Abstract
The snake venom rhodocytin has been reported to bind to integrin α2β1 and glycoprotein (GP) Ibα on platelets, but it is also able to induce activation independent of the 2 receptors and of GPVI. Using rhodocytin affinity chromatography, we have identified a novel C-type lectin receptor, CLEC-2, in platelets that confers signaling responses to rhodocytin when expressed in a cell line. CLEC-2 has a single tyrosine residue in a YXXL motif in its cytosolic tail, which undergoes tyrosine phosphorylation upon platelet activation by rhodocytin or an antibody to CLEC-2, but not to collagen, thrombin receptor agonist peptide (TRAP), or convulxin. Tyrosine phosphorylation of CLEC-2 and other signaling proteins by rhodocytin is inhibited by the Src family kinase inhibitor PP2. Further, activation of murine platelets by rhodocytin is abolished in the absence of Syk and PLCγ2, and partially reduced in the absence of LAT, SLP-76, and Vav1/Vav3. These findings define a novel signaling pathway in platelets whereby activation of CLEC-2 by rhodocytin leads to tyrosine phosphorylation of its cytosolic tail, binding of Syk and initiation of downstream tyrosine phosphorylation events, and activation of PLCγ2. CLEC-2 is the first C-type lectin receptor to be found on platelets which signals through this novel pathway.
Introduction
Snake venoms contain a vast number of toxins that target a rich diversity of proteins in the vasculature and elsewhere.1,2 Identification of the molecular targets of many of these toxins has made an enormous contribution to our understanding of key physiological processes. This includes the discovery of new classes of signaling receptors that regulate the function of cells in the vasculature. For example, the C-type lectin snake venom toxin, convulxin, which elicits powerful activation of platelets, was instrumental in the purification and cloning of the immunoglobulin glycoprotein VI (GPVI), which is the major signaling receptor for collagen on platelets.3 Further, convulxin has played a critical role in our understanding of the molecular basis of platelet activation by GPVI4,5 and has been an important tool for analyzing GPVI expression on platelets and on cell lines.6,7 Significantly, it is now realized that many other snake venom toxins also target GPVI, including other C-type lectins,8,9 and a metalloproteinase, alborhagin.10 Snake venom toxins have also been reported to target other classes of platelet receptors, including mocarhagin, which cleaves GPIb/IX/V, a receptor for von Willebrand factor (VWF), and disintegrins, which inhibit the major platelet integrin, αIIbβ3.1,2
The C-type lectin snake venom toxin, rhodocytin (also called aggretin) was purified from the venom of Calloselasma rhodostoma by 2 separate research groups on the basis of its ability to elicit powerful activation of platelets, with a characteristic lag phase.11,12 Initial studies provided evidence that rhodocytin mediates platelet activation through binding to a collagen receptor, integrin α2β1, and to GPIb/IX/V.12-14 A subsequent study, however, using α2-deficient murine platelets that also lack the extracellular domain of GPIbα, demonstrated that neither receptor is essential for activation by rhodocytin.15 Further, rhodocytin is able to activate murine platelets deficient in the GPVI/Fc receptor (FcR) γ-chain complex.13-15 Thus, the cell-surface receptor underlying platelet activation by rhodocytin, and the roles played by integrin α2β1 and GPIb/IX/V in this interaction, are not known.
In this study, we used rhodocytin affinity chromatography and mass spectrometry in an attempt to identify 1 or more further receptors that could underlie platelet activation by rhodocytin. This approach led to the identification of a novel 32-kDa surface receptor, the C-type lectin, CLEC-2, that is able to confer signaling responses to rhodocytin when expressed in a cell line. Significantly, CLEC-2 has a single tyrosine in its cytosolic tail that represents one-half of an immunoreceptor tyrosine-based activation motif (ITAM), YXXL, and which undergoes tyrosine phosphorylation upon activation by rhodocytin downstream of Src kinases. Further, tyrosine-phosphorylated CLEC-2 is precipitated by the tandem SH2 domains of Syk, and activation of platelets by rhodocytin is abolished in the absence of Syk. These results demonstrate that CLEC-2 is a novel platelet activation receptor that is likely to underlie activation by the snake toxin rhodocytin. These results are also consistent with a model in which CLEC-2 signals through sequential activation of Src and Syk families of tyrosine kinases and a novel YXXL motif. These results have previously been reported in abstract form.16
Materials and methods
Animals and materials
Genetically modified mice deficient in PLCγ2,17 Syk,18 LAT,19 SLP-76,20 and Vav1/321 were obtained as previously described. Rhodocytin was purified from the venom of Calloselasma rhodostoma.11,22 Polyclonal anti–CLEC-2 antibody was from R&D Systems (Minneapolis, MN). Polyclonal rabbit anti-rhodocytin antibody was generated by T.M. and G.D.L. Gly-Arg-Gly-Asp-Ser (GRGDS) peptide was obtained from Peptide Institute (Osaka, Japan). Ser-Phe-Leu-Leu-Arg-Asn (SFLLRN) peptide (thrombin receptor agonist peptide [TRAP]) was from Bachem Biochemica (Heidelberg, Germany). Biotin-labeled phosphorylated or nonphosphorylated peptide containing the YXXL motif in CLEC-2 (biotin-6-aminohexanoic acid [Ahx]–MQDEDGY(PO3H2)ITLNIK-OH, or biotin-6-aminohexanoic acid [Ahx]–MQDEDGYITLNIK-OH) was synthesized by Kurabo Industries (Osaka, Japan). All other reagents were from previously named sources.6,17,21
Platelet preparation
Venous blood from healthy drug-free volunteers was taken into 10% sodium citrate. This study was approved by the ethical committees in University of Yamanashi and University of Birmingham, and informed consent was provided according to the Declaration of Helsinki. Murine blood was drawn from CO2 asphyxiated mice by portal vein puncture and taken into 100 μL of acid citrate dextrose. Washed human and murine platelets were obtained by centrifugation as previously described, using prostacyclin to prevent activation during the isolation procedure.17 Both sets of washed platelets were resuspended in modified Tyrode buffer17 at a cell density of 2 × 108 or 1 × 109/mL.
Pull-down with rhodocytin-coated beads
Purified rhodocytin (200 μg) was covalently coupled to 122 mg cyanogen bromide (CNBr)–activated Sepharose 4B beads (Amersham Biosciences, Piscataway, NJ) according to the manufacturer's instructions. Glycine-coated beads were used as a negative control. Surface proteins in washed platelets or rhodocytin were labeled with biotin using ECL Protein Biotinylation System (Amersham Biosciences). One milliliter biotin-labeled or -unlabeled washed platelets (1 × 109/mL) was lysed by an equal volume of 2 × ice-cold lysis buffer17 and precleared by 100 μL Sepharose 4B (50% slurry) for 1 hour. After the detergent-insoluble debris was clarified by centrifugation at 16 000g for 10 minutes, the supernatant was incubated with 200 μL rhodocytin-bound or glycine-bound Sepharose 4B for 4 hours at 4°C. The beads were washed 5 times in 1 × lysis buffer, and proteins were eluted from the beads with 40 μL of sodium dodecyl sulfate (SDS)–reducing sample buffer and boiled for 5 minutes. Precipitated platelet proteins were separated by 4% to 20% SDS–polyacrylamide gel electrophoresis (PAGE) and electrotransferred to polyvinylidene difluoride (PVDF) membrane. Biotin-labeled surface proteins were detected by HRP (horseradish peroxidase)–conjugated streptavidin. Unlabeled platelet proteins electrotransferred to PVDF membrane were ligand blotted with biotin-labeled rhodocytin and HRP-conjugated streptavidin, followed by reprobing with anti–CLEC-2 antibody. For mass spectrometric analysis, unlabeled washed platelets were used and gels were stained with Coomassie Brilliant Blue.
Protein digestion and mass spectrometric analysis
The gel pieces were dried in a speed-vac and in-gel trypsinolysis was carried out as described.23 The high-performance liquid chromatography (HPLC) (CapLC; Waters, Milford, MA) was coupled to a Q-TOF mass spectrometer (Micromass, Manchester, United Kingdom) equipped with a nanoelectrospray Z-spray source. Mobile phases for the HPLC were 5% acetonitrile, 0.1% formic acid (Solvent A), and 95% acetonitrile, 0.1% formic acid (Solvent B). The tryptic peptides were loaded and desalted on a 300-μm internal diameter (id)/5-mm length C18 PepMap pre-column (LC Packings, San Francisco, CA), and then eluted at a flow of 200 nL/min onto a75 μm id/25-cm length C18 PepMap nano column, using a gradient of 5% to 40% Solvent B in 40 minutes and then 40% to 80% Solvent B in 3 minutes. Tandem mass spectrometry (MS/MS) analysis was carried out as previously described.24 The database search was performed with the MASCOT search tool (Matrix Science, London, United Kingdom) screening SWISS-PROT.42
N-glycosidase treatment
Immunoprecipitation with anti–CLEC-2 from platelets was performed as described in “Immunoprecipitation and Western blotting.” After washing, immunoprecipitated samples were incubated with 1.5% 2-mercaptoethanol for 3 minutes at 100°C. After addition of 1.4% Nonidet P-40, samples were incubated with or without 55 mU/mL N-glycosidase F (TaKaRa Bio, Shiga, Japan) at 37°C overnight. Immunoprecipitated and enzyme-treated CLEC-2 protein was dissolved with SDS sample buffer, separated by SDS-PAGE, electrotransferred, and Western blotted using the CLEC-2 antibody.
Cell culture and stimulation
CLEC-2 was expressed under a tet repressor protein in 293T-REx cell line (Invitrogen, Carlsbad, CA) and grown as described.25 CLEC-2 expression was induced by addition of 1 μg/mL doxycycline to the medium 24 to 48 hours before experimentation. Vehicle-added cells were used for control. Cells were detached and resuspended in modified Tyrode buffer as described6 and stimulated with vehicle or 500 nM rhodocytin for 10 minutes at 37°C. Reactions were stopped by addition of 2 × SDS sample buffer. Tyrosine phosphorylation and CLEC-2 expression were detected by antiphosphotyrosine (4G10) and anti–CLEC-2 antibodies, respectively, as described.17
Flow cytometry studies
Washed human platelets (1 × 108/mL) or 293T-REx cells (5 × 106/mL) were incubated with 2 μg/mL of anti–CLEC-2 antibody for 15 minutes followed by staining with fluoroscein isothiocyanate (FITC)–conjugated anti-goat immunoglobulin G (IgG) for 15 minutes at room temperature. After diluting 3-fold with phosphate-buffered saline (PBS), samples were analyzed with a FACScan flow cytometer and CellQuest software (Becton Dickinson, San Jose, CA) as described.17 To detect direct binding of rhodocytin, 293T-REx cells (5 × 106/mL) pretreated with or without doxycycline were preincubated with 100 nM rhodocytin for 15 minutes at room temperature. After excess of rhodocytin was removed by centrifugation, cells were incubated with 10 μg/mL of control rabbit IgG or antirhodocytin antibody for 20 minutes at room temperature followed by removing unbound antibodies. Then, the samples were stained with FITC-conjugated anti–rabbit IgG and analyzed as described.
Immunoprecipitation and Western blotting
Washed human platelets (1 × 109/mL) pretreated with 1 mM GRGDS peptide or washed murine platelets (2 × 108/mL) pretreated with 10 μM lotrafiban to inhibit platelet aggregation were stimulated with 50 nM rhodocytin, 100 μM SFLLRN, or 50 μg/mL collagen. Where indicated, 30 μM PP2 (a Src kinase inhibitor) or PP3 (an inactive analog of PP2) were incubated for 10 minutes at 37°C. Reactions were terminated by addition of 2 × ice-cold lysis buffer. Platelet lysates were precleared and detergent-insoluble debris was clarified as described.17 A small aliquot was dissolved with SDS sample buffer for detection of total tyrosine phosphorylation. Antibodies against CLEC-2, PLCγ2, Syk, Btk, SLP-76, LAT, Vav1, or Vav3 were added to the resultant supernatant and incubated overnight. Where indicated, 10 μg of CLEC-2 YXXL-containing peptide plus avidin-Sepharose or GST-fusion protein–associated glutathione Sepharose were incubated with the lysates. In the indicated experiments, human washed platelets (1 × 109/mL) were stimulated by 10 μg/mL of anti–CLEC-2 antibody or control goat IgG in the presence of F(ab)′2 fragment of anti-FcγRIIA antibody (IV.3; 10 μg/mL). The samples were lysed with 2× lysis buffer before and 5 minutes after stimulation. Anti–CLEC-2 antibody (5 μg/mL) and control goat IgG were added to the sample lysed before stimulation. Anti–CLEC-2 (5 μg/mL) or control goat IgG was added to the sample stimulated by control IgG and that stimulated by CLEC-2 antibody, respectively. After removing insoluble debris by centrifugation, CLEC-2 was immunoprecipitated by addition of protein G. Precipitated proteins or whole-cell lysates were separated by SDS-PAGE, electrotransferred, and Western blotted by the indicated antibodies.
Platelet aggregation
Human washed platelets (2 × 108/mL) were stimulated by 10 μg/mL of anti–CLEC-2 antibody or control goat IgG in the presence of F(ab)′2 fragment of anti-FcγRIIA antibody (IV.3; 10 μg/mL). Washed murine or human (2 × 108/mL) platelets were stimulated with low and intermediate concentrations of rhodocytin as indicated in “Critical role of LAT, SLP-76, Vav1/3, and PLCγ2 in signaling by rhodocytin.” Aggregation was monitored by light transmission using a Born aggregometer (Chronolog, Havertown, PA) as described.17
Results
CLEC-2 binds to rhodocytin-coated beads
We used Sepharose 4B beads that were covalently coupled with rhodocytin or glycine to isolate binding proteins for the snake toxin from platelet lysates, whose surface proteins had been labeled with biotin. Blotting using streptavidin demonstrated that the rhodocytin-but not glycine-coupled Sepharose 4B beads precipitated a 32-kDa protein that was pulled down along with several other proteins that associated nonspecifically with both sets of beads (Figure 1A). The same 32-kDa band was also precipitated by rhodocytin-but not glycine-coupled beads from non–biotin-labeled platelets, as detected by ligand blotting using rhodocytin (Figure 1B). The portion of the gel corresponding to the 32-kDa band was excised, digested with trypsin, and subjected to MS/MS analysis. This approach identified CLEC-2 as a component of the 32-kDa band (Table 1). The expression of CLEC-2 on platelets was confirmed by flow cytometry and Western blotting using a specific antibody (Figure 1C,D). Interestingly, CLEC-2 was detected as a doublet and sometimes as a triplet in platelets by Western blotting, with a major band migrating at 32 kDa and a minor band at 40 kDa (Figure 1D,G). The 32-kDa band corresponds to that precipitated by the rhodocytin-coupled beads. The apparent absence of the 40-kDa band in the rhodocytin-affinity eluates may be because of comigration with a nonspecific protein (Figure 1A only) or because of a lower level of expression of the 40-kDa band combined with a limitation in the sensitivity of the ligand-blotting assay. Significantly, precipitation of 32- and 40-kDa bands by rhodocytin-but not by glycine-coupled beads could be detected using a specific antibody to CLEC-2, although the 40-kDa band was present at a much lower level than the 32-kDa band, consistent with the results in platelets (Figure 1E). Densitometric analysis of anti–CLEC-2 Western blots from several platelet samples demonstrated that the level of expression of the 40-kDa is 23.1% ± 5.3% of 32-kD band (Figure 1F). The presence of the 32- and 40-kDa forms of CLEC-2 in platelets suggests that it may undergo differential glycosylation, consistent with the presence of 3 potential sites of N-glycosylation. To address this, we treated CLEC-2 immunoprecipitates with N-glycosidase, which cleaves N-glycosylation. As shown in Figure 1G, the 32- and 40-kDa bands, and a third band that was seen in some experiments, collapse to a single band of 27 kDa, the molecular weight that is deduced from the amino acid sequence of CLEC-2.26 These findings demonstrate that the different molecular weights are due to different glycosylation.
Compiled data output file for MASCOT MS searches
Protein name and peptide sequences . |
|---|
| (Q9P126) C-type lectin-like receptor 2 |
| TGTLQQLAK (0) |
| YYGDSCYGFFR (0) |
| THLIR (0) |
| NYLQDENENR (0) |
| RFCQYVVK (1) |
| FCQYVVK (0) |
| HYLMCER (0) + 1 oxidation (M) |
| MHPTFCENK (0) + 1 oxidation (M) |
Protein name and peptide sequences . |
|---|
| (Q9P126) C-type lectin-like receptor 2 |
| TGTLQQLAK (0) |
| YYGDSCYGFFR (0) |
| THLIR (0) |
| NYLQDENENR (0) |
| RFCQYVVK (1) |
| FCQYVVK (0) |
| HYLMCER (0) + 1 oxidation (M) |
| MHPTFCENK (0) + 1 oxidation (M) |
The 8 peptides that matched are shown. SWISS-PROT search results with 25.76% sequence coverage. Numbers in parentheses indicate number of tryptic miscleavage sites.
Association of CLEC-2 with rhodocytin-coated beads. (A) Washed platelets were labeled with biotin and lysed with an equal volume of 2 × lysis buffer. They were precleared and incubated with rhodocytin-bound (rhod-Seph) or glycin-bound (gly-Seph) Sepharose 4B for 4 hours at 4°C. After extensive washing, proteins were eluted from the beads with SDS-reducing sample buffer. Precipitated platelet proteins were separated by 4% to 20% SDS-PAGE and detected by horseradish peroxidase–conjugated streptavidin (avidin-HRP). Data are representative of 2 experiments. (B) Pull-down and electrophoresis were performed as described in panel A using unlabeled washed platelets. Ligand blotting was performed by biotin-conjugated rhodocytin (rhodocytin-biotin) and avidin-HRP. Arrows indicate 32-kDa protein that was precipitated specifically by the rhodocytin-coupled beads. (C) Washed human platelets were incubated with control goat IgG or anti–CLEC-2 antibody, followed by staining with FITC-conjugated anti-goat IgG. Samples were analyzed with a Becton Dickinson FACScan. (D) Washed platelets were dissolved in 4 × SDS sample buffer, separated by SDS-PAGE, and blotted with anti–CLEC-2 antibody (left) or control goat IgG (right). The data are representative of 5 experiments. (E) The membrane used in panel B was reprobed with anti–CLEC-2 antibody. (F) Densitometric analysis of 32- and 40-kDa CLEC-2 bands in panel D was performed by Molecular Imager FX and Quantity One software (Bio-Rad Laboratories, Hercules, CA). Relative intensity of the 40-kDa band compared with the 32-kDa band was expressed as mean ± SE (n = 4). (G) CLEC-2 immunoprecipitates were incubated with 1.5% 2-mercaptoethanol for 3 minutes at 100°C. After addition of 1.4% Nonidet P-40, the proteins were incubated with or without N-glycosidase F at 37°C overnight. Immunoprecipitated and enzyme-treated CLEC-2 protein was dissolved with SDS sample buffer, separated by SDS-PAGE, electrotransferred, and Western blotted by the CLEC-2 antibody.
Association of CLEC-2 with rhodocytin-coated beads. (A) Washed platelets were labeled with biotin and lysed with an equal volume of 2 × lysis buffer. They were precleared and incubated with rhodocytin-bound (rhod-Seph) or glycin-bound (gly-Seph) Sepharose 4B for 4 hours at 4°C. After extensive washing, proteins were eluted from the beads with SDS-reducing sample buffer. Precipitated platelet proteins were separated by 4% to 20% SDS-PAGE and detected by horseradish peroxidase–conjugated streptavidin (avidin-HRP). Data are representative of 2 experiments. (B) Pull-down and electrophoresis were performed as described in panel A using unlabeled washed platelets. Ligand blotting was performed by biotin-conjugated rhodocytin (rhodocytin-biotin) and avidin-HRP. Arrows indicate 32-kDa protein that was precipitated specifically by the rhodocytin-coupled beads. (C) Washed human platelets were incubated with control goat IgG or anti–CLEC-2 antibody, followed by staining with FITC-conjugated anti-goat IgG. Samples were analyzed with a Becton Dickinson FACScan. (D) Washed platelets were dissolved in 4 × SDS sample buffer, separated by SDS-PAGE, and blotted with anti–CLEC-2 antibody (left) or control goat IgG (right). The data are representative of 5 experiments. (E) The membrane used in panel B was reprobed with anti–CLEC-2 antibody. (F) Densitometric analysis of 32- and 40-kDa CLEC-2 bands in panel D was performed by Molecular Imager FX and Quantity One software (Bio-Rad Laboratories, Hercules, CA). Relative intensity of the 40-kDa band compared with the 32-kDa band was expressed as mean ± SE (n = 4). (G) CLEC-2 immunoprecipitates were incubated with 1.5% 2-mercaptoethanol for 3 minutes at 100°C. After addition of 1.4% Nonidet P-40, the proteins were incubated with or without N-glycosidase F at 37°C overnight. Immunoprecipitated and enzyme-treated CLEC-2 protein was dissolved with SDS sample buffer, separated by SDS-PAGE, electrotransferred, and Western blotted by the CLEC-2 antibody.
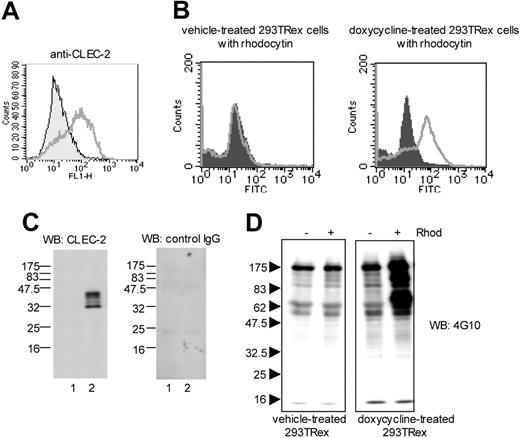
Selective response of CLEC-2–expressing 293T-REx cells to rhodocytin. (A) 293T-REx cells, which express CLEC-2 under a tet repressor, were incubated with vehicle or 1 μg/mL of doxycycline for 24 to 48 hours. 5 × 106/mL cells were incubated with an antibody to CLEC-2 or an isotype matched control and analyzed by FACscan. Filled areas represent vehicle-treated 293TRex cells; lines, doxycycline-treated 293TRex cells. (B) 293T-REx cells (5 × 106/mL) pretreated with or without doxycycline were preincubated with 100 nM rhodocytin. After excess of rhodocytin was removed by centrifugation, cells were incubated with control rabbit IgG (filled areas) or antirhodocytin antibody (lines), followed by FITC-conjugated anti–rabbit IgG. (C) 1 × 107 cells were dissolved with 4 × SDS sample buffer. Proteins were separated by SDS-PAGE and blotted with anti–CLEC-2 antibody (left panel) or control–goat IgG (right panel). Lane 1: vehicle-treated 293TRex cells; lane 2: doxycycline-treated 293TRex cells. (D) Cells were stimulated with or without 500 nM rhodocytin for 10 minutes, dissolved with SDS sample buffer, and separated by SDS-PAGE. Protein tyrosine phosphorylation was detected by Western blotting with antiphosphotyrosine antibody (4G10). The data are representative of 2 to 3 experiments.
Selective response of CLEC-2–expressing 293T-REx cells to rhodocytin. (A) 293T-REx cells, which express CLEC-2 under a tet repressor, were incubated with vehicle or 1 μg/mL of doxycycline for 24 to 48 hours. 5 × 106/mL cells were incubated with an antibody to CLEC-2 or an isotype matched control and analyzed by FACscan. Filled areas represent vehicle-treated 293TRex cells; lines, doxycycline-treated 293TRex cells. (B) 293T-REx cells (5 × 106/mL) pretreated with or without doxycycline were preincubated with 100 nM rhodocytin. After excess of rhodocytin was removed by centrifugation, cells were incubated with control rabbit IgG (filled areas) or antirhodocytin antibody (lines), followed by FITC-conjugated anti–rabbit IgG. (C) 1 × 107 cells were dissolved with 4 × SDS sample buffer. Proteins were separated by SDS-PAGE and blotted with anti–CLEC-2 antibody (left panel) or control–goat IgG (right panel). Lane 1: vehicle-treated 293TRex cells; lane 2: doxycycline-treated 293TRex cells. (D) Cells were stimulated with or without 500 nM rhodocytin for 10 minutes, dissolved with SDS sample buffer, and separated by SDS-PAGE. Protein tyrosine phosphorylation was detected by Western blotting with antiphosphotyrosine antibody (4G10). The data are representative of 2 to 3 experiments.
These results identify CLEC-2 as a novel rhodocytin binding protein on the platelet surface. Intriguingly, CLEC-2 is a type II transmembrane protein of the C-type lectin superfamily with a single tyrosine residue in its cytoplasmic tail that is present in a YXXL motif,26 suggesting that it may have the capacity to signal through a tyrosine kinase–based pathway. There are no previous reports of the presence of CLEC-2 on platelets or its ability to function as a signaling receptor.
Rhodocytin stimulates tyrosine phosphorylation in a CLEC-2–expressing cell line
To confirm CLEC-2 as a receptor for rhodocytin and its ability to generate tyrosine kinase–based intracellular signals, we used a previously reported cell line in which CLEC-2 was expressed under the control of a tet repressor protein.25 The addition of doxycycline to transfected 293T-REx cells induces surface expression of CLEC-2, as measured using a specific antibody to the lectin receptor (Figure 2A) or a combination of rhodocytin and a rhodocytin antibody (Figure 2B). Further, Western blotting for CLEC-2 using a specific antibody but not with a control IgG identified 2 major bands of approximately 32 and 40 kDa and a minor band of 34 kDa in doxycycline-treated but not control cells (Figure 2C). The presence of more than 1 band corresponding to CLEC-2 is similar to the situation in platelets, although 3 bands were detected in the cell line and the 40-kDa band was expressed at a similar level to the 32-kDa band. Significantly, rhodocytin stimulation induced an increase in tyrosine phosphorylation of several proteins in whole-cell lysates from doxycycline-treated (CLEC-2–expressing) but not from vehicle-treated cells (Figure 2D). Together, these results confirm that CLEC-2 is a functional receptor for rhodocytin.
Activation of platelets using an antibody to CLEC-2
We used an antibody to CLEC-2 to investigate whether the lectin receptor is able to induce activation of platelets in the absence of signals from other receptors. This experiment is necessary bearing in mind that rhodocytin binds to at least 2 other platelet receptors, the integrin α2β1 and GPIbα.12-14 As shown in Figure 3A, an antibody to CLEC-2 induces platelet aggregation after a characteristic delay. This experiment was carried out in the presence of monoclonal antibody IV.3 to block binding of Fc portion of the antibody to FcγRIIA. A control goat IgG did not stimulate aggregation. Significantly, the CLEC-2 antibody, but not a control goat IgG, induced a significant increase in tyrosine phosphorylation above basal of both the 32- and 40-kDa forms of CLEC-2, as demonstrated by immunoprecipitation of CLEC-2 and Western blotting for phosphotyrosine and then for CLEC-2 (Figure 3B). Since CLEC-2 contains only a single tyrosine in its cytosolic tail, phosphorylation must have taken place at this YXXL motif. These results demonstrate that engagement of CLEC-2 is sufficient to mediate platelet activation in the absence of signals from other receptors. Further, the demonstration that the lectin receptor undergoes tyrosine phosphorylation provides a possible mechanism underlying CLEC-2–mediated platelet activation.
Platelet aggregation and CLEC-2 tyrosine phosphorylation induced by cross-linking of CLEC-2 by anti–CLEC-2 antibody. (A) Human washed platelets (2 × 108/mL) were stimulated by 10 μ g/mL of anti–CLEC-2 antibody or control goat IgG in the presence of F(ab)′2 fragment of anti-FcγRIIA antibody (IV.3) and platelet aggregation was monitored with an aggregometer. (B) Human washed platelets (1 × 109/mL) were lysed with 2 × lysis buffer after stimulation as described. CLEC-2 was immunoprecipitated and sequentially Western blotted with antiphosphotyrosine antibody (4G10) or polyclonal anti–CLEC-2 antibody. The data are representative of 4 experiments. Lane 1: basal; lane 2: goat anti–CLEC-2; lane 3: control goat IgG.
Platelet aggregation and CLEC-2 tyrosine phosphorylation induced by cross-linking of CLEC-2 by anti–CLEC-2 antibody. (A) Human washed platelets (2 × 108/mL) were stimulated by 10 μ g/mL of anti–CLEC-2 antibody or control goat IgG in the presence of F(ab)′2 fragment of anti-FcγRIIA antibody (IV.3) and platelet aggregation was monitored with an aggregometer. (B) Human washed platelets (1 × 109/mL) were lysed with 2 × lysis buffer after stimulation as described. CLEC-2 was immunoprecipitated and sequentially Western blotted with antiphosphotyrosine antibody (4G10) or polyclonal anti–CLEC-2 antibody. The data are representative of 4 experiments. Lane 1: basal; lane 2: goat anti–CLEC-2; lane 3: control goat IgG.
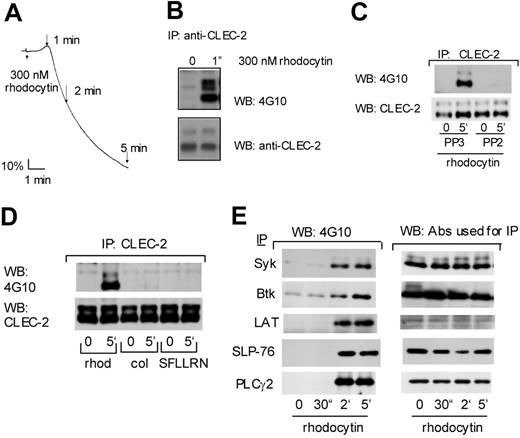
Tyrosine phosphorylation of CLEC-2 and signaling molecules downstream GPVI upon rhodocytin stimulation. (A) Human washed platelets (2 × 108/mL) were stimulated by 300 nM rhodocytin and platelet aggregation was monitored using an aggregometer. (B-E) Washed human platelets (300 or 500 μL at 1 × 109/mL) were stimulated with (B) 300 nM rhodocytin, (C-E) 50 nM rhodocytin, 50 μ g/mL collagen, or 100 μM SFLLRN for the indicated times. In panel D, platelets were pretreated with 30 μM PP3 or PP2, prior to stimulation with rhodocytin. Reactions were terminated by addition of an equal volume of 2 × lysis buffer. Platelet lysates were precleared and detergent-insoluble debris was removed by centrifugation. Antibodies against CLEC-2 (B-D), PLCγ2, Syk, Btk, SLP-76, or LAT (E) were added to the resultant supernatant and incubated overnight with protein A or G Sepharose. Precipitated proteins were separated by SDS-PAGE and Western blotted with the indicated antibodies. The data are representative of 2 to 5 experiments.
Tyrosine phosphorylation of CLEC-2 and signaling molecules downstream GPVI upon rhodocytin stimulation. (A) Human washed platelets (2 × 108/mL) were stimulated by 300 nM rhodocytin and platelet aggregation was monitored using an aggregometer. (B-E) Washed human platelets (300 or 500 μL at 1 × 109/mL) were stimulated with (B) 300 nM rhodocytin, (C-E) 50 nM rhodocytin, 50 μ g/mL collagen, or 100 μM SFLLRN for the indicated times. In panel D, platelets were pretreated with 30 μM PP3 or PP2, prior to stimulation with rhodocytin. Reactions were terminated by addition of an equal volume of 2 × lysis buffer. Platelet lysates were precleared and detergent-insoluble debris was removed by centrifugation. Antibodies against CLEC-2 (B-D), PLCγ2, Syk, Btk, SLP-76, or LAT (E) were added to the resultant supernatant and incubated overnight with protein A or G Sepharose. Precipitated proteins were separated by SDS-PAGE and Western blotted with the indicated antibodies. The data are representative of 2 to 5 experiments.
Src kinases mediate tyrosine phosphorylation of CLEC-2
Rhodocytin stimulates platelet activation with a characteristic lag phase, which reduces as the concentration of the toxin is increased. At a relatively high concentration of rhodocytin (300 nM), activation begins after a delay of approximately 30 seconds (Figure 4A), although this is much longer with lower concentrations of the snake venom toxin. In line with this, the stimulation of tyrosine phosphorylation in whole-cell lysates by rhodocytin occurs over a similar time scale to that of platelet aggregation, occurring more rapidly with higher concentrations of rhodocytin (not shown). Significantly, substantial phosphorylation of CLEC-2 can be seen in response to rhodocytin at 60 seconds, which corresponds to the peak in the shape change response and the onset of aggregation (Figure 4A,B). This is reminiscent of the time course of platelet aggregation by collagen, which also occurs after a dose-dependent delay, and together with the increase in tyrosine phosphorylation of platelet lysates and Fc receptor γ-chain, which forms part of the collagen receptor.27
The significance of tyrosine phosphorylation events in platelet activation by rhodocytin is illustrated by the ability of the Src family kinase inhibitors PP1 or PP2 to block tyrosine phosphorylation in whole cells and all functional responses in response to stimulation by rhodocytin (Suzuki-Inoue et al13 and not shown). Significantly, PP2, but not its inactive analog PP3, also blocks tyrosine phosphorylation of both the 32- and 40-kDa forms of CLEC-2 (Figure 4C), consistent with the possibility that tyrosine phosphorylation of CLEC-2 initiates platelet activation. In contrast, CLEC-2 does not undergo tyrosine phosphorylation in response to stimulation by collagen or by a thrombin receptor PAR1-activating peptide, SFLLRN, over a time course of up to 5 minutes (Figure 4D), demonstrating that the lectin receptor is only phosphorylated upon direct activation.
We have previously reported that rhodocytin stimulates tyrosine phosphorylation of Syk in platelets.13 The observation that CLEC-2 undergoes tyrosine phosphorylation on a single YXXL motif, which forms one-half of an ITAM sequence, raises the possibility that the lectin receptor signals through recruitment of Syk to the cytosolic tail and initiating a signaling cascade that is similar to that used by the collagen receptor GPVI. In line with this, rhodocytin stimulates tyrosine phosphorylation of Syk and many of the major signaling proteins in the GPVI cascade, including the tyrosine kinase Btk, the adapters LAT and SLP-76, the GTP exchange factors Vav1 and Vav3, and PLCγ2 (Figures 4E, 5A). The time course of phosphorylation of all of these proteins is similar to that for Syk (Figure 4E). Tyrosine phosphorylation of all of these proteins is abolished in the presence of the Src kinase inhibitor PP2 (not shown).
Syk mediates platelet activation by rhodocytin
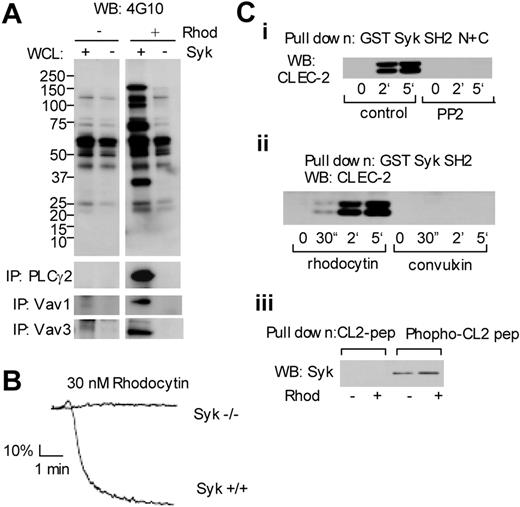
The functional role of Syk in signaling by rhodocytin was further investigated using murine platelets deficient in the tyrosine kinase. Rhodocytin stimulates robust tyrosine phosphorylation in whole-cell lysates of murine platelets, which is completely inhibited in the absence of Syk (Figure 5A). The critical role of Syk in signaling by rhodocytin is further illustrated by the complete loss of phosphorylation of PLCγ2 and the 2 members of the Vav family, Vav1 and Vav3, in the Syk-deficient platelets (Figure 5A). Furthermore, shape change and aggregation induced by rhodocytin are completely inhibited in the absence of Syk (Figure 5B). These results thereby confirm a proximal role for Syk in platelet activation by rhodocytin.
Crucial role of Syk in platelet activation downstream of CLEC-2. (A) Washed murine platelets were stimulated with 50 nM rhodocytin for the indicated times. Whole-cell lysates or immunoprecipitates with antibodies against PLCγ2, Vav1, or Vav3 were separated by SDS-PAGE and Western blotted with the indicated antibodies. (B) Control or Syk-deficient platelets were stimulated with 30 nM rhodocytin and platelet aggregation was monitored using an aggregometer. (Ci-ii) Washed human platelets, pretreated with or without 30 μM PP2, were stimulated with 50 nM rhodocytin or 10 μg/mL convulxin for the indicated times. Reactions were terminated by addition of an equal volume of 2 × lysis buffer. Platelet lysates were precleared and detergent-insoluble debris was clarified by centrifugation. The resultant supernatant was incubated with 40 μL glutathione beads associated with GST fusion protein containing tandem Syk SH2 domains. Precipitated proteins were separated by SDS-PAGE and Western blotted with an antibody to CLEC-2. (Ciii) CLEC-2 associated with 10 μg of CLEC-2 phospho-YXXL–containing peptide plus avidin-sepharose was detected by CLEC-2 antibody. The data are representative of 2 to 5 experiments.
Crucial role of Syk in platelet activation downstream of CLEC-2. (A) Washed murine platelets were stimulated with 50 nM rhodocytin for the indicated times. Whole-cell lysates or immunoprecipitates with antibodies against PLCγ2, Vav1, or Vav3 were separated by SDS-PAGE and Western blotted with the indicated antibodies. (B) Control or Syk-deficient platelets were stimulated with 30 nM rhodocytin and platelet aggregation was monitored using an aggregometer. (Ci-ii) Washed human platelets, pretreated with or without 30 μM PP2, were stimulated with 50 nM rhodocytin or 10 μg/mL convulxin for the indicated times. Reactions were terminated by addition of an equal volume of 2 × lysis buffer. Platelet lysates were precleared and detergent-insoluble debris was clarified by centrifugation. The resultant supernatant was incubated with 40 μL glutathione beads associated with GST fusion protein containing tandem Syk SH2 domains. Precipitated proteins were separated by SDS-PAGE and Western blotted with an antibody to CLEC-2. (Ciii) CLEC-2 associated with 10 μg of CLEC-2 phospho-YXXL–containing peptide plus avidin-sepharose was detected by CLEC-2 antibody. The data are representative of 2 to 5 experiments.
The possibility that tyrosine phosphorylation of CLEC-2 leads to recruitment of Syk, thereby initiating the downstream tyrosine phosphorylation-signaling cascade, was investigated using a GST fusion protein encoding the tandem SH2 domains of Syk.28 The Syk fusion protein precipitated similar levels of the 32- and 40-kDa forms of CLEC-2 from stimulated but not control platelets, whereas the ability to precipitate CLEC-2 was abolished in the presence of the Src kinase inhibitor PP2 (Figure 5Ci). Further, the Syk fusion protein was able to precipitate CLEC-2 within 30 seconds of stimulation by rhodocytin, showing that it occurs in parallel with the onset of shape change/aggregation, but not by the GPVI agonist convulxin (Figure 5Cii), confirming the specificity of regulation. In line with these results, Syk could be precipitated by a phosphorylated peptide containing the YXXL motif from the cytosolic tail of CLEC-2, whereas there was no interaction with the equivalent, nonphosphorylated peptide (Figure 5Ciii). These results thereby support a model in which Syk interacts with the phosphorylated tail of CLEC-2, thereby initiating downstream signaling events. We have not been unable to confirm this association in stimulated platelets, however, through immunoprecipitation studies using antibodies to CLEC-2 or to Syk, possibly because of a relatively low affinity of the Syk SH2 domain for a single phospho-YXXL motif, thereby rendering the complex unstable on solubilization.
Critical role of LAT, SLP-76, Vav1/3, and PLCγ2 in signaling by rhodocytin
We used platelets from mutant mice to establish the role of a number of the proteins that undergo tyrosine phosphorylation upon stimulation by rhodocytin. Low (3-10 nM) and intermediate (20-30 nM) concentrations of rhodocytin were used to establish whether any inhibitory effects could be overcome with increasing concentrations of the snake toxin. The response to a low concentration of rhodocytin was abolished in the absence of PLCγ2, whereas an intermediate concentration induced shape change, which is likely to reflect the presence of a low level of PLCγ117 (Figure 6A). A marked inhibition of the response to a low concentration of rhodocytin was also seen in the absence of LAT, SLP-76, and Vav1/Vav3, although an almost full recovery of aggregation was seen in response to higher concentrations of the snake toxin (Figure 6B-D). The results with LAT and PLCγ2 are similar to those obtained following stimulation of mutant murine platelets by GPVI, using either the snake venom toxin convulxin or the GPVI-specific collagen-related peptide (CRP) as agonists.17,19 In contrast, the observation that a high concentration of rhodocytin is able to stimulate maximal aggregation in the absence of SLP-76, or the combined absence of Vav1 and Vav3,21,29 contrasts those seen for convulxin or CRP, where aggregation is largely abolished. These observations therefore further distinguish the CLEC-2–dependent signaling pathway from that used by the collagen receptor, GPVI.
Discussion
In the present study, we have identified a novel class of signaling receptor on platelets, the C-type lectin receptor CLEC-2, and demonstrated that this serves as a receptor for the snake venom toxin rhodocytin. CLEC-2 is the first member of the C-type lectin family of receptors identified to regulate platelets through sequential activation of Src and Syk family tyrosine kinases, thereby initiating a signaling cascade that culminates in tyrosine phosphorylation and activation of PLCγ2. A unique feature in this signaling cascade, which distinguishes it from that used by other platelet glycoprotein receptors, is that Src kinase–dependent tyrosine phosphorylation of CLEC-2 on a YXXL motif appears to be sufficient to confer binding to Syk and thereby initiate downstream signaling events. This contrasts with the signaling cascade used by the platelet collagen receptor GPVI, which recruits Syk to a doubly phosphorylated ITAM on FcR γ-chain complex. Further, these 2 signaling pathways can be distinguished by their dependency on the adapter protein SLP-76 and the GTP exchange factors Vav1 and Vav3. An intermediate concentration of rhodocytin is able to induce full aggregation of platelets deficient in these proteins, whereas responses to the snake toxin convulxin and CRP show a much greater level of reduction. The observation that platelets express a new class of signaling receptor that appears to signal through a unique pathway opens up a new line of research concerned with establishing its role in health and disease.
Inhibition of rhodocytin-induced platelet aggregation in mice deficient in PLCγ2, LAT, SLP-76, or Vav1/3. Murine washed platelets from wild-type mice or mice deficient in PLCγ2, LAT, SLP-76, or Vav1/3 were stimulated with indicated concentrations of rhodocytin and platelet aggregation was monitored using an aggregometer. Results are representative of 3 to 6 experiments.
Inhibition of rhodocytin-induced platelet aggregation in mice deficient in PLCγ2, LAT, SLP-76, or Vav1/3. Murine washed platelets from wild-type mice or mice deficient in PLCγ2, LAT, SLP-76, or Vav1/3 were stimulated with indicated concentrations of rhodocytin and platelet aggregation was monitored using an aggregometer. Results are representative of 3 to 6 experiments.
The present study goes a long way in establishing CLEC-2 as the receptor that mediates activation by the snake venom toxin rhodocytin in platelets that lack integrin α2β1 and functional GPIb/IX/V.15 However, definitive proof of this will require generation of platelets that are deficient in the lectin receptor, as it is possible that rhodocytin binds to other surface proteins in platelets, in addition to α2β1, GPIb/IX/V,12-14 and CLEC-2. Nevertheless, the observation that an antibody to CLEC-2 is able to mediate activation in the presence of monoclonal antibody IV.3, which blocks FcγRIIA, confirms that CLEC-2 on its own is able to mediate platelet activation and strengthens the possibility that it is the major signaling receptor for the snake toxin in platelets. Rhodocytin also activates endothelial cells, although it is unclear whether this response also requires CLEC-2, as activation is blocked by antibodies to integrin α2β1.30
C-type lectins are proteins that bind carbohydrates in a calcium-dependent manner through their carbohydrate recognition domains. Carbohydrate recognition domains have a distinctive fold that is shared with domains in other proteins that do not necessarily bind carbohydrates. This domain is known as a C-type lectin–like domain. At least 70 proteins in humans contain a C-type lectin or lectin-like domain although their overall architecture is diverse. The family can be subdivided into 14 groups based on the domain organization within the whole molecule. CLEC-2 belongs to subgroup V of the C-type lectin–like family of receptors.31,32 Nineteen members of the group V family of C-type lectin–like receptors have been identified in the human genome and form a diverse group of proteins that appear to perform a number of distinct functions. Of particular interest in the context of the present study is that several of these have been shown to mediate activation in hematopoietic cells. NKG2D and MDL-1, for example, signal by associating with the adapter polypeptides DAP10 and DAP12, respectively.33 DAP10 contains a YXXM motif and generates PI3-kinase–dependent, but Syk-independent, activation signals. DAP-12 contains an ITAM, which is analogous in function to that of the FcRγ-chain, which is part of the collagen receptor GPVI and a number of Fc receptors. Interestingly, the type II C-type lectin–like receptors, Dectin-1, asialoglycoprotein, and DC-SIGN, also have a single YXXL motif in their cytoplasmic tails, similar to that found in CLEC-2. Dectin-1 has been recently shown to signal through this motif upon yeast stimulation,34 although this sequence is not required for Dectin-1–mediated phagocytosis35 or endocytosis of asialoglycoprotein receptor.36,37 Interestingly, platelets express 5 other members of the C type lectin–like family of receptors, namely P-selectin, Lox-1, AA4 antigen, CD23, and CD69.32,38-41 None of the other receptors contain a cytosolic YXXL motif, although a closely related YXXI motif is present in the cytosolic tail of CD23. It is therefore of interest to establish if this receptor is also able to mediate platelet activation.
CLEC-2 was first cloned from human bone marrow at the same time as CLEC-1.26 These are 2 very different proteins, however, with distinct distributions despite the same overall architecture. CLEC-1 is selectively expressed in dendritic cells but is not present on the cell surface when expressed in a cell line model26 and does not contain a cytoplasmic YXXL motif. CLEC-2 cDNA was originally reported to be selectively expressed in the liver and in some blood cells of myeloid origin, including monocytes, dendritic cells, and granulocytes.26 Interestingly, however, comparison of a serial analysis of gene expression (SAGE) library from mouse megakaryocytes with 30 other SAGE libraries of hematopoietic origin identified CLEC-2 as a megakaryocyte/platelet-specific protein (Michael G. Tomlinson, Victoria L. Heath, Stephen P. Cobbold, and S.P.W., unpublished data, July 2004). Furthermore, CLEC-2 had the largest number of SAGE tags within the group of megakaryocyte/platelet-specific proteins suggesting that it may be expressed at a high level and that it may play a unique role in the megakaryocyte/platelet lineage.
An understanding of the physiological significance of CLEC-2 on platelets and megakaryocytes will require the genetic deletion of CLEC-2 and identification of its endogenous ligand. The powerful stimulatory action of CLEC-2 on platelets, however, strongly indicates that it may play an important role in supporting platelet activation. In this context, it is noteworthy that neither mice nor humans deficient in the major signaling receptor for collagen, GPVI, have a major bleeding phenotype, thereby indicating the presence of a compensatory mechanism. The possibility that this is mediated by CLEC-2, which signals in a similar way to GPVI, and is likely to be activated by crosslinking either by binding to an unidentified extracellular matrix protein or possibly a transmembrane protein on another cell, seems worthy of consideration. Potentially, CLEC-2 could also play an important role in mediating thrombus formation in response to stimulation by exogenous stimuli, bearing in mind the ability of C-type lectin-like receptors to bind a diverse range of stimuli. In either case, the restricted distribution of CLEC-2 would make it a good target for a novel class of antithrombotics.
Prepublished online as Blood First Edition Paper, September 20, 2005; DOI 10.1182/blood-2005-05-1994.
Supported in part by grants from the Ministry of Education, Culture, Sports, Science and Technology of Japan (grant no. 16790533), from the British Heart Foundation (BHF) and Wellcome Trust, and Biotechnology and Biological Sciences Research Council (BBSRC). S.P.W. holds a BHF Professorship. G.L.J.F., J.A.E., and S.P. were supported by a BHF Studentship, DFG grant Eb177/4-2, and SFB 466, respectively. S.C.H. holds a National Health and Medical Research Council (Australia) CJ Martin Fellowship.
K.S.-I. designed research, performed research, analyzed data, and wrote the paper; G.L.J.F. performed research and analyzed data; A.G. contributed vital analytical tools and analyzed data; J.A.E. contributed vital new reagents; S.P. contributed vital new reagents; O.I. performed research; T.K.G. performed research; S.C.H. performed research; A.C.P. performed research; G.D.L. contributed vital new reagents; D.G.T. contributed vital new reagents; E.S. contributed vital new reagents; N.Z. contributed vital analytical tools; T.M. contributed vital new reagents; V.L.J.T. contributed vital new reagents; Y.O. designed research; and S.P.W. designed research, analyzed data, and wrote the paper.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
We are very grateful to Dr Sripadi Prabhakar for the MS/MS analysis, to Dr Toshiro Takafuta for his helpful suggestions, to Mrs Chiaki Komatsu and Mrs Yumi Sakamoto for their excellent technical assistance, and to Drs Martin Turner and Elena Vigorito for the supply of Vav–/– mice.




This feature is available to Subscribers Only
Sign In or Create an Account Close Modal