Abstract
Deficiency of ADAMTS13 is found in patients with thrombotic thrombocytopenic purpura (TTP), and the genetic defects in the ADAMTS13 gene or the autoantibody against ADAMTS13 is thought to be responsible for the development of TTP. The clinical correlation and mechanisms of secondary ADAMTS13 deficiency in other disease states were investigated. In addition to TTP, ADAMTS13 levels were severely decreased in patients with sepsis-induced disseminated intravascular coagulation (DIC). The incidence of acute renal failure and serum creatinine levels in patients with ADAMTS13 activity levels lower than 20% (incidence, 41.2%; creatinine, 160 ± 150 μM [1.81 ± 1.70 mg/dL]) (P < .05) were significantly higher than they were in patients with ADAMTS13 activity levels higher than 20% (incidence, 15.4%; creatinine, 84 ± 67 μM [0.95 ± 0.76 mg/dL]) (P < .01). Additionally, unusually large von Willebrand factor multimers were detected in 26 (51.0%) of 51 patients with ADAMTS13 activity levels lower than 20%. Lower molecular weight forms of ADAMTS13 were found in the plasma of patients with sepsis-induced DIC, suggesting that the deficiency of ADAMTS13 was partially caused by its cleavage by proteases in addition to decreased synthesis in the liver. These data suggested that severe secondary ADAMTS13 deficiency can be associated with sepsis-induced DIC and may contribute to the development of renal failure.
Introduction
Deficiency of the von Willebrand factor (VWF)–cleaving protease,1-5 ADAMTS13 (a disintegrin-like metalloprotease with thrombospondin type 1 repeats) is found in most patients with thrombotic thrombocytopenic purpura (TTP), and this deficiency is thought to be responsible for platelet aggregation and microthrombi formation in the circulation, which in turn cause typical thrombotic microangiopathies (TMAs) to develop.6-9 Deficiency of ADAMTS13 in patients with TTP is caused by genetic defects in the ADAMTS13 gene (familial TTP, Upshaw-Schulman syndrome) or by autoantibodies against ADAMTS13. Although hemolytic uremic syndrome (HUS) is clinically similar to TTP, the role of ADAMTS13 deficiency in the development of HUS is controversial because reports conflict about whether ADAMTS13 activity remains unchanged6-8 or decreases.10-13 It also is possible that secondary deficiency of ADAMTS13 may account for the development of microthrombi formation in disease states other than TTP. To search for the clinical correlation of secondary ADAMTS13 deficiency in disease states, we measured ADAMTS13 activity levels by the standard method14 and determined antigen levels by our newly developed monoclonal antibody–based enzyme-linked immunosorbent assay (ELISA) for ADAMTS13 in patients with TTP and in patients with sepsis-induced disseminated intravascular coagulation (DIC). We found that severe secondary ADAMTS13 deficiency could occur in patients with sepsis-induced DIC and that it had a clinical correlation with the development of renal failure.
Patients, materials, and methods
Blood samples
All samples were obtained with informed consent from patients according to the Declaration of Helsinki. Blood was drawn from 113 patients (65 men, aged 17-83; 44 women, aged 21-81; idiopathic TTP, 3 patients; Upshaw-Schulman syndrome, 1 patient; sepsis-induced DIC, 109 patients). The diagnosis of TTP was made with note of the presence of typical clinical features (fever, bleeding tendency, neurologic symptoms) laboratory examination results (thrombocytopenia, hemolytic anemia with red blood cell fragmentation, increased levels of LDH, increased levels of serum creatinine), and effectiveness of plasma exchange treatment. Patients with definite infection, such as bacteremia, pneumonia, urinary tract infection, biliary tract infection, or pathogenic Escherichia coli O-157 infection, were excluded from the TTP group. The patient with Upshaw-Schulman syndrome had TTP, and plasma transfusion was effective in preventing recurrence.
The diagnosis of DIC was made according to the criteria established in 1988 by the Japanese Ministry of Health and Welfare. Criteria for DIC were reported previously.15 Briefly, the presence of underlying disease—such as infection and malignancies—specific clinical conditions (bleeding symptoms, organ dysfunction), and results of laboratory examinations (platelet counts, prothrombin time, fibrinogen, fibrin degradation products) were quantified based on score. If the score was 7 or more, the diagnosis of DIC was made. In patients with hematologic malignancy, scores on the bleeding symptom and platelet counts were excluded, and the diagnosis of DIC was made if the total score was 4 or more.
The diagnosis of sepsis was made according to the guidelines of the Society of Critical Care Medicine Consensus Conference Committee.16 Briefly, patients had to meet at least 3 of the 4 criteria for systemic inflammatory response and had to have a known infection or a suspected infection, as evidenced by one or more of the following: bacteremia, pathologic microorganisms or white blood cells in a normally sterile body fluid such as urine or joint fluid; purulent sputum; radiographic evidence of pneumonia; clinical signs associated with high risk for infection (eg, cholangitis, peritonitis) or increased levels of endotoxin, β-d-glucan, or Candida antigen.
Thirty-nine patients with DIC were shown to have bacteremia, as evidenced by their blood cultures. Twelve patients, whose bacteremia was not evidenced by blood culture, had increased levels of endotoxin, β-d-glucan, or Candida antigen. Twenty-eight patients who were negative for bacteria in blood culture or who did not have increased levels of endotoxin, β-d-glucan, or Candida antigen, had pneumonia as evidenced by radiography, 11 patients had urinary tract infection, 4 patients had wound infection during postoperative periods, 1 patient had biliary tract infection, 1 patient had bacterial arthritis, 1 patient had bacterial osteomyelitis, and 12 patients had suspected respiratory infection with the presence of pathogenic microorganisms, such as methicillin-resistant Staphylococcus aureus in sputum cultures.
Citrated platelet-poor plasma samples were prepared and stored at –80°C until use. Blood was also drawn from 12 healthy volunteers (7 men, aged 25-53; 5 women, aged 25-48) for the preparation of normal pooled plasma. Laboratory analyses of patients' blood were performed by the standard methods using automated analyzers. Complete blood cell counts, serum creatinine (normal range, 35-97 μM [0.4-1.1 mg/dL]), serum bilirubin (normal range, 3-21 μM [0.2-1.2 mg/dL]), aspartate aminotransferase (AST; normal range, 8-35 IU/L), alanine aminotransferase (ALT; normal range, 5-40 IU/L), serum albumin (normal range, 39-51 g/L [3.9-5.1 g/dL]), and C-reactive protein (CRP; normal range, less than 5 mg/L [0.5 mg/dL]) were measured in this study.
Determination of ADAMTS13 antigen and activity levels
The human ADAMTS13 cDNA used in this study was described previously.5 Human ADAMTS13 was expressed in human embryo kidney 293 cells stably transfected with pCAG-ADAMTS13 Neo and was purified. Murine monoclonal antibodies (mAbs) to human ADAMTS13 were generated by the standard method17 after immunization of BALB/c mice with recombinant human ADAMTS13. Two mAbs, WH10 and WH2-22-1A, were selected for ELISA, which was shown to bind to the third TSP-1 motif and to the disintegrin domain of ADAMTS13 by the binding study to recombinant ADAMTS13 mutants, respectively.5,14,18 WH10 (2 μg/mL) was used for microtiter plate coating (Maxi Sorp plate; Nalge Nunc International, Rochester, NY). After blocking with 1% casein, plasma samples from healthy subjects and patients were diluted in phosphate-buffered saline, pH 7.2/0.1% casein, and then incubated in WH10-coated plates. ADAMTS13 bound to the microtiter plates was detected by peroxidase-conjugated WH2-22-1A. Purified recombinant ADAMTS13 was used as the standard to determine ADAMTS13 antigen levels in normal plasma. The ADAMTS13 level in each patient's plasma was expressed as the percentage of that in normal pooled plasma. ADAMTS13 activity levels in plasma were measured according to the previously described method.14 Briefly, 10 μL plasma was mixed with purified VWF (1 μg) in 100 μL reaction buffer (5 mM Tris [pH 8.0]/1.5 M urea/10 mM BaCl2/0.4 mM Pefabloc SC [Roche Diagnostics, Mannheim, Germany]) at 37 °C for 24 hours. Reaction was terminated by the addition of 10 μL of 500 mM EDTA, pH 8.0.14 Portions of samples were subjected to 1.4% sodium dodecyl sulfate–agarose gel electrophoresis to determine the extent of VWF degradation. After electrophoresis, proteins were transferred to polyvinylidene fluoride (PVDF) membranes, and VWF multimers were detected by peroxidase-labeled rabbit anti–human VWF antibodies (Dako, Glostrup, Denmark).12-14
Quantification of molecular markers of DIC
Plasma levels of fibrin degradation products (FDPs) were quantified with commercial kits (Roche Diagnostics, Tokyo, Japan) used for laboratory examinations. Given that the quantification of free thrombin concentration in plasma is technically difficult, we used ELISA (Sysmex, Kobe, Japan) to quantify plasma levels of thrombin/antithrombin III (TAT) complexes. Similarly, plasma levels of plasmin/α2 plasmin inhibitor complexes (PICs) were measured using ELISA with commercial kits (Sysmex) used for laboratory examinations. Plasma plasminogen activator inhibitor 1 (PAI-1) levels were quantified by the latex photometric immunoassay by using a commercial kit (Mitsubishi Kagaku Iatron, Tokyo, Japan), as described previously.19 The granulocyte elastase digests of cross-linked fibrin (granulocyte elastase–dependent fibrin degradation products [E-XDPs]) were measured by the automated latex photometric immunoassay using IF-123 monoclonal antibody, which is specific for the fibrin fragment D species generated by granulocyte-elastase digestion.20 Monoclonal antibody IF-123–bound latex particles (Mitsubishi Kagaku Iatron) were used for the assay. A 2.4-μL aliquot of sample plasma was mixed with 32 μL latex reagents in 250 μL Tris-buffered saline, and then absorbance changes were analyzed with an automated analyzer for latex photometric immunoassay (model LPIA-NV7; Mitsubishi Kagaku Iatron). The standard E-XDP was purified according to the method of Kohno et al.20 The normal range of plasma E-XDP levels is less than 3 U/mL.
Effect of granulocyte elastase on ADAMTS13
Recombinant ADAMTS13 (250 nM) was incubated in 20 μL Tris-buffered saline, pH 7.4, in the absence or presence of granulocyte elastase (Elastin Products, Owensville, MO) at 5 nM and 50 nM. Aliquots (5 μL each) were harvested after incubation at 37°C for 5, 15, and 30 minutes. The reaction of each aliquot was terminated by addition of the sodium dodecyl sulfate–polyacrylamide gel electrophoresis (SDS-PAGE) sample buffer containing 2% SDS. Samples were then analyzed by SDS-PAGE followed by Western blotting with anti-ADAMTS13 monoclonal antibody WH2-22-1A and peroxidase-labeled anti–mouse IgG.
Detection of ADAMTS13 molecular forms in plasma
Western blot analysis of ADAMTS13 in plasma by mAb WH2-22-1A was performed after immunoprecipitation with anti-ADAMTS13 polyclonal antibody immobilized to protein G-Sepharose.
Analysis of VWF multimers in patient plasma
VWF multimers in patient plasma were analyzed by SDS-agarose gel electrophoresis according to the method described previously.12-14
Results
ELISA for ADAMTS13
We generated mAbs against recombinant human ADAMTS13 and used them to develop an mAb-based ADAMTS13 ELISA. To determine the specificity of this assay, plasma obtained from a patient with Upshaw-Schulman syndrome was mixed with normal plasma at various ratios, and the ADAMTS13 activity and antigen levels were measured. As shown in Figure 1, ADAMTS13 activity and ADAMTS13 antigen levels in the plasma of the patient with Upshaw-Schulman syndrome were less than 1%, and the ADAMTS13 antigen level in the patient plasma increased linearly in parallel with the ADAMTS13 activity in the presence of increasing amounts of normal plasma. The correlation coefficient between ADAMTS13 antigen and ADAMTS13 activity was 0.997. The ADAMTS13 level in normal pooled plasma was 1.57 μg/mL when recombinant human ADAMTS13 was used as the standard. The calibration curve was linear (r = 0.999), and the ELISA could distinguish absorbance changes of ADAMTS13 at 0.3% of the normal plasma level from ADAMTS13-depleted plasma. Interassay variability in samples containing 50% and 100% of ADAMTS13 were 7.9% and 5.2%, respectively.
Analysis of ADAMTS13 activity and antigen levels in plasma of patients with Upshaw-Schulman syndrome. ADAMTS13 activity and antigen levels were determined in the plasma of a patient with Upshaw-Schulman syndrome (USS) mixed with normal pooled plasma at various ratios. (A) Result of ADAMTS13 activities in the plasma of the USS patient mixed with normal plasma at various ratios (0:10-10:0). (B) Correlation of ADAMTS13 activity and antigen levels in these samples.
Analysis of ADAMTS13 activity and antigen levels in plasma of patients with Upshaw-Schulman syndrome. ADAMTS13 activity and antigen levels were determined in the plasma of a patient with Upshaw-Schulman syndrome (USS) mixed with normal pooled plasma at various ratios. (A) Result of ADAMTS13 activities in the plasma of the USS patient mixed with normal plasma at various ratios (0:10-10:0). (B) Correlation of ADAMTS13 activity and antigen levels in these samples.
ADAMTS13 levels in disease states
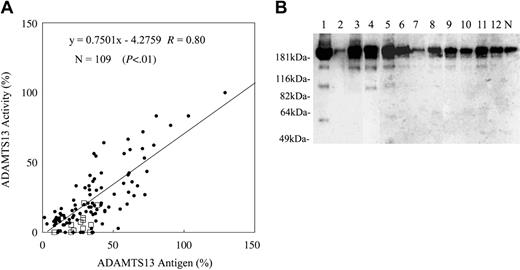
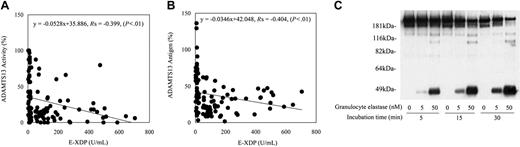
ADAMTS13 antigen and activity levels in the plasma of patients with sepsis-induced DIC or TTP were studied (Figure 2). The correlation coefficient of ADAMTS13 antigen and ADAMTS13 activity was 0.80. As shown in Figure 2A, discrepancies between ADAMTS13 antigen levels and activity levels were observed in many samples. These discrepancies mainly were caused by the decreased level of specific ADAMTS13 activity compared with the ADAMTS13 antigen level. Some samples had higher specific activity of ADAMTS13. To explore the possibility that decreased levels of the ADAMTS13-specific activity correlated with disease states, Western blot analysis of ADAMTS13 molecular forms in patient plasma was performed. Low molecular–weight ADAMTS13 species were observed in DIC patient plasma by Western blotting (Figure 2B), indicating that proteolytic cleavage of ADAMTS13 could occur in this disease state. The recent report showed that ADAMTS13 could be digested in vitro by proteases such as thrombin and plasmin.21 Because thrombin and plasmin can be generated in DICs, we tested the correlation between ADAMTS13 levels and molecular markers of coagulation and fibrinolysis. There was no correlation of ADAMTS13 activity, antigen, or specific activity level with levels of fibrinogen, FDP, TAT, PIC, PAI-1, or platelet counts (Table 1). We could only find a negative correlation between activity levels and antigen levels of ADAMTS13 and plasma levels of granulocyte elastase digests of fibrin (E-XDP) (Table 1; Figure 3A-B). Based on these results, we studied the effects of granulocyte elastase on ADAMTS13 in vitro. In accordance with previous reports, recombinant ADAMTS13 was determined to migrate at approximately 190 kDa by SDS-PAGE, followed by Western blotting.14,21 As shown in Figure 3C, recombinant ADAMTS13 migrating at approximately 190 kDa was converted to the 120-kDa and 100-kDA fragments and finally to the approximately 40-kDa fragment on incubation with granulocyte elastase in a dose-dependent and a time-dependent manner in vitro. A variety of lower molecular–weight ADAMTS13 fragments were detected in DIC patient plasma by Western blot (Figure 2B). According to the previous report, the ADAMTS13 fragments migrating approximately 150 to 170 kDa could be generated by thrombin.21 ADAMTS13 fragments migrating approximately 120 kDa and 100 kDa in patient plasma might correspond to the granulocyte elastase digests of ADAMTS13. However, the 120-kDa ADAMTS13 fragment and the 100-kDa ADAMTS13 fragment could be generated by thrombin and plasmin, respectively.21 It also is possible that thrombin-cleaved ADAMTS13 or plasmin-cleaved ADAMTS13 could be digested by granulocyte elastase or vice versa. These data may suggest that granulocyte elastase, together with other proteases (thrombin and plasmin plays a role in ADAMTS13 cleavage under certain pathologic conditions), may partially account for the decrease of the ADAMTS13-specific activity observed in DIC patients.
Correlation between the ADAMTS13 levels and molecular markers of DIC in patients with sepsis-induced DIC
. | ADAMTS13* . | . | . | ||
|---|---|---|---|---|---|
. | Activity . | Antigen . | Activity-antigen ratio . | ||
| Fibrinogen | -0.347 | -0.244 | -0.219 | ||
| FDP | 0.354 | 0.242 | 0.274 | ||
| TAT | 0.246 | 0.379 | 0.367 | ||
| PIC | 0.370 | 0.357 | 0.327 | ||
| PAI-1 | -0.230 | -0.300 | -0.006 | ||
| Platelet | 0.260 | 0.245 | 0.239 | ||
| E-XDP | -0.399† | -0.404† | -0.229 | ||
. | ADAMTS13* . | . | . | ||
|---|---|---|---|---|---|
. | Activity . | Antigen . | Activity-antigen ratio . | ||
| Fibrinogen | -0.347 | -0.244 | -0.219 | ||
| FDP | 0.354 | 0.242 | 0.274 | ||
| TAT | 0.246 | 0.379 | 0.367 | ||
| PIC | 0.370 | 0.357 | 0.327 | ||
| PAI-1 | -0.230 | -0.300 | -0.006 | ||
| Platelet | 0.260 | 0.245 | 0.239 | ||
| E-XDP | -0.399† | -0.404† | -0.229 | ||
n = 109 patients.
Values are rs determined by Spearman rank correlation test.
Statistically significant (P < .01).
Analysis of ADAMTS13 activity, antigen, and molecular forms in plasma of patients with sepsis-induced DIC. (A) ADAMTS13 activity and antigen levels in the plasma of patients with sepsis-induced DIC were determined as described in “Patients, materials and methods.” Samples (□) were subjected to immunoprecipitation followed by Western blotting to investigate the cleavage of ADAMTS13, as described in “Patients, materials, and methods.” (B) Typical Western blot of degraded ADAMTS13 found in the patients' plasma indicated in panel A (□) is shown. Western blotting of ADAMTS13 antigen in normal pooled plasma (N) is shown as the control. ADAMTS13 molecules in normal plasma migrated at approximately 190 kDa.
Analysis of ADAMTS13 activity, antigen, and molecular forms in plasma of patients with sepsis-induced DIC. (A) ADAMTS13 activity and antigen levels in the plasma of patients with sepsis-induced DIC were determined as described in “Patients, materials and methods.” Samples (□) were subjected to immunoprecipitation followed by Western blotting to investigate the cleavage of ADAMTS13, as described in “Patients, materials, and methods.” (B) Typical Western blot of degraded ADAMTS13 found in the patients' plasma indicated in panel A (□) is shown. Western blotting of ADAMTS13 antigen in normal pooled plasma (N) is shown as the control. ADAMTS13 molecules in normal plasma migrated at approximately 190 kDa.
Correlation between the ADAMTS13 levels and the granulocyte elastase digests of cross-linked fibrin (E-XDP) levels in plasma of patients with sepsis-induced DIC and the effect of granulocyte elastase on ADAMTS13 in vitro. Correlations between the activity levels of ADAMTS13 and the plasma levels of granulocyte-elastase digests of fibrin (E-XDP) (A) and between the antigen levels of ADAMTS13 and the plasma levels of granulocyte-elastase digests of fibrin (E-XDP) (B) in patients with sepsis-induced DIC are shown. Values were analyzed by Spearman correlation coefficient by rank test. Recombinant ADAMTS13 was incubated with granulocyte elastase at 5 nM or 50 nM, and degradation of ADAMTS13 by granulocyte elastase was studied after the indicated time and analyzed as described in “Patients, materials, and methods” (C).
Correlation between the ADAMTS13 levels and the granulocyte elastase digests of cross-linked fibrin (E-XDP) levels in plasma of patients with sepsis-induced DIC and the effect of granulocyte elastase on ADAMTS13 in vitro. Correlations between the activity levels of ADAMTS13 and the plasma levels of granulocyte-elastase digests of fibrin (E-XDP) (A) and between the antigen levels of ADAMTS13 and the plasma levels of granulocyte-elastase digests of fibrin (E-XDP) (B) in patients with sepsis-induced DIC are shown. Values were analyzed by Spearman correlation coefficient by rank test. Recombinant ADAMTS13 was incubated with granulocyte elastase at 5 nM or 50 nM, and degradation of ADAMTS13 by granulocyte elastase was studied after the indicated time and analyzed as described in “Patients, materials, and methods” (C).
ADAMTS13 deficiency in disease states
ADAMTS13 antigen and activity levels in patient groups and in healthy subjects are shown in Figure 4. The plasma ADAMTS13 antigen and activity levels in untreated patients with TTP (no plasma exchange treatment, no fresh frozen plasma transfusion) were 13.5% ± 7.1% (range, 5.1%-19.6%) and 6.3% ± 5.7% (range, 0%-12.5%), respectively (idiopathic TTP 3, Upshaw-Schulman syndrome 1). Decreased levels of ADAMTS13 antigen and activity were observed in patients with sepsis-induced DIC compared with healthy subjects (P < .01) in this study, and severe decreases of ADAMTS13 activity and antigen levels were observed in patients with sepsis-induced DIC. Of the 109 patients with sepsis-induced DIC, decreases in ADAMTS13 activity levels (less than 5%) were found in 17 (15.6%) patients; clinical features and laboratory data of these patients are summarized in Table 2. Consciousness disturbance, thrombocytopenia, decreased hemoglobin levels, and increased LDH levels were commonly found in these patients. Clinical features were indistinguishable from those of patients with TTP, though patients with sepsis-induced DIC had evidence of the infection. Given that the highest ADAMTS13 activity level in patients with TTP without plasma exchange or blood transfusion was 12.5%, patients with sepsis-induced DIC were divided into 2 groups. One included patients with decreased ADAMTS13 activity levels (less than 20%; n = 51), and the other included patients with ADAMTS13 activity levels greater than 20% (n = 52). Patients with chronic renal failure before infection were excluded from this analysis. Patients were in severe condition; 25 (49.0%) of 51 patients in the former group and 35 (67.3%) of 52 patients of the latter group received transfusions of fresh frozen plasma, platelet concentrates, or both within 5 days of the determination of ADAMTS13 levels. This might have affected the activity and antigen levels of ADAMTS13.
Clinical profiles of patients with sepsis-induced DIC whose ADAMTS13 activity levels were lower than 5%
Characteristic . | Value . |
|---|---|
| Age, y | 56.9 ± 21.3 |
| Consciousness disturbance, no. (%) | 8 (47.1) |
| Blood transfusion, no. (%) | 11 (64.7) |
| ADAMTS13 antigen, % | 25.5 ± 13.6 |
| Creatinine, mg/dL | 1.88 ± 2.06 |
| Albumin, g/dL | 2.2 ± 0.5 |
| WBC count, cells/μL | 11 200 ± 7 500 |
| RBC count, × 104/μL | 260 ± 86 |
| Hemoglobin, g/dL | 8.3 ± 2.0 |
| Platelet count, × 104/μL | 6.7 ± 5.3 |
| LDH, IU/L | 2481.3 ± 4107.8 |
| CRP, mg/dL | 18.11 ± 13.41 |
Characteristic . | Value . |
|---|---|
| Age, y | 56.9 ± 21.3 |
| Consciousness disturbance, no. (%) | 8 (47.1) |
| Blood transfusion, no. (%) | 11 (64.7) |
| ADAMTS13 antigen, % | 25.5 ± 13.6 |
| Creatinine, mg/dL | 1.88 ± 2.06 |
| Albumin, g/dL | 2.2 ± 0.5 |
| WBC count, cells/μL | 11 200 ± 7 500 |
| RBC count, × 104/μL | 260 ± 86 |
| Hemoglobin, g/dL | 8.3 ± 2.0 |
| Platelet count, × 104/μL | 6.7 ± 5.3 |
| LDH, IU/L | 2481.3 ± 4107.8 |
| CRP, mg/dL | 18.11 ± 13.41 |
n = 17 patients.
Values for all categories except consciousness disturbance and blood transfusion are mean ± SD.
To convert creatinine from milligrams per deciliter to micromoles per liter, multiply milligrams per deciliter by 88.4.
To convert albumin from grams per deciliter to grams per liter, multiply grams per deciliter by 10.
To convert WBC count from cells per microliter to × 109 cells per liter, divide cells per microliter by 1000.
To convert RBC count from × 104 cells per microliter to × 1012 cells per liter, divide × 104 cells per microliter by 100.
To convert hemoglobin from grams per deciliter to grams per liter, multiply grams per deciliter by 10.
To convert platelet count from × 104 platelets per microliter to × 109 per liter, multiply × 104 platelets per microliter by 10.
To convert CRP from milligrams per deciliter to milligrams per liter, multiply milligrams per deciliter by 10.
Correlation between secondary ADAMTS13 deficiency and organ failure
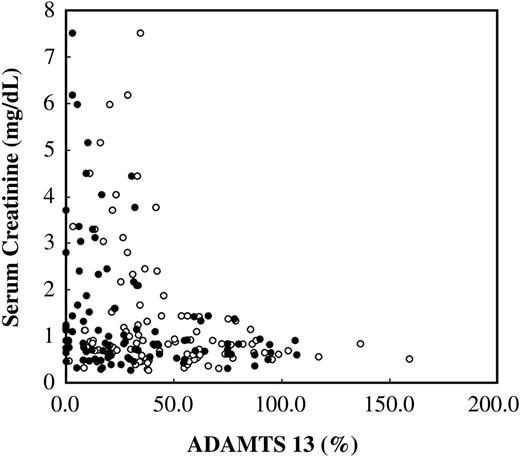
Analyses of clinical and laboratory data showed that the patients with severe ADAMTS13 deficiency (ADAMTS13 activity less than 20%) had elevated serum creatinine levels (Figure 5) that were significantly higher than those in patients with ADAMTS13 levels higher than 20% (Table 3). The incidence of renal injuries in patients with severe ADAMTS13 deficiency (ADAMTS13 activity less than 20%) was significantly higher than in patients with ADAMTS13 activity levels higher than 20% (Table 3). However, there were no differences in the incidence of liver dysfunction or serum levels of bilirubin, AST, and ALT among these groups (Table 3), suggesting that severe ADAMTS13 deficiency in these patients may be linked to the development of renal injuries. There was a significant difference in serum albumin levels between both groups, suggesting that the decrease of ADAMTS13 activity and antigen levels in patients was at least partially caused by reduced synthesis in the liver.
Correlation between ADAMTS13 levels and organ injury in patients with sepsis-induced DIC
. | ADAMTS13 activity less than 20%, n = 51 . | ADAMTS13 activity greater than 20%, n = 52 . | P . |
|---|---|---|---|
| Creatinine, mg/dL | 1.81 ± 1.70 | 0.95 ± 0.76 | < .01* |
| AST, IU/L | 106 ± 128 | 182 ± 290 | NS |
| ALT, IU/L | 72 ± 109 | 122 ± 160 | NS |
| Bilirubin, mg/dL | 2.70 ± 3.13 | 2.20 ± 2.53 | NS |
| Albumin, g/dL | 2.3 ± 0.4 | 2.9 ± 0.7 | < .05* |
| CRP, mg/dL | 13.50 ± 10.51 | 6.90 ± 8.61 | < .01* |
| Organ injury, no. (%) | |||
| Renal injury | 21 (41.2) | 8 (15.4) | < .05† |
| Liver injury | 40 (78.4) | 38 (73.1) | NS |
. | ADAMTS13 activity less than 20%, n = 51 . | ADAMTS13 activity greater than 20%, n = 52 . | P . |
|---|---|---|---|
| Creatinine, mg/dL | 1.81 ± 1.70 | 0.95 ± 0.76 | < .01* |
| AST, IU/L | 106 ± 128 | 182 ± 290 | NS |
| ALT, IU/L | 72 ± 109 | 122 ± 160 | NS |
| Bilirubin, mg/dL | 2.70 ± 3.13 | 2.20 ± 2.53 | NS |
| Albumin, g/dL | 2.3 ± 0.4 | 2.9 ± 0.7 | < .05* |
| CRP, mg/dL | 13.50 ± 10.51 | 6.90 ± 8.61 | < .01* |
| Organ injury, no. (%) | |||
| Renal injury | 21 (41.2) | 8 (15.4) | < .05† |
| Liver injury | 40 (78.4) | 38 (73.1) | NS |
Values for all categories except organ injury are mean ± SD. Renal injury: serum creatinine greater than 1.2 mg/dL.
Liver injury: elevation of bilirubin (> 2.0 mg/dL), AST (> 40 IU/L), or ALT (> 40 IU/L).
To convert creatinine from milligrams per deciliter to micromoles per liter, multiply milligrams per deciliter by 88.4.
To convert bilirubin from milligrams per deciliter to micromoles per liter, multiply milligrams per deciliter by 17.1.
To convert albumin from grams per deciliter to grams per liter, multiply grams per deciliter by 10.
To convert CRP from milligrams per deciliter to milligrams per liter, multiply milligrams per deciliter by 10.
NS indicates not significant.
Statistically significant (Welch t test).
Statistically significant (Fisher exact probability test).
Plasma ADAMTS13 levels in patients and healthy subjects. ADAMTS13 activity levels (A) and antigen levels (B) of healthy subjects, patients with TTP (idiopathic TTP, 3; Upshaw-Schulman syndrome, 1) before plasma exchange treatment, and patients with sepsis-induced DIC (n = 109) are shown. Differences in the mean values (horizontal lines) between the healthy subject group and patient groups were statistically significant (nonrepeated measures ANOVA and Dunnett test; P < .01).
Plasma ADAMTS13 levels in patients and healthy subjects. ADAMTS13 activity levels (A) and antigen levels (B) of healthy subjects, patients with TTP (idiopathic TTP, 3; Upshaw-Schulman syndrome, 1) before plasma exchange treatment, and patients with sepsis-induced DIC (n = 109) are shown. Differences in the mean values (horizontal lines) between the healthy subject group and patient groups were statistically significant (nonrepeated measures ANOVA and Dunnett test; P < .01).
Correlation between the plasma ADAMTS13 levels and the serum creatinine levels. Correlation between serum creatinine levels and ADAMTS13 activity (•) levels or antigen (○) levels in patients with sepsis-induced DIC is shown (n = 103). Patients with a history of chronic renal failure were excluded from the study.
Correlation between the plasma ADAMTS13 levels and the serum creatinine levels. Correlation between serum creatinine levels and ADAMTS13 activity (•) levels or antigen (○) levels in patients with sepsis-induced DIC is shown (n = 103). Patients with a history of chronic renal failure were excluded from the study.
Analysis of VWF multimers in patients with severe secondary ADAMTS13 deficiency
Additionally, unusually large VWF multimers were detected in the plasma of patients with severe secondary ADAMTS13 deficiency (ADAMTS13 activity less than 20%), as shown in Figure 6. Serum creatinine levels in patients in whom unusually large VWF multimers and severe ADAMTS13 deficiency were detected were significantly higher than in patients in whom the unusually large VWF multimers were absent (Table 4). There was no significant difference in ADAMTS13 activity (Table 4) and ADAMTS13-specific activity (activity-antigen ratio) between these patient groups (not shown).
Correlation between presence of unusually large multimers of VWF and serum creatinine levels of patients with sepsis-induced DIC and ADAMTS13 activity levels lower than 20%
. | Presence, n = 26 . | Absence, n = 25 . | P . |
|---|---|---|---|
| Creatinine, mg/dL | 2.39 ± 2.24 | 1.34 ± 1.35 | < .05* |
| ADAMTS13 activity, % | 6.6 ± 6.8 | 8.9 ± 6.0 | NS |
. | Presence, n = 26 . | Absence, n = 25 . | P . |
|---|---|---|---|
| Creatinine, mg/dL | 2.39 ± 2.24 | 1.34 ± 1.35 | < .05* |
| ADAMTS13 activity, % | 6.6 ± 6.8 | 8.9 ± 6.0 | NS |
Values are mean ± SD.
To convert creatinine from milligrams per deciliter to micromoles per liter, multiply milligrams per deciliter by 88.4.
Presence indicates unusually large VWF multimers present in the plasma of patients; absence, unusually large VWF multimers absent in the plasma of patients. NS, not significant.
Statistically significant (Welch t test).
There was a significant difference in CRP levels between the ADAMTS13 activity less than 20% group and the ADMATS13 activity greater than 20% group, but their platelet counts were not significantly different (not shown), indicating that the decrease in ADAMTS13 may be related to inflammatory responses. These results are consistent with the data showing a negative correlation between the activity and antigen levels of ADAMTS13 and the plasma levels of granulocyte elastase digests of fibrin (E-XDP).
Discussion
ADAMTS13 has been shown to play an important role in VWF processing.1-14,22,23 As shown previously, ADAMTS13 may cleave the unusually large multimers of VWF on the endothelial cell surface, preventing entrance of such unusually large multimers into the circulation.8,24 Without this processing of VWF multimers, the unusually large multimers of VWF secreted from endothelial cells would enter the circulation and initiate platelet thrombus formation, which in turn would cause the development of TMA.8,24 Patients with primary ADAMTS13 deficiency caused by defects in the ADAMTS13 gene or with autoantibodies against ADAMTS13 have been shown to develop TTP, suggesting the important physiologic role of ADAMTS13-catalyzed cleavage of these unusually large VWF multimers in humans. TTP is a fatal thrombotic microangiopathic disease if patients are not treated appropriately, but the incidence of TTP is low.8,22 While searching for the role of ADAMTS13 in common thromboembolic diseases, we found severe secondary ADAMTS13 deficiency in patients with sepsis-induced DIC and showed its clinical correlation to the development of renal failure in this study.
Analysis of VWF multimers of patients with sepsis-induced DIC. VWF multimers in the plasma of patients with sepsis-induced DIC with ADAMTS13 activity levels lower than 20% were analyzed by SDS-agarose gel electrophoresis, as described in “Patients, materials, and methods.” VWF multimer patterns of patients and healthy subjects (N) were analyzed simultaneously. *Representative unusually large multimers of VWF found in the plasma of patients with ADAMTS13 activity levels lower than 20%.
Analysis of VWF multimers of patients with sepsis-induced DIC. VWF multimers in the plasma of patients with sepsis-induced DIC with ADAMTS13 activity levels lower than 20% were analyzed by SDS-agarose gel electrophoresis, as described in “Patients, materials, and methods.” VWF multimer patterns of patients and healthy subjects (N) were analyzed simultaneously. *Representative unusually large multimers of VWF found in the plasma of patients with ADAMTS13 activity levels lower than 20%.
DIC is associated with a variety of disease states such as sepsis, advanced malignancy, severe tissue damage, and pregnancy-related complications. Sepsis may be the most common pathogenic disease that leads to the development of DIC, and the endotoxemia and high cytokine levels in the circulation are thought to induce tissue factor expression that in turn initiates fibrin thrombus formation in the circulation. Microthrombi formed in the circulation cause ischemia of and damage to a variety of organs. Lines of evidence have suggested that proteases released from white blood cells may also be involved in the development of organ injuries. This study showed that patients with sepsis-induced DIC frequently exhibited decreased antigen and activity levels of ADAMTS13 and that severe ADAMTS13 deficiency was found in these patients at high incidence. Many patients in this study had undergone transfusion with ADAMTS13-containing blood products, such as fresh frozen plasma and platelet concentrates, soon before blood sample collection for the determination of ADAMTS13 levels, suggesting that the levels of ADAMTS13 in the plasma samples of these patients might not reflect the severity of ADAMTS13 deficiency before blood transfusion. Thus, severe secondary ADAMTS13 deficiency in sepsis-induced DIC might be more common. Clinical manifestations and laboratory data of these patients with sepsis and secondary severe ADAMTS13 deficiency were nearly indistinguishable from those of patients with TTP, though the former had evidence of infection (Table 2), indicating that there exists a subset of patients who have secondary severe ADAMTS13 deficiency caused by sepsis and in whom the disease course is clinically similar to that of TTP. In addition, they might also have the same ADAMTS13 deficiency pathophysiology for the development of TMA seen in patients with idiopathic TTP.
Organ failure might be caused by tissue factor–dependent fibrin thrombus formation and platelet aggregation because of severe ADAMTS13 deficiency in the patients with sepsis-induced DIC with ADAMTS13 activity levels lower than 20%. This notion was supported by the correlation between severe secondary ADAMTS13 deficiency and renal failure in patients with sepsis-induced DIC with ADAMTS13 activity levels lower than 20%. We could not find any significant difference in the ADAMTS13-specific activity levels between these 2 groups (not shown). One possibility is that small molecular forms of ADAMTS13 could be lost in urine because of renal injuries. However, we could not determine whether this was the case because no urine samples were available for study.
In a previous report by Reife et al,25 patients with TMA who did not have DIC were analyzed for the correlation between ADAMTS13 activity levels and serum creatinine levels without distinguishing TTP from HUS. They found that creatinine levels in patients with severely decreased ADAMTS13 activity levels were significantly lower than those in patients without severely decreased ADAMTS13 activity levels. These data are contrary to our findings that patients with severe ADAMTS13 deficiency (ADAMTS13 activity less than 20%) had significantly higher serum creatinine levels than did patients with the ADAMTS13 activity levels higher than 20%. Given that patients with HUS were not distinguished from patients with TTP in the report by Reife et al,25 it is possible that the patients without severe ADAMTS13 deficiency in that study included patients with HUS. We studied patients with sepsis-induced DIC, and this difference in patient groups explains the opposing findings. There was no apparent difference between the platelet counts of patients with ADAMTS13 activity levels less than 20% and those of patients with ADAMTS13 activity levels greater than 20%. The combination of underlying DIC and platelet transfusion in these patients may account for the data.
The presence of the unusually large multimers of VWF in the plasma of patients with severe secondary ADAMTS13 deficiency and its correlation with serum creatinine levels supports the notion that severe secondary ADAMTS13 deficiency may correlate with the development of renal failure in sepsis-induced DIC. There was no significant correlation between the unusually large multimers of VWF and ADAMTS13 activity levels, possibly because of technical difficulties in determining the unusually large VWF multimers and the differences in endothelial cell damage among these patients.
Decreased specific activity of ADAMTS13, presumably caused by its cleavage by proteases, was a mechanism for severe secondary ADAMTS13 deficiency in patients with sepsis-induced DIC. Various proteases have been shown to degrade ADAMTS13 in vitro.21 Thrombin and plasmin are generated in DIC, and these enzymes may cleave ADAMTS13, resulting in the inactivation of ADAMTS13. Our data suggest that granulocyte elastase may be one of the proteases that cleave ADAMTS13, together with thrombin and plasmin, under in vivo pathologic conditions. In this regard, the case report by Galbusera et al26 of chronically relapsing TTP—which showed that α1-antitrypsin (the physiologic granulocyte elastase inhibitor) therapy was effective at preventing the appearance of unusually large VWF multimers in the circulation but not at preventing TTP relapse—was interesting and suggested the link between granulocyte elastase and cleavage of ADAMTS13. Correlation between ADAMTS13 activity and antigen levels and E-XDP levels, not only in patients with TTP but also in patients with pathogenic E coli infection–related HUS, would be a further study to investigate the role of granulocyte elastase in TMA development. Specific inhibitors of these proteases are present at high concentrations in blood, indicating that cleavage of ADAMTS13 by these proteases may depend on the kinetic balance between ADAMTS13, the proteases, and their inhibitors. Thus, cleavage of ADAMTS13 by these proteases may not proceed completely in vivo. It is possible that other proteases could also digest ADAMTS13 in the disease state. This possibility should be investigated in a future study.
Because serum albumin levels decreased in most patients, liver injuries associated with the underlying disease might be an additional mechanism for decreasing ADAMTS13 antigen levels given that this enzyme is synthesized in the liver. Mutations or polymorphisms of the ADAMTS13 gene are another possible cause of a decrease or an increase of ADAMTS13-specific activity. These possibilities should also be explored in future studies.
In conclusion, the precise analysis of ADAMTS13 antigen and activity levels in disease states offers insight into the roles of ADAMTS13 in thromboembolic diseases. Severe ADAMTS13 deficiency takes place secondarily in disease states such as sepsis-induced DIC, and it may not be specific for idiopathic TTP and may not have a solo diagnostic value for idiopathic TTP. Although the mechanisms of severe ADAMTS13 deficiency in sepsis are different from those of idiopathic TTP, the clinical features of patients with sepsis-induced DIC and severe ADAMTS13 deficiency are similar to those of patients with idiopathic TTP. Sepsis may have the same pathophysiology of severe ADAMTS13 deficiency for TMA development as idiopathic TTP, raising the possibility of novel supportive therapies for patients with sepsis and severe ADAMTS13 deficiency, such as ADAMTS13 supplementation, α1-antitrypsin administration, and use of synthetic granulocyte elastase inhibitors. Given that severe secondary ADAMTS13 deficiency might correlate with the development of organ injury in patients with sepsis-induced DIC, determining the ADAMTS13 levels of patients in severe condition at the time of hospital admission would provide better understanding of the extent of disease. Current analyses of ADAMTS13 levels in disease states are retrospective; thus, prospective study is needed for the timely execution of ADAMTS13 supplementation for patients not only with TTP but also with secondary ADAMTS13 deficiency.
Prepublished online as Blood First Edition Paper, September 27, 2005; DOI 10.1182/blood-2005-03-1087.
Supported by Grants-in-Aid for Scientific Research from the Japanese Ministry of Education and Science; Health and Labor Science Research Grants for Research from the Japanese Ministry of Health, Labor, and Welfare; and Grants for “High-Tech Center Research” Projects for Private Universities: matching fund subsidy from MEXT (Japanese Ministry of Education, Culture, Sports, Science, and Technology), 2002-2006.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.




This feature is available to Subscribers Only
Sign In or Create an Account Close Modal