heparin or enoxaparin. Shown are means ± SEM. (Most error bars are too small to be seen.)
The effect of sTF-annV or factor VIIa on the aPTT of heparin-treated plasma. (A) aPTT assays of plasma containing heparin sodium (1 unit/mL) were measured in the presence of various concentrations of factor VIIa (•) or sTF-annV (▪). (B) The aPTT of plasma containing enoxaparin sodium (1 unit/mL) was measured in the presence of various concentrations of factor VIIa (•) or sTF-annV (▪). The dotted lines show the mean clotting time for the aPTT assay in the absence of heparin or enoxaparin. Shown are means ± SEM. (Most error bars are too small to be seen.)
The tail-bleeding time was measured in additional groups of mice. sTF-annV shortened the bleeding time of animals not treated with enoxaparin (1.5 ± 0.7 minute versus 3.6 ± 0.2 minute, P > .02, unpaired t test with Welch correction; Figure 7B). Naive animals were then treated with enoxaparin (20 mg/kg, subcutaneously) 2 hours before they were injected intravenously with sTF-annV (90 μg/kg) or equimolar amounts of sTF, annexin V, or the combination of sTF and annexin V. As shown in Figure 7B, shortening of the bleeding time was seen only in animals treated with sTF-annV (P < .001, 2-tailed unpaired t test with Welch correction), showing that the chimera itself, and not its individual constituents, was responsible for the shortening of the bleeding time.
Discussion
Previous studies have shown that the isolated extracellular domain of TF (sTF) retains a conformation sufficient to allow binding to factor VIIa and enhancement of its cleavage of a small tripeptidyl synthetic substrate.3,37 sTF is much less efficient in promoting the activation of factor X by factor VIIa, however. This appears to be due to relatively low affinity of sTF and the sTF-VIIa complex for membrane surfaces.29 By substituting a glycosylphosphatidylinositol anchor for the TF transmembrane domain, Paborsky et al were able to create a membrane-bound sTF variant that had full procoagulant activity, indicating the ability of sTF to function as well as native TF if appropriately tethered to a cell surface.4
The effect of sTF-annV on the tail-bleeding time. (A) Mice (n = 13) were given a subcutaneous injection of enoxaparin sodium (20 mg/kg), and 2 hours later they were anesthetized and the tail-bleeding time was determined as described in “Materials and methods.” Immediately upon the cessation of bleeding, the animals were injected intravenously with sTF-annV (90 μg/kg) in TBS. Five minutes later, the bleeding time was measured again. The differences were significant at a level of P = .01 (paired 2-tailed t test). (B) Tail-bleeding times were measured as described in panel A. Two hours after receiving subcutaneous enoxaparin (or saline), animals were injected intravenously with sTF-annV (90 μg/kg) or equivalent molar amounts of sTF, annexin V, or both sTF and annexin V. Tail-bleeding times were measured 10 minutes later. sTF-annV shortened the bleeding time of mice that did not receive enoxaparin (P > .2) as well as those that did (P < .001, 2-tailed unpaired t test with Welch correction). Two animals were studied in each of the groups that did not receive enoxaparin, and 4 in each of the enoxaparin-treated groups. Shown are the means ± SEM.
The effect of sTF-annV on the tail-bleeding time. (A) Mice (n = 13) were given a subcutaneous injection of enoxaparin sodium (20 mg/kg), and 2 hours later they were anesthetized and the tail-bleeding time was determined as described in “Materials and methods.” Immediately upon the cessation of bleeding, the animals were injected intravenously with sTF-annV (90 μg/kg) in TBS. Five minutes later, the bleeding time was measured again. The differences were significant at a level of P = .01 (paired 2-tailed t test). (B) Tail-bleeding times were measured as described in panel A. Two hours after receiving subcutaneous enoxaparin (or saline), animals were injected intravenously with sTF-annV (90 μg/kg) or equivalent molar amounts of sTF, annexin V, or both sTF and annexin V. Tail-bleeding times were measured 10 minutes later. sTF-annV shortened the bleeding time of mice that did not receive enoxaparin (P > .2) as well as those that did (P < .001, 2-tailed unpaired t test with Welch correction). Two animals were studied in each of the groups that did not receive enoxaparin, and 4 in each of the enoxaparin-treated groups. Shown are the means ± SEM.
Others have coupled sTF to peptide moieties and demonstrated activation of the coagulation system in vitro and in vivo.38-42 Each used a targeting domain specific for a particular cell type or anatomic region, and all of these studies were directed toward developing anticancer agents, rather than novel hemostatic compounds.
In this study, a chimeric protein that contained the sTF domain was created with several novel features: (1) it was designed to be “polyspecific” with regard to the cell types that play a role in hemostasis; (2) it used a membrane-targeting domain that has anticoagulant properties, and therefore raised questions about the likelihood that the chimera would be procoagulant; and (3) it was anticipated that its effectiveness as a procoagulant would depend upon the local concentration of anionic extracellular phospholipids. Thus, although annexin V's potency as a PS-binding protein made it attractive as a targeting moiety, its anticoagulant action cautioned against its use in a construct designed to be a procoagulant hemostatic agent.
The initial studies of sTF-annV were therefore directed toward determining whether it was in fact procoagulant, and if so, how its procoagulant activity compared with that of both sTF and native TF. Because we recognized that the ratio of annexin V to phospholipid would be important, we used phospholipid suspensions, rather than activated cells, to maintain consistency, during our analysis. Once we found that sTF-annV was procoagulant (Figure 2A), we established that the procoagulant activity was specific to the chimera, and could not be reproduced by adding equimolar amounts of a mixture of sTF and annexin V (Figure 2B). We also prepared a chimera in which amino acids 165 and 166 of sTF were mutated from lysine to alanine, a change known to significantly reduce the procoagulant activity of TF,24,27 and found that it had diminished procoagulant activity (Figure 2C). Finally, we explored the consequences of altering the ratio of the chimera to the PS present in the reaction mixture. We found that for both sTF-annV and sTFAA-annV, an increase in the protein/PS ratio beyond a certain point resulted in an anticoagulant effect (Figure 2C). Thus, targeting of sTF to a membrane surface by the annexin V domain is procoagulant when there is a functional excess of PS-bearing sites that can bind other coagulation factors. As the amount of annexin V present increases, those sites become less accessible to coagulation factors VII/VIIa, X, VIII, IX, V, and II, and coagulation slows.
We then conducted analyses of the functional domains comprising sTF-annV to determine if linking them together caused a loss or gain of function of either. Tests of the chimera's ability to bind PS-containing vesicles (Figure 3), promote factor VII autoactivation (Figure 4), and enhance factor VIIa's amidolytic and factor X-cleaving activity (Figure 5) indicated that both the sTF and annexin V domains were functional, although native TF promoted the catalytic efficiency of factor VIIa toward factor X twice as well as did sTF-annV (Table 1).
These studies indicated that sTF-annV could function as a procoagulant, but its procoagulant activity was heavily dependent upon the phospholipid concentration. Since the local concentration of PS at a wound site in vivo is not precisely known, in vitro experiments have limited ability to predict the behavior of a PS binding protein (such as sTF-annV) in vivo. In order to test sTF-annV in vivo under conditions where the PS concentration would be determined by the body's response to injury, we performed tail-bleeding times in mice, a method known to be sensitive to defective thrombin generation36,43,44 in the setting of the prior administration of enoxaparin, a low-molecular-weight heparin used to impair thrombin generation.31 In preparation for this experiment, we studied the effect of sTF-annV on plasma coagulation in the presence of enoxaparin, as well as unfractionated heparin, and contrasted it with the effects of factor VIIa, a protein currently used to treat patients with a variety of bleeding disorders.32 Both factor VIIa and sTF-annV shortened the coagulation time of heparin- and enoxaparin-treated plasma, although sTF-annV was several orders of magnitude more potent than factor VIIa (Figure 6).
We treated mice with increasing doses of enoxaparin, establishing a dose that increased 2 sequential bleeding times reproducibly. We then injected sTF-annV by intravenous tail-vein injection between the first and second tail-bleeding time. In all animals tested, the second bleeding time was significantly shorter than the first. Using additional animals, we found that the effect of sTF-annV could not be reproduced by injecting sTF, annexin V, or a mixture of the two. Because human factor VIIa is not fully active in mouse plasma,45 it could not be used for direct comparisons. The dose of sTF-annV was chosen because it is equivalent (on a weight basis) to a commonly recommended recombinant factor VIIa dose. Further studies will be required to determine the optimal dose of sTF-annV to be used, which may well vary in different clinical situations, depending upon the local PS concentration and the magnitude of the vascular injury.
These studies demonstrate that seemingly antagonistic protein domains may be combined in a chimeric molecule to generate a protein whose activity is not simply determined by the net effect of its dominant domain. sTF-annV exhibits an effect that is reflective of either of its opposing domains, depending upon the conditions. The ability of sTF-annV to exhibit both procoagulant and anticoagulant activity makes it unique among hemostatic molecules. Further work in vivo may demonstrate that sTF-annV can be used as a probe of the local PS concentration, as reflected, for example, by its effect on thrombin generation in shed blood. Such a determination may be of therapeutic, as well as experimental, importance. For example, if the local PS concentration is very low (as may be the case at sites of bleeding in thrombocytopenic individuals), a procoagulant dose of sTF-annV might be unduly anticoagulant, thereby worsening an individual's bleeding. Alternatively, if some stimuli, such as massive trauma, result in high local concentrations of PS, higher doses of sTF-annV might be safely used to rapidly obtain hemostasis and prevent the subsequent deleterious effects of major blood loss. If the hypothesis that local concentrations of PS differ (depending upon the clinical circumstances) is correct, studies of the microenvironment unique to different types and sites of vascular injury may stimulate the development of new hemostatic compounds.
Others have shown that targeting sTF to tumors with coaguloligands,39 chimeric proteins that target sTF to specific vascular beds by tethering it to tumor-specific antibodies or ligands, can lead to obstruction of the tumor vasculature and cell death.38-42,46 Since PS is not present on normal blood vessels, but is found on cells within tumor blood vessels,47,48 as well as on the surface of apoptotic tumor49-51 and endothelial52 cells, sTF-annV may also find use as an anticancer agent when given alone or in conjugation with other therapies.
Prepublished online as Blood First Edition Paper, September 29, 2005; DOI 10.1182/blood-2005-07-2733.
Supported by the University of Oklahoma Bioengineering Center and NIH grant R01 HL47104 (J.H.M.).
An Inside Blood analysis of this article appears at the front of this issue.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.


This feature is available to Subscribers Only
Sign In or Create an Account Close Modal