Abstract
Three general classes of small, nonpeptide “antigens” activate Vγ9Vδ2 T cells: pyrophosphomonoesters, such as isopentenyl diphosphate (IPP), nitrogen-containing bisphosphonates (N-BPs), and alkylamines. However, we have shown recently that N-BPs indirectly activate Vγ9Vδ2 T cells as a consequence of inhibition of farnesyl diphosphate synthase (a key enzyme of the mevalonate pathway) and the intracellular accumulation of IPP. We now show that alkylamines activate Vγ9Vδ2 T cells by the same mechanism. Alkylamines were found to be weak inhibitors of farnesyl diphosphate synthase and caused accumulation of unprenylated Rap1A in peripheral blood mononuclear cells and macrophages, indicative of inhibition of the mevalonate pathway. Furthermore, as with N-BPs, the stimulatory effect of the alkylamines on Vγ9Vδ2T cells was abrogated by simultaneous treatment with mevastatin. These findings suggest that only pyrophosphomonoesters such as IPP are true Vγ9Vδ2 T-cell agonists, whereas alkylamines and N-BPs indirectly activate Vγ9Vδ2 T cells through a common mechanism involving the accumulation of IPP.
Introduction
Vγ9Vδ2 (also termed Vγ2Vδ2) T cells are activated by a diverse array of nonpeptide, stimulatory molecules. The first antigen of Vγ9Vδ2 T cells to be fully described was a pyrophosphomonoester, isopentenyl pyrophosphate (IPP),1 an intermediate in the mevalonate biosynthetic pathway. Although other pyrophosphomonoester antigens have been described,2,4 Vγ9Vδ2 T cells are also activated by 2 further classes of stimulatory molecules: the nitrogen-containing bisphosphonates (N-BPs), such as pamidronate (PAM) and zoledronic acid (ZOL),5,6 and alkylamines, such as iso-butylamine (IBA) and sec-butylamine (SBA).7,10 However, we and others have recently reported that N-BPs indirectly stimulate Vγ9Vδ2 T cells in peripheral blood mononuclear cell (PBMC) cultures through inhibition of farnesyl diphosphate (FPP) synthase,11,12 a key enzyme in the mevalonate pathway. Inhibition of FPP synthase by N-BPs prevents the biosynthesis of isoprenoid lipids required for the prenylation of small guanosine triphosphatases (GTPases) such as Rap1A, but also results in the accumulation of upstream isoprenoid intermediates, such as IPP,11,13,14 which are then able to activate Vγ9Vδ2 T cells.
Given the similar structure of alkylamines and N-BPs, we sought to determine whether the alkylamines, like N-BP drugs, induce Vγ9Vδ2 T-cell activation through a similar, indirect mechanism involving inhibition of the mevalonate pathway.
Study design
Materials
ZOL (the hydrated disodium salt) was kindly provided by Novartis Pharma (Basel, Switzerland). All reagents were obtained from Sigma (Poole, Dorset, United Kingdom), unless otherwise stated. Mevastatin was converted from the lactone form to the free acid as previously described.15 Stock solutions of farnesol (FOH) and geranylgeraniol (GGOH) were prepared in ethanol, and ZOL was prepared as described previously.16 [14C]-mevalonolactone (52 mCi [1924 MBq]/mmol) was from NEN Life Science Products (Boston, MA). Cell culture reagents were from Life Technologies (Paisley, United Kingdom).
PBMC isolation and culture conditions
This study was approved by the Grampian Local Research Ethics Committee review board. Informed consent was obtained for the collection of peripheral blood from healthy volunteers in accordance with the Declaration of Helsinki. PBMCs were isolated and cultured as previously described.12 Cells were routinely cultured at a concentration of 1 × 106 cells/mL in the presence of 10 U/mL recombinant human interleukin 2 (rhIL-2).
Western blot analysis
J774 cells were cultured as previously described17 and treated with 10 mM n-butylamine (BA), IBA, iso-propylamine (IPA), or SBA for 24 hours (J774 cells) or 48 hours (PBMCs) with or without 10 μM FOH or GGOH. Lysates were prepared and subjected to immunoblotting for unprenylated Rap1A, as described previously.17,18 To ensure lanes were evenly loaded, blots were also incubated with 6 μg/mL rabbit anti-actin antibody (Sigma) followed by 0.2 μg/mL anti–rabbit IgG-horseradish peroxidase (HRP) conjugate (Calbiochem, Nottingham, United Kingdom).
Incorporation of [14C]-mevalonate into prenylated proteins in J774 cells
FPP synthase assay
Flow cytometric analysis
PBMCs were prepared for flow cytometric analysis as previously described.12 For Vγ9Vδ2 T cells, PBMCs were dual-stained with anti–CD3-peridinin chlorophyll protein (PerCP) antibody (BD Biosciences, Milan, Italy) and anti–Vδ2-fluorescein isothiocyanate (FITC) antibody (Coulter-Immunotech, Palo Alto, CA).
Quantification of IFN-γ release
Conditioned media was harvested from PBMCs and analyzed for interferon γ (IFN-γ) content with Quantikine human IFN-γ immunoassays (R&D Systems, Wiesbaden, Germany).
Results and discussion
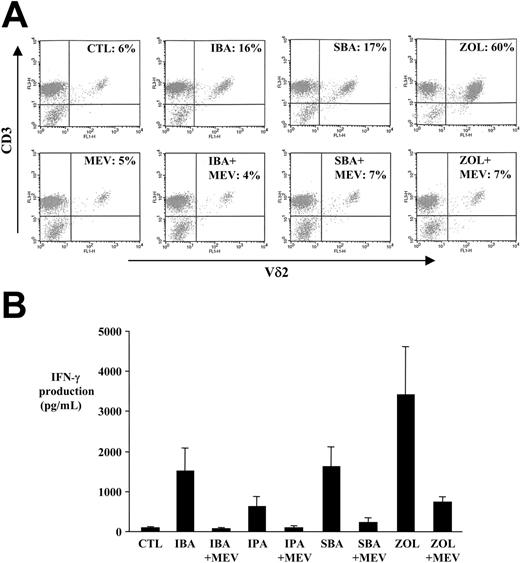
Treatment of human PBMCs for 7 days with 0.5 mM of the alkylamines IBA and SBA increased the proportion of γδ T cells (specifically, Vγ9Vδ2 T cells) in the CD3+ population (Figure 1A and Figure S1A, available on the Blood website; see the Supplemental Figures link at the top of the online article). Treatment of PBMCs for 48 hours with 0.5 mM IPA, BA, and particularly IBA and SBA, caused a marked release of IFN-γ (a more sensitive indication of T-cell activation) into conditioned media (Figure 1B and Figure S1B) consistent with previous studies that have reported a stimulatory effect of 0.5 to 10 mM alkylamines.7,10 The concentration of alkylamines required to induce Vγ9Vδ2 T-cell activation and proliferation in PBMC cultures was 500-fold greater than that required of the N-BP ZOL, because ZOL was more effective at a concentration of 1 μM than 0.5 mM of the alkylamines (Figure 1A-B and Figure S1A-B).
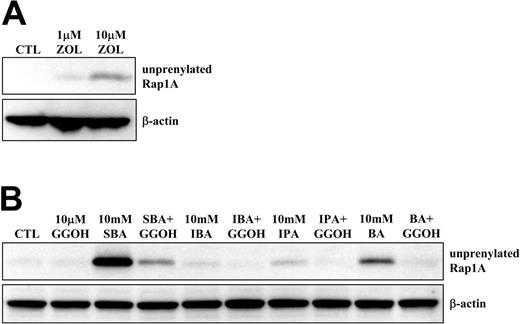
Although 1 μM N-BP (eg, ZOL) is sufficient to strongly activate Vγ9Vδ2 T cells (Figure 1B and Figure S1B), by inhibiting FPP synthase and causing accumulation of upstream IPP,12 higher concentrations (eg, 10 μM ZOL; Figure 2A) are required to cause detectable inhibitory effects on downstream protein prenylation. To determine whether alkylamines, like N-BPs, can inhibit the mevalonate pathway, we examined their effects on protein prenylation. When J774 macrophages were treated with 1 to 10 mM alkylamines for 24 hours, 5 mM or more IBA or SBA, or 10 mM IPA or BA, caused clear accumulation of unprenylated Rap1A detectable by Western blot analysis (Figure S2A). When human PBMCs were treated with 10 mM alkylamines, IPA, BA, and particularly SBA, clear accumulation of unprenylated Rap1A was noted, whereas IBA appeared less effective (Figure 2B). The apparent lesser effect of IBA on protein prenylation in PBMCs versus J774 cells is likely because IBA is more cytotoxic than other alkylamines toward PBMCs at concentrations greater than 1 mM.20 Nevertheless, these observations indicate that concentrations of alkylamines less than 1 mM (ie, that activate Vγ9Vδ2 T cells) are likely to perturb the mevalonate pathway, with detectable inhibitory effects on protein prenylation at concentrations of 5 to 10 mM.
Mevastatin prevents alkylamine-induced activation and proliferation of Vγ9Vδ2 T cells. (A) PBMCs were cultured for 7 days with 0.5 mM IBA, SBA, or 1 μM ZOL with or without 1 μM mevastatin (MEV), in the presence of rhIL-2, prior to dual staining with anti–Vδ2-FITC and anti–CD3-PerCP antibodies, and then were subjected to FACS analysis of the T cell–gated population. Data shown are from one experiment and representative of 2 further experiments from independent donors. (B) PBMCs were treated with 0.5 mM IBA, IPA, or SBA with or without 1 μM mevastatin, in the presence of rhIL-2, for 48 hours. Conditioned media was harvested and IFN-γ levels measured by enzyme-linked immunosorbent assay (ELISA). Data shown are the mean of experiments with PBMCs from 4 independent donors ± SEM. CTL indicates control.
Mevastatin prevents alkylamine-induced activation and proliferation of Vγ9Vδ2 T cells. (A) PBMCs were cultured for 7 days with 0.5 mM IBA, SBA, or 1 μM ZOL with or without 1 μM mevastatin (MEV), in the presence of rhIL-2, prior to dual staining with anti–Vδ2-FITC and anti–CD3-PerCP antibodies, and then were subjected to FACS analysis of the T cell–gated population. Data shown are from one experiment and representative of 2 further experiments from independent donors. (B) PBMCs were treated with 0.5 mM IBA, IPA, or SBA with or without 1 μM mevastatin, in the presence of rhIL-2, for 48 hours. Conditioned media was harvested and IFN-γ levels measured by enzyme-linked immunosorbent assay (ELISA). Data shown are the mean of experiments with PBMCs from 4 independent donors ± SEM. CTL indicates control.
GGOH rescues the inhibition of Rap1A prenylation by alkylamines. PBMCs were treated with (A) 1 or 10 μM ZOL, or (B) 10 mM SBA, IBA, IPA, or BA with or without 10 μM GGOH, for 48 hours prior to Western blot analysis for unprenylated Rap1A and β-actin. Data shown are from one experiment and representative of 2 further experiments from independent donors.
GGOH rescues the inhibition of Rap1A prenylation by alkylamines. PBMCs were treated with (A) 1 or 10 μM ZOL, or (B) 10 mM SBA, IBA, IPA, or BA with or without 10 μM GGOH, for 48 hours prior to Western blot analysis for unprenylated Rap1A and β-actin. Data shown are from one experiment and representative of 2 further experiments from independent donors.
To confirm that inhibition of Rap1A prenylation by alkylamines was due to inhibition of the mevalonate pathway and the loss of downstream geranylgeranyl diphosphate (GGPP) synthesis (the substrate required for Rap1A prenylation), rather than a nonspecific effect, we used cell-permeable forms of the isoprenoid lipids FPP and GGPP (FOH and GGOH, respectively) in an attempt to restore protein prenylation.21,22 When J774 cells were treated with 10 mM IBA or SBA, 10 μM GGOH completely prevented the IBA- or SBA-induced accumulation of unprenylated Rap1A (Figure S2B), whereas 10 μM FOH had no effect. Similarly, 10 μM GGOH completely prevented the accumulation of unprenylated Rap1A induced by SBA, BA, and IPA treatment in PBMC cultures (Figure 2B). We have previously demonstrated that GGOH, but not FOH, also overcomes the effects of N-BPs on osteoclasts and macrophages in vitro22,23 and restores the prenylation of Rap1A in N-BP–treated cells (Figure S2C).
To further confirm that alkylamines can inhibit the mevalonate pathway, we cultured J774 macrophages with [14C]-mevalonate in the presence of 10 mM BA, IBA, IPA, and SBA. All of the alkylamines decreased the incorporation of [14C]-mevalonate into prenylated 21- to 26-kDa small GTPase proteins (Figure S2D). Together, these observations demonstrate for the first time that alkylamines, like N-BPs, can inhibit one or more enzymes of the mevalonate pathway, thereby preventing the synthesis of downstream isoprenoid lipids such as GGPP and causing loss of prenylated proteins.
We next examined the effects of alkylamines on recombinant human FPP synthase, the molecular target of N-BP drugs.13,14,16 All of the alkylamines tested inhibited rhFPP synthase, with IBA and SBA more effective than BA or IPA (Table 1), although inhibition of FPP synthase by alkylamines occurs at much higher concentration (∼106-fold higher) than that observed with N-BPs (50% inhibitory concentration [IC50] ∼3 nM for ZOL).16 The lower potency of the alkylamines for inhibiting FPP synthase is consistent with the lower potency for inhibiting Rap1A prenylation, requiring millimolar concentrations (Figure 2B and Figure S2A-C) compared to the micromolar concentrations of N-BPs required to inhibit Rap1A prenylation.17,18
Effect of alkylamines on rhFPP synthase activity
Alkylamine . | rhFPP synthase activity remaining at 1 mM, % . | rhFPP synthase activity remaining at 10 mM, % . |
|---|---|---|
| iso-Propylamine | 75.5 ± 2.7 | 43.6 ± 4.1 |
| n-Butylamine | 72.9 ± 3.4 | 40.6 ± 3.4 |
| sec-Butylamine | 67.5 ± 2.2 | 32.8 ± 1.4 |
| iso-Butylamine | 60.6 ± 1.6 | 28.8 ± 1.9 |
Alkylamine . | rhFPP synthase activity remaining at 1 mM, % . | rhFPP synthase activity remaining at 10 mM, % . |
|---|---|---|
| iso-Propylamine | 75.5 ± 2.7 | 43.6 ± 4.1 |
| n-Butylamine | 72.9 ± 3.4 | 40.6 ± 3.4 |
| sec-Butylamine | 67.5 ± 2.2 | 32.8 ± 1.4 |
| iso-Butylamine | 60.6 ± 1.6 | 28.8 ± 1.9 |
Enzyme activity was determined in the presence of 1 or 10 mM IPA, BA, SBA, or IBA. Data shown are the means of 3 independent experiments ± SEM.
As well as preventing protein prenylation, inhibition of FPP synthase by N-BPs causes the accumulation of the upstream metabolites IPP/dimethylallyl diphosphate (DMAPP),11,13 an effect that can be prevented by simultaneously inhibiting the proximal enzyme in the mevalonate pathway, HMG-CoA reductase, with statins.11 Consistent with this, the stimulatory effect of IBA and SBA on Vγ9Vδ2 T-cell activation and proliferation was completely abrogated by simultaneous treatment with 1 μM mevastatin (Figure 1A-B). This was not due to a cytotoxic effect of the mevastatin, because PBMCs treated with SBA plus mevastatin or mevastatin alone responded to the further addition of IPP with an increase in IFN-γ release and Vγ9Vδ2 T-cell proliferation (Figure S3), in accord with a previous study with mevastatin and N-BPs.12 Furthermore, addition of 100 μM mevalonate largely restored the stimulatory effect of SBA in the presence of mevastatin (Figure S3). In addition, consistent with our previous study with N-BPs,12 1 μM lovastatin (but not des-oxo-lovastatin, an analog that does not inhibit HMG-CoA reductase) also completely prevented the stimulatory effect of SBA on Vγ9Vδ2 T-cell activation and proliferation (Figure S4). Together, these observations indicate that alkylamines, like N-BP drugs, indirectly stimulate Vγ9Vδ2 T-cell activation and proliferation by inhibiting FPP synthase (and perhaps also other enzymes of the mevalonate pathway) in PBMCs, thereby causing an accumulation of IPP/DMAPP. It is possible that the accumulation of IPP/DMAPP might be further potentiated by feedback up-regulation of HMG-CoA reductase, because our unpublished studies demonstrate that N-BPs cause an increase in HMG-CoA reductase in J774 cells. Nevertheless, statins, by inhibiting at a proximal point in the mevalonate pathway, can prevent the accumulation of IPP/DMAPP and thereby completely prevent the ability of alkylamines or N-BPs to activate Vγ9Vδ2 T cells. The low potency of alkylamines for activating Vγ9Vδ2 T cells compared with N-BPs (Figure S1A-B) reflects the low potency of alkylamines for inhibiting FPP synthase.
Dietary ingestion of plant products rich in ethylamine, and its precursor l-theanine, such as tea,7,10 apples,24 and wine25 may “prime” Vγ9Vδ2 T cells and contribute to the health properties of these foodstuffs.7 Furthermore, because alkylamines and N-BPs act through a similar mechanism, extensive dietary ingestion of alkylamines may explain, in part, why some patients (perhaps those with a greater dietary intake of alkylamines), but not all, suffer an acute phase response to intravenous N-BP therapy.26,28
In summary, we demonstrate in this study that alkylamines, although previously considered to be direct antigens for Vγ9Vδ2 T cells, act indirectly to stimulate Vγ9Vδ2 T-cell activation and proliferation by inhibiting FPP synthase and causing the subsequent intracellular accumulation of IPP, a well-characterized agonist of Vγ9Vδ2 T cells.1,6,29,30 This strongly suggests that only pyrophosphomonoesters such as IPP are true Vγ9Vδ2-TCR agonists, whereas alkylamines and N-BPs act in a similar, indirect manner to activate Vγ9Vδ2 T cells through inhibition of the mevalonate pathway.
Prepublished online as Blood First Edition Paper, September 22, 2005; DOI 10.1182/blood-2005-03-1025.
The online version of the article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
We are grateful to Dr Jim Dunford for advice on assaying FPP synthase activity.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal