Comment on Liersch et al, page 1214
Research on lymphatic vasculature development has made exciting progress over the past years, yet suitable in vitro systems to study the formation of the lymphatic endothelium were unavailable. Now, Liersch and colleagues report that lymphatic endothelial cells differentiate in mouse embryonic stem cell-derived embryoid bodies.
The lymphatic vasculature is vital for maintaining fluid homeostasis in the body and for absorbing macromolecules from the interstitial space. Lymph vessels also serve as a route for circulating immune cells involved in immune surveillance. A number of clinical disorders are associated with the lymphatic vasculature: abnormal development or acquired dysfunction of the lymphatic vasculature causes lymphedema, and metastatic tumor cells may disseminate in the body via the lymphatic vasculature. Hence, there is great interest to understand the mechanisms that regulate the growth of lymphatic vessels (lymphangiogenesis).
The embryonic development of the lymphatic vasculature was described by Florence Sabin as early as 1902.1 However, until recently, the molecular mechanisms involved remained elusive. Progress in the field awaited the discovery of molecular markers for lymphatic endothelial cells, notably the vascular endothelial growth factor receptor-3 (VEGFR-3), the transcription factor prospero-related homeobox 1 (Prox-1), and the lymphatic vessel hyaluronan receptor 1 (LYVE-1) (for review, see Hong et al2 and Oliver and Alitalo3 ). Genetic studies performed in mouse embryos revealed that the expression of Prox-1 in a subpopulation of endothelial cells in the anterior cardinal vein, and their subsequent stimulation by VEGF-C, is required for the subsequent budding and sprouting of lymphatic vessels. A number of additional factors were implicated in lymphangiogenesis; not surprisingly, several of them are also involved in blood-vessel angiogenesis.2,3
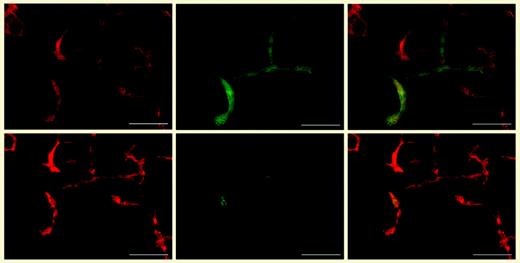
Development of lymphatic endothelial cells in embryoid bodies. Differential immunofluorescence stains for CD31 (red; leftmost panels) and LYVE-1 (green; top middle panel) revealed that the CD31+/LYVE-1+ lymphatic vessels also expressed Prox-1 (green; bottom middle panel). Merged images appear in the rightmost panels. See the complete figure in the article beginning on page 1214.
Development of lymphatic endothelial cells in embryoid bodies. Differential immunofluorescence stains for CD31 (red; leftmost panels) and LYVE-1 (green; top middle panel) revealed that the CD31+/LYVE-1+ lymphatic vessels also expressed Prox-1 (green; bottom middle panel). Merged images appear in the rightmost panels. See the complete figure in the article beginning on page 1214.
Although it is now possible to isolate lymphatic endothelial cells, in vitro systems to study their development were lacking. Embryonic stem (ES) cells are extremely useful to study cellular differentiation processes involved in early-stage organ development because they have the amazing potential to differentiate into a variety of different cell types in vitro, including the cellular constituents of the cardiovascular system.4 Such experiments were instrumental for studying the differentiation of blood-vessel endothelial cells from mesodermal progenitors.
In this issue of Blood, Liersch and colleagues report the induction of lymphatic endothelial-cell differentiation in ES cell-derived embryoid bodies. These cells were positive for the panendothelial marker CD31/PECAM-1 and the lymphatic endothelial markers Prox-1 and LYVE-1 (see figure), but negative for the blood vascular marker MECA-32. Lymphatic vessels frequently sprouted from blood-vessel structures, consistent with the in vivo observations. Of interest, the addition of certain growth factors known to induce lymphangiogenesis in vivo revealed significant differences in their potential to promote lymphatic endothelial-cell differentiation in vitro: VEGF-C, and, to a lesser extent, VEGF-A, promoted lymphatic vessel formation, whereas basic fibroblast growth factor did not. Whether these differences indicate that lymphangiogenesis in vivo involved indirect effects, or simply reflect limitations of the in vitro system, remains to be clarified.
One interesting question that is not addressed by the study is whether lymphatic endothelium forms solely by sprouting from blood-vessel endothelium, or whether it differentiates also de novo from endothelial progenitor cells, as blood-vessel endothelium does. In other words, is (lymph-) vasculogenesis involved in lymph vessel formation? The authors plan to address this question in the future by time-lapse studies. Thus, the availability of an in vitro system for studying the differentiation of lymphatic endothelium is a major step toward the elucidation of the molecular events that govern the formation of the lymphatic vasculature. ▪

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal