Abstract
High–molecular-weight kininogen (HK) is an abundant plasma protein that plays a central role in activation of the kallikrein-kinin system. Cleavage of HK by plasma kallikrein results in release of the nonapeptide bradykinin (BK), leaving behind cleaved high–molecular-weight kininogen (HKa). Previous studies have demonstrated that HKa induces apoptosis of proliferating endothelial cells and inhibits angiogenesis in vivo, activities mediated primarily through its domain 5. However, the mechanisms by which these effects occur are not well understood. Here, we demonstrate that HKa induces apoptosis of endothelial cells cultured on gelatin, vitronectin, fibronectin, or laminin but not collagen type I or IV. The ability of HKa to induce endothelial-cell apoptosis is dependent on the generation of intracellular reactive oxygen species and associated with depletion of glutathione and peroxidation of endothelial-cell lipids, effects that occur only in cells cultured on matrix proteins permissive for HKa-induced apoptosis. Finally, the ability of HKa to induce endothelial-cell apoptosis is blocked by the addition of reduced glutathione or N-acetylcysteine. These studies demonstrate a unique role for oxidant stress in mediating the activity of an antiangiogenic polypeptide and highlight the importance of the extracellular matrix in regulating endothelial-cell survival.
Introduction
Angiogenesis, the process by which new capillaries develop from preexisting vessels, plays an important role in embryonic development, wound healing, the female reproductive cycle, and pathophysiologic processes such as cancer and inflammation. Inhibition of angiogenesis is a useful therapy in some neoplastic disorders,1 and stimulation of angiogenesis demonstrates promise in the treatment of ischemic vascular disease.2
High–molecular-weight kininogen (HK) is a 120-kDa single-chain glycoprotein composed of 6 domains.3 Cleavage of HK by plasma kallikrein results in the release of the nonapeptide bradykinin (BK) from HK domain 4 and the generation of 2-chain kininogen (HKa).4-6 While BK has been reported to stimulate angiogenesis,7-9 HKa is a potent angiogenesis inhibitor, inducing selective apoptosis of proliferating endothelial cells.10 Studies from our laboratory10 and others11,12 have demonstrated that the antiangiogenic activity of HKa is mediated through its domain 5, although the mechanism by which HKa inhibits angiogenesis remains controversial.
In investigating the antiangiogenic mechanism(s) of HKa, we observed that HKa-induced apoptosis of proliferating endothelial cells was modulated by the extracellular matrix (ECM) protein upon which the cells were cultured. HKa induced apoptosis of human umbilical vein endothelial cells (HUVECs) cultured on gelatin, vitronectin, fibronectin, or laminin, whereas cells cultured on type I or type IV collagen were resistant to the proapoptotic effects of HKa.10 These observations support the paradigm that while fragments or conformationally altered forms of some extracellular matrix proteins inhibit angiogenesis,13 intact extracellular matrix proteins may regulate the activity of antiangiogenic polypeptides by promoting endothelial-cell survival. Such interactions might underlie, in part, resistance of some neoplasms to antiangiogenic therapy.
Collagen is an abundant component of the extracellular matrix that regulates cellular growth and viability.14-17 The protective effect of collagen against HKa-induced endothelial-cell apoptosis might result from several mechanisms including enhancement of endothelial-cell survival through engagement of integrin α2β1 and activation of integrin-mediated cell-survival pathways.17-19 Alternatively, should generation of reactive oxygen species (ROSs) be involved in HKa-induced apoptosis, collagen might block apoptosis through quenching of ROSs, as has been previously reported during HeLa-cell apoptosis induced by oxidative products of the Fenton reaction.20
In this report, we demonstrate that culture of endothelial cells on collagen type I or IV prevents HKa-induced endothelial-cell apoptosis but that soluble collagen, small peptidic α2β1 integrin agonists, or activating α2β1 antibodies do not. Moreover, endothelial-cell apoptosis on noncollagenous extracellular matrix proteins is dependent upon ROS generation and is blocked by reduced glutathione (GSH) or N-acetylcysteine (NAC). HKa-induced apoptosis is also associated with rapid nuclear translocation of thioredoxin, a redox-sensitive signaling factor.21 These results implicate a ROS-dependent pathway in mediating HKa-induced endothelial-cell apoptosis. This critical dependency on ROS generation has not been reported for naturally occurring angiogenesis inhibitors and provides new insight into the antiangiogenic mechanism of HKa.
Materials and methods
Materials
Cleaved human high–molecular-weight kininogen (HKa) was from Enzyme Research Laboratories (South Bend, IN). Recombinant bFGF and type IV collagen were from Becton Dickinson Biosciences (Franklin Lakes, NJ). Gelatin, type I collagen, reduced glutathione (GSH), N-acetylcysteine (NAC), and venom from the Malayan pit viper, Calloselasma rhodostoma (used as a source of aggretin, an activator of integrin α2β1), were obtained from Sigma (St Louis, MO). Laminin was from Alexis (San Diego, CA). A monoclonal antithioredoxin antibody was obtained from American Diagnostica (Stamford, CT). Fibronectin and vitronectin were purified as described,21,22 and purity of the intact proteins was confirmed by sodium dodecyl sulfate–polyacrylamide gel electrophoresis (SDS-PAGE) and immunoblotting. The monoclonal antibody (mAb) JBS2, which activates integrin α2β1,23 was a kind gift of Dr John Wilkins (University of Manitoba, Canada). The β1 integrin–activating mAb TS2/1624 was obtained from Pierce (Rockford, IL).
Cell culture
Human umbilical vein endothelial cells (HUVECs) were isolated and cultured as described in our previous reports.10 All cells used in these studies were of passage 3 or lower.
Detection of endothelial-cell apoptosis
HKa-induced apoptosis of proliferating endothelial cells was measured by assessing DNA fragmentation according to the method of Steinfelder et al,25 as in our previous studies.10 Briefly, extracts of control and HKa-exposed cells (50 nM, 12 hours) were prepared in 0.1 M Tris-EDTA (pH 7.5) containing 1.2% SDS and 1 μg/mL RNAse. After CsCl precipitation of protein and genomic DNA, cytoplasmic DNA was isolated using a Qiaprep spin column (Qiagen, Valencia, CA). DNA was collected in 35 μL 10 mM Tris-HCl (pH 8.5) and visualized using 1% agarose gel electrophoresis followed by ethidium bromide staining.
Cell proliferation assay
For some experiments, a cell-proliferation assay was used to enumerate relative numbers of viable endothelial cells following exposure to HKa, as previously described10 ; in these studies the proliferation assay was used as a surrogate marker of apoptosis. Briefly, HUVECs (3 × 104/mL) were plated in individual wells of 96-well microplates coated with specific ECM proteins at a concentration of 2.5 μg/mL. After firm adhesion and spreading was established, the medium was replaced with fresh medium containing 2% serum, 10 ng/mL bFGF, 10 μM ZnCl2, 50 nM HKa, and specific test reagent(s). Relative numbers of cells remaining in each well after incubation for an additional 48 hours were determined using the AQueous cell-proliferation assay (Promega, Madison, WI). Results are presented as the percent inhibition of bFGF-induced endothelial proliferation, as defined in our previous studies.10 In selected experiments involving reduced glutathione (GSH), which affects the colorimetric end points of the AQueous cell-proliferation assay, relative cell numbers were assessed using the CyQuant cell-proliferation system (Molecular Probes/Invitrogen, Carlsbad, CA).
Purification of aggretin
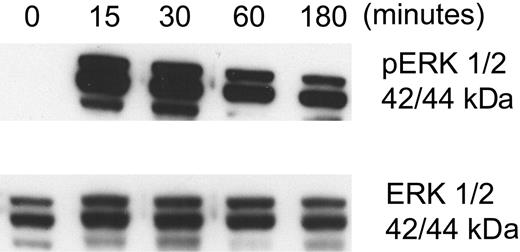
Aggretin, an activator of integrin α2β1, was purified from the venom of the Malayan pit viper, Calloselasma rhodostoma.26,27 Briefly, C rhodostoma crude venom (100 mg; Sigma) was dissolved in 0.1 M HEPES buffer (pH 8.0) and centrifuged at 12 000g at 4°C for 10 minutes. The supernatant was fractionated by chromatography on a Superdex 75 column equilibrated with the same buffer, using an Amersham-Pharmacia fast protein liquid chromatography (FPLC) system (Amersham-Pharmacia, Piscataway, NJ). Fractions containing aggretin were pooled and applied to a Mono-Q HR column pre-equilibrated with 20 mM Tris-HCl (pH 8.2). Elution was performed using a linear gradient of 0 to 0.5 M NaCl. The purity of aggretin was confirmed using 12% SDS-PAGE and its functional activity confirmed by assessing its ability to activate ERK 1/2 in endothelial cells.26
Determination of intracellular GSH content
Intracellular GSH content was measured according to the method of Hissin and Hilf,28 with minor modifications. Briefly, cell lysates were prepared from control and HKa-exposed cells using 2 freeze-thaw cycles, and proteins were precipitated by the addition of 1% (final concentration) meta-phosphoric acid. After centrifugation of lysates at 13 700g for 10 minutes, the GSH content of the supernatant was measured by assessing the fluorescence of o-phthaldialdehyde (final concentration 24 mM) at 465 nm (excitation wavelength, 350 nm). The protein content was measured in parallel using the bicinchoninic acid (BCA) method (Bio-Rad, Cupertino, CA). The concentration of intracellular GSH was expressed as nanomoles per mg of protein.
Measurement of lipid peroxidation
Peroxidation of endothelial-cell lipids was assessed by measuring the cellular content of malondialdehyde (MDA) and 4-hydroxy-2-nonenal (4-HNE),29 using the Lipid Peroxidation Assay Kit (Calbiochem/EMD Biosciences, San Diego, CA). This chromogenic assay measures the stable chromophores that form upon combination of the chromogenic reagent R1 with MDA or 4-HNE. Lysates were prepared in an identical manner as for GSH assays, and MDA and 4-HNE concentrations were determined per the manufacturer's protocol. Protein content was measured in parallel using the BCA method, and the concentration of lipid peroxides was expressed as nanomoles per mg protein.
Nuclear translocation of thioredoxin
To examine the effect of HKa on nuclear translocation of thioredoxin, endothelial cells were prepared as described for proliferation assays and then stimulated with 10 ng/mL bFGF in the absence or presence of 50 nM HKa.30 At various time points, cells were washed with PBS, then scraped from the plate and centrifuged at 2900g for 5 minutes at 4°C. The cell pellet was resuspended in 200 μL of 10 mM HEPES (pH 7.9) containing 10 mM KCl, 0.1 mM EDTA, 0.1 mM EGTA, 1 mM DTT, and 0.5 mM PMSF and left on ice for 15 minutes. Nonidet P-40 (final concentration 0.4%) was added, followed by vortexing and centrifugation at 13 700g for 2 minutes to pellet intact nuclei. The supernatant was saved as the cytoplasmic fraction and the nuclear pellet was suspended in 20 mM HEPES (pH 7.9) containing 0.422 M NaCl, 1 mM EGTA, 1 mM DTT, and 0.5 mM PMSF, then incubated at 4°C for 15 minutes with periodic vortexing. After centrifugation at 13 700g for 5 minutes, the supernatant was saved as the nuclear fraction and the protein concentration of the nuclear and cytoplasmic fractions was determined.30 Equal amounts of protein from each fraction were separated using 15% SDS-PAGE and transferred to polyvinylidene difluoride (PVDF) membranes (Millipore, Billerica, MA). Immunoblotting was then performed using a monoclonal antithioredoxin antibody.
Results
Type I and IV collagens protect proliferating endothelial cells from HKa-induced apoptosis
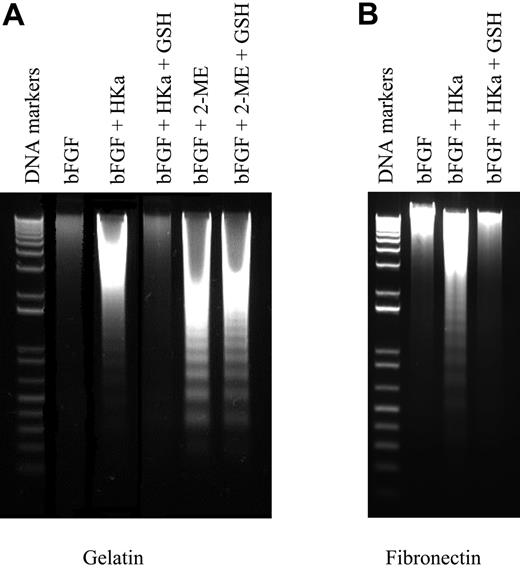
Several binding sites for HK and/or HKa on endothelial cells have been identified, including cytokeratin,31,32 the receptor for the globular heads of C1q,33 chondroitin34 (but not heparan)35 sulfate proteoglycans, the urokinase receptor (uPAR),36 and tropomyosin37 ; involvement of the latter 2 sites in the antiangiogenic activity of HKa has been proposed. The urokinase receptor mediates cellular adhesion to vitronectin,38-40 and it has been suggested that the ability of HKa to induce apoptosis of endothelial cells cultured on vitronectin reflects its inhibition of uPAR-mediated adhesion of endothelial cells to this substrate.41,42 We hypothesized that if this were the case, then the activity of HKa should be limited to, or at least primarily directed toward, endothelial cells cultured on vitronectin. In contrast, if other mechanisms were of importance, we would expect HKa to also induce apoptosis of endothelial cells on other matrices.10 To test this hypothesis, we assessed the ability of HKa to induce apoptosis of proliferating endothelial cells cultured on several purified ECM proteins. As depicted in Figure 1, HKa caused endonucleolytic degradation of endothelial-cell DNA, consistent with apoptosis, in endothelial cells cultured on gelatin, laminin, vitronectin, or fibronectin. However, the most remarkable finding was that HKa-induced apoptosis was prevented when endothelial cells were cultured on type I or IV collagen. Identical findings were obtained when a proliferation assay was used as a surrogate measure of HKa-induced apoptosis (not shown).
Effect of extracellular matrix on HKa-induced endonucleolytic DNA cleavage. Endothelial cells cultured on different extracellular matrix proteins were stimulated with 10 ng/mL bFGF in the absence or presence of 50 nM HKa for 12 hours. Cytoplasmic DNA was then isolated as described under “Detection of endothelial-cell apoptosis” in “Materials and methods” and analyzed using 1% agarose gel electrophoresis. Agarose gels were stained with ethidium bromide to detect fragmented DNA. Vn indicates vitronectin; Fn, fibronectin; Ln, laminin; col I, type I collagen; and col IV, type IV collagen.
Effect of extracellular matrix on HKa-induced endonucleolytic DNA cleavage. Endothelial cells cultured on different extracellular matrix proteins were stimulated with 10 ng/mL bFGF in the absence or presence of 50 nM HKa for 12 hours. Cytoplasmic DNA was then isolated as described under “Detection of endothelial-cell apoptosis” in “Materials and methods” and analyzed using 1% agarose gel electrophoresis. Agarose gels were stained with ethidium bromide to detect fragmented DNA. Vn indicates vitronectin; Fn, fibronectin; Ln, laminin; col I, type I collagen; and col IV, type IV collagen.
HKa-induced ROS generation is necessary for apoptosis and is diminished on collagen
To explore the mechanism(s) by which type I or IV collagen prevented HKa-induced endothelial-cell apoptosis, we considered several possibilities. First, we hypothesized that the effect of collagen on HKa-induced apoptosis might reflect activation of a specific survival pathway following engagement of the primary endothelial-cell collagen receptor integrin α2β1.18,19,43 To investigate this possibility, we assessed the ability of purified aggretin, a collagen-like α2β1 integrin agonist isolated from Calloselasma rhodostoma venom,26 to prevent HKa-induced apoptosis of proliferating endothelial cells cultured on gelatin. An endothelial-cell proliferation assay was used as a surrogate marker of apoptosis to quantitate relative numbers of viable cells present at the end of the 48-hour experiment.10 However, despite the ability of aggretin to induce phosphorylation of endothelial-cell ERK 1/2, confirming its ability to activate α2β1-dependent signaling26 (Figure 2), aggretin did not inhibit HKa-induced endothelial-cell apoptosis. Identical results were obtained using mAb JBS2, a specific α2β1-activating monoclonal antibody,44,45 and mAb TS2/16, a specific β1 integrin– activating antibody, either in solution or as a coating for microplates on which cells were cultured (not shown). Although these results do not exclude a role for integrin α2β1 in mediating collagen-dependent endothelial-cell survival, they suggest that if such an interaction is important, a more complex ligand-receptor interaction is involved than the monovalent or bivalent interactions associated with aggretin or monoclonal antibodies, respectively. More complex interactions with intact collagen might activate additional processes such as mechanochemical signaling,46,47 which may not be activated by smaller ligands.
Effect of aggretin on endothelial ERK 1/2 phosphorylation. The integrin α2β1 agonist aggretin was purified from the venom of Calloselasma rhodostoma as described under “Purification of aggretin” in “Materials and methods.” To assess the activity of the purified protein, HUVECs were incubated with 0.15 μM aggretin for various times. Cell extracts were prepared and separated using 10% SDS-PAGE, then transferred to PVDF membranes. Membranes were immunoblotted with antibodies specific for phospho-ERK 1/2 (A) or total ERK 1/2 (B). Aggretin induced rapid phosphorylation of ERK 1/2. Similar observations were noted when mAbs JSB2 or TS2/16 were used as agonists.
Effect of aggretin on endothelial ERK 1/2 phosphorylation. The integrin α2β1 agonist aggretin was purified from the venom of Calloselasma rhodostoma as described under “Purification of aggretin” in “Materials and methods.” To assess the activity of the purified protein, HUVECs were incubated with 0.15 μM aggretin for various times. Cell extracts were prepared and separated using 10% SDS-PAGE, then transferred to PVDF membranes. Membranes were immunoblotted with antibodies specific for phospho-ERK 1/2 (A) or total ERK 1/2 (B). Aggretin induced rapid phosphorylation of ERK 1/2. Similar observations were noted when mAbs JSB2 or TS2/16 were used as agonists.
Another mechanism by which collagen could protect against HKa-induced apoptosis may involve quenching of reactive oxygen species (ROSs).20 ROSs may play an important role in apoptosis48,49 and type I collagen blocks ROS-induced apoptosis of HeLa cells caused by products of the Fenton reaction, primarily hydroxyl radicals.20 To investigate the relevance of this activity to our system, we again used a proliferation assay to determine whether soluble collagen inhibited HKa-induced apoptosis of endothelial cells cultured on other ECM proteins. However, even when present at high concentrations (150 μg/mL), soluble collagen was ineffective in this regard (Figure 3A).
To determine whether oxidative stress was involved in HKa-induced apoptosis, we assessed the effect of GSH on this process,50 using a proliferation assay to determine relative numbers of viable endothelial cells remaining after exposure to HKa in the absence or presence of GSH. GSH (150 μg/mL) inhibited HKa-induced endothelial-cell apoptosis on all ECM proteins (except collagen, on which no apoptosis occurred; Figure 3B). GSH alone did not affect the proliferation or viability of endothelial cells, nor did it cause phosphorylation of ERK 1/2 (not shown). Identical results were obtained when NAC, a cell-permeable precursor of GSH, was employed (Figure 3C).
Since intracellular ROS generation may be a common feature of apoptosis,51,52 we assessed the specificity of the antiapoptotic effect of GSH or NAC on HKa-induced apoptosis by determining whether they blocked apoptosis in response to another antiangiogenic agent, 2-methoxyestradiol (2-ME).53 However, endothelial-cell apoptosis caused by 2-ME was not affected by either GSH (Figure 3D) or NAC (not shown), demonstrating that the inhibition of HKa-induced apoptosis by these antioxidants was specific. Identical results were obtained when endonucleolytic fragmentation of DNA from HKa-exposed cells cultured on different matrices was analyzed (Figure 4).
Effect of soluble collagen I and antioxidants on endothelial-cell apoptosis caused by HKa and 2-ME. An endothelial-cell proliferation assay was used to quantitate viable cells remaining after 48 hours of stimulation with 10 ng/mL bFGF in the absence or presence of 50 nM HKa. Greater than 100% inhibition of proliferation, as defined in our previous studies,9 means that fewer viable cells remain at the end of the experiment than at the beginning. (A) Cells were stimulated with bFGF in the presence of HKa and in the absence (□) or presence (▪) of 150 μg/mL soluble type I collagen. Collagen did not block HKa-induced endothelial-cell apoptosis. (B) Cells were stimulated with bFGF in the presence of HKa and in the absence (□) or presence (▪) of 150 μg/mL GSH, which provided substantial protection against HKa-induced apoptosis. (C) Cells were stimulated with bFGF in the presence of HKa and in the absence (□) or presence (▪) of 150 μg/mL of NAC. (D) Cells were stimulated with bFGF in the presence of 4 μM 2-methoxyestradiol (2-ME) and in the absence or presence of 150 μg/mL GSH. GSH did not protect cells from 2-ME–induced apoptosis. Error bars indicate standard deviation of triplicate points.
Effect of soluble collagen I and antioxidants on endothelial-cell apoptosis caused by HKa and 2-ME. An endothelial-cell proliferation assay was used to quantitate viable cells remaining after 48 hours of stimulation with 10 ng/mL bFGF in the absence or presence of 50 nM HKa. Greater than 100% inhibition of proliferation, as defined in our previous studies,9 means that fewer viable cells remain at the end of the experiment than at the beginning. (A) Cells were stimulated with bFGF in the presence of HKa and in the absence (□) or presence (▪) of 150 μg/mL soluble type I collagen. Collagen did not block HKa-induced endothelial-cell apoptosis. (B) Cells were stimulated with bFGF in the presence of HKa and in the absence (□) or presence (▪) of 150 μg/mL GSH, which provided substantial protection against HKa-induced apoptosis. (C) Cells were stimulated with bFGF in the presence of HKa and in the absence (□) or presence (▪) of 150 μg/mL of NAC. (D) Cells were stimulated with bFGF in the presence of 4 μM 2-methoxyestradiol (2-ME) and in the absence or presence of 150 μg/mL GSH. GSH did not protect cells from 2-ME–induced apoptosis. Error bars indicate standard deviation of triplicate points.
HKa exposure decreases intracellular GSH content and causes peroxidation of endothelial lipids
To assess the effects of HKa on intracellular redox status, we measured the levels of GSH, an important intracellular antioxidant,50,54-56 in control and HKa-treated endothelial cells. Exposure of proliferating endothelial cells cultured on gelatin to HKa led to a rapid fall in intracellular GSH, which was not observed in untreated endothelial cells or in cells undergoing apoptosis after exposure to 2-methoxyestradiol (Figure 5A).
Reactive oxygen species react with polyunsaturated membrane lipids to cause lipid peroxidation,57 yielding malondialdehyde (MDA) and 4-hydroxylalkenals (4-HNE). To assess the effect of HKa on this process in proliferating endothelial cells, we measured the MDA and 4-HNE content of control and HKa-exposed cells. As shown in Figure 5B, HKa caused a 3-fold increase in MDA and 4-HNE content in cells cultured on gelatin within 12 hours of exposure.
To further explore the relationship between the matrix dependence of HKa-induced endothelial-cell apoptosis, GSH depletion, and lipid peroxidation, we assessed these parameters in HKa-exposed cells cultured on other extracellular matrix proteins, specifically fibronectin and type I collagen. As observed with cells cultured on gelatin, HKa induced GSH depletion and lipid peroxidation in cells cultured on fibronectin (Figure 6A-B). However, when cells were cultured on type I collagen, no such changes were observed (Figure 6C-D). These results demonstrate that depletion of endothelial-cell GSH and cellular lipid peroxidation occur in parallel with HKa-induced apoptosis and, taken together with the observation that exogenous GSH or NAC blocks HKa-mediated apoptosis, suggest an important role for oxidative stress in this process.58
Effect of antioxidants on HKa-induced endothelial-cell apoptosis. Endothelial cells cultured on gelatin (A) or fibronectin (B) were stimulated with 10 ng/mL bFGF in the absence or presence of 50 nM HKa or 4 μM 2-ME and 150 μg/mL GSH for 12 hours. Cytoplasmic DNA was then isolated as described under “Detection of endothelial-cell apoptosis” in “Materials and methods” and analyzed using 1% agarose gel electrophoresis. Agarose gels were stained with ethidium bromide to detect fragmented DNA as a marker of apoptosis. GSH prevented DNA fragmentation caused by HKa, but not 2-ME, in cells cultured on either gelatin or fibronectin.
Effect of antioxidants on HKa-induced endothelial-cell apoptosis. Endothelial cells cultured on gelatin (A) or fibronectin (B) were stimulated with 10 ng/mL bFGF in the absence or presence of 50 nM HKa or 4 μM 2-ME and 150 μg/mL GSH for 12 hours. Cytoplasmic DNA was then isolated as described under “Detection of endothelial-cell apoptosis” in “Materials and methods” and analyzed using 1% agarose gel electrophoresis. Agarose gels were stained with ethidium bromide to detect fragmented DNA as a marker of apoptosis. GSH prevented DNA fragmentation caused by HKa, but not 2-ME, in cells cultured on either gelatin or fibronectin.
HKa causes consumption of cellular GSH and phospholipid peroxidation in endothelial cells. (A) To assess GSH consumption, endothelial cells were cultured in 10 cm2 gelatin-coated dishes at a concentration of 3 × 104 cells/mL and stimulated with 10 ng/mL bFGF in the absence or presence of 50 nM HKa. At various time points, cell lysates were prepared as described under “Determination of cellular GSH content” in “Materials and methods” and, after precipitation of cellular proteins, were analyzed for GSH content by measuring the fluorescence of added o-phthaldialdehyde (24 mM final concentration). Residual protein content was measured in parallel, and the concentration of intracellular GSH was expressed as nanomoles per mg of protein. (B) To assess lipid peroxidation, cells were cultured as described for panel A and stimulated with bFGF in the absence or presence of 50 nM HKa. Lysates were prepared as in panel A, and total MDA and 4-HNE content was determined using a commercial Lipid Peroxidation Assay Kit, as described in “Materials and methods.” Protein content was measured in parallel, and the concentration of lipid peroxides was expressed as nanomoles per mg protein. Error bars indicate standard deviation of triplicate points.
HKa causes consumption of cellular GSH and phospholipid peroxidation in endothelial cells. (A) To assess GSH consumption, endothelial cells were cultured in 10 cm2 gelatin-coated dishes at a concentration of 3 × 104 cells/mL and stimulated with 10 ng/mL bFGF in the absence or presence of 50 nM HKa. At various time points, cell lysates were prepared as described under “Determination of cellular GSH content” in “Materials and methods” and, after precipitation of cellular proteins, were analyzed for GSH content by measuring the fluorescence of added o-phthaldialdehyde (24 mM final concentration). Residual protein content was measured in parallel, and the concentration of intracellular GSH was expressed as nanomoles per mg of protein. (B) To assess lipid peroxidation, cells were cultured as described for panel A and stimulated with bFGF in the absence or presence of 50 nM HKa. Lysates were prepared as in panel A, and total MDA and 4-HNE content was determined using a commercial Lipid Peroxidation Assay Kit, as described in “Materials and methods.” Protein content was measured in parallel, and the concentration of lipid peroxides was expressed as nanomoles per mg protein. Error bars indicate standard deviation of triplicate points.
HKa induces translocation of thioredoxin to the nucleus
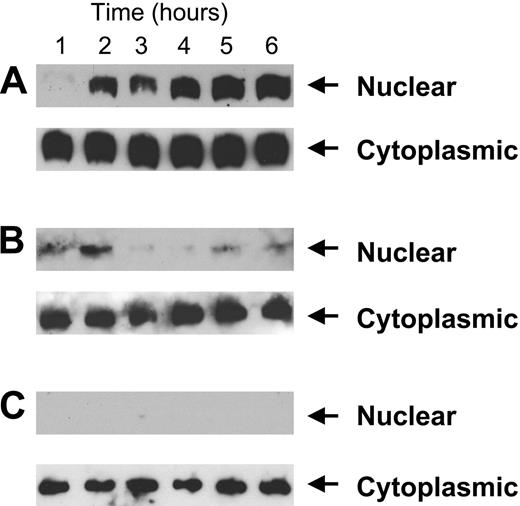
Thioredoxin, a ubiquitous intracellular protein with 2 redox-active half-cysteine residues in its catalytic center, localizes primarily to the cytoplasm under normal homeostatic conditions.21 However, in response to oxidative stress such as that induced by radiation or tumor necrosis factor alpha, thioredoxin translocates to the nucleus, facilitating the activity of specific transcription factors such as NF-κB and AP-1.58,59 Given our observations that HKa induces ROS generation, depletion of intracellular antioxidants, and lipid peroxidation in proliferating endothelial cells, we hypothesized that HKa might also induce nuclear translocation of endothelial-cell thioredoxin. As depicted in Figure 7A, nuclear translocation of thioredoxin occurred within 1 hour of exposure of proliferating endothelial cells cultured on gelatin to HKa; thioredoxin continued to accumulate in the nucleus for the remainder of the 6-hour experiment. In contrast, nuclear translocation of thioredoxin did not occur in cells cultured on gelatin in the presence of GSH or NAC (Figure 7B) or in cells cultured on collagen (Figure 7C). These results suggest a strong association between nuclear translocation of thioredoxin, ROS generation, and apoptosis in proliferating endothelial cells exposed to HKa.
GSH consumption and lipid peroxidation in bFGF-stimulated endothelial cells cultured on fibronectin or collagen and exposed to HKa. GSH consumption and lipid peroxidation were measured using protocols identical to those described in Figure 5. Panels A and B depict GSH consumption and lipid peroxidation, respectively, in cells cultured on fibronectin. Panels C and D depict GSH consumption and lipid peroxidation, respectively, in endothelial cells cultured on type I collagen. The differences in GSH consumption and phospholipid peroxidation were statistically significant only for cells cultured on fibronectin (P = .008 and .032, respectively). Error bars indicate standard deviation of triplicate points.
GSH consumption and lipid peroxidation in bFGF-stimulated endothelial cells cultured on fibronectin or collagen and exposed to HKa. GSH consumption and lipid peroxidation were measured using protocols identical to those described in Figure 5. Panels A and B depict GSH consumption and lipid peroxidation, respectively, in cells cultured on fibronectin. Panels C and D depict GSH consumption and lipid peroxidation, respectively, in endothelial cells cultured on type I collagen. The differences in GSH consumption and phospholipid peroxidation were statistically significant only for cells cultured on fibronectin (P = .008 and .032, respectively). Error bars indicate standard deviation of triplicate points.
Discussion
We and others have previously reported that HKa induces apoptosis of proliferating endothelial cells and inhibits angiogenesis.10,11 However, the mechanisms by which this occurs remain uncertain. The activity of HKa is Zn2+ dependent and is mediated through HKa domain 5, suggesting that specific binding of HKa to the cell is required.10,60 However, there is little information available concerning the signaling pathways involved in induction of endothelial-cell apoptosis by HKa.
Here, we demonstrate that HKa-induced endothelial-cell apoptosis is closely linked to the generation of intracellular reactive oxygen species. ROS generation in HKa-exposed endothelial cells was associated with depletion of intracellular GSH and membrane lipid peroxidation, suggesting generalized intracellular oxidant stress. These changes were specific for HKa and did not occur during endothelial-cell apoptosis induced by 2-methoxyestradiol. A critical role for ROSs in the induction of apoptosis by HKa is supported by the observation that repletion of intracellular GSH, either directly or through provision of its precursor NAC, completely blocked HKa-induced endothelial-cell apoptosis. Likewise, HKa did not induce apoptosis of endothelial cells cultured on extracellular matrices (collagen) on which ROSs were not generated. Endothelial-cell apoptosis induced by HK domain 5 was also inhibited by antioxidants (not shown).
Nuclear translocation of thioredoxin following exposure of proliferating endothelial cells to HKa. (A) Cells were cultured in 10 cm2 fibronectin-coated plates at a concentration of 3 × 104 cells/mL and stimulated with 10 ng/mL bFGF and 50 nM HKa. At increasing intervals, cell extracts were prepared and separated using 15% SDS-PAGE, as described in “Materials and methods.” After transfer to PVDF, membranes were incubated with peroxidase-conjugated goat anti–mouse thioredoxin antibodies and developed using enhanced chemiluminescence. No thioredoxin translocation occurred in cells exposed to bFGF alone in the absence of HKa (not shown). (B) Experiments were conducted as described in panel A but HKa-exposed cells were incubated concurrently with 150 μg/mL GSH. No nuclear translocation of thioredoxin occurred under these conditions. (C) Experiments were performed as described in panel A but using cells cultured on type I collagen.
Nuclear translocation of thioredoxin following exposure of proliferating endothelial cells to HKa. (A) Cells were cultured in 10 cm2 fibronectin-coated plates at a concentration of 3 × 104 cells/mL and stimulated with 10 ng/mL bFGF and 50 nM HKa. At increasing intervals, cell extracts were prepared and separated using 15% SDS-PAGE, as described in “Materials and methods.” After transfer to PVDF, membranes were incubated with peroxidase-conjugated goat anti–mouse thioredoxin antibodies and developed using enhanced chemiluminescence. No thioredoxin translocation occurred in cells exposed to bFGF alone in the absence of HKa (not shown). (B) Experiments were conducted as described in panel A but HKa-exposed cells were incubated concurrently with 150 μg/mL GSH. No nuclear translocation of thioredoxin occurred under these conditions. (C) Experiments were performed as described in panel A but using cells cultured on type I collagen.
ROSs may contribute to apoptosis in several situations,61,62 such as in response to TNFα61 or cell detachment.63 However, we are unaware of any prior reports in which endothelial-cell apoptosis induced by a naturally occurring antiangiogenic polypeptide has shown a critical dependence on ROS generation. Moreover, as our previous studies suggest an important role for tropomyosin in mediating the antiangiogenic activity of HKa64 and other reports suggest involvement of tropomyosin in the antiangiogenic effects of polypeptides such as endostatin65 and histidine-proline–rich glycoprotein,66,67 the mechanisms outlined in this study may be applicable to a family of antiangiogenic polypeptides that activate a similar apoptotic pathway.68
HKa led to rapid ROS generation in endothelial cells only on specific extracellular matrix proteins that were permissive for HKa-induced apoptosis, including gelatin, fibronectin, vitronectin, and laminin. Neither ROS generation nor apoptosis occurred following exposure of cells cultured on collagen type I or IV to HKa. We considered 2 primary mechanisms to explain the ability of collagen to block HKa-induced apoptosis. First, adhesion of endothelial cells to collagen may lead to activation of specific signaling pathways that promote endothelial-cell survival, perhaps through engagement of the primary endothelial-cell collagen receptor integrin α2β1.17-19,45,69 While our results are consistent with such a mechanism, we were not able to demonstrate protection from apoptosis by soluble collagen or by smaller ligands such as aggretin or monoclonal antibodies that activate α2β1.26,27,44 Thus, we hypothesize that the ability of immobilized collagen to prevent HKa-induced apoptosis may reflect a need for receptor aggregation, or at least engagement by a multivalent ligand, perhaps accompanied by activation of mechanochemical signaling responses not induced by smaller, soluble ligands.46,47,70 A second hypothesis, that the ability of collagen to quench ROSs may contribute to its antiapoptotic activity, is not supported by our data, as addition of soluble type I collagen did not inhibit HKa-induced endothelial-cell apoptosis. However, in prior studies in which collagen inhibited hydroxyl radical–induced apoptosis, apoptosis was induced by direct application of hydroxyl radicals, generated via the Fenton reaction, to the extracellular milieu.20 In contrast, HKa-induced ROSs appear to be generated intracellularly, a site inaccessible to soluble collagen. Hence, GSH, an efficient intracellular free-radical scavenger that is transported into the cell71 and contributes to the generation of other antioxidant species55,72 that protect cellular components from oxidative damage,50,54,73 or NAC, a membrane-permeable GSH precursor, were efficient inhibitors of HKa-mediated apoptosis.
Our results differ in several regards from those recently published by Guo et al.74 These investigators assessed the ability of HKa to induce apoptosis of endothelial cells cultured on vitronectin or fibronectin, observing that apoptosis occurred on vitronectin only and concluding that the apoptotic activity of HKa was dependent upon binding of HKa to the urokinase receptor (uPAR) and inhibition of uPAR-dependent adhesion of endothelial cells to vitronectin.42 In contrast, we have reproducibly observed that HKa induces apoptosis of endothelial cells cultured on fibronectin, gelatin, and laminin, as well as vitronectin.10 The reasons for these discrepancies are not evident at this point. Although interactions between uPAR, HKa, and vitronectin have been defined,38,75,76 such interactions may not fully explain the proapoptotic effects of HKa toward proliferating endothelial cells on extracellular matrix proteins other than vitronectin or on biologic matrices composed of a broad array of proteins.17,43,77-79 Taken together, it is likely that more than a single mechanism explaining the antiangiogenic activity of HKa may exist, and different apoptotic pathways may be initiated dependent upon the specific extracellular environment.
Our studies highlight the important role of collagen in the regulation of angiogenesis, particularly when its abundance and critical localization in the vascular wall and stroma are considered.77 In the early stages of angiogenesis, endothelial cells must degrade and escape the confines of the endothelial basement membrane, which is rich in type IV collagen.77 Angiogenic endothelial cells must then migrate through the interstitial matrix, composed largely of type I collagen,77 prior to forming new vessels and establishing a new type IV collagen–rich basement membrane.77 Hence, endothelial cells are in intimate contact with these and other collagen species throughout the angiogenic process, suggesting that the activity of collagen in supporting endothelial-cell viability may be of physiologic importance. We speculate that such interactions might explain, in part, the inconsistent results of some angiogenesis inhibitors in the therapy of human neoplasms.80
The rapid translocation of thioredoxin to the nucleus in response to oxidative stress has been reported by several groups. Normally present in the cytoplasm, where it may inhibit apoptosis signal-regulating kinase (ASK-1)81 and function as an antioxidant,82-84 nuclear thioredoxin may facilitate the DNA binding activity of NF-κB and AP-1.30,59 While defining the significance of thioredoxin translocation in HKa-induced apoptosis of endothelial cells will require additional study, the rapid translocation (< 1 hour after addition of HKa) and the fact that translocation was blocked in the presence of GSH or in cells cultured on type I collagen suggests an intimate association of this event with HKa-induced apoptosis of endothelial cells and may reflect an attempt by the cell to respond to oxidant stress.21
The extracellular matrix plays a critical role in the regulation of angiogenesis.17,79 Extracellular matrix proteins within the tumor milieu expose cryptic epitopes following cleavage by matrix metalloproteases and other enzymes, and unique interactions between these sites and endothelial cells lead to profound alterations in endothelial-cell function.13,79,85 Fragments of ECM proteins such as endostatin derived from type XVIII collagen,86 tumstatin and canstatin derived from type IV collagen,87,88 and other polypeptides inhibit angiogenesis. In contrast, intact ECM proteins, often in concert with endothelial-cell growth factors, promote cellular adhesion and differentiation and stimulate integrin-dependent pathways that lead to enhanced-cell survival.43,69,89 In the tumor milieu, a complex environment exists in which the contrasting effects of proangiogenic and antiangiogenic proteins and polypeptides determine whether angiogenesis will occur.17,78,79 Our studies provide one example of a simplified system in which a specific extracellular matrix protein blocks the activity of an antiangiogenic polypeptide, though this example may illustrate a more generalized paradigm for the regulation of angiogenesis.
Prepublished online as Blood First Edition Paper, January 17, 2006; DOI 10.1182/blood-2005-09-3584.
Supported by a postdoctoral fellowship award from the American Heart Association (D.S.) and National Institutes of Health (NIH) grants CA83134 and HL076810 (K.R.M.).
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.





This feature is available to Subscribers Only
Sign In or Create an Account Close Modal