Abstract
The zinc finger transcription factor GATA-1 is essential for both primitive (embryonic) and definitive (adult) erythropoiesis. To define the roles of GATA-1 in the production and differentiation of primitive and definitive erythrocytes, we established GATA-1-null embryonic stem cell lines in which GATA-1 was able to be conditionally expressed by using the tetracycline conditional gene expression system. The cells were subjected to hematopoietic differentiation by coculturing on OP9 stroma cells. We expressed GATA-1 in the course of primitive and definitive erythropoiesis and analyzed the ability of GATA-1 to rescue the defective erythropoiesis caused by the GATA-1 null mutation. Our results show that GATA-1 functions in the proliferation and maturation of erythrocytes in a distinctive manner. The early-stage expression of GATA-1 during both primitive and definitive erythropoiesis was sufficient to promote the proliferation of red blood cells. In contrast, the late-stage expression of GATA-1 was indispensable to the terminal differentiation of primitive and definitive erythrocytes. Thus, GATA-1 affects the proliferation and differentiation of erythrocytes by different mechanisms.
Introduction
Hematopoiesis is the sequential proliferation and differentiation process that produces more than 8 distinct, mature blood cells. The process is tightly controlled by various lineage-specific transcription factors.1 Among them, GATA transcription factors are one of the most well-studied transcription factors. The GATA protein is in the zinc finger transcription factor family and is categorized by recognizing the consensus motif WGATAR in a conserved multifunctional domain consisting of 2 C4-type zinc fingers.2-6 The GATA family includes GATA-1, GATA-2,5,7-9 and GATA-3,10 which are essential hematopoietic factors, and GATA-4, GATA-5, and GATA-6, which regulate heart, lung, and gut cell development.11,12 GATA-1, the founding member of the GATA family, has been mapped to the X chromosome, and the GATA-1 protein has been detected in erythroid, megakaryocytic, eosinophilic, and mast cell lines within the hematopoietic system.13-19
From a developmental point of view, erythropoiesis consists of 2 waves of red blood cell production, primitive and definitive erythropoiesis.20 The differences between primitive and definitive erythropoiesis are attributable to not only the emerging time but also to the origin, morphology, and globin gene expression. Primitive erythropoiesis, the first wave of erythroid production, generates nucleated, primitive erythrocytes (EryPs) that express embryonic hemoglobin at the blood island in the embryonic yolk sac. A recent work reported that murine primitive erythroblasts also enucleated and continued to circulate through late gestation and even into the postnatal period, indicating that the primitive erythropoiesis in mammals shared many processes with its definitive counterpart.21 Definitive erythropoiesis, the second wave of erythropoiesis, commences in the fetal liver and then migrates to the bone marrow beginning around birth. The erythrocytes produced during definitive erythropoiesis (definitive erythrocytes [EryDs]) are enucleated and synthesize the adult forms of globin. A cooperative cohort of broad-spectrum and specific transcription factors regulates the formation, survival, proliferation, and differentiation of a multipotent progenitor into the erythroid lineage as well as into other hematopoietic cell lines.22,23
GATA-1 participates in the expression of numerous genes involved in primitive and definitive erythropoiesis.2,13,14,24,25 The use of targeted mutagenesis in embryonic stem (ES) cells has revealed the crucial roles of GATA-1 in erythropoiesis. GATA-1–null embryonic stem cells gave rise to definitive erythroid precursors that were arrested at the proerythroblast stage in vitro and failed to contribute to adult red blood cells in chimeric mice.26,27 GATA-1–null mice showed complete ablation of primitive and definitive erythropoiesis resulting from the arrested maturation and apoptosis of erythroid precursors at the proerythroblast stage.28 These results clearly demonstrate that GATA-1 is indispensable for maturation and survival in both primitive and definitive erythropoiesis.
As represented by the case of GATA-1, targeted gene disruption is a useful approach for analyzing the function of transcription factors. However, when the mutation severely affects erythropoiesis, as occurs with the GATA-1–null mutation, embryonic lethality is inevitable, and the analysis of the function of transcription factors becomes difficult. In these cases, the in vitro differentiation of ES cells to blood cells is a powerful tool because it enables us to analyze the direct consequences of the effects of gene disruption. There are basically 2 methods used for in vitro differentiation: the conventional embryoid body formation method and the coculture of ES cells on a stromal cell line, such as OP9. The OP9 system has several advantages, which include allowing the quantitative analysis of terminally differentiated blood cells.29-33 In the OP9 system, primitive and definitive erythrocytes are sequentially developed, and the time course of the development precisely recapitulates normal erythroid development. Another advantage of the system is the synchronized, stepwise manner with which differentiation proceeds. Therefore, the OP9 system is a convenient and physiologically relevant system for studying various aspects of erythroid biology.
To define the roles of GATA-1 in erythropoiesis, we established an experimental system in which the defective erythropoiesis of the GATA-1 mutant was rescued by the conditional expression of the Gata1 gene. This system was constructed by combining the OP9 system with the tetracycline-regulated conditional gene expression method (Tet-off system).34,35 The regulation of the expression level and the expression period of GATA-1 in GATA-1–deficient erythroid cells allowed us to examine the precise function of GATA-1 in primitive and definitive erythropoiesis. Our results show that GATA-1 regulates the proliferation and differentiation of erythroid cells by different mechanisms.
Materials and methods
Targeting vector and GATA-1–null ES cells
The targeting vector contained 8.2 kilobases (kb) of the Gata-1 locus (6.2 kb upstream of the XbaI site in intron 1 and a 2-kb fragment between the EcoRI site in exon 3 and the BstEII site in intron 5). The sequences between the XbaI site in intron 1 and the EcoRI site in exon 3, including the initiation codon in exon 2, were replaced by a phosphoglycerate kinase (PGK) promoter–driven neomycin-resistance gene cassette in a reverse orientation. A herpes simplex virus thymidine kinase (TK) cassette was added at the 5′ boundary of the construct for negative selection. This construct was linearized with NotI and electroporated into P2-1 ES cells, which constitutively express the modified Tet-regulated transactivator driven by the CAG promoter.35 Homologous recombination events were screened by polymerase chain reaction (PCR) and confirmed by Southern blot analysis of EcoRI-digested genomic DNA with a probe located between the 2 EcoRI sites, which included exon 3-6.
Establishment of Tet-regulated and G1HRD promoter–regulated GATA-1 expression in GATA-1–null ES cells and OP9 differentiation induction
P2-1 ES cells and their clones were maintained as previously described.36 To employ the Tet-off system, a bicistronic Tet-regulated GATA-1. IRESEGFP construct was made, linearized, and coelectroporated with the plasmid pPGKhygro containing the hygromycin-resistant gene into the GATA-1–null ES cells. The Tet-regulated clones were selected through the application of 160 μg/mL hygromycin (Wako Chemicals, Osaka, Japan) for 7 to 10 days in the presence of 1 μg/mL tetracycline (Sigma, St Louis, MO) and then were screened with enhanced green fluorescent protein (EGFP) and GATA-1 expression by tetracycline as previously described.37
A GATA-1 construct regulated by the G1HRD (GATA-1 hematopoietic regulatory domain) promoter, which is sufficient to direct Gata1 gene transcription in both primitive and definitive erythroid cells,38-40 was produced and coelectroporated with the pPGKhygro plasmid into GATA-1–null ES cells. The appropriate gene regulation by G1HRD was confirmed by the rescue of erythroid differentiation in GATA-1–null cells cocultured on OP9 stroma cells and by Western blotting of GATA-1 expression after erythroid differentiation.
The culturing of the OP9 stroma cells and the in vitro differentiation induction to hematopoietic cells from ES cells on the OP9 cells were performed as previously described.29,30 Either human recombinant erythropoietin (EPO; a kind gift from Chyugai Pharmaceutical, Tokyo, Japan), human macrophage colony-stimulating factor (M-CSF; a kind gift from Morinaga Milk, Tokyo, Japan), or murine granulocyte–macrophage colony-stimulating factor (GM-CSF; Peprotech, London, United Kingdom) was added from day 5 of differentiation at a concentration of 2 IU/mL, 10 ng/mL, or 10 ng/mL, respectively.
Flow cytometry analysis and cell sorting
The primary antibodies used were biotin-anti–Gr-1 (BD Pharmingen, San Diego, CA), biotin-anti–Mac-1 (BD Pharmingen), biotin-anti–TER-119 (a kind gift from Dr T. Kina, Kyoto University, Japan), and PE–anti-CD71 (eBioscience, San Diego, CA). Non-specific binding was blocked by the anti–mouse CD16/32 antibody (BD Pharmingen). Streptavidin–Cy-chrome (BD Pharmingen) was used as a secondary antibody. The samples were analyzed and sorted using a FACSCalibur flow cytometer (Becton Dickinson, Franklin Lakes, NJ). DNA content analysis was performed using propidium iodide (PI) as described previously.41 Cell-cycle distribution was analyzed using ModFit LT software (Becton Dickinson).
Western blotting
Western blotting was performed as previously described.37 The blotted membranes were probed with rat monoclonal anti–GATA-1 antibody (N6; Santa Cruz Biotechnology, Santa Cruz, CA) and reprobed with mouse monoclonal anti–β-actin (AC-15; Sigma). Horseradish peroxidase (HRP)–conjugated anti–rat and anti–mouse IgG antibodies (Zymed, San Francisco, CA) were used as secondary antibodies. The membranes were stained with an enhanced chemiluminescence (ECL) detection kit (Amersham Pharmacia, Buckinghamshire, United Kingdom).
May-Grünwald Giemsa and benzidine staining
The cells were cytospun on glass slides (approximately 5 × 104 cells per slide) at 600 rpm (45g) for 4 minutes with a Cytospin 4 (Thermo Electron, United Kingdom) and then stained with either May-Grünwald followed by Giemsa (Nacalai Tesque, Kyoto, Japan) or with a benzidine dye mixture (3,3′-diaminobenzidine; Nacalai Tesque) according to standard protocols. Light microscopy images were obtained using an Olympus IX70 microscope equipped with a × 20 objective lens, an Olympus DP50 CCD camera, and Viewfinder Lite and Studio Lite software (Olympus, Tokyo, Japan). Images were processed using Adobe Photoshop (Adobe Systems, San Jose, CA).
RT-PCR analysis
The total RNA was recovered with an RNeasy mini kit (Qiagen, Valencia, CA). The cDNA synthesis from RNA was performed using the Thermoscript reverse transcription (RT)–PCR system (Invitrogen, Rockville, MD) with oligo(dT)20 as a primer. Reactions were performed using primers specific for β-H1 globin, β-major globin, ζ globin, GATA-1, and β-actin. The primer sequences and PCR conditions were previously described.27,28
Production and infection of GATA-1–expressing retrovirus
The Gata-1 cDNA sequence was cloned into the pMY.IRESEGFP vector and designated as pMY.GATA-1.IRESEGFP. pMY.GATA-1.IRESEGFP was transfected into the PlatE packaging cell line by using Lipofectamine 2000 (Invitrogen) to produce high-titer retroviral supernatants. The plasmid pMY.IRE.EGFP and Plat-E cells were kind gifts from T. Kitamura (Tokyo University, Japan). The differentiation-induced cells were harvested at day 8 and were suspended in the 0.22-μm–filtered retrovirus supernatant containing 10 μg/mL Polybrene (Sigma). The cells were spin infected at 2500 rpm (1100g) for 2 hours at room temperature, centrifuged again to remove the retrovirus supernatant, suspended in fresh medium, and then seeded onto a fresh OP9 layer. The infection efficiency was approximately 50%.
Results
Conditional expression of GATA-1 in the GATA-1–deficient ES cell lines
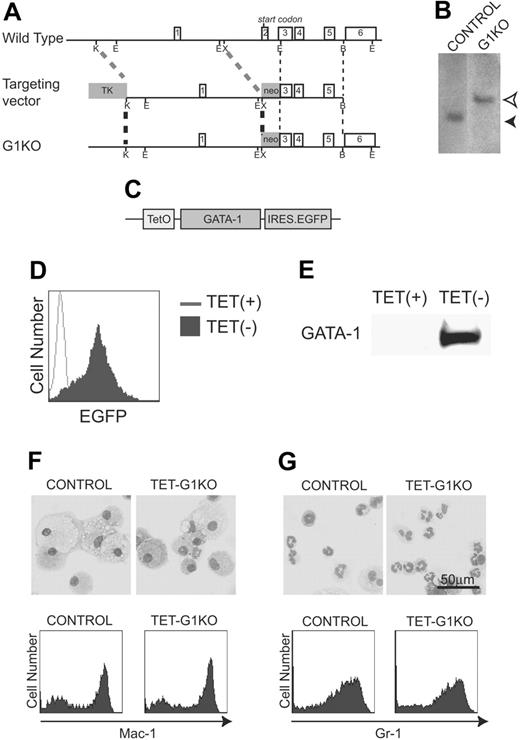
To analyze the function of GATA-1, we established GATA-1–null ES cell lines in which GATA-1 expression was controlled by the Tet-off conditional gene expression system. The null mutation of GATA-1 was introduced to an ES cell line expressing the Tet regulator (control ES cells) using a gene-targeting strategy that was essentially the same as previously described (Figure 1A-B),28 and the cells were named G1KO cells. A plasmid carrying the Gata-1 cDNA and a reporter IRES EGFP gene under the control of a tetracycline-regulated promoter was introduced into the GATA-1–deficient ES cells (Figure 1C). The on and off conditional switching of GATA-1 and EGFP expression was achieved by the removal and addition of tetracycline, respectively. Three independent cell lines in which GATA-1 expression was tightly regulated by tetracycline were selected, and all 3 produced essentially the same results. Hereafter, the data of a representative line, TET-G1KO, are shown (Figure 1D-E). Using the method of coculturing ES cells on OP9 stromal cells (the OP9 system),29,30 we found that the ability of TET-G1KO cells to differentiate into macrophages or granulocytes in the presence of 100 ng/mL tetracycline was identical to that of the control cells in the presence of M-CSF or GM-CSF, respectively (Figure 1F-G). Thus, the ability for hematopoietic differentiation was maintained in TET-G1KO cells despite the genetic manipulations.
Establishment of TET-G1KO cells. (A) Targeted GATA-1 allele. Schematic maps of the Gata-1 locus, the targeting vector, and the targeted locus are shown from top to bottom, respectively. Exon numbers are indicated. Restriction enzyme cleavage sites are shown as follows: K, KpnI; E, EcoRI; X, XbaI; B, BstEII. (B) Southern blot analysis. Homologous recombination was detected by Southern blot analysis of EcoRI-digested genomic DNA using a probe between 2 EcoRI sites of GATA-1 cDNA, which included exons 3-6. Homologous recombination brought about the band of 4.7 kb (open arrow head) instead of the 2.9-kb band (filled arrow head) in the control cells. (C) Construct of GATA-1 (HRES) EGFP. TetO stands for the tetracycline-responsive element. (D-E) Fluorescence-activated cell-sorting (FACS) analysis of EGFP and Western blot of GATA-1 expression in ES cells induced by the deprivation of tetracycline. (F-G) No effects are seen of the GATA-1–null mutation on myeloid differentiation of ES cells. Control and TET-G1KO ES cells were differentiated in the presence of 100 ng/mL tetracycline into macrophages (F) or granulocytes (G) in the presence of M-CSF or GM-CSF, respectively. May-Giemsa staining and FACS analysis of myeloid cell surface markers are shown in the top and bottom panels, respectively.
Establishment of TET-G1KO cells. (A) Targeted GATA-1 allele. Schematic maps of the Gata-1 locus, the targeting vector, and the targeted locus are shown from top to bottom, respectively. Exon numbers are indicated. Restriction enzyme cleavage sites are shown as follows: K, KpnI; E, EcoRI; X, XbaI; B, BstEII. (B) Southern blot analysis. Homologous recombination was detected by Southern blot analysis of EcoRI-digested genomic DNA using a probe between 2 EcoRI sites of GATA-1 cDNA, which included exons 3-6. Homologous recombination brought about the band of 4.7 kb (open arrow head) instead of the 2.9-kb band (filled arrow head) in the control cells. (C) Construct of GATA-1 (HRES) EGFP. TetO stands for the tetracycline-responsive element. (D-E) Fluorescence-activated cell-sorting (FACS) analysis of EGFP and Western blot of GATA-1 expression in ES cells induced by the deprivation of tetracycline. (F-G) No effects are seen of the GATA-1–null mutation on myeloid differentiation of ES cells. Control and TET-G1KO ES cells were differentiated in the presence of 100 ng/mL tetracycline into macrophages (F) or granulocytes (G) in the presence of M-CSF or GM-CSF, respectively. May-Giemsa staining and FACS analysis of myeloid cell surface markers are shown in the top and bottom panels, respectively.
Defective primitive erythropoiesis resulting from the GATA-1–null mutation, and rescue by the conditional expression of GATA-1
Two waves of erythroid cell production were observed when ES cells were cocultured with OP9 stromal cells. The first wave of erythropoiesis appeared at day 6 of the induction and matured till day 8, showing characteristics of EryPs. Subsequently, the second wave of erythroid lineage cells appeared around day 10 and matured until day 12, remarkably identical to EryDs. Morphologic analysis and immunostaining using antibodies against embryonic and adult hemoglobins showed that, in the presence of EPO, about 90% of the day 8 and day 12 nonadherent cells belonged to the EryP and EryD lineages, respectively.42,43
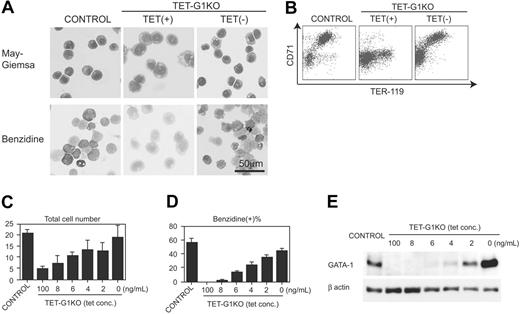
First, we examined the EryP development at day 8 of induction. In contrast to the mature EryPs differentiated from the control ES cells, only immature and benzidine-negative primitive erythroid cells developed from the TET-G1KO ES cells in the presence of 100 ng/mL tetracycline (Figure 2A), which showed that differentiation was arrested during primitive erythropoiesis in TET-G1KO. The fact that no obviously apoptotic EryP cells were observed during the course of differentiation (data not shown) is notable. When GATA-1 was expressed from day 5 to day 8 during the differentiation, morphologically mature and benzidine-positive EryPs appeared (Figure 2A).
Next, we examined the surface expression of TER-119 and CD71, which are reportedly useful for analyzing the differentiation stages of erythroid cells.44-46 The vast majority of TER-119–positive cells induced from control ES cells also expressed CD71 at high levels (Figure 2B). In contrast, only a few percent of TER-119–positive, TET-G1KO–derived cells showed high CD71 expression in the presence of 100 ng/mL tetracycline (Figure 2B). The cell-sorting experiment revealed that the vast majority of TER-119+CD71hi cells were benzidine-positive and expressed embryonic β-H1 globin (Figure S1A-B; see the Supplemental Figures link at the top of the online article, at the Blood website). The TER-119–/CD71low cells were morphologically erythroid lineage cells and up-regulated the expression of globin after the deprivation of tetracycline, suggesting that the cells belonged to erythroid linege (data not shown). The TER-119+CD71lo cells were morphologically immature and benzidine-negative (Figure S1A-B). These results show that the commitment to the primitive erythroid lineage occurred even in the GATA-1–null condition but that the subsequent maturation was severely impaired. Conditional GATA-1 expression produced TER-119+CD71hi EryPs from TET-G1KO cells (Figure 2B).
Rescue of primitive erythropoiesis by conditional expression of GATA-1. (A) May-Giemsa and benzidine staining of EryPs differentiated from control ES cells and TET-G1KO cells at day 8 of induction. TET-G1KO cells were cultured in the presence (100 ng/mL) or absence of tetracycline from day 5. (B) FACS analysis of TER-119 and CD71 of the day-8 EryPs. TET-G1KO cells were cultured in the presence (100 ng/mL) or absence of tetracycline from day 5. (C-D) Number of the cells and percentage of benzidine-positive cells at day 8 of induction. Control ES cells and TET-G1KO cells were cultured with medium containing tetracycline as shown. Data are means ± SD of 6 samples. (E) Western blot of GATA-1 at day 8 of induction. TET-G1KO cells were cultured in the media containing tetracycline as indicated.
Rescue of primitive erythropoiesis by conditional expression of GATA-1. (A) May-Giemsa and benzidine staining of EryPs differentiated from control ES cells and TET-G1KO cells at day 8 of induction. TET-G1KO cells were cultured in the presence (100 ng/mL) or absence of tetracycline from day 5. (B) FACS analysis of TER-119 and CD71 of the day-8 EryPs. TET-G1KO cells were cultured in the presence (100 ng/mL) or absence of tetracycline from day 5. (C-D) Number of the cells and percentage of benzidine-positive cells at day 8 of induction. Control ES cells and TET-G1KO cells were cultured with medium containing tetracycline as shown. Data are means ± SD of 6 samples. (E) Western blot of GATA-1 at day 8 of induction. TET-G1KO cells were cultured in the media containing tetracycline as indicated.
The number of EryP cells produced from the TET-G1KO cells in the absence of tetracycline was essentially similar to that from the control ES cells (Figure 2C), showing that GATA-1 expression rescued EryP production in TET-G1KO cells not only qualitatively but also quantitatively. The expression level of GATA-1 was inversely correlated with the concentration of tetracycline in the culture medium (Figure 2E). As shown in Figure 2C, the number of cells at day 8 increased in proportion to the expression level of GATA-1. Considering that there was no significant increase in apoptotic cells, the increase at day 8 must be attributable to the proliferation of primitive erythropoiesis. The percentage of benzidine-positive cells was also proportional to the GATA-1 expression level (Figure 2D).
Differential effects of GATA-1 on the proliferation and differentiation of EryPs
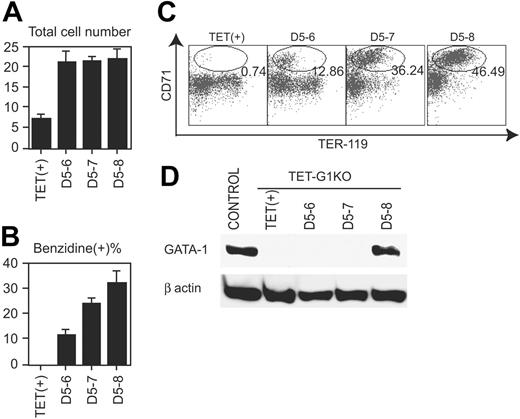
To reveal the action point of GATA-1 during EryP production, GATA-1 was conditionally expressed for limited time periods. Even when GATA-1 was expressed only for 1 day, between days 5 and 6, in the course of TET-G1KO cell differentiation, the number of cells was similar to that obtained when GATA-1 was expressed for 3 days (Figure 3A). Thus, full recovery of the number of EryP cells was achieved within 1 day of expression. The percentage of benzidine-positive cells was significantly increased within 1 day of GATA-1 expression (Figure 3B). Similarly, the expression profiles of TER-119 and CD71 indicated that 1 day of GATA-1 expression produced significant effects on differentiation (Figure 3C). However, both experiments showed that expression for 1 day was not sufficient for full recovery of differentiation, compared with that for 3 days (Figure 3B-C).
The amount of GATA-1 in EryP cells was analyzed at day 8 by Western blotting after the expression of GATA-1 for 1 to 3 days, beginning at day 5. As shown in Figure 3D, GATA-1 protein was not detectable at day 8 if the Gata1 gene was expressed only until day 6 or 7, demonstrating that GATA-1 was degraded within a day in primitive erythroid cells. Taken together with the above-mentioned data (Figure 3A-C), the results indicate that GATA-1 expression for only a limited duration was sufficient for the proliferation but not the differentiation of EryPs. In other words, although both the proliferation and differentiation of EryPs were reproduced by the conditional expression of GATA-1, the effectiveness of the conditional expression was different on proliferation and differentiation.
Effects of the conditional expression of GATA-1 on defective definitive erythropoiesis resulting from the GATA-1–null mutation
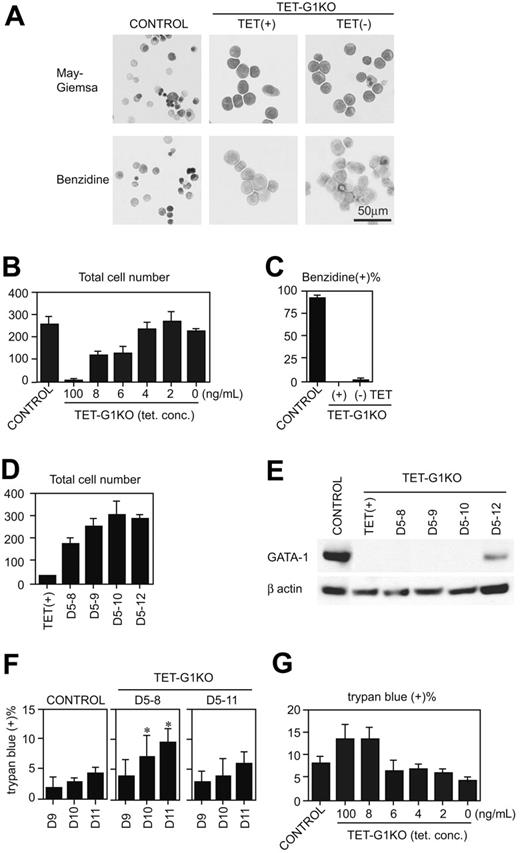
Next, we examined the EryD development at day 12 of induction (Figure 4A-C). TET-G1KO cells were differentiated to benzidine-negative proerythroblasts, and the number of cells was less than 10% of the control cells in the presence of 100 ng/mL tetracycline. When GATA-1 was continuously expressed from day 5 until day 12, the cell number derived from the TET-G1KO cells increased in a manner equal to that from the control cells (Figure 4B), indicating that definitive erythropoiesis was rescued from the numeric point of view. It is conceivable that the rescue of the number was attributable to the proliferation of erythroid cells. Because the percentages of the erythroid cells in S phase were 43.4% ± 1.6% and 30.5% ± 5.9% in the absence and the presence of tetracycline (P < .05 by Student t test; n = 3). The incremental increase in the total number of cells was proportional to the amount of GATA-1 expression (Figure 4B). About 85%-90% of the cells were erythroid cells, which was estimated by morphologic analysis and retroviral introduction of GATA-1 cDNA (data not shown). The increase of the erythroid cells in individual colonies was also proportional to the amount of GATA-1 (data not shown). However, unexpectedly, the percentage of benzidine-positive cells was quite low, and the vast majority of the cells remained at the proerythroblast stage (Figure 4A,C). Although orthochromatic erythroblasts and enucleated erythrocytes were detected, the proportion of these cells was less than 1%. These cells were EryD cells, not residual EryPs, as the cells expressed only adult-type globin genes (Figure S2).
Effects of GATA-1 on primitive erythropoiesis. (A) Number of cells at day 8 of induction after the conditional expression of GATA-1 for the indicated periods. Data are means ± SD of 6 samples. The numbers of cells showed no significant differences among those of D5-6, D5-7, and D5-8. (B) Percentage of benzidine-positive cells at day 8 of induction after the conditional expression of GATA-1 for the indicated periods. Data are means ± SD of 6 samples. The data show significant intervalue differences (P < .01 by Student t test). (C) FACS analysis of TER-119 and CD71 on day 8 induced cells after the conditional expression of GATA-1 for the indicated periods. Ovals demonstrate the fraction of TER119+CD71hi cells, and the percentages of the cells in the fraction are indicated. (D) Western blot of GATA-1 at day 8 after the conditional expression of GATA-1 for the indicated periods.
Effects of GATA-1 on primitive erythropoiesis. (A) Number of cells at day 8 of induction after the conditional expression of GATA-1 for the indicated periods. Data are means ± SD of 6 samples. The numbers of cells showed no significant differences among those of D5-6, D5-7, and D5-8. (B) Percentage of benzidine-positive cells at day 8 of induction after the conditional expression of GATA-1 for the indicated periods. Data are means ± SD of 6 samples. The data show significant intervalue differences (P < .01 by Student t test). (C) FACS analysis of TER-119 and CD71 on day 8 induced cells after the conditional expression of GATA-1 for the indicated periods. Ovals demonstrate the fraction of TER119+CD71hi cells, and the percentages of the cells in the fraction are indicated. (D) Western blot of GATA-1 at day 8 after the conditional expression of GATA-1 for the indicated periods.
Rescue of definitive erythropoiesis by conditional expression of GATA-1. (A) May-Giemsa and benzidine staining of EryDs differentiated from control ES cells and TET-G1KO cells at day 12 of induction. TET-G1KO cells were cultured in the presence (100 ng/mL) or absence of tetracycline from day 5. (B) Number of cells at day 12 of induction. Control ES cells and TET-G1KO cells were cultured with medium containing tetracycline as shown. Data are means ± SD of 6 samples. The numbers of cells at 6 to 100 ng/mL tetracycline were significantly lower than the number at 0 ng/mL tetracycline (P < .001 by Student t test). (C) Percentage of benzidine-positive cells at day 12 of induction. TET-G1KO cells were cultured in the presence (100 ng/mL) or absence of tetracycline as shown. Data are means ± SD of 6 samples. (D) Number of cells at day 12 of induction after the conditional expression of GATA-1 for shorter periods, as shown. Data are means ± SD of 6 samples. The cell numbers for TET(+), D5-8, and D5-9 differ significantly from that of D5-12 (P < .05 by Student t test). The cell numbers for D5-8 to D5-12 differ significantly from that of TET(+) (P < .001 by Student t test). (E) Western blot of GATA-1 at day 11 after the conditional expression of GATA-1 for the indicated periods. (F) Percentages of dead cells after the expression of GATA-1. GATA-1 was expressed from day 5 to 8, or from day 5 to 11, as shown. The percentage of dead cells was counted by trypan blue staining at days 9 to 11. Data are means ± SD of 6 samples. *Data that are significantly different from those of control and D5-11 (P < .01 by Student t test). (G) Percentages of dead cells at day 11 of induction. Control ES cells and TET-G1KO cells were cultured with medium containing tetracycline as shown. Data are means ± SD of 6 samples. There are no significant differences between the percentages at 2 to 6 ng/mL of tetracycline and that of control.
Rescue of definitive erythropoiesis by conditional expression of GATA-1. (A) May-Giemsa and benzidine staining of EryDs differentiated from control ES cells and TET-G1KO cells at day 12 of induction. TET-G1KO cells were cultured in the presence (100 ng/mL) or absence of tetracycline from day 5. (B) Number of cells at day 12 of induction. Control ES cells and TET-G1KO cells were cultured with medium containing tetracycline as shown. Data are means ± SD of 6 samples. The numbers of cells at 6 to 100 ng/mL tetracycline were significantly lower than the number at 0 ng/mL tetracycline (P < .001 by Student t test). (C) Percentage of benzidine-positive cells at day 12 of induction. TET-G1KO cells were cultured in the presence (100 ng/mL) or absence of tetracycline as shown. Data are means ± SD of 6 samples. (D) Number of cells at day 12 of induction after the conditional expression of GATA-1 for shorter periods, as shown. Data are means ± SD of 6 samples. The cell numbers for TET(+), D5-8, and D5-9 differ significantly from that of D5-12 (P < .05 by Student t test). The cell numbers for D5-8 to D5-12 differ significantly from that of TET(+) (P < .001 by Student t test). (E) Western blot of GATA-1 at day 11 after the conditional expression of GATA-1 for the indicated periods. (F) Percentages of dead cells after the expression of GATA-1. GATA-1 was expressed from day 5 to 8, or from day 5 to 11, as shown. The percentage of dead cells was counted by trypan blue staining at days 9 to 11. Data are means ± SD of 6 samples. *Data that are significantly different from those of control and D5-11 (P < .01 by Student t test). (G) Percentages of dead cells at day 11 of induction. Control ES cells and TET-G1KO cells were cultured with medium containing tetracycline as shown. Data are means ± SD of 6 samples. There are no significant differences between the percentages at 2 to 6 ng/mL of tetracycline and that of control.
GATA-1 was expressed for shorter periods, and the increase of day-12 cells was analyzed (Figure 4D). Even when GATA-1 was expressed only until day 8, the number of cells significantly recovered. Next, the percentage of dead cells was analyzed when GATA-1 was expressed from day 5 to 8 or from day 5 to 11. First, the amount of GATA-1 in EryD cells was analyzed by Western blot at day 11 after the conditional expression of GATA-1 for shorter periods (Figure 4E). GATA-1 protein was not detectable at day 11 when the Gata-1 gene was expressed until day 8, 9, or 10, demonstrating that GATA-1 was also degraded within a day in definitive erythroid cells. When GATA-1 expression was maintained until day 11, the percentage of dead cells was not significantly higher than that of the control cells (Figure 4F). When the expression was turned off at day 8 in the TET-G1KO cells, the percentage of dead cells was twice that of the control (Figure 4F). From these data, we suspected that antiapoptotic effects could not account for the increase of EryDs, and analyzed the percentage of apoptotic cell death at day 11 in the presence of various tetracycline concentrations (Figure 4G). At a tetracycline concentration of 6 ng/mL, although the percentage of apoptotic cell death in the TET-G1KO cells was the same as in the control (Figure 4G), the number of EryDs was significantly lower than in the control (Figure 4B). Therefore, GATA-1 plays a role not only in antiapoptosis but also in proliferation during definitive erythropoiesis.
Rescue of definitive erythropoiesis by the late-stage expression of GATA-1
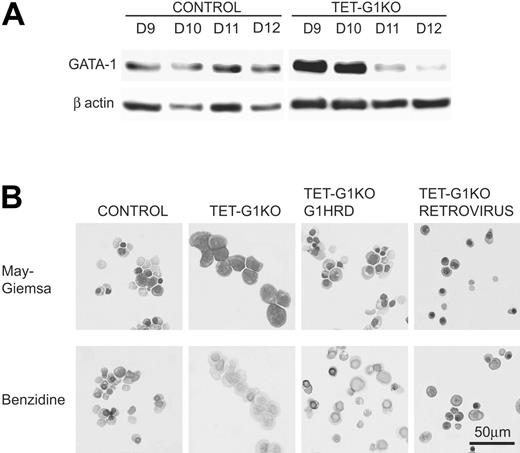
The inefficient EryD differentiation in the GATA-1–expressing TET-G1KO cells is potentially attributable to the inappropriate expression of GATA-1. The amount of GATA-1 protein induced by the conditional expression was analyzed by Western blotting (Figure 5A). Until day 10 of differentiation, an equivalent or greater amount of GATA-1 protein was detected in the TET-G1KO erythroid cells compared with that in the control erythroid cells. However, the amount of GATA-1 was significantly lower after day 11 (Figure 5A). Considering that this decrease was detectable in all of the lines examined, the decrease was presumed to be the result of chromatin condensation throughout erythroid differentiation.
Expression of GATA-1 by the conditional regulation by tetracycline, GATA-1 promoter, and retrovirus vector. (A) Western blot of GATA-1 protein expression at days 9 to 12 of induction after the conditional expression of the Gata-1 gene from day 5. (B) Effects of GATA-1 expression driven by the Gata-1 promoter and by the retrovirus vector on definitive erythropoiesis. May-Giemsa and benzidine staining of EryDs differentiated from control ES cells and TET-G1KO cells at day 12 of induction. TET-G1KO cells were cultured in the presence (100 ng/mL) of tetracycline. G1HRD.GATA-1 and RETROVIRUS show the data for TET-G1KO cells in which GATA-1 was expressed using the Gata-1 promoter and the retroviral vector, respectively.
Expression of GATA-1 by the conditional regulation by tetracycline, GATA-1 promoter, and retrovirus vector. (A) Western blot of GATA-1 protein expression at days 9 to 12 of induction after the conditional expression of the Gata-1 gene from day 5. (B) Effects of GATA-1 expression driven by the Gata-1 promoter and by the retrovirus vector on definitive erythropoiesis. May-Giemsa and benzidine staining of EryDs differentiated from control ES cells and TET-G1KO cells at day 12 of induction. TET-G1KO cells were cultured in the presence (100 ng/mL) of tetracycline. G1HRD.GATA-1 and RETROVIRUS show the data for TET-G1KO cells in which GATA-1 was expressed using the Gata-1 promoter and the retroviral vector, respectively.
To verify that complete terminal differentiation could be achieved from the TET-G1KO cells by other regulatory methods, we adapted 2 strategies. One was the expression of GATA-1 under a G1HRD promoter that was sufficient to direct Gata-1 gene transcription in both primitive and definitive erythroid cells.38,40 The other was the retroviral expression of GATA-1. Under the G1HRD promoter, about 70% of the cells became benzidine-positive and about 30% became enucleated at day 12 of differentiation (Figure 5B), and the number of erythroid cells was comparable to those of the control and TET-G1KO–expressing GATA-1 cells (data not shown).
In the other experiment, to achieve a high expression level of GATA-1 only in the late stage of culture, we infected TET-G1KO cells with a murine stem cell virus (MSCV)–based retroviral vector expressing a bicistronic mRNA encoding GATA-1 and EGFP. Differentiated TET-G1KO cells were spin-infected at day 8 with the GATA-1–EGFP virus and analyzed at day 12 of differentiation induction. As shown in Figure 5B, the infected cells developed into benzidine-positive mature EryDs.
Discussion
In this study, we analyzed the function of GATA-1 by rescuing defective erythropoiesis caused by a GATA-1–null mutation, by combining the OP9 in vitro hematopoietic differentiation of ES cells with tetracycline-regulated conditional gene expression. The conditional rescue of GATA-1 function revealed that the requirement of GATA-1 for proliferation in erythroid cells was different from the requirement for differentiation in both primitive and definitive erythropoiesis.
Complete rescue of proliferation and differentiation of primitive erythropoiesis
In previous studies involving the GATA-1–null mutation, primitive erythroid cells reportedly underwent apoptotic cell death at an immature stage.27,28,47 However, without GATA-1, no obvious cell death was detected throughout the differentiation of EryPs (data not shown) in our study. Therefore, it is conceivable that the reduction of the EryP numbers was caused by impaired proliferation in this experimental system, and the discrepancy is probably resulting from the difference between the microenvironment of EryP differentiation under in vivo conditions and in our in vitro system. One possibility is the concentration of EPO given that immature EryPs are susceptible to EPO withdrawal.43
When the conditional expression of GATA-1 was carried out between days 5 and 8, both the proliferation and differentiation of EryPs were similarly rescued (Figure 2). However, the effects were different when GATA-1 was expressed for shorter periods. Western blot analysis showed that the induced GATA-1 protein was undetectable 1 day after the cessation of Gata-1 gene expression (Figure 3D). Only a short duration of Gata-1 expression was necessary for proliferation, but relatively longer and presumably late-phase expression of GATA-1 was indispensable for the full maturation of EryPs (Figure 3A-C). Thus, the requirement for GATA-1 was different between the proliferation and differentiation of primitive erythropoiesis.
Partial rescue of definitive erythropoiesis by GATA-1
Unlike in primitive erythropoiesis, very few benzidine-positive EryD cells, which arise through terminal differentiation, developed in definitive erythropoiesis from TET-G1KO cells with the conditional expression of GATA-1, even in the absence of tetracycline (Figure 4). However, the expression of GATA-1, driven by a Gata-1 promoter or retrovirus vector, could bring about the terminal differentiation of EryD cells from TET-G1KO cells in the presence of 100 ng/mL tetracycline (Figure 5B). The expression of tetracycline-regulated GATA-1 was similar to or slightly higher than that of GATA-1 expression in control erythroid differentiation until day 10, but was significantly lower than control levels after day 11 of differentiation induction (Figure 5A). Considering these results in the context of the data involving the Gata-1 promoter- and retrovirus-driven GATA-1 expression, it appears that the late-phase expression of GATA-1 is indispensable for the terminal differentiation of EryD cells.
In contrast to the differentiation of cells, the number of definitive erythroid cells fully recovered with GATA-1 conditional expression. That is, the expression of GATA-1 at an earlier stage of EryD differentiation is sufficient to maintain the number of definitive erythroid lineage cells (Figure 4D). In this case, however, terminal differentiation did not occur, and the phenotype remained immature. Based on the expression pattern of tetracycline-regulated GATA-1 in TET-G1KO cells, it appears that transient GATA-1 expression at an appropriate time point allows GATA-1–deficient definitive erythroid cells to survive without suffering apoptotic cell death. Even if GATA-1 is essential for the survival of proerythroblasts, the pivotal time period for avoiding apoptosis should be short. After erythroid cells pass the critical period, GATA-1 would no longer be necessary for their survival. A GATA-1–deficient, immortalized proerythroblastic cell line, G1E, was established by in vitro differentiation from GATA-1–null ES cells. Overcoming the critical period by using transient Bcl-2 expression may have been the key for establishing the G1E cell line.48
Differential effects of GATA-1 on proliferation and differentiation
The production of erythroid cells is controlled by the balance between proliferation and apoptosis.49,50 The proliferation induced by GATA-1 was a major factor in rescuing primitive erythropoiesis because no obvious apoptotic cell death was observed. It is conceivable that both proliferation and the avoidance of apoptosis resulting from GATA-1 are similarly involved in recovering definitive erythropoiesis. Previous reports suggested that apoptosis was a major cause of deficient definitive erythropoiesis.27,47 In our experiments, GATA-1 deficiency produced increased apoptotic cell death in definitive erythropoiesis as well (Figure 4F-G). However, at the same time, our data show that apoptosis is not sufficient to explain the effect of GATA-1 deficiency in definitive erythropoiesis. As shown in Figure 4G, in the presence of 6 ng/mL of tetracycline, apoptotic cells were completely rescued, but the number of EryD cells at day 12 (Figure 4B) was significantly lower than in the control. Therefore, although GATA-1 is important for the survival of definitive erythroid cells, apoptotic cell death is not the only cause of EryD deficiency. For this conclusion, it was necessary to exclude the possibility that impaired commitment to erythroid lineage occurred by the ablation of GATA-1. Therefore, colony formation analysis using day-8 induced cells was undertaken and no differentiation skewing was observed in erythroid colony formation (data not shown). Taken together, GATA-1 is at least partially necessary for the proliferation of EryD precursors.
The differential effects of GATA-1 on production and differentiation may be explained by differences in the timing associated with the requirement of GATA-1.9,51-53 The other interpretation is that different transcriptional complexes are necessary for production and differentiation, given that GATA-1 has been reported to function by forming complexes with other transcription factor(s).22,23,54,55 The third and nonexclusive possibility is epigenetic alteration caused by GATA-1. Histone acetylation occurred rapidly after GATA-1 activation in the G1E cells.56,57 The short-term expression of GATA-1 may bring about some epigenetic change that induces resistance to apoptosis.
Merits and limitations of the experimental system using ES cell differentiation and conditional gene expression
A limitation is present in our experimental system. The expression pattern of GATA-1 by tetracycline deprivation would be different from the pattern of physiologic GATA-1 expression. GATA-1 was not expressed well at the late stage of definitive erythroid differentiation in our system. However, we believe that the present experimental system contains many merits in spite of this limitation. The functions of transcription factors are presumably diversified based on the cell differentiation status.41 An experimental system combining a null mutation with the conditional expression of the gene is the most suitable way to analyze these functions. In vivo rescue experiments have been reported for only a few genes, presumably because the experiments are difficult to perform.58-60 The overexpression experiments using cell lines did not necessarily draw decisive conclusions because cell lines generally reflect only limited aspects of gene functions. Compared with those experimental procedures, the present experimental system is biologically relevant and relatively easy to perform. In addition, cessation of the expression of the genes can be induced, and the gene effects can be analyzed. The present study is a good example of the applicability of the system.
Prepublished online as Blood First Edition Paper, September 20, 2005; DOI 10.1182/blood-2005-04-1385.
Supported in part by grants from the Ministry of Education, Science, Sports, and Culture; Support Program for Technology Development on the Basis of Academic Findings (NEDO); Uehara Memorial Foundation; and the 21st Century Center of Excellence “CICET.”
J.Z., K.K., E.S., and T.K. performed research on both molecular and cell biology; N.M. and M.Y. contributed to constructing the plasmids used in this research; and T.N. designed this research and wrote this manuscript.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
We would like to thank Dr T. Kitamura (Tokyo University), Dr T. Kina (Kyoto University), Chyugai Pharmaceutical, and Morinaga Milk for providing us with research materials. We would also like to thank Ms M. Ikeuchi, Y. Fujita, T. Asada, and A. Mizokami for their excellent assistance.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal