Abstract
Tissue factor (TF) is the cellular receptor for clotting factor VIIa (FVIIa). The formation of TF-FVIIa complexes on cell surfaces triggers the activation of coagulation cascade and cell signaling. In the present study, we characterized the subcellular distribution of TF and its transport in fibroblasts by dual immunofluorescence confocal microscopy and biochemical methods. Our data show that a majority of TF resides in various intracellular compartments, predominantly in the Golgi. Tissue factor at the cell surface is localized in cholesterol-rich lipid rafts and extensively colocalized with caveolin-1. FVIIa binding to TF induces the internalization of TF. Of interest, we found that TF-FVIIa complex formation at the cell surface leads to TF mobilization from the Golgi with a resultant increase in TF expression at the cell surface. This process is dependent on FVIIa protease activity. Overall, the present data suggest a novel mechanism for TF expression at the cell surface by FVIIa. This mechanism could play an important role in hemostasis in response to vascular injury by increasing TF activity where and when it is needed.
Introduction
Tissue factor (TF) is a 45-kDa membrane glycoprotein with a short intracellular COOH-terminal tail and a transmembrane domain that anchors the TF to the membrane surface.1 TF extracellular domain binds to factor VII (FVII) and factor VIIa (FVIIa) with high affinity. The formation of TF-FVIIa complexes triggers the coagulation cascade by activating both factors IX and X, which ultimately leads to thrombin generation, platelet activation, and fibrin deposition.2 In addition to its role in coagulation, TF-FVIIa may also have nonhemostatic functions. TF-FVIIa and the coagulation proteases generated by TF-FVIIa (ie, factor Xa and thrombin) are shown to activate proteinase-activated receptors (PARs).3 The TF-dependent signaling pathways contribute to a variety of pathophysiologic processes, including inflammation, atherosclerosis, angiogenesis, and tumor metastasis.4,5 Thus, a proper regulation of TF expression is critical not only for the maintenance of hemostatic balance but also for other pathophysiologic processes that influence health in general.
TF is constitutively expressed in many extravascular cells, including fibroblasts and pericytes in and surrounding blood-vessel walls and lung epithelial cells, but absent from blood cells and endothelial cells that line blood-vessel walls.6,7 However, TF expression is induced in vivo in monocyte and endothelial cells under various pathologic conditions.8-12 Thus, vessel wall injury that disrupts the endothelial-cell barrier or the pathologic expression of TF in monocytes and endothelial cells will result in the circulating blood coming into contact with TF on cell surfaces, allowing TF-FVIIa complex formation and the subsequent activation of coagulation pathway.
Although much is known about the biochemistry of TF-FVIIa and transcriptional regulation of TF expression, the way cellular dynamics affect TF and TF-FVIIa expression at the plasma membrane is unknown. Since TF activity measured with intact cells is always less than when measured with lysed cells,13-17 it was interpreted by some that TF activity and antigen were stored intracellularly.16,17 Since blocking the surface TF of the intact cells with anti-TF antibody was shown to inhibit more than 90% of TF activity of the intact cells as well as of lysed cells, it was believed that TF expression was entirely localized on cell surfaces.18,19 However, immunohistochemical studies of stimulated smooth muscle cells showed only a small fraction of TF antigen on the cell surface, with the majority of TF antigen in intracellular pools with a distinct perinuclear locus.17 Our recent studies with BHK cells transfected with TF also showed a similar pattern (ie, diffuse membrane staining and bright spots in vesicular perinuclear compartments).20 At present, the specific localization of intracellular TF pools is unclear. Further, there is little information on whether intracellular TF is transported to the cell surface and, if so, what brings it to the cell surface. A clear understanding of the nature of intracellular TF is essential for understanding the regulation of TF expression as the intracellular pools of TF may contribute to the activation of coagulation pathway following cell injury.
In the present study, we precisely characterize the localization of TF in fibroblasts by confocal microscopy and define specific changes in cellular localization of TF in response to FVIIa. Novel data presented herein show that a substantial fraction of intracellular TF is localized in the Golgi, and FVIIa binding to cell-surface TF mobilizes intracellular TF from the Golgi and increases TF expression at the cell surface.
Material and methods
Reagents
Monospecific polyclonal antibodies against human TF were prepared as described earlier.21 TF monoclonal antibodies (TF9-10H10 and TF9-9C3) were kindly provided by Wolfram Ruf (Scripps Research Institute, La Jolla, CA). Anticaveolin antibodies were from Transduction Laboratories (San Diego, CA); anti–human golgin-97, secondary antibodies conjugated to Oregon Green or Rhodamine Red, and SYTOX Green nucleic acid stain were from Molecular Probes (Eugene, OR); and anti-LAMP1, anti-rab5, and anticalreticulin were from Abcam (Cambridge, MA). Recombinant human FVIIa22 and active site–inactivated FVIIa (FFR-FVIIa)23 were obtained from Novo Nordisk A/S (Maaloev, Denmark). Factors X and Xa were from either Enzyme Research Laboratories (South Bend, IN) or Haematological Technologies (Essex Junction, VT).
Cell culture
A human fibroblast cell line (WI-38), derived from normal embryonic lung tissue, was obtained from ATCC (Rockville, MD). Primary lung fibroblasts were obtained from BioWhittaker (Walkersville, MD). Fibroblasts were maintained in DMEM with Glutamax and high-glucose medium supplemented with 1% penicillin/streptomycin/glutamine, and 10% FBS. The cells were cultured at 37°C and 5% CO2 in a humidified incubator.
Radiolabeling of proteins
Factor VIIa and TF mAb were labeled by using Iodo-Gen (Pierce Biotechnology, Rockford, IL)–coated tubes and Na125I according to the manufacturer's technical bulletin and as described previously.24 Free iodine was removed by extensive dialysis against 10 mM Hepes, pH 7.5, 150 mM NaCl. Our earlier studies24,25 established that the radiolabeled proteins were intact with no apparent degradation, and 125I-labeled FVIIa retained 80% or more of its functional activity.
Binding studies
Cell surface binding of 125I-FVIIa or 125I-TF mAb (TF9H10) was performed essentially as described previously.26
Determination of cell-surface TF-FVIIa activity
Monolayers of WI-38 cells were incubated with FVIIa (10 nM) in buffer B (10 mM Hepes, 0.15 M NaCl, 4 mM KCl, 11 mM glucose, pH 7.5 buffer [buffer A] containing 5 mM CaCl2 and 1 mg/mL bovine serum albumin) for 5 minutes at 37°C, followed by the addition of substrate factor X (175 nM). Unless otherwise specified, the reaction was stopped at 10 minutes, which is in the linear phase of FX activation, by transferring an aliquot into stop buffer (TBS containing 1 mg/mL BSA and 10 mM EDTA), and factor Xa in the sample was measured in a chromogenic assay as described earlier.27
Internalization and recycling
Tissue factor endocytosis and recycling were evaluated by biotinylation of cell-surface TF and its protection from cleavage by a membrane-impermeable reagent based on previously published protocols.28-30 Cell-surface biotinylation was performed as described earlier30 with few modifications. Monolayers of fibroblasts cultured in T-25 flask were washed twice with phosphate-buffered saline (PBS) and incubated for 30 minutes on ice in the presence of 0.5 mg/mL sulfo NHS-SS-biotin (Pierce Biotechnology) in PBS. The biotinylation reaction was terminated by washing the monolayers twice with PBS and incubating them on ice for 10 minutes with 10 mM glycine. Biotin-labeled cells were then treated with buffer B or buffer B containing FVIIa or FFR-FVIIa (10 nM) for varying periods at 37°C to allow internalization. Following the treatment, the monolayers were washed 3 times with PBS; cell-surface biotin was cleaved by incubating them with a cell-impermeable reducing buffer (50 mM glutathione, 1 mM MgCl2, 1.0 mM EDTA, 0.2% BSA, and 75 mM NaCl, pH 8.0) for 10 minutes at 37°C. Cells were washed with PBS and lysed in 300 μL lysis buffer (20 mM Tris-HCl, pH 7.4, containing 1 mM EDTA, 150 mM NaCl, 1% Triton X-100, and a cocktail of protease inhibitors). Cell lysates were incubated overnight at 4°C with anti-TF beads (20 μL, 7 mg anti–TF IgG/mL packed beads). Following the removal of unbound material, the beads were washed 3 times with 10 mM Hepes buffer, and the bound material was eluted using 40 μL sodium dodecyl sulfate–polyacrylamide gel electrophoresis (SDS-PAGE) sample buffer. Eluted samples (15 μL) were subjected to SDS-PAGE, followed by Western blot analysis with streptavidin-HRP conjugate. For recycling studies, biotinylated cells were first incubated with FVIIa (10 nM) for 60 minutes to allow internalization of TF, and then cell-surface–biotinylated proteins were removed by reduction, and the internalized receptors were chased by reincubation at 37°C for various time periods (see “Results” and Figure 3 legend for further details).
Immunofluorescence microscopy
Fibroblasts were cultured on 8-chamber glass slide system (Nunc, Rochester, NY). After 1 day of culturing, cells were treated with FVIIa or other agents as indicated in “Results,” and then fixed for 1 hour at 4°C in PBS containing 4% paraformaldehyde and 0.1% glutaraldehyde. The fixed cells were permeabilized with 0.1% Triton X-100 in PBS for 10 minutes and blocked with 3% goat serum in PBS for 1 hour at room temperature. The cells were stained overnight at 4°C with polyclonal rabbit anti-TF and organelle-specific antibodies as indicated in the figure legends, followed by incubation with Oregon Green–conjugated (excitation/emission wavelengths, 490/510 nm) or Rhodamine Red–conjugated (excitation/emission wavelength, 590/620 nm) anti–rabbit or anti–mouse IgG for 60 minutes at room temperature. For negative controls, cells were incubated with non–immune IgG in place of specific primary antibodies.
Image acquisition
The immunostained cells were viewed under a Nikon Eclipse TE2000-5 (Tokyo, Japan) microscope equipped with a 60×/1.4 oil-immersion objective lens at room temperature. Images were acquired by scanning the cells sequentially in 2-μm increments using the UltraVIEW LCI scanning confocal system (Perkin Elmer, Boston, MA) with 488- and 568-nm excitation laser lines. A digital CCD camera (Ultra Pix; Hamamatsu Photonics, Okayama City, Japan) with a resolution of 1344 × 1024 × 12 was used to capture images. Perkin Elmer's ImagingSuite (version 5.0) Acquisition & Processing Software was used for the acquisition of images and measuring the colocalization by determining the correlation coefficient of overlap of the green and the red fluorescence. The scanned images were imported into PowerPoint (Office 2000 software; Microsoft, Redmond, WA) for compilation of figures. All experiments were repeated a minimum of 3 times.
Results
Tissue factor is localized at the plasma membrane as well as intracellularly
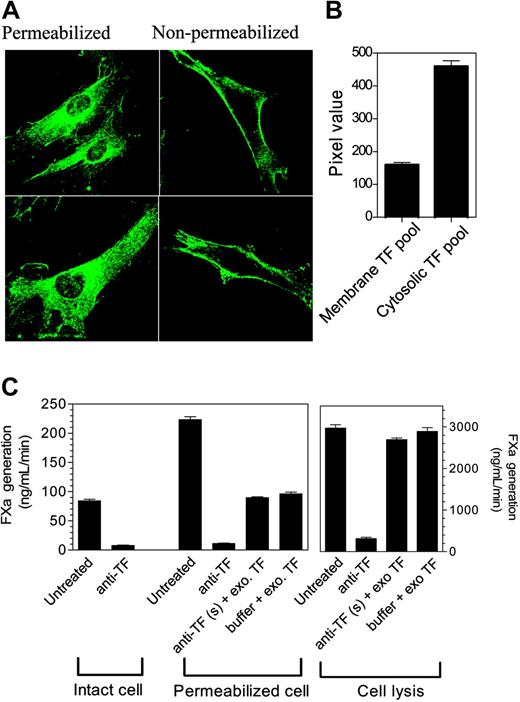
To characterize the distribution pattern of TF, we immunostained fibroblasts (both nonpermeabilized and permeabilized) with monospecific polyclonal rabbit anti–human TF antibody. As expected, the TF antibody brightly stained nonpermeabilized cells along the periphery with no intracellular staining (Figure 1A). Most of the cell surface was stained uniformly, but occasionally we observed increased staining at cell leading edges. In the case of permeabilized cells, in addition to cell-surface staining, TF staining was scattered throughout the cell with intense staining around the nucleus (Figure 1B). When anti-TF IgG was replaced with non–immune IgG, no fluorescence signal was detected in either nonpermeabilized or permeabilized cells (data not shown), indicating the specificity of staining to TF antibodies. Analysis of the mean fluorescence intensity of 3-D reconstructed images of permeabilized and nonpermeabilized cells in paired experiments using ImagingSuite (Perkin Elmer) software suggested that only one fourth of cellular TF is present at the cell surface, whereas the majority of TF resides in the cytosol (mean fluorescence intensity of permeabilized cells, 618.56 ± 17.72; mean fluorescence intensity of nonpermeabilized cells, 159.41 ± 7.45 [n = 31]). It is unlikely that a potential loss of antibody reactivity to TF upon fixation, where intracellular pools of TF are relatively protected from the fixative, biased our quantification toward intracellular TF since we found a similar fluorescence intensity at the cell surface in both live and fixed cells stained with anti-TF antibody at 4°C. Further, we observed a similar TF distribution pattern in fibroblasts transfected with TF-GFP, in which TF expression was evaluated by GFP fluorescence (data not shown). Presence of substantial amounts of TF in intracellular compartments is not unique to WI-38 fibroblasts as we have observed a similar distribution pattern of TF in other cell types, including primary lung fibroblasts, cytokine-stimulated primary endothelial cells, serum-induced primary smooth muscle cells, and tumor cells (MDA-MB-231) (data not shown).
Cellular distribution of tissue factor in fibroblasts. (A) Immunostaining of tissue factor. Nonpermeabilized and Triton X-100–permeabilized WI-38 lung fibroblasts were immunostained with polyclonal rabbit anti–human TF IgG (10 μg/mL), followed by Oregon Green–conjugated anti–rabbit IgG. Fluorescence was viewed with a Perkin Elmer UltraVIEW laser scanning confocal microscope. (B) Quantification of the plasma membrane and intracellular tissue factor. TF levels at the plasma membrane and in the intracellular pool were determined by measuring pixel density of the fluorescence of immunostained cells using 3-D reconstructed images of z slices. The difference in the pixel density between the nonpermeabilized and permeabilized cells was taken as an estimate of the TF in the intracellular compartment (n = 31 cells from 4 different experiments, mean ± SEM). (C) Functional activities of the cell surface and the intracellular tissue factor. Tissue factor activity at the cell surface was blocked by incubating the intact cells with anti-TF IgG (10 μg/mL) for 1 hour at 4°C. Unbound antibodies were removed and the cells were washed 3 times before they were permeabilized by Triton X-100 (0.01% for 10 minutes) or lysed by freeze-thawing. TF activity was measured by adding FVIIa (10 nM) and factor X (175 nM), and measuring the generation of FXa in a chromogenic assay. To demonstrate that there were no free/excess anti-TF antibodies in the experimental system, a small amount of purified relipidated TF (to permeabilized cells) or fibroblast cell lysate (to lysed cells) was added to cells whose surface TF activity was blocked by anti-TF antibodies or to a buffer (n = 3; mean ± SEM).
Cellular distribution of tissue factor in fibroblasts. (A) Immunostaining of tissue factor. Nonpermeabilized and Triton X-100–permeabilized WI-38 lung fibroblasts were immunostained with polyclonal rabbit anti–human TF IgG (10 μg/mL), followed by Oregon Green–conjugated anti–rabbit IgG. Fluorescence was viewed with a Perkin Elmer UltraVIEW laser scanning confocal microscope. (B) Quantification of the plasma membrane and intracellular tissue factor. TF levels at the plasma membrane and in the intracellular pool were determined by measuring pixel density of the fluorescence of immunostained cells using 3-D reconstructed images of z slices. The difference in the pixel density between the nonpermeabilized and permeabilized cells was taken as an estimate of the TF in the intracellular compartment (n = 31 cells from 4 different experiments, mean ± SEM). (C) Functional activities of the cell surface and the intracellular tissue factor. Tissue factor activity at the cell surface was blocked by incubating the intact cells with anti-TF IgG (10 μg/mL) for 1 hour at 4°C. Unbound antibodies were removed and the cells were washed 3 times before they were permeabilized by Triton X-100 (0.01% for 10 minutes) or lysed by freeze-thawing. TF activity was measured by adding FVIIa (10 nM) and factor X (175 nM), and measuring the generation of FXa in a chromogenic assay. To demonstrate that there were no free/excess anti-TF antibodies in the experimental system, a small amount of purified relipidated TF (to permeabilized cells) or fibroblast cell lysate (to lysed cells) was added to cells whose surface TF activity was blocked by anti-TF antibodies or to a buffer (n = 3; mean ± SEM).
Next, we investigated whether the intracellular pool of TF is functionally active by measuring TF activity in permeabilized cells where cell-surface TF was blocked with the antibodies before the cells were permeabilized either with 0.01% Triton X-100 treatment or by freeze-thawing. TF activity was 2- to 3-fold higher in Triton X-100–permeabilized cells compared with nonpermeabilized cells, whereas disrupting the cells by freeze-thaw increased TF activity by more than 30-fold (Figure 1C). However, blocking cell-surface TF activity prior to permeabilization fully attenuated the increase in TF activity of both Triton X-100–permeabilized and the freeze-thawed cells. Addition of traces of purified TF or fibroblast cell lysate to the permeabilized cells in which cell-surface TF activity was blocked by the antibodies increased the TF activity as expected, indicating that there were no excessive anti-TF antibodies to block intracellular TF in our experimental system. These data suggest that the intracellular TF is not functionally active.
Tissue factor at the cell surface is localized primarily in cholesterol-rich membrane microdomains
Our recent electron microscopy and biochemical studies31 suggest that TF in fibroblasts is localized in cholesterol-rich membrane microdomains, both caveolae and noncaveolar lipid rafts. In the present study, we further investigated this by analyzing TF immunofluorescence in control fibroblasts and fibroblasts treated with either mβCD or filipin. Untreated, mβCD-treated, and filipin-treated cells were stained for dual immunofluorescence using anti-TF and anti–caveolin-1, followed by Oregon Green– and Rhodamine Red–conjugated secondary antibodies. In control fibroblasts, both TF and caveolin-1 were stained intensely at the plasma membrane (Figure S1 [top panel], available on the Blood website; see the Supplemental Figure link at the top of the online article). Although one expects a punctuate staining for caveolin-1, this was not clearly discernible. Overlap of TF and caveolin-1 fluorescence showed a high degree of colocalization of TF and caveolin-1 (0.8822 ± 0.02, n = 14), indicating TF localization in caveolae. Removal of cholesterol from the plasma membrane by mβCD treatment markedly reduced the intensity of caveolin-1 staining, which reflects the diffusion of caveolin-1 on the plasma membrane following the loss of caveolar structure. Cholesterol depletion markedly reduced the colocalization of TF and caveolin-1 (0.2014 ± 0.01, n = 16), which is consistent with localization of TF in caveolae. In contrast to mβCD, filipin treatment had no effect on TF and caveolin-1 colocalization (0.9024 ± 0.021, n = 15). Moreover, the TF and caveolin staining in filipin-treated cells shows discontinuous colocalization patches compared with uniform colocalization in control cells (Figure S1, insets in the right panel). These patches may represent cholesterol aggregates in the plasma membrane following complex formation with filipin.
Intracellular localization of tissue factor
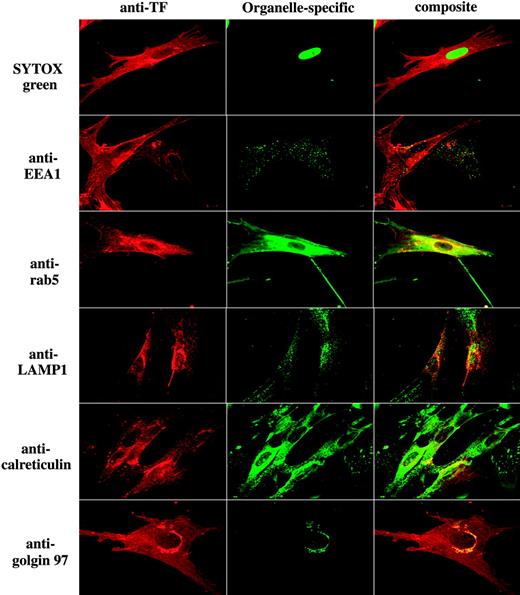
Data shown in Figure 1 suggest that TF is localized at the plasma membrane as well as intracellularly. To identify the intracellular compartments that contain TF, fibroblasts were immunostained with antibodies to TF and a specific intracellular compartment protein marker. We have used antibodies to EEA1 (early endosomal antigen 1) for early endosomes, rab5 for endosomes, LAMP1 for lysosomes, calreticulin for endoplasmic reticulum, and golgin-97 for the Golgi. As shown in Figure 2 (row 2), TF is colocalized with EEA1 in punctuate intracellular structures, indicating TF localization in early endosomes. Extensive colocalization of TF and rab5 also supports the localization of TF in endosomes (Figure 2 row 3). A small fraction of intracellular TF is also colocalized with a subset of intracellular vesicles containing LAMP1, suggesting that a fraction of TF is localized in lysosomes (Figure 2 row 4). TF does not appreciably localize in reticular structures that are stained with anticalreticulin (Figure 2 row 5). Intense perinuclear staining of TF suggests that TF may reside in the Golgi apparatus. Consistent with this notion, TF is colocalized with golgin-97 in perinuclear compartment (Figure 2 row 6). Brefeldin A treatment, which leads to dispersal of the Golgi, abolished the perinuclear staining of TF and the colocalization of TF and golgin-97, confirming the TF distribution in the Golgi (data not shown).
Intracellular distribution of tissue factor in fibroblasts. Fibroblasts were fixed, permeabilized, and stained with anti–human TF and an organelle-specific antibody. Left column represents TF staining; middle column, organelle-specific staining; and the right column, the overlay image (colocalization) of TF and organelle-specific marker. Organelle-specific antibodies/stain used as follows: SYTOX Green for nucleus, anti-EEA1 and anti-rab5 for early endosomes, anti-LAMP1 for lysosomes, anticalreticulin for endoplasmic reticulum, and anti–golgin-97 for the Golgi. Note: In contrast to EEA1, whose localization is limited to early endosomes, rab5 is also known to present in the cytoplasmic side of the plasma membrane and clathrin-coated vesicles.
Intracellular distribution of tissue factor in fibroblasts. Fibroblasts were fixed, permeabilized, and stained with anti–human TF and an organelle-specific antibody. Left column represents TF staining; middle column, organelle-specific staining; and the right column, the overlay image (colocalization) of TF and organelle-specific marker. Organelle-specific antibodies/stain used as follows: SYTOX Green for nucleus, anti-EEA1 and anti-rab5 for early endosomes, anti-LAMP1 for lysosomes, anticalreticulin for endoplasmic reticulum, and anti–golgin-97 for the Golgi. Note: In contrast to EEA1, whose localization is limited to early endosomes, rab5 is also known to present in the cytoplasmic side of the plasma membrane and clathrin-coated vesicles.
TF trafficking between the plasma membrane and intracellular compartments
To investigate whether TF recycles between the plasma membrane and intracellular compartments, we first analyzed whether the plasma membrane TF is endocytosed. Fibroblasts were labeled with sulfo-NHS-SS-biotin on ice and then incubated with a control buffer or the buffer containing FVIIa or inactive FVIIa (FFR-FVIIa) for various times at 37°C to allow internalization of TF (radiolabeled FVIIa binding studies showed that the biotinylation of cell-surface TF had no effect on its binding to FVIIa or mediating the internalization of FVIIa; data not shown). Thereafter, the cells were exposed to the membrane-impermeable reducing agent to cleave the biotin label from cell-surface proteins (endocytosed pool of proteins are protected from the reduction and remain biotinylated). Analysis of TF immunoprecipitates from fibroblasts that were treated with the control buffer or FFR-FVIIa showed only a minimal increase of biotinylated TF in the intracellular pool of proteins (Figure 3A), indicating that TF is not internalized actively under these conditions or that the internalized TF is immediately recycled back to the cell surface. However, a significant and time-dependent TF internalization was observed in fibroblasts that were exposed to FVIIa (Figure 3A). Accumulation of biotinylated TF inside the cell reached maximum at 60 minutes. To investigate whether the internalized TF recycles back to the cell surface, we first allowed TF to internalize for 60 minutes at 37°C in the presence of FVIIa before treating the cells with the reducing agent to remove the surface biotin. Cells were then reincubated at 37°C for varying time periods to chase the internalized receptors, and then treated with or without the reducing agent to remove the biotin label from the receptors that are recycled back to the cell surface. Under this experimental setup, the difference in the biotinylation signal in cells treated with the reducing agent or none gives the measure of recycling. We found no significant differences in the biotinylation signals between the reduced and nonreduced samples (Figure 3B). These data indicate that the internalized TF does not appreciably recycle back to the cell surface.
Factor VIIa–induced internalization and recycling of tissue factor. (A) Fibroblasts were surface-labeled with sulfo NHS-SS-biotin at 4°C and then incubated at 37°C for varying times with a control buffer or the buffer containing FVIIa or FFR-FVIIa (10 nM). The cells were then treated with the reducing agent, lysed, and immunoprecipitated with anti-TF beads. The immunoprecipitated samples were analyzed for the biotin to identify the internalized TF. TF biotin signal detected in cells that were treated with the reducing agent immediately following the biotinylation was taken as 100%. To show the extent of cell-surface TF biotinylation, immunoprecipitates of cells that were not treated with the reducing agent were analyzed for the biotin label. *Values significantly differ from the TF internalized in the absence of FVIIa (P < .02). (B) To measure the recycling of the internalized cell-surface TF, biotin-labeled cells were first incubated with FVIIa at 37°C for 60 minutes to allow TF internalization. Cells were washed with PBS, and the internalized receptors were chased by reincubation at 37°C for various time periods in duplicate samples. After the incubation, only 1 of 2 samples was reduced again. The cells were lysed and subjected to TF immunoprecipitation, followed by immunoblotting. Differences between the chased cells that were treated or not treated with the reducing agent represent the amount of TF recycled back to the cell surface. NS denotes no statistically significant difference. The data shown in the figure represent mean ± SEM (n = 3 to 6 for A, n = 3 for B).
Factor VIIa–induced internalization and recycling of tissue factor. (A) Fibroblasts were surface-labeled with sulfo NHS-SS-biotin at 4°C and then incubated at 37°C for varying times with a control buffer or the buffer containing FVIIa or FFR-FVIIa (10 nM). The cells were then treated with the reducing agent, lysed, and immunoprecipitated with anti-TF beads. The immunoprecipitated samples were analyzed for the biotin to identify the internalized TF. TF biotin signal detected in cells that were treated with the reducing agent immediately following the biotinylation was taken as 100%. To show the extent of cell-surface TF biotinylation, immunoprecipitates of cells that were not treated with the reducing agent were analyzed for the biotin label. *Values significantly differ from the TF internalized in the absence of FVIIa (P < .02). (B) To measure the recycling of the internalized cell-surface TF, biotin-labeled cells were first incubated with FVIIa at 37°C for 60 minutes to allow TF internalization. Cells were washed with PBS, and the internalized receptors were chased by reincubation at 37°C for various time periods in duplicate samples. After the incubation, only 1 of 2 samples was reduced again. The cells were lysed and subjected to TF immunoprecipitation, followed by immunoblotting. Differences between the chased cells that were treated or not treated with the reducing agent represent the amount of TF recycled back to the cell surface. NS denotes no statistically significant difference. The data shown in the figure represent mean ± SEM (n = 3 to 6 for A, n = 3 for B).
FVIIa mobilizes TF pool from the Golgi
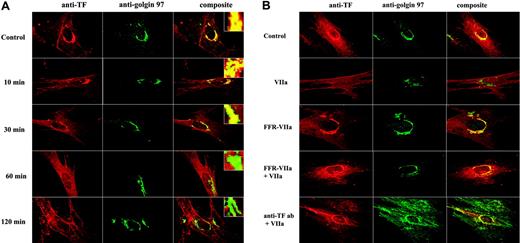
Although FVIIa induced TF endocytosis, even prolonged incubation of FVIIa failed to deplete the TF receptors from the cell surface.26 This suggests that TF at the cell surface is replenished through the recycling of the endocytosed TF and/or the mobilization of TF from the intracellular compartments in response to FVIIa. To investigate the latter possibility, we investigated the effect of FVIIa on TF distribution and trafficking by immunofluorescence microscopy. Fibroblasts were treated with FVIIa for varying times, and the distribution of TF into various intracellular compartments was analyzed by immunofluorescence microscopy after staining the cells with antibodies to TF and an organelle-specific marker. As discussed earlier, a substantial amount of intracellular TF was found in the Golgi as demonstrated by the colocalization of TF and golgin-97. FVIIa treatment mobilized TF out of the Golgi. FVIIa, in a time-dependent manner, reduced the colocalization of TF and golgin-97 (Figure 4A). At the end of 2 hours of treatment with FVIIa, most of the TF was moved out of the Golgi as implicated by marked reduction in the extent of TF and golgin-97 colocalization compared with the fibroblasts that were treated with a control vehicle (correlation coefficient: control, 0.84 ± 0.066; FVIIa treated, 0.43 ± 0.077; n = 28 to 33, P < .001).
To investigate whether FVIIa binding to TF alone is sufficient to induce TF mobilization from the Golgi or whether it requires the protease activity of FVIIa, we tested the effect of active site–inhibited FVIIa on TF mobilization. Fibroblasts were treated with FFR-FVIIa for 2 hours and the mobilization of TF from the Golgi was evaluated as described in Figure 4A. In contrast to FVIIa, FFR-FVIIa failed to deplete TF from the Golgi (Figure 4B row 3). No significant differences were detected between the control- and FFR-FVIIa–treated cells in the extent of TF and golgin-97 colocalization in the perinuclear compartment (correlation coefficient: control, 0.84 ± 0.066; FFR-FVIIa treated, 0.83 ± 0.055; n = 24 to 33). To demonstrate that the mobilization of TF from the Golgi is a specific response to FVIIa binding to cell-surface TF, we pretreated fibroblasts with 20-fold molar excess of FFR-FVIIa (Figure 4B row 4) or anti-TF IgG (Figure 4B row 5) prior to the addition of FVIIa to block FVIIa binding to cell-surface TF. As expected, both anti-TF IgG and FFR-FVIIa blocked the FVIIa-induced TF mobilization from the Golgi pool (Figure 4B).
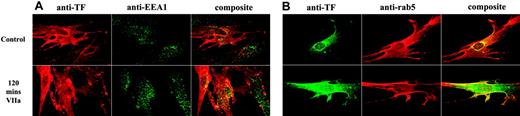
Treatment of FVIIa not only dispersed TF from the Golgi but also increased the TF levels in endosomes as evidenced by the increased colocalization of TF and rab5 in the endocytotic vesicles (Figure 5). The correlation coefficient of TF and rab5 staining in cells treated for 2 hours was as follows: control vehicle, 0.69 ± 0.029; FVIIa, 0.90 ± 0.009; and FFR-FVIIa, 0.71 ± 0.02 (n = 8 to 10; P < .001 for FVIIa vs control; P = .58 for FFR-FVIIa vs control). Similar data were obtained in cells stained with EEA1 (control, 0.42 ± 0.02; FVIIa treated, 0.59 ± 0.02; P < .001, n = 23).
A close examination of TF staining at the cell surface showed an increased TF expression at the cell surface in cells treated with FVIIa and not FFR-FVIIa (Figures 4-5). Quantification of TF expression at the cell surface by image analysis of TF fluorescence (compared with matched controls, which was taken as 100%) was as follows: FVIIa treated, 178 ± 15.5; FFR-FVIIa treated, 104 ± 2.4 (n = 12).
Factor VIIa mobilizes tissue factor from the Golgi. (A) Fibroblasts were exposed to FVIIa (10 nM) for different time intervals (0 to 120 minutes). Cells were fixed, permeabilized, and immunostained with rabbit polyclonal anti–human TF and monoclonal anti–human golgin-97 antibodies, followed by Rhodamine Red–labeled anti–rabbit IgG and Oregon Green–labeled anti–mouse IgG as secondary reporter antibodies. (B) Factor VIIa's protease activity and the binding to cell-surface tissue factor are essential for the trafficking of tissue factor from the Golgi. WI-38 cells were exposed to FVIIa (10 nM) or FFR-FVIIa (10 nM) alone for 2 hours at 37°C or first incubated with 20-fold molar excess of FFR-FVIIa or anti-TF IgG (10 μg/mL) for 30 minutes before FVIIa (10 nM) was added to the cells. The cells were stained with anti-TF and anti–golgin-97 antibodies and analyzed as described in panel A. Left panel images represent TF staining, middle panel images represent the Golgi staining, and right panel images represent the overlay of TF and the Golgi staining (colocalization). Insets in panel A show a magnified view of TF localization in the Golgi.
Factor VIIa mobilizes tissue factor from the Golgi. (A) Fibroblasts were exposed to FVIIa (10 nM) for different time intervals (0 to 120 minutes). Cells were fixed, permeabilized, and immunostained with rabbit polyclonal anti–human TF and monoclonal anti–human golgin-97 antibodies, followed by Rhodamine Red–labeled anti–rabbit IgG and Oregon Green–labeled anti–mouse IgG as secondary reporter antibodies. (B) Factor VIIa's protease activity and the binding to cell-surface tissue factor are essential for the trafficking of tissue factor from the Golgi. WI-38 cells were exposed to FVIIa (10 nM) or FFR-FVIIa (10 nM) alone for 2 hours at 37°C or first incubated with 20-fold molar excess of FFR-FVIIa or anti-TF IgG (10 μg/mL) for 30 minutes before FVIIa (10 nM) was added to the cells. The cells were stained with anti-TF and anti–golgin-97 antibodies and analyzed as described in panel A. Left panel images represent TF staining, middle panel images represent the Golgi staining, and right panel images represent the overlay of TF and the Golgi staining (colocalization). Insets in panel A show a magnified view of TF localization in the Golgi.
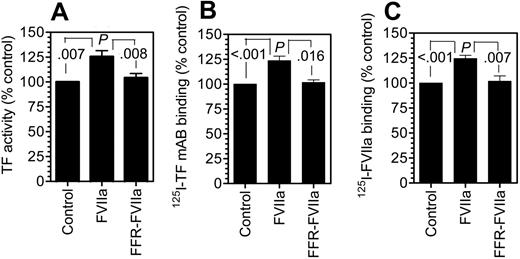
Factor VIIa treatment increased TF levels at the cell surface
To confirm the data obtained with immunofluorescence microscopy that FVIIa treatment not only depletes TF from the Golgi but also increases TF levels at the cell surface by trafficking it to the cell surface, we measured TF levels at the cell surface using 3 independent assay methods—measurement of cell-surface TF activity, TF-specific FVIIa binding, and TF mAb binding to cells. As shown in Figure 6, FVIIa treatment increased the TF levels at the cell surface by 20% to 30%. Although the increase appears to be modest, it is highly consistent, statistically significant (P < .01), and specific to FVIIa. FFR-FVIIa treatment failed to increase TF levels at the cell surface. This small increase is an expected finding since only a fraction of the total TF is located in the Golgi, and thus the transport of TF from the Golgi to the plasma membrane, in response to FVIIa, would increase TF concentration at the plasma membrane at most only by the amount of TF in the Golgi. It may be pertinent to note here that FVIIa did not induce new TF synthesis as evaluated by measurement of TF mRNA in Northern blot analysis (data not shown).
Increase in tissue factor concentration in endosomes in response to factor VIIa. Fibroblasts were exposed to control vehicle or FVIIa (10 nM) for 2 hours at 37°C. The cells were fixed, permeabilized, and immunostained with anti–human TF and antibodies against either EEA1 (A) or rab5 (B). Left panel represents TF staining, middle panel represents the staining for early endosomes (EEA1 or rab5), and the right panel represents the overlay of TF and EEA1 (or rab5) staining.
Increase in tissue factor concentration in endosomes in response to factor VIIa. Fibroblasts were exposed to control vehicle or FVIIa (10 nM) for 2 hours at 37°C. The cells were fixed, permeabilized, and immunostained with anti–human TF and antibodies against either EEA1 (A) or rab5 (B). Left panel represents TF staining, middle panel represents the staining for early endosomes (EEA1 or rab5), and the right panel represents the overlay of TF and EEA1 (or rab5) staining.
Discussion
Vascular injury results in the exposure of blood to TF-expressing cells within the vessel wall, allowing FVIIa binding to TF. The resultant TF-FVIIa triggers the activation of blood coagulation cascade. Although much is known as to how TF-FVIIa activity is regulated at the cell surface, information on TF secretion, its subcellular localization, and its surface expression is scant. Further, it is unknown whether FVIIa binding to cell-surface TF induces the internalization and recycling of TF or alters its subcellular distribution. In the present study, we defined cell-surface and subcellular localization of TF in native fibroblasts and fibroblasts that were exposed to FVIIa, and we report several novel observations.
Earlier studies suggested that TF antigen and its activity are entirely localized at the cell surface.18,19,32 However, recent studies found that a substantial amount of TF is localized intracellularly in several cell types.17,20,33-35 Consistent with these data, we found that approximately 75% of total TF antigen is localized intracellularly in fibroblasts. The intracellular TF is not functionally active. These data fit with the earlier observations made by our group as well as by others18,19 but contrast with the findings of Schecter et al,17 who reported that the intracellular TF pool contains as much as 37% of the total TF activity in stimulated smooth muscle cells. Reasons for this discrepancy are unknown, but if the affinity of TF antibodies used by Schecter et al17 for blocking cell-surface TF was lower, it might have compromised the methodology used to evaluate the functionality of intracellular TF.
Factor VIIa binding to fibroblasts increases tissue factor expression at the cell surface. Fibroblasts were incubated with a control buffer, FVIIa (10 nM), or FFR-FVIIa (10 nM) for 2 hours at 37°C. Thereafter, the cells were washed with 5 mM EDTA to remove the bound FVIIa/FFR-FVIIa, and cell-surface TF levels were determined by incubating the cells for 2 hours at 4°C with 125I-TF mAB (TF9H10) or 125I-FVIIa (10 nM) (± polyclonal anti–human TF) and then determining TF mAB binding (B) or TF-specific FVIIa binding (C) to the cells. To measure TF functional activity, fresh FVIIa (10 nM) and factor X (175 nM) were added to the cells and the rate of factor X activation was measured (A). Cell-surface TF antigen and activity were significantly higher statistically (P < .003 to .008) in FVIIa-treated cells compared with control vehicle–treated cells or cells treated with FFR-FVIIa. No significant differences were found between control vehicle– and FFR-FVIIa–treated cells (n = 3 to 6, mean ± SEM).
Factor VIIa binding to fibroblasts increases tissue factor expression at the cell surface. Fibroblasts were incubated with a control buffer, FVIIa (10 nM), or FFR-FVIIa (10 nM) for 2 hours at 37°C. Thereafter, the cells were washed with 5 mM EDTA to remove the bound FVIIa/FFR-FVIIa, and cell-surface TF levels were determined by incubating the cells for 2 hours at 4°C with 125I-TF mAB (TF9H10) or 125I-FVIIa (10 nM) (± polyclonal anti–human TF) and then determining TF mAB binding (B) or TF-specific FVIIa binding (C) to the cells. To measure TF functional activity, fresh FVIIa (10 nM) and factor X (175 nM) were added to the cells and the rate of factor X activation was measured (A). Cell-surface TF antigen and activity were significantly higher statistically (P < .003 to .008) in FVIIa-treated cells compared with control vehicle–treated cells or cells treated with FFR-FVIIa. No significant differences were found between control vehicle– and FFR-FVIIa–treated cells (n = 3 to 6, mean ± SEM).
Ultrastructural localization of TF in smooth muscle cells showed that about 20% of TF on the cell surface is associated with caveolae.36 Our recent studies with fibroblasts suggest that TF is localized in caveolae as well as noncaveolar cholesterol-sphingolipid rafts.31 However, TF partitioning into rafts varied according to the detergent used for the partition, making it difficult to evaluate the true extent of TF localization in cholesterol-sphingolipid rafts. The present immunofluorescence confocal microscopic studies reveal that TF is highly enriched in cholesterol-rich lipid rafts, and the removal of cholesterol from the membrane, as expected, markedly reduced its association with the lipid rafts/caveolin-1. Of interest, filipin, which disrupts caveolar structures by forming cholesterol aggregates in the plasma membrane, did not reduce the extent of TF colocalization with caveolin-1. These data suggest, albeit indirectly, that TF, as with caveolin-1, associates with lipid rafts probably through its direct interaction with cholesterol.
Although earlier studies demonstrated intracellular localization of TF in stimulated smooth muscle cells, monocytes, and other cell types,17,34,35 to our knowledge, the specific localization of the intracellular TF pool is not identified. The present study reveals that TF is localized in distinct intracellular compartments. A major fraction of intracellular TF is localized in the perinuclear compartment, and this compartment is stained intensely with the Golgi-specific marker, suggesting TF localization in the Golgi. Tissue factor pool in the Golgi appears to be newly synthesized TF since cycloheximide treatment diminished the presence of TF in the Golgi (data not shown). In addition to the Golgi, a substantial amount of TF is localized in early endosomes, and a small fraction in lysosomes. The presence of TF in the Golgi and endosomes may represent newly synthesized TF and the TF in the transportation pathway to the cell surface rather than the constitutive recycling of TF. A biochemical assay based on NHS-SS biotin-labeling of surface molecule, in which SS-biotin link on the surface could be cleaved by cell-impermeable reducing agent, revealed a minimal TF internalization in the absence of ligand FVIIa. FVIIa induced the internalization of TF in a time-dependent manner; however, the process is not very rapid. Further, only a small fraction of total cell-surface TF appears to be endocytosed. Our data do not provide any evidence for appreciable recycling of internalized TF back to the cell surface. The present data are the first direct evidence for FVIIa-induced internalization of TF. In this context, it is interesting to note that endocytosed TF was not detected in fibroblasts exposed to FFR-FVIIa despite the prior evidence that FFR-FVIIa bound to TF was internalized in a similar fashion as FVIIa.26 These data suggest that the TF internalized with FFR-FVIIa recycles back to the cell surface at a much faster rate than the TF internalized with FVIIa. It is difficult to demonstrate such fast internalization and recycling with the present methodology. The differences we observed between FVIIa and FFR-FVIIa in TF internalization in the present study would also explain other differences we found between FVIIa and FFR-FVIIa in earlier studies25 (ie, internalized FFR-FVIIa recycles back to the cell surface much faster than FVIIa, and FVIIa and not FFR-FVIIa was retained in a specific intracellular compartment).
The most important and interesting observation of the present study is that FVIIa binding to TF at the cell surface mobilizes TF from the Golgi. At least some of the mobilized TF appears to be transported to the outer cell-surface plasma membrane via the endosome pathway. This conclusion was supported by 3 sequential observations made in response to FVIIa—the decreased TF antigen levels in the Golgi, the increased TF antigen in the endosomes, and the increased TF antigen and activity at the cell surface. Further, FVIIa treatment increased the colocalization of TF and rab5 at the inner side of the plasma membrane, suggesting that some of the mobilized TF is transported nearer to the plasma membrane, but not necessarily to the outer surface. It is important to note that although the increase in TF at the cell surface is modest, it was consistent and measurable by all 4 independent assay methods—immunofluorescence of TF, FVIIa binding, TF mAB binding, and TF-FVIIa functional activity. Although the intracellular TF is functionally inactive, its transport back to the plasma membrane containing phospholipids will restore its functional activity. Binding of FVIIa to cell-surface TF and the catalytic activity of FVIIa are essential for the mobilization of TF from the Golgi, as evidenced by the failure of active site–inhibited FVIIa (FFR-FVIIa) to deplete TF from the Golgi or to increase TF expression or activity at the cell surface. Addition of FFR-FVIIa (in a 20-fold molar excess to FVIIa) or anti–TF IgG, before adding FVIIa, blocked the FVIIa-induced TF mobilization. At present, the mechanism by which FVIIa mobilizes TF from the Golgi and transports it to the plasma membrane is unknown. Further, it is unclear whether FVIIa-induced intracellular TF mobilization depends solely on TF-FVIIa protease activity at the cell surface or is influenced by TF-FVIIa internalization. Recent studies from us and others show that TF-FVIIa can transmit cell signaling via activation of PAR2 (for a review, see Rao and Pendurthi4 and Belting et al5 ). Therefore, it is possible that FVIIa-induced PAR2-mediated cell signaling could play a role in the observed TF cellular transport. Further studies are needed to address whether TF-FVIIa–induced PAR2-mediated cell signaling is responsible for TF-FVIIa–induced mobilization.
Transport of intracellular TF to the outer cell surface in response to stimuli could play an important role in regulating TF expression at the cell surface. By measuring TF activity in intact, sonicated, and shed vesicles at varying times (6 to 36 hours) following endotoxin stimulation, Bona et al14 suggested that TF was transported selectively to the cell membrane and shed into conditioned media in membrane vesicles in short-term cultures of endotoxin-stimulated monocytes. In a recent study, Egorina et al34 reported an increased TF antigen expression on stimulation with LPS onto the monocyte surface with a clear depletion of TF staining from the cytoplasm. As these studies were not focused on characterizing and identifying the intracellular localization of TF, it is unclear whether TF staining in the cytoplasm observed in monocytes reflects TF pool in the Golgi. Nonetheless, these data suggest that LPS may act very similarly to FVIIa in mediating the TF cellular transport. Alternatively, it is possible that the reported effect of LPS on TF transport in monocytes actually stems from plasma FVIIa binding to TF on monocytes, since whole blood was used in these studies to stimulate monocytes with LPS. It will be interesting to investigate this possibility in future studies.
Overall, the present data characterize distribution of TF in fibroblasts and demonstrate the ability of FVIIa to transport intracellular TF to the cell surface. These data bring forth a novel mechanism by which TF expression at the cell surface could be enhanced by its ligand in response to injury. This mechanism allows increase in TF concentration at the cell surface when it is needed and thus could play an important role in hemostasis.
Prepublished online as Blood First Edition Paper, February 21, 2006; DOI 10.1182/blood-2005-11-4674.
Supported by grants from the National Institutes of Health HL58869 and HL65550, and the American Heart Association, Texas Affiliate (0355096Y). S.K.M. is a recipient of a postdoctoral fellowship award from the American Heart Association, Texas Affiliate.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.


This feature is available to Subscribers Only
Sign In or Create an Account Close Modal