Abstract
Transfusion-related acute lung injury (TRALI) is a hazardous complication of transfusion and has become the leading cause of transfusion-related death in the United States and United Kingdom. Although leukoagglutinating antibodies have been frequently shown to be associated with the syndrome, the mechanism by which they induce TRALI is poorly understood. Therefore, we reproduced TRALI in an ex vivo rat lung model. Our data demonstrate that TRALI induction by antileukocyte antibodies is dependent on the density of the cognate antigen but does not necessarily require leukoagglutinating properties of the antibody or the presence of complement proteins. Rather, antibody-mediated activation of neutrophils seems to initiate TRALI, a process that could be triggered by neutrophil stimulation with fMLP. Antibody-mediated neutrophil activation and subsequent release of reactive oxygen species may thus represent key events in the pathophysiologic cascade that leads to immune TRALI.
Introduction
Transfusion-related acute lung injury (TRALI) is a serious, life-threatening complication of hemotherapy. In the United States and United Kingdom, TRALI is considered the most common cause of transfusion-related fatalities.1,2 It typically presents within 6 hours after transfusion as a clinical syndrome characterized by acute respiratory distress, hypoxemia, and a bilateral pulmonary edema on chest x-ray.3 Most investigators have postulated that TRALI is an antibody-mediated event because recipient-specific antileukocyte antibodies of donor origin are frequently associated with the syndrome.4-7 Leukocyte antibodies of different specificity have been implicated, including, HLA-related and granulocyte-specific antibodies (eg, anti-HNA-2a). These findings have been corroborated with the clinical observation that plasma-rich products are most commonly implicated in TRALI. In the recent report from the British hemovigilance system, 17 of 36 suspected cases of TRALI received fresh frozen plasma (FFP) and 10 received platelets. Antileukocyte antibodies supportive for the diagnosis of TRALI were detected in two thirds of these cases.2 Despite the accumulation of data strongly suggesting an etiologic link between transfused antileukocyte antibodies and TRALI, little information has been gained regarding pathophysiology. In this study we demonstrate that antileukocyte antibodies can induce TRALI in an ex vivo lung model via neutrophil activation, a process that is independent from the presence of complement or leukoagglutinating properties of the antibody.
Study design
Antibodies recognizing CD177 (HNA-2a) have been implicated in TRALI.7 A monoclonal mouse anti-human IgG antibody (mAb) against CD177, 7D8, was used instead of human anti-HNA-2a in all experiments.8 Neutrophils were isolated from whole blood and the size of the HNA-2a+ subpopulation was determined by flow cytometry.9 Granulocytes were suspended in Krebs-Henseleit buffer (KHB) and added to the perfusion circuit of isolated rat lungs. The rat lung model has been described elsewhere.10 Briefly, isolated rat lungs were ventilated with a gas mixture of 5.3% CO2, 21.0% O2, and 73.7% N2 and perfused via cannulas in the pulmonary artery and the left ventricle with KHB containing 5% hydroxy-ethyl starch. Pulmonary artery pressure (PAP) and lung weight gain were recorded continuously. Capillary filtration coefficient (Kf,c) and total vascular compliance (TVC) were repeatedly determined from sudden venous pressure elevation maneuvers of 10 cm H2O for 8 minutes. After an isogravimetric steady-state period of 30 minutes, a first maneuver (t = 0) was performed. Subsequent repetitive maneuvers were performed as indicated (Figure 1). Neutrophils (108) were added to the buffer following the first maneuver and 75 μg mAb 7D8 or control IgG following the second maneuver. In a second set of experiments, fMLP was added to the buffer fluid at time point 120 minutes. Histologic sections from all lungs were stained and examined by light microscopy. Priming activity of anti-HNA-2a and its inhibition by diphenylene iodonium was measured using the BurstTest kit from Becton Dickinson (Heidelberg, Germany) as described previously.11,12
Results and discussion
Normal physiologic responses are maintained in the isolated rat lung
Under baseline conditions, PAP, Kf,c data, and lung weight ranged within normal values. Application of neutrophils plus control IgG (Figure 1A-B, open circles) and the addition of neutrophils, IgG, and fMLP (Figure 1C-D, open circles) did not induce any biophysical changes, as did application of control IgG or anti-HNA-2a alone (not shown). In addition, isolated rat lungs responded normally to the application of angiotensin II at time point 200 minutes (data not shown), indicating that normal physiologic responses are maintained throughout the experiment.
Venous pressure elevation maneuvers.. Capillary filtration coefficient (g/[min × 100 g lung weight. × ΔPc]) and challenge-induced weight gain (g/g lung weight) are shown in the absence or presence of anti-HNA-2a. Following an isogravimetric steady-state phase, venous pressure elevations were performed at indicated time points. Neutrophils derived from either high-expressing (A,C) or low-expressing (B,D) individuals were administered following the first hydrostatic challenge at minute 15. Anti-HNA-2a (black squares) or control antibody (open circles) was added at minute 50. After administration of CD177 high-expressing granulocytes and anti-HNA-2a, TRALI develops after the sixth pressure elevation (A), whereas no significant changes occur after administration of low-expressing granulocytes and anti-HNA-2a (B). Addition of fMLP to the buffer fluid at minute 120 (C,D) promotes TRALI induction in the presence of either high-expressing (C) or low-expressing (D) neutrophils. All results are presented as mean values + SD for 5 independent experiments. Note the discontinuous y-axis for. Kf,c. Pc denotes capillary pressure.
Venous pressure elevation maneuvers.. Capillary filtration coefficient (g/[min × 100 g lung weight. × ΔPc]) and challenge-induced weight gain (g/g lung weight) are shown in the absence or presence of anti-HNA-2a. Following an isogravimetric steady-state phase, venous pressure elevations were performed at indicated time points. Neutrophils derived from either high-expressing (A,C) or low-expressing (B,D) individuals were administered following the first hydrostatic challenge at minute 15. Anti-HNA-2a (black squares) or control antibody (open circles) was added at minute 50. After administration of CD177 high-expressing granulocytes and anti-HNA-2a, TRALI develops after the sixth pressure elevation (A), whereas no significant changes occur after administration of low-expressing granulocytes and anti-HNA-2a (B). Addition of fMLP to the buffer fluid at minute 120 (C,D) promotes TRALI induction in the presence of either high-expressing (C) or low-expressing (D) neutrophils. All results are presented as mean values + SD for 5 independent experiments. Note the discontinuous y-axis for. Kf,c. Pc denotes capillary pressure.
Antileukocyte antibodies induce TRALI in the absence of complement
In contrast, when neutrophils from individuals expressing 70% or more CD177 and anti-HNA-2a were added to the buffer fluid, a delayed, severe increase in lung vascular permeability was observed. A venous pressure elevation performed after 200 minutes was followed by a virtually 18-fold increase in Kf,c in all lungs (Figure 1A, left panel), accompanied by a dramatic increase in lung weight (Figure 1A, right panel). Vascular tone and TVC values remained unaltered (not shown), indicating that the increase in Kf,c must be due to an increase in conductivity of the lung microvasculature. Notably, this reaction was independent from the presence of complement because it occurred in a plasma-free environment.
TRALI induction depends on the presence of the cognate antigen
Next, neutrophils from individuals expressing 30% or less CD177 were administered, followed by the addition of anti-HNA-2a. Under these conditions, we were unable to observe any significant changes of the lung weight at any time point. A minor increase of Kf,c was observed after 200 minutes, but seems negligible when compared to the Kf,c data obtained in other experiments (Figure 1B). Thus, changes in the lung microvasculature depend on the presence of both anti-HNA-2a antibodies and the number of neutrophils expressing the cognate antigen.
Costimulation of neutrophils promotes the induction of TRALI
In vivo responses of neutrophils are known to be modulated in the presence of bioactive substances, such as fMLP. When anti-HNA-2a neutrophils from individuals expressing CD177 on 70% or more of their cells and fMLP were administered, the increase in lung vascular permeability was dramatically accelerated (Figure 1C). This effect of neutrophil costimulation was also present when neutrophils from individuals expressing CD177 on less than 30% of their cells were administered. Whereas only a minor increase in lung vasculature permeability was observed in the absence of fMLP when anti-HNA-2a was administered (Figure 1B), addition of fMLP led to a virtually 14-fold increase in Kf,c and an 8-fold increase in lung weight (Figure 1D). These data demonstrate that the response of neutrophils to antileukocyte antibodies can be potentiated when neutrophils are stimulated with bioactive substances such as fMLP, a situation that resembles clinical conditions of surgery or infection.13
The fact that in control experiments fMLP did not induce TRALI (Figure 1C-D) needs to be mentioned, because plastic tubing has been reported to prime neutrophils in certain clinical situations such as hemodialysis.13 Although we cannot generally exclude that such nonspecific priming due to contact with plastic tubing partially adds to effects seen in our ex vivo lung model, it seems unlikely that it adds substantially to effects seen herein.
Two major events discussed in the literature that may eventually lead to endothelial damage and, subsequently, induction of TRALI are complement activation and leukocyte agglutination.14 Anti-HNA-2a antibodies used in our study did not induce leukoagglutination in standard in vitro assays (data not shown). Furthermore, in the rat lung model, complement activation was not a prerequisite for TRALI induction. Our findings indicate that certain antileukocyte antibodies may induce a direct response of the neutrophil once they have bound. Such direct cellular responses have been elegantly demonstrated by Nardi et al,15 who gave evidence that antiplatelet IgG was able to cause complement-independent platelet fragmentation via the induction of reactive oxygen species (ROS). We conclude from our data that direct antibody-mediated cellular responses contribute to the pathogenesis of immune TRALI. This hypothesis is corroborated by the finding that fMLP was able to accelerate TRALI induction in our model. Furthermore, the mAb 7D8 as well as IgG fractions obtained from patients with anti-HNA-2a induced the production of ROS in CD177+ neutrophils in vitro (Figure 2A). Preincubation of neutrophils with the flavin antagonist diphenylene iodonium led to a 64% to 98% reduction in ROSs production (not shown), indicating that radicals may be produced by NAD(P)H-like oxidase systems of the neutrophil rather than by other oxygenases.12
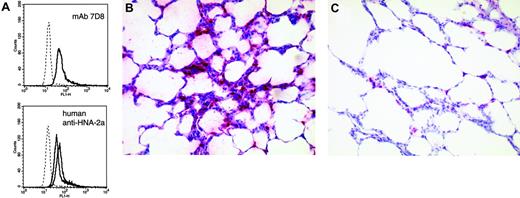
Anti-HNA-2a leads to neutrophil activation and clustering of leukocytes in the capillaries of the lung. (A) Production of ROS as determined by flow cytometry. Left panel shows response to mAb 7D8; right panel shows response to IgG fractions obtained from 2 of 3 analyzed human sera containing anti-HNA-2a. Appropriate controls (murine and human IgG, respectively) are given as histograms with dotted lines. All histograms are representative of 3 independent experiments. (B,C) Clustering of leukocytes in pulmonary capillaries following the administration of anti-HNA-2a (B), but not of control antibody (C). After the final venous pressure elevation, rat lungs were instilled with formaldehyde solution (4.5%, pH 7.2) through the trachea. Fixation was allowed to proceed overnight at room temperature. Subsequently, tissue samples were embedded in paraffin and sections of 5 μm from all lungs were stained with hematoxylin-eosin and antimyeloperoxidase. Pictures are representative for experiments and controls (antimyeloperoxidase, APAAP). A Zeiss Axioskop 40 (Carl Zeiss, Jena, Germany) with Plan-NEO Fluar 20×/0.5 NA objective lenses, in connection with a JVC KY-S75U digital camera (JVC, Friedberg, Germany) was used to acquire the micrographs. Images were processed with Diskus acquisition software, version 4.50 (Hilgers, Königswinter, Germany).
Anti-HNA-2a leads to neutrophil activation and clustering of leukocytes in the capillaries of the lung. (A) Production of ROS as determined by flow cytometry. Left panel shows response to mAb 7D8; right panel shows response to IgG fractions obtained from 2 of 3 analyzed human sera containing anti-HNA-2a. Appropriate controls (murine and human IgG, respectively) are given as histograms with dotted lines. All histograms are representative of 3 independent experiments. (B,C) Clustering of leukocytes in pulmonary capillaries following the administration of anti-HNA-2a (B), but not of control antibody (C). After the final venous pressure elevation, rat lungs were instilled with formaldehyde solution (4.5%, pH 7.2) through the trachea. Fixation was allowed to proceed overnight at room temperature. Subsequently, tissue samples were embedded in paraffin and sections of 5 μm from all lungs were stained with hematoxylin-eosin and antimyeloperoxidase. Pictures are representative for experiments and controls (antimyeloperoxidase, APAAP). A Zeiss Axioskop 40 (Carl Zeiss, Jena, Germany) with Plan-NEO Fluar 20×/0.5 NA objective lenses, in connection with a JVC KY-S75U digital camera (JVC, Friedberg, Germany) was used to acquire the micrographs. Images were processed with Diskus acquisition software, version 4.50 (Hilgers, Königswinter, Germany).
We suggest that binding of antileukocyte antibodies can activate neutrophils, a process that might lead to an oxidative response, cytotoxicity, and subsequent endothelial damage in the lung microvasculature. This process can be potentiated by neutrophil coactivation. Only recently, preliminary evidence has been presented that anti-HNA-3a might also be able to augment the oxidative response of neutrophils to a subsequent stimulus with fMLP,16 indicating that a direct effect of antileukocyte antibodies is not specific to anti-HNA-2a. Interestingly, it was also demonstrated that nonimmune TRALI can be induced in a rat lung model by the application of lipopolysaccharide (LPS) and plasma or lipids from stored blood,17 where lipids are thought to activate LPS-primed neutrophils. A common pathway for both immune and nonimmune TRALI will likely emerge in future.
Taken together, we have demonstrated that (1) antileukocyte antibodies can induce TRALI in the presence of the cognate antigen; (2) complement activation is not an absolute requirement for TRALI induction; (3) TRALI can be promoted by neutrophil coactivation; and (4) antileukocyte antibodies can activate neutrophils to produce ROS.
These results may focus our view on the activating capability of transfused antibodies as an important pathomechanism in TRALI and subsequent ROS production as a central process in endothelial damage and capillary leakage.
Prepublished online as. Blood First Edition Paper, October 6, 2005; DOI 10.1182/blood-2005-04-1744.
Supported by the Deutsche Forschungsgemeinschaft (SFB 547 and DFG Bu770/3-6) and the Foundation for Haemotherapeutic Research (Bonn, Germany), of which U.J.H.S. is a fellow. The manuscript contains parts of T.W.'s doctoral thesis.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.

![Figure 1. Venous pressure elevation maneuvers. Capillary filtration coefficient (g/[min × 100 g lung weight. × ΔPc]) and challenge-induced weight gain (g/g lung weight) are shown in the absence or presence of anti-HNA-2a. Following an isogravimetric steady-state phase, venous pressure elevations were performed at indicated time points. Neutrophils derived from either high-expressing (A,C) or low-expressing (B,D) individuals were administered following the first hydrostatic challenge at minute 15. Anti-HNA-2a (black squares) or control antibody (open circles) was added at minute 50. After administration of CD177 high-expressing granulocytes and anti-HNA-2a, TRALI develops after the sixth pressure elevation (A), whereas no significant changes occur after administration of low-expressing granulocytes and anti-HNA-2a (B). Addition of fMLP to the buffer fluid at minute 120 (C,D) promotes TRALI induction in the presence of either high-expressing (C) or low-expressing (D) neutrophils. All results are presented as mean values + SD for 5 independent experiments. Note the discontinuous y-axis for. Kf,c. Pc denotes capillary pressure.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/107/3/10.1182_blood-2005-04-1744/4/m_zh80030690410001.jpeg?Expires=1767924779&Signature=m1qE9aU5gYIJ~G82x3Brdno8cAE7VGd0TdnE~vTWEtx5nuBhP78eUuIerk1CKfSoM3QkMvt4fhfdmA12c4Wo9huQHW8-oDN7FFkGLeWNoNxpRznvVselGfp4I1nukYres9ibTNqPmh6g8eprnlWt00n592A3EBHqyk8bVqmooPzA25n3nuruzHmn2epgujApvnk9M0kvbydP2oqL0xeJ1-0MyHQCgsuqThw54r0~GHtHvxoRQ7OIPW2FfLB1qjzQV~7WQHHbt7ihonYRCucvXrebsk1lTRKjHk3xh1cy5BDLIluLDMSjg3PyMeDtcyAGUzeX5fmOnmDXJyws7ex5KA__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
This feature is available to Subscribers Only
Sign In or Create an Account Close Modal