Comment on Arimura et al, page 661
Renal failure in multiple myeloma complicates treatment and shortens life span. Arimura and colleagues introduce the concept that a member of the vasoactive intestinal peptide family might serve a potential renoprotective role in cast nephropathy.
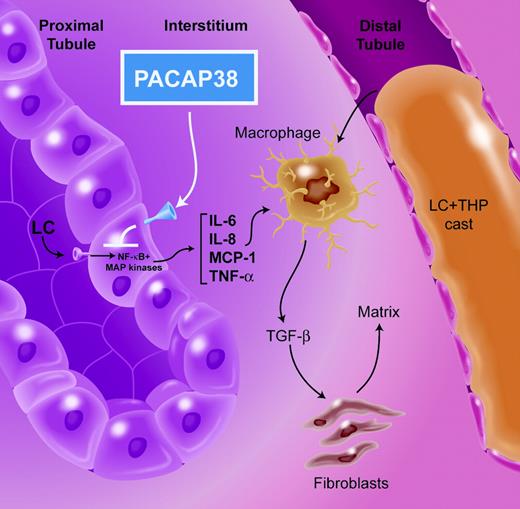
One of the severe and common complications of multiple myeloma is renal failure. The underlying etiology is cast nephropathy, also known as myeloma kidney, in more than two thirds of myeloma-associated renal diseases. The central feature of this process is the immunoglobulin light chain, which is a low-molecular-weight protein that readily undergoes glomerular filtration and can appear in the tubular lumen in significant concentrations, particularly in the setting of multiple myeloma. A prominent feature of cast nephropathy is tubulointerstitial fibrosis, which involves 2 processes (see figure). Proximal tubular reabsorption of light chains sparks the activation of NF-κB and mitogen-activated protein (MAP) kinases; these signaling pathways cooperate to promote chemokine and cytokine production by these cells.1,2 Intrarenal production of these agents facilitates the infiltration of inflammatory cells, which in turn increase TGF-β production, resulting in renal interstitial matrix protein deposition. The second process involves coprecipitation of the light chain in the distal nephron with Tamm-Horsfall protein,3,4 a secreted and apically anchored glycoprotein synthesized by cells of the thick ascending limb of the loop of Henle. Cast formation in the distal nephron obstructs the flow of tubular fluid and promotes breaks in the epithelial lining, facilitating interstitial inflammation. It is likely that both processes contribute to the interstitial fibrosis of cast nephropathy.FIG1
Following glomerular ultrafiltration, immunoglobulin light chains (LCs) bind to a receptor on the apical surface of proximal tubule epithelial cells and undergo endocytosis. Through a process not yet understood, NF-κB and the MAP kinase pathways are activated, resulting in production of chemokines and cytokines that include IL-6, IL-8, MCP-1, and TNF-α. Local production of these chemoattractants results in renal interstitial inflammation, TGF-β activation, and matrix protein production by fibroblasts. In the distal nephron, LC coprecipitates with Tamm-Horsfall protein (THP) to produce an intraluminal cast that obstructs tubule fluid flow and produces breaks in the epithelial cell lining, compounding the interstitial scarring. PACAP38 prevents the activation of the proximal tubule epithelium by LC and inhibits production of TNF-α and IL-6. Illustration by A. Y. Chen.
Following glomerular ultrafiltration, immunoglobulin light chains (LCs) bind to a receptor on the apical surface of proximal tubule epithelial cells and undergo endocytosis. Through a process not yet understood, NF-κB and the MAP kinase pathways are activated, resulting in production of chemokines and cytokines that include IL-6, IL-8, MCP-1, and TNF-α. Local production of these chemoattractants results in renal interstitial inflammation, TGF-β activation, and matrix protein production by fibroblasts. In the distal nephron, LC coprecipitates with Tamm-Horsfall protein (THP) to produce an intraluminal cast that obstructs tubule fluid flow and produces breaks in the epithelial cell lining, compounding the interstitial scarring. PACAP38 prevents the activation of the proximal tubule epithelium by LC and inhibits production of TNF-α and IL-6. Illustration by A. Y. Chen.
In this issue of Blood, Arimura and colleagues extended their earlier observations involving proximal tubular epithelial cell activation by light chains, by identifying a novel inhibitor of cytokine production. The investigators demonstrated that pituitary adenylate cyclase-activating polypeptide with 38 residues (PACAP38), a member of the vasoactive intestinal peptide family, effectively inhibited signal transduction events and associated secretion of IL-6 and TNF-α mediated by incubation of a human proximal tubule cell line with a human immunoglobulin light chain. Furthermore, the inhibitory effect of PACAP38 on light chain–induced renal production of TNF-α was demonstrated in vivo in rats. Because PACAP38 also inhibited in vitro light chain–mediated epithelial cell injury and suppressed growth of myeloma cells, the authors concluded that PACAP38 therapy might serve as a renoprotective agent in multiple myeloma.
Of clinical importance is the observation that cast nephropathy represents a potentially reversible form of renal failure. More importantly, early identification and treatment may prevent the progression to end-stage kidney failure commonly seen in cast nephropathy. By decreasing the circulating levels of monoclonal light chain, cytoreduction therapies are mainstays of treatment. However, eradication of the clone of plasma cells can be challenging, and circulating (and potentially nephrotoxic) light chains often remain detectable for some time after initiation of therapy. While additional studies are required, the original findings of Arimura and associates have merit and support a potential role for PACAP38 as adjunctive therapy in myeloma with associated cast nephropathy.
Dr Sanders is supported by the Medical Research Service of the Department of Veterans Affairs and by a grant from the Multiple Myeloma Research Foundation. ▪

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal