Abstract
Hepatitis C virus (HCV) poses a global health problem because it readily establishes persistent infection and a vaccine is not available. CD4+CD25+ T cells have been implicated in HCV persistence because their frequency is increased in the blood of HCV-infected patients and their in vitro depletion results in increased IFN-γ production by HCV-specific T cells. Studying a well-characterized cohort of 16 chimpanzees, the sole animal model for HCV infection, we here demonstrate that the frequency of Foxp3+CD4+CD25+ regulatory T cells (TRegs) and the extent of suppression was as high in spontaneously HCV-recovered chimpanzees as in persistently HCV-infected chimpanzees. Foxp3+CD4+CD25+ TRegs suppressed IFN-γ production, expansion, and activation-induced cell death of HCV-specific T cells after recovery from HCV infection and in persistent HCV infection. Thus, TReg cells control HCV-specific T cells not only in persistent infection but also after recovery, where they may regulate memory T-cell responses by controlling their activation and preventing apoptosis. However, Foxp3+CD4+CD25+ TReg cells of both HCV-recovered and HCV-infected chimpanzees differed from Foxp3+CD4+CD25+TReg cells of HCV-naive chimpanzees in increased IL-2 responsiveness and lower T-cell receptor excision circle content, implying a history of in vivo proliferation. This result suggests that HCV infection alters the population of Foxp3+CD4+CD25+ TReg cells.
Introduction
Several CD4+ T-cell populations have been shown to regulate activation, differentiation, and effector functions of T cells.1-5 Naturally occurring CD4+ regulatory T cells (TRegs), which constitutively express Foxp3 and the IL-2Rα chain (CD25), are generated during normal T-cell development in the thymus and mediate suppression via a cytokine-independent, contact-dependent mechanism.1,6 Others, such as IL-10–secreting Tr-1 cells7 and TGF-β–secreting Th3 cells,8-10 constitute induced TRegs as they are generated in the periphery and exert cytokine-mediated suppression.
Hepatitis C virus (HCV) readily establishes persistent infection in the majority of infected persons and cannot yet be prevented by vaccines.11,12 The small percentage of patients (20%-40%) who spontaneously clear the virus and recover from hepatitis C mount vigorous HCV-specific CD4+ and CD8+ T-cell responses.13-19 Memory T-cell responses are maintained for decades after recovery19 and mediate protective immunity in HCV-recovered chimpanzees on rechallenge with homologous20-24 and heterologous25 HCV. Unfortunately, most patients (60%-80%) either do not mount an HCV-specific CD4+ and CD8+ T-cell response of sufficient vigor and breadth or their response is rapidly lost during HCV infection.
The identification of those factors and mechanisms that contribute to the high incidence of HCV persistence is a field of intense investigation (for a review, see Racanelli and Rehermann12 ). Most recently, an increased frequency of CD4+CD25+ T cells has been described in the blood of patients with persistent HCV infection compared with those who spontaneously cleared HCV.26-29 Based on this finding and the observation that in vitro depletion of CD25+ T cells resulted in increased HCV-specific T-cell responsiveness, it has been proposed that CD4+CD25+ cells contribute to HCV persistence by suppressing HCV-specific T-cell responses26-29 and that they are absent or less functional after recovery from hepatitis C.
The latter conclusion is based on limited information, however, because time and route of infection, length of recovery, and genotype sequence of the previously infecting virus are often unknown and not comparable among patients, so the immune status after recovery cannot be precisely assessed. Here, we compare frequency and function of Foxp3+-CD4+CD25+ T cells with regulatory activity between HCV-recovered and persistently infected chimpanzees, the sole nonhuman species susceptible to HCV infection. The chimpanzees included in this study are well characterized with regard to the clinical, virologic, and immunologic course of infection, including the demonstration of protective, T cell–based immunity in those chimpanzees that recovered spontaneously.22,23
This study provides the first formal demonstration of CD4+CD25+ TRegs that express Foxp3, display hypoproliferation (anergy), and suppress anti-CD3–stimulated proliferation of CD4+CD25– T cells in nonhuman primates. In contrast to previous reports in humans, which focused on the role of CD4+CD25+ T cells in established chronic HCV infection,26-29 we show that CD4+CD25+ TRegs can also be detected after HCV clearance. The data suggest that Foxp3+CD4+CD25+ TRegs contribute to the maintenance of HCV-specific memory T cells by regulating proliferation, ex vivo effector functions, and activation-induced death (AICD) of HCV-specific memory T cells. In contrast to their counterparts in HCV-naive animals, Foxp3+CD4+CD25+ TRegs of HCV-recovered and of persistently HCV-infected chimpanzees exhibited increased IL-2 responsiveness and relatively lower numbers of T-cell receptor excision circles (TRECs), suggesting that TRegs can be induced or expanded in vivo during HCV infection. The study thereby extends previous data obtained in HCV-infected humans and sets the stage for experimental intervention and modulation of TReg activity in this nonhuman primate model.
Materials and methods
Chimpanzees
Chimpanzees were housed under standard conditions for humane care and in compliance with NIH guidelines at facilities accredited by the Association for Assessment of Laboratory Animal Care (AALAC). Study protocols were approved by the Animal Care and Use Committee and the Public Health Service Interagency Model Committee. The clinical and virological course of HCV infection in these chimpanzees has been described.22,23,30-33
Peptides, proteins, and antibodies
Six hundred HCV genotype 1a pentadecamer peptides with 10-amino acid overlap (Mimotopes, Clayton, Australia) were used in 18 mixes (1 μg/mL per peptide). Flow cytometry antibodies were anti–CD4-FITC, anti–CD4-PerCP, anti–CD8-FITC, anti–CD3-FITC, anti–CD62L-FITC, anti–CD25-PE (M-A251), anti–CD8-PerCP, anti–CD69-PerCP, anti–CD3-APC, anti–CTLA4-APC (all from BD Pharmingen, San Diego, CA), anti–CD27-FITC, anti–CD28-FITC, anti–CD45RO-APC (all from Caltag Laboratories, Burlingame, CA), anti–Foxp3-FITC (eBiosciences, San Diego, CA), purified anti-CCR7 (BD PharMingen), and goat anti–mouse IgM μ chain-FITC (Jackson ImmunoResearch Laboratories, West Grove, PA).
Intranuclear staining for Foxp3
After staining with anti–CD25-PE, anti–CD4-PerCP, and anti–CD3-APC, cells were fixed and permeabilized with Fix/Perm buffer (eBioscience), washed with permeabilization buffer (eBioscience), blocked with normal rat serum, stained with anti–Foxp3-FITC (Foxp3 Staining Set, clone PCH101, eBioscience), and analyzed on a fluorescence-activated cell sorter (FACSCalibur) using CellQuest (BD Biosciences) and FlowJo (TriStar, San Carlos, CA) software.
Cloning and sequencing of Patr-class I alleles and synthesis of Patr-tetramers
Total RNA was isolated from EBV-transformed B-cell lines or from peripheral blood mononuclear cells (PBMCs) of each chimpanzee.34 First-strand cDNA synthesis and polymerase chain reaction (PCR) amplification of Patr-class I sequences were performed as previously described.34,35 PCR-amplified Patr-class I cDNA was cloned into the pGEM11Zf(+) vector (Promega, Madison, WI)35 and sequenced using primers T7, 3S, 3N, 4S, and 4N.35 Error-free Patr-inserts were subcloned into pCMV-Script (Stratagene, La Jolla, CA) and human β2-microglobulin was expressed from the plasmid pHN1-2m.22,36 Folding, purification, and biotinylation of the following Patr-tetramers was performed by the NIAID Tetramer Facility as described37 : HCVcore41 (GPRLGVRAT)/Patr-B*1301, HCV-E1306 (CSIYPGHITG)/Patr-A*0401, HCV-E2542 (TRPPLGNWF)/Patr-B*1301, HCV-E2588 (KHPEATYSR)/Patr-A*0401, HCV-NS31379 (IPFYG-KAI)/Patr-B*1301, and HCV-NS31444 and (FTGDFDSVI)/Patr-B*0101.
Patr-tetramer staining of PBMCs and T-cell lines
PBMCs were isolated via density gradient centrifugation.34 T-cell lines were established by stimulating PBMCs or CD25-depleted PBMCs with 10 μg/mL indicated HCV peptide, 10 ng/mL IL-7 (PeproTech, Rocky Hill, NJ), and 300 pg/mL IL-12 (PeproTech) for 10 to 12 days. Then, 20 U/mL IL-2 and 0.5 μg/mL IL-15 were added every 3 to 4 days. PBMCs and T-cell lines were stained with a 1:100 dilution of the respective tetramer for 20 minutes at room temperature, followed by anti–CD8-PerCP and anti–CD3-APC staining.
Isolation of PBMC subpopulations
CD4+ and CD8+ cells were purified from PBMCs with magnetic beads (Miltenyi Biotec, Bergisch-Gladbach, Germany) according to the manufacturer's protocol. Consistent with other publications26,27,38 purified CD8+ cells contained on average 3.1% CD4+ cells (range, 0.6%-3.5%, with one exception of 6.2%). Irradiated CD4–CD8– cells or CD3-depleted PBMCs were used as autologous antigen-presenting cells (APCs). For depletion of CD25+ cells, CD4+ cells or total PBMCs were incubated with anti-CD25 beads, washed, and subjected to a sensitive auto–magnetic-activated cell sorting (autoMACS) depletion program (Miltenyi Biotec). To isolate CD4+CD25+ cells, positively isolated CD4+ cells were cleaved off the CD4 beads, incubated with CD25-PE antibody (clone M-A251, Becton Dickinson, San Jose, CA) and with anti-PE beads and isolated with MS columns (Miltenyi Biotec). The purity of isolated CD4+CD25+ cells was 93% to 98% (Figure 2B).
ELISPOT assays
IFN-γ enzyme-linked immunospot (ELISPOT) assays were performed as described19,22 using HCV peptide pools (1 μg/mL per peptide) or DMSO or a 1:200 dilution PHA-M (Invitrogen, Carlsbad, CA) or 10 ng/mL Staphylococcus aureus enterotoxin B (SEB; Toxin Technology, Sarasota, FL) to stimulate (1) 105 PBMCs or (2) 105 CD25-depleted PBMCs or (3) CD8+ T cells and autologous, CD3-depleted or CD4/CD8-depleted, irradiated (3000 rad) PBMCs in the indicated numbers. Duplicate or triplicate cultures were incubated in complete medium (RPMI 1640, 5% AB serum, 2 mM l-glutamine) for 22 to 38 hours. Antigen-specific responses greater than 10 spots (after subtraction of background spots in the absence of peptides) were considered significant, according to a cut-off that we established by stimulating PBMCs from 10 HCV-naive chimpanzees with peptides.22
Suppression assay
The 96-well flat bottom plates were coated with 0.625 to 10 μg/mL (as indicated) anti-CD3 (Immunotech/Beckman Coulter, Fullerton, CA), and washed twice prior to adding the indicated number of CD4+CD25– or CD4+CD25+ cells in complete medium with or without 200 U/mL IL-2 or 10 μg/mL neutralizing anti–TGF-β1 (clone 141322; R&D Systems, Minneapolis, MN). At the time of maximal proliferation (day 2-7), cultures were pulsed with 1 μCi (0.037 MBq) 3H-thymidine (ICN, Costa Mesa, CA) and harvested 12 to 16 hours later. Forty-eight hours after set-up or immediately prior to addition of 3H-thymidine, 75 to 100 μL supernatant was harvested for TGF-β1 and IL-10 enzymatic immunoassays (EIAs; R&D Systems).
Induction and detection of apoptosis
For antigen-nonspecific stimulation (condition 1), 105 CD8+ T cells were stimulated with or without 2.5 μg/mL soluble anti-CD3 in the presence of 5 × 104 APCs (ie, either CD3- or CD4/CD8-depleted PBMCs) and 5 × 104 CD4+CD25– or CD4+CD25+ cells. For antigen-specific stimulation (condition 2), an HCVcore41-specific T-cell line was established from PBMCs as described,34 but in the presence of 0.1 μg/mL IL-15. After 10 days of culture, 105 CD8+ T cells were isolated from this line and restimulated with or without 1 μg/mL HCVcore41 peptide in the presence of 5 × 104 APCs and 5 × 104 CD4+CD25– or CD4+CD25+ cells. After 20 hours for condition 1 and 6 hours for condition 2, respectively, cells were washed, stained with the indicated Patr-tetramer and subsequently with antibodies against cell surface markers, washed, resuspended in 500 μL Ca2+-containing binding buffer (BD PharMingen), and stained with 2.5 μL annexin V-FITC (BD PharMingen).
Quantification of Foxp3 mRNA and TRECs
To quantitate Foxp3 mRNA, total RNA was isolated from purified CD4+CD25+ and CD4+CD25– T cells using the RNeasy Mini Kit (Qiagen, Valencia, CA) with an on-column DNase digestion step. RNA (200-400 ng) was reverse transcribed with the First Strand cDNA Synthesis Kit (Marligen Biosciences, Ijamsville, MD) and real-time PCR was performed using the primers and probe of TaqMan Gene Expression Assays from Applied Biosystems (Foster City, CA).
TREC copy numbers were quantitated by real-time PCR using genomic DNA of T-cell subsets, primers CACATCCCTTTCAACCATGCT and GCCAGCTGCAGGGTTTAGG, and FAM-ACACCTCTGGTTTTTGTAAAGGTGC-CCACT-TAMRA probe (Applied Biosystems). One denaturation cycle (95°C for 10 minutes) was followed by 40 amplification cycles (95°C for 15 seconds, 60°C for 1 minute) in an ABI PRISM 7700 (Applied Biosystems). Serial dilutions of quantitated numbers of TREC-DNA plasmid served as standard. Foxp3 mRNA quantity and TREC copy number were calculated with Sequence Detector v1.6.3 software (Applied Biosystems), normalized to β-actin mRNA levels as endogenous reference.
Statistical analysis
The Kruskal-Wallis nonparametric analysis of variance test and the Mann-Whitney nonparametric 2-sample rank test were used to compare the frequency of CD25+ cells in the different chimpanzee groups (Figure 5A) and the absolute and relative increase of T-cell responsiveness after CD25 depletion between chimpanzees with different courses of HCV infection (Figure 5B). The 2-tailed Student t test was used to assess the significance of suppression in cocultures (Figures 1C and 3), the effect of IL-2 on the proliferation of CD25+ T cells (Figure 3C), and the differences in IFN-γ responses between PBMCs and CD25-depleted PBMCs (Figure 5B). Analyses were performed using GraphPad Prism 3.0 software (GraphPad Software, San Diego, CA). A 2-sided P value below .05 was considered statistically significant.
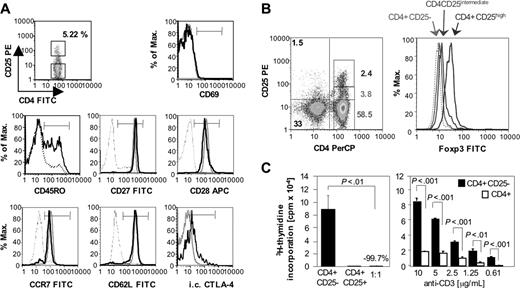
Frequency, phenotype, and function of CD4+CD25+ T cells of HCV-naive chimpanzees. (A) CD4+CD25+ T cells (bold histogram line) of a representative HCV-naive chimpanzee (Ch6497) displayed a nonactivated (CD69–), undifferentiated (CD27+CD28+) central memory (CD45RO+CCR7+CD62L+) phenotype with higher levels of CD45RO and intracellular CTLA-4 expression than CD4+CD25– T cells (dotted histogram line). The thin histogram line to the left in each graph indicates the negative control. (B) CD4+CD25+ but not CD4+CD25– T cells express Foxp3. Numbers in the left graph indicate the percentage of events in the respective gate. (C) Purified CD4+CD25+ T cells do not proliferate in response to plate-bound anti-CD3, but suppress the proliferation of CD4+CD25– T cells (left panel). Moreover, CD4+CD25– T cells proliferate more vigorously in response to plate-bound anti-CD3 than total CD4+ T cells (right panel). Mean and SD are indicated.
Frequency, phenotype, and function of CD4+CD25+ T cells of HCV-naive chimpanzees. (A) CD4+CD25+ T cells (bold histogram line) of a representative HCV-naive chimpanzee (Ch6497) displayed a nonactivated (CD69–), undifferentiated (CD27+CD28+) central memory (CD45RO+CCR7+CD62L+) phenotype with higher levels of CD45RO and intracellular CTLA-4 expression than CD4+CD25– T cells (dotted histogram line). The thin histogram line to the left in each graph indicates the negative control. (B) CD4+CD25+ but not CD4+CD25– T cells express Foxp3. Numbers in the left graph indicate the percentage of events in the respective gate. (C) Purified CD4+CD25+ T cells do not proliferate in response to plate-bound anti-CD3, but suppress the proliferation of CD4+CD25– T cells (left panel). Moreover, CD4+CD25– T cells proliferate more vigorously in response to plate-bound anti-CD3 than total CD4+ T cells (right panel). Mean and SD are indicated.
Results
Identification and functional characterization of CD4+CD25+ TRegs in HCV-naive chimpanzees
Because CD25+ TRegs have not been described in chimpanzees, we first characterized CD4+CD25+ T cells in 2 HCV-naive chimpanzees (Ch6457 and Ch6497). In both chimpanzees, CD4+CD25+ cells constituted approximately 5% of the circulating CD4+ T-cell population and displayed a nonactivated (CD69–), undifferentiated (CD27+CD28+), central memory (CCR7+CD62L+) phenotype with notably higher expression of CD45RO+ and intracellular CTLA-4, than CD4+CD25– cells (Figure 1A). CD4+CD25+, but not CD4+CD25– T cells expressed Foxp3 (Figure 1B).
To assess their proliferative potential, CD4+CD25+ cells were then magnetically purified and stimulated with plate-bound anti-CD3 (Figure 1C). In contrast to CD4+CD25– T cells, CD4+CD25+ cells did not proliferate in response to plate-bound anti-CD3 (Figure 1C left panel). To characterize their regulatory function, CD4+CD25+ cells were then added to anti-CD3–stimulated CD4+CD25– cells in a 1:1 coculture, which resulted in significant suppression of CD4+CD25– T-cell proliferation (Figure 1C left panel; P < .01). Similarly, depletion of CD25+ cells from the CD4+ T-cell population resulted in significantly enhanced proliferation in response to a wide range of anti-CD3 concentration (Figure 1C right panel; P < .01). Collectively, these data provide the first formal demonstration of functional CD4+CD25+ TRegs in nonhuman primates.
FoxP3+, CD4+CD25+ T cells with suppressor function are present in the blood of HCV-naive, HCV-recovered, and persistently HCV-infected chimpanzees
Next, we analyzed the presence and regulatory potential of CD4+CD25+ T cells in 2 HCV-naive, 8 HCV-recovered, and 6 persistently HCV-infected chimpanzees (Table 1). All recovered chimpanzees tested HCV-RNA negative in serum and liver biopsies by nested reverse transcription (RT)–PCR prior to our immunologic analysis and HCV-tetramer+ memory T cells displayed a resting (CD69–DR–CD38–) phenotype (not shown), indicating that they had not recently been activated in vivo. Several of these chimpanzees (Ch1552, Ch4X0186, Ch4X0132, and Ch1605), had recovered not only from the primary HCV infection, but also from 1 to 2 HCV rechallenges (Table 1). All persistently HCV-infected chimpanzees tested consistently HCV-RNA+ with 103 to 105 HCV genome equivalents per milliliter serum (not shown).
HCV infection history of the recovered or chronically HCV-infected chimpanzees included in this study
. | HCV infection, HCV genotype/treatment . | . | . | . | ||
|---|---|---|---|---|---|---|
| Chimpanzee . | Primary infection . | First HCV rechallenge . | Second rechallenge . | Reference . | ||
| HCV-recovered chimpanzees | ||||||
| Ch1552 | 1a/IH, cleared after 16 wk | 1a/IV, cleared after 4 wk | 1a/IV, sterilizing immunity | Nascimbeni et al22 ; Major et al23 | ||
| Ch4×0186 | 1a/IV, cleared after 9 wk | 1a/IV, cleared after 6 wk | 1b/IV, sterilizing immunity | Nascimbeni et al22 ; Major et al23 | ||
| Ch4×0132 | 1b/IH, cleared after 8 wk | 1b/IV, cleared after 10 wk | NA | Thomson et al31,32 | ||
| Ch1605 | 1a/IV, cleared after 12 wk | 1a/IV, cleared after 3 wk | NA | Nascimbeni et al22 ; Major et al23 | ||
| Ch4×0190 | 1b/IV, cleared after 10 wk | NA | NA | Thomson et al31,32 | ||
| Ch4×0355 | IV, recovered after 9 wk | NA | NA | NA | ||
| Ch6455 | 1a/IV, recovered after 14 wk | NA | NA | Major et al30 | ||
| Ch6461 | 1a/IV, recovered after 18 wk | NA | NA | Major et al30 | ||
| Persistently HCV-infected chimpanzees | ||||||
| Ch6411 | 1a/IV | NA | NA | Major et al30 | ||
| Ch6412 | 1a/IV | NA | NA | Major et al30 | ||
| Ch6475 | 1a/IV | NA | NA | Major et al30 | ||
| Ch1629 | 1a/IV | NA | NA | Major et al30 | ||
| Ch1535 | 1a/IH | NA | NA | Kolykhalov et al33 | ||
| Ch1536 | 1a/IH | NA | NA | Kolykhalov et al33 | ||
. | HCV infection, HCV genotype/treatment . | . | . | . | ||
|---|---|---|---|---|---|---|
| Chimpanzee . | Primary infection . | First HCV rechallenge . | Second rechallenge . | Reference . | ||
| HCV-recovered chimpanzees | ||||||
| Ch1552 | 1a/IH, cleared after 16 wk | 1a/IV, cleared after 4 wk | 1a/IV, sterilizing immunity | Nascimbeni et al22 ; Major et al23 | ||
| Ch4×0186 | 1a/IV, cleared after 9 wk | 1a/IV, cleared after 6 wk | 1b/IV, sterilizing immunity | Nascimbeni et al22 ; Major et al23 | ||
| Ch4×0132 | 1b/IH, cleared after 8 wk | 1b/IV, cleared after 10 wk | NA | Thomson et al31,32 | ||
| Ch1605 | 1a/IV, cleared after 12 wk | 1a/IV, cleared after 3 wk | NA | Nascimbeni et al22 ; Major et al23 | ||
| Ch4×0190 | 1b/IV, cleared after 10 wk | NA | NA | Thomson et al31,32 | ||
| Ch4×0355 | IV, recovered after 9 wk | NA | NA | NA | ||
| Ch6455 | 1a/IV, recovered after 14 wk | NA | NA | Major et al30 | ||
| Ch6461 | 1a/IV, recovered after 18 wk | NA | NA | Major et al30 | ||
| Persistently HCV-infected chimpanzees | ||||||
| Ch6411 | 1a/IV | NA | NA | Major et al30 | ||
| Ch6412 | 1a/IV | NA | NA | Major et al30 | ||
| Ch6475 | 1a/IV | NA | NA | Major et al30 | ||
| Ch1629 | 1a/IV | NA | NA | Major et al30 | ||
| Ch1535 | 1a/IH | NA | NA | Kolykhalov et al33 | ||
| Ch1536 | 1a/IH | NA | NA | Kolykhalov et al33 | ||
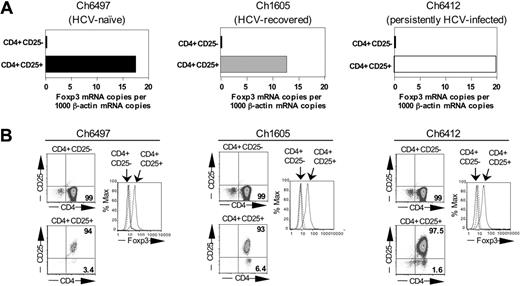
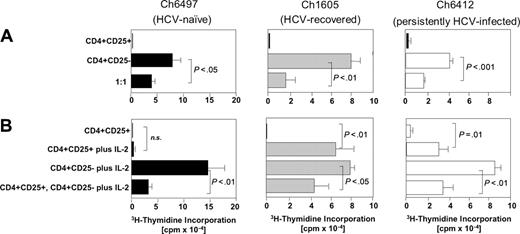
As shown in Figures 2 and 3 for representative chimpanzees of each group, CD4+CD25+ T cells expressed Foxp3 at the RNA (Figure 2A) and protein level (Figure 2B), did not proliferate to anti-CD3 stimulation, and significantly suppressed the proliferation of CD4+CD25– T cells (P < .01; Figure 3A). Thus, Foxp3+CD4+CD25+ TRegs were detectable not only in the blood of persistently HCV-infected chimpanzees, but also in HCV-recovered chimpanzees and displayed comparable regulatory function to Foxp3+ TRegs of HCV-naive chimpanzees. Of note, however, the detection of CD4+CD25+ TRegs with regulatory function was more variable in persistently HCV-infected chimpanzees than in HCV-naive and HCV-recovered chimpanzees. Whereas an individual persistently HCV-infected chimpanzee (Ch6412) consistently displayed clear evidence of functional regulatory CD4+CD25+ T cells (Figure 3A right panel), purified CD4+CD25+ T cells of other persistently infected chimpanzees (Ch6475, Ch6411, not shown) displayed suppressive activity that varied from bleed date to bleed date and correlated inversely to anti-CD3–mediated proliferation of CD4+CD25+ T cells (not shown). These findings suggest that the circulating pool of CD4+CD25+ cells of persistently HCV-infected chimpanzees contained not only CD25+ TRegs but also varying numbers of activated CD25-expressing effector cells.
In all HCV-naive and HCV-recovered chimpanzees and in the persistently HCV-infected chimpanzee Ch6412, CD4+CD25+ TReg-mediated suppression was neither associated with detectable IL-10 or TGF-β1 in the culture supernatants (not shown) nor reversible by neutralizing anti–TGF-β1 (Figure S1, available on the Blood website; see the Supplemental Figures link at the top of the online article) rendering suppression by membrane-bound TGF-β1 an unlikely mechanism.
CD4+CD25+ TRegs of HCV-recovered and of persistently HCV-infected chimpanzees differ from CD4+CD25+ TRegs of HCV-naive chimpanzees in their responsiveness to IL-2 and in their TRECs copy number
CD4+CD25+ TRegs of HCV-recovered chimpanzees and CD4+CD25+ TRegs of persistently HCV-infected chimpanzees differed from CD4+CD25+ TRegs of HCV-naive chimpanzees in 2 aspects. First, whereas hypoproliferation (anergy) of CD4+CD25+ TRegs of HCV-naive chimpanzees was maintained in the presence of high amounts of IL-2 (Figure 3B left panel), anergy of CD4+CD25+ TRegs of HCV-recovered chimpanzees and the persistently HCV-infected chimpanzee Ch6412 could partially be overcome (Figure 3B center and right panels). Even when hypoproliferation (anergy) was overcome, the suppressive activity of CD4+CD25+ TRegs was maintained in the presence of IL-2 (Figure 3B center and right panels; P < .05).
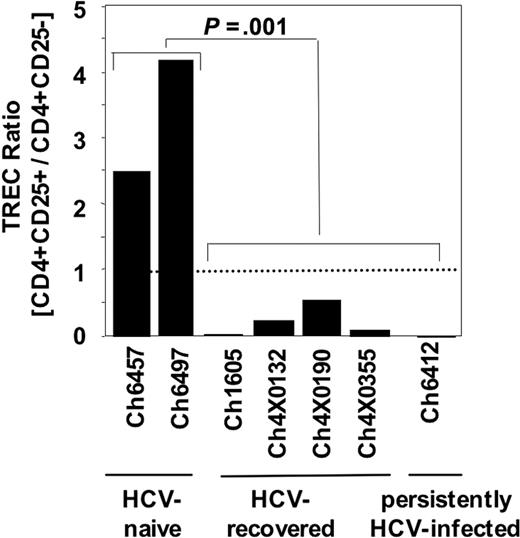
Second, CD4+CD25+ TRegs of HCV-recovered chimpanzees and CD4+CD25+ TRegs of persistently HCV-infected chimpanzees displayed a lower TREC ratio in CD4+CD25+ to CD4+CD25– cell populations than CD4+CD25+ TRegs of HCV-naive chimpanzees (P < .001; Figure 4). For each chimpanzee, the TREC copy number was determined ex vivo in aliquots of those CD4+CD25+ cells that were also tested in the suppression assays in Figure 3 and normalized to the TREC content in the CD4+CD25– T-cell population. TRECs do not amplify during cell division and the TREC copy number therefore indicates the proliferative history of the CD4+CD25+ TReg population. Because the TREC content of the CD25– PBMC population is unlikely to change during HCV infection due to the low frequency of HCV-specific T cells in this population (< 2% of PBMCs26 ), the data suggest that HCV infection stimulates either the proliferation of CD4+CD25+ natural TRegs or the induction of CD4+CD25+ TRegs from CD4+CD25– T cells. Thus, HCV infection appears to alter the population of regulatory T cells in vitro.
Expression of FoxP3. CD4+CD25+ T cells of HCV-naive, HCV-recovered, and persistently HCV-infected chimpanzees express FoxP3 at the RNA (A) and protein (B) levels. The data are representative of 12 experiments, performed with T cells from 2 HCV-naive, 4 HCV-recovered, and 3 persistently HCV-infected chimpanzees. Functional analyses of the same T-cell subpopulations are shown in Figure 3.
Expression of FoxP3. CD4+CD25+ T cells of HCV-naive, HCV-recovered, and persistently HCV-infected chimpanzees express FoxP3 at the RNA (A) and protein (B) levels. The data are representative of 12 experiments, performed with T cells from 2 HCV-naive, 4 HCV-recovered, and 3 persistently HCV-infected chimpanzees. Functional analyses of the same T-cell subpopulations are shown in Figure 3.
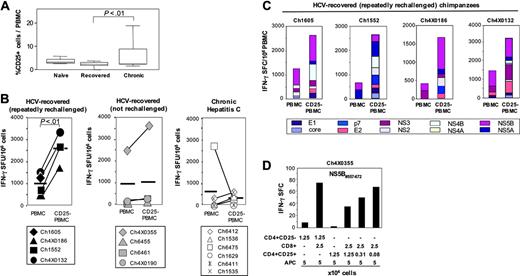
Depletion of CD4+CD25+ TRegs increases IFN-γ responses of HCV-specific T cells of HCV-recovered chimpanzees
Because the percentage of CD25+ T cells was found to be higher in chronic HCV infection than after recovery, previous in vitro depletion studies focused on PBMCs from patients with chronic hepatitis C.26,27,29 Consistent with these published studies, we found the overall frequency of circulating CD25+ cells to be significantly higher in the group of 6 persistently HCV-infected chimpanzees than in the group of 8 HCV-recovered chimpanzees (Figure 5A; P < .01). However, based on the demonstration of Foxp3 expression and regulatory activity of the CD4+CD25+ T cells of HCV-recovered chimpanzees in Figures 2 and 3, we decided not to limit the analysis of CD4+CD25+ T cells to persistent HCV infection but to specifically study their role and function after recovery from hepatitis C.
Using a set of 600 overlapping HCV peptides that covered the complete sequence of the HCV polyprotein, we first compared the HCV-specific IFN-γ response of CD25-depleted PBMCs with that of undepleted PBMCs in ex vivo ELISPOT assays. Depletion of CD25+ cells with magnetic beads was effective (Figure S2A) and the proportion of each cell population was comparable before and after depletion (Figure S2B). As shown in Figure 5B, depletion of CD25+ cells from PBMCs increased the number of HCV-specific IFN-γ ELISPOTs most notably in those chimpanzees that had recovered from multiple sequential infections (Figure 5B left panel). The comparison of the response of PBMCs and CD25-depleted PBMCs within this group was statistically significant (P < .01). In contrast, the increase in IFN-γ responses after depletion of CD25+ T cells was significantly weaker in chimpanzees that had recovered from a single infection (Figure 5B middle panel) and in chimpanzees that were persistently HCV infected (Figure 5B right panel; P < .01 for the comparison of the increase in IFN-γ responses between each of the 2 right panels and the left panel, respectively).
CD4+CD25+ TRegs of HCV-naive, HCV-recovered, and persistently HCV-infected chimpanzees all exert suppressive function but differ in their response to exogenous IL-2. (A) Purified CD4+CD25+ T cells (2.5 × 104/well) of HCV-naive, HCV-recovered, and persistently HCV-infected chimpanzees did not proliferate in response to plate-bound anti-CD3, but suppressed the proliferation of CD4+CD25– T cells. The data are representative of 15 experiments, performed with T cells from 2 HCV-naive, 4 HCV-recovered, and 3 persistently HCV-infected chimpanzees. CD4+CD25+ and CD4+CD25– T-cell populations are identical to those in Figure 2. (B) IL-2 responsiveness differed between CD4+CD25+ T cells of HCV-naive chimpanzees and those of chimpanzees that had experienced HCV infection (here shown for Ch1605, Ch6412). The results are representative for 2 HCV-naive and 4 HCV-recovered chimpanzees. Error bars indicate SD. n.s. indicates not significant.
CD4+CD25+ TRegs of HCV-naive, HCV-recovered, and persistently HCV-infected chimpanzees all exert suppressive function but differ in their response to exogenous IL-2. (A) Purified CD4+CD25+ T cells (2.5 × 104/well) of HCV-naive, HCV-recovered, and persistently HCV-infected chimpanzees did not proliferate in response to plate-bound anti-CD3, but suppressed the proliferation of CD4+CD25– T cells. The data are representative of 15 experiments, performed with T cells from 2 HCV-naive, 4 HCV-recovered, and 3 persistently HCV-infected chimpanzees. CD4+CD25+ and CD4+CD25– T-cell populations are identical to those in Figure 2. (B) IL-2 responsiveness differed between CD4+CD25+ T cells of HCV-naive chimpanzees and those of chimpanzees that had experienced HCV infection (here shown for Ch1605, Ch6412). The results are representative for 2 HCV-naive and 4 HCV-recovered chimpanzees. Error bars indicate SD. n.s. indicates not significant.
In all HCV-recovered chimpanzees, depletion of CD25+ cells increased the immune response to multiple pools of overlapping HCV peptides (Figure 5C) rather than to few, dominant pools. Results were comparable for IFN-γ production (Figure 5C) and granzyme B secretion (not shown), indicating that more than a single function of HCV-memory T cells was affected. To prove that the depletion was responsible for this increase in HCV-specific memory T-cell responsiveness, we added increasing numbers of the depleted cells back to the T-cell culture, thereby reconstituting the suppressive effect (Figure 5D and Figure S3A). Using purified CD8+ and CD4+CD25+ T-cell populations in the absence of CD4+CD25– accessory cells, we also found that CD4+CD25+ TRegs did not themselves produce IFN-γ (Figure 5D third bar from left) and did not require CD4+CD25– accessory cells to suppress HCV-specific CD8 T cells (Figure 5D and Figure S3A). Collectively, these results demonstrate that CD4+CD25+ TRegs regulate effector functions of HCV-specific T cells of HCV-recovered chimpanzees.
CD4+CD25+ TRegs of HCV-recovered chimpanzees and CD4+CD25+ TRegs of persistently HCV-infected chimpanzees differ from CD4+CD25+ TRegs of HCV-naive chimpanzees in their TREC content. The TREC copy number of CD4+CD25+ cells was divided by that of CD4+CD25– cells for normalization. The dotted line indicates an equal TREC copy number in CD4+CD25+ and CD4+CD25– populations. The TREC copy number was assessed in the same cell populations as in the suppression assays (Figure 3).
CD4+CD25+ TRegs of HCV-recovered chimpanzees and CD4+CD25+ TRegs of persistently HCV-infected chimpanzees differ from CD4+CD25+ TRegs of HCV-naive chimpanzees in their TREC content. The TREC copy number of CD4+CD25+ cells was divided by that of CD4+CD25– cells for normalization. The dotted line indicates an equal TREC copy number in CD4+CD25+ and CD4+CD25– populations. The TREC copy number was assessed in the same cell populations as in the suppression assays (Figure 3).
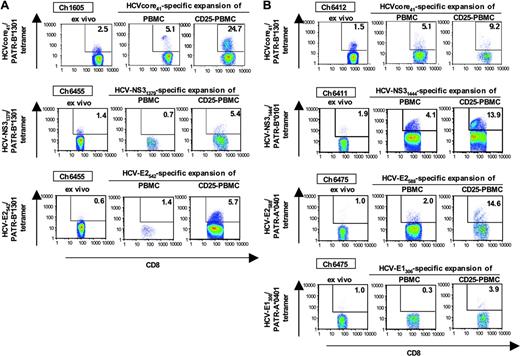
CD4+CD25+ TRegs control the proliferation of HCV-specific CD8+ T cells in HCV-recovered and in persistently HCV-infected chimpanzees
Because the capacity to rapidly proliferate on antigen recall is a key characteristic of memory T cells, we next studied the effect of CD4+CD25+ TRegs on in vitro proliferation of HCV-specific T cells. The expansion of HCV-specific tetramer-positive CD8 T-cell populations was assessed over a course of 10 to 12 days in the presence of full PBMCs or CD25-depleted PBMCs. Seven representative experiments of a total of 15 experiments, including both HCV-recovered and persistently HCV-infected chimpanzees, are shown in Figure 6. In each case, the HCV-specific tetramer-positive CD8 T-cell population expanded to the greatest extent when CD25+ T cells were depleted prior to stimulation with the cognate HCV peptide. This effect was observed in HCV-recovered (Figure 6A) and in persistently HCV-infected (Figure 6B) chimpanzees.
CD4+CD25+ TRegs suppress HCV-specific CD8+ T cells. (A) Frequency of CD25+ T cells in the blood of HCV-naive, HCV-recovered, and persistently HCV-infected chimpanzees. (B) Depletion of CD25+ T cells from PBMCs increased HCV-specific IFN-γ responses of HCV-recovered chimpanzees, especially if they had recovered from repeated HCV rechallenges. The data were calculated from duplicate cultures and are representative of 16 of 18 experiments, the remaining 2 of 18 experiments showed no increase in IFN-γ responses. In contrast, the increase of IFN-γ responses was weaker or absent in persistently HCV-infected chimpanzees (P < .01 when the increase in IFN-γ responses was compared between the recovered/rechallenged and the persistently HCV-infected chimpanzees). The data are representative of 12 experiments in persistently HCV-infected chimpanzees. Horizontal bold lines indicate the mean response. (C) Increase of HCV-specific IFN-γ responses after depletion of CD25+ T cells from PBMCs. The depleted T-cell populations exerted suppressor function when added back to the culture (Figure S3). (D) CD4+CD25+ TRegs of an HCV-recovered chimpanzees inhibited IFN-γ secretion of autologous NS5B peptide-specific CD8+ T cells, did not produce IFN-γ and did not require CD4+CD25– cells to exert suppressive function (for additional experiments, see Figure S3). APC indicates antigen-presenting cells (irradiated, CD4- and CD8-depleted PBMCs).
CD4+CD25+ TRegs suppress HCV-specific CD8+ T cells. (A) Frequency of CD25+ T cells in the blood of HCV-naive, HCV-recovered, and persistently HCV-infected chimpanzees. (B) Depletion of CD25+ T cells from PBMCs increased HCV-specific IFN-γ responses of HCV-recovered chimpanzees, especially if they had recovered from repeated HCV rechallenges. The data were calculated from duplicate cultures and are representative of 16 of 18 experiments, the remaining 2 of 18 experiments showed no increase in IFN-γ responses. In contrast, the increase of IFN-γ responses was weaker or absent in persistently HCV-infected chimpanzees (P < .01 when the increase in IFN-γ responses was compared between the recovered/rechallenged and the persistently HCV-infected chimpanzees). The data are representative of 12 experiments in persistently HCV-infected chimpanzees. Horizontal bold lines indicate the mean response. (C) Increase of HCV-specific IFN-γ responses after depletion of CD25+ T cells from PBMCs. The depleted T-cell populations exerted suppressor function when added back to the culture (Figure S3). (D) CD4+CD25+ TRegs of an HCV-recovered chimpanzees inhibited IFN-γ secretion of autologous NS5B peptide-specific CD8+ T cells, did not produce IFN-γ and did not require CD4+CD25– cells to exert suppressive function (for additional experiments, see Figure S3). APC indicates antigen-presenting cells (irradiated, CD4- and CD8-depleted PBMCs).
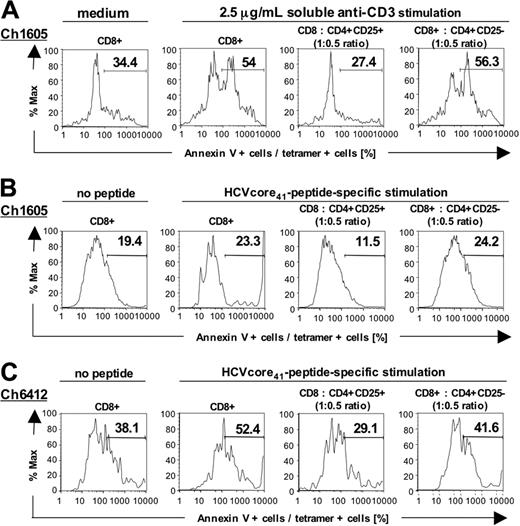
CD4+CD25+ TRegs protect memory CD8+ T cells from AICD
Because T-cell receptor activation and IFN-γ production are associated with AICD40-42 and because CD4+CD25+ TRegs blocked IFN-γ production (Figure 5 and Figure S3), we asked whether CD4+CD25+ TRegs prevented AICD of HCV-specific memory CD8+ T cells. To answer this question, we took advantage of a relatively high percentage of CD8+ effector memory cells in the HCV-recovered chimpanzee Ch1605 that was readily detectable ex vivo using the HCVcore41/Patr-B*1301 tetramer. CD8+ T cells were cultured for 20 hours in the presence or absence of either anti-CD3 (Figure 7A) or PMA/ionomycin (not shown). Apoptosis of tetramer-positive cells was assessed by annexin V binding. Interestingly, addition of CD4+CD25+ T cells but not addition of CD4+CD25– T cells reduced apoptosis of HCV tetramer-positive CD8+ cells (Figure 7A). These experiments were extended to additional recovered or chronic chimpanzees and to stimulation with the cognate HCV peptide. Consistent with the results of anti-CD3 stimulation, apoptosis was diminished in the presence of CD4+CD25+ TRegs compared with addition of the same number of CD4+CD25– T cells (Figure 7B-C). The data suggest that CD4+CD25+ TRegs may contribute to the regulation of HCV-specific T-cell memory by regulating their effector functions and by reducing AICD.
Effect of CD4+CD25+ cells on expansion of HCV-specific, peptide-stimulated CD8 T-cell populations. Depletion of CD25+ T cells from PBMCs of HCV-recovered chimpanzees (A) and persistently HCV-infected chimpanzees (B) resulted in increased expansion of tetramer-positive, HCV-specific CD8+ T cells on stimulation with cognate peptide. Seven representative experiments of a total of 15 are shown.
Effect of CD4+CD25+ cells on expansion of HCV-specific, peptide-stimulated CD8 T-cell populations. Depletion of CD25+ T cells from PBMCs of HCV-recovered chimpanzees (A) and persistently HCV-infected chimpanzees (B) resulted in increased expansion of tetramer-positive, HCV-specific CD8+ T cells on stimulation with cognate peptide. Seven representative experiments of a total of 15 are shown.
Discussion
To date, only few studies describe the function of CD4+CD25+ TRegs in HCV infection and all focus on their role in established, chronic hepatitis C.26-29 Patient studies are difficult, however, because HCV-recovered patients are rare and because the time point of infection and recovery as well as the genotype and sequence of the previously infecting HCV are rarely known and likely heterogeneous within the study cohort. In contrast to patient studies, the chimpanzee model permits the assessment of T-cell responses to defined HCV sequences with defined time points of HCV infection and HCV clearance.
Using this valuable model of HCV infection, we here demonstrate that Foxp3+CD4+CD25+ TRegs with suppressor function are present not only in persistently HCV-infected chimpanzees but also in HCV-recovered chimpanzees. Furthermore, we provide evidence that the CD4+CD25+ TReg population changes during HCV infection. In healthy, HCV-naive chimpanzees, CD4+CD25+ TRegs displayed the same phenotype and function as CD4+CD25+ TRegs of uninfected humans43 and mice.44 In comparison with Foxp3+CD4+CD25+ TReg populations of HCV-naive chimpanzees, Foxp3+CD4+CD25+ TReg populations of HCV-recovered and persistently HCV-infected chimpanzees displayed lower TREC contents. In addition, in vitro hypoproliferation (anergy) of CD4+CD25+ TRegs of HCV-recovered and persistently HCV-infected chimpanzees, but not those of HCV-naive chimpanzees, could partially be overcome by IL-2, a cytokine that is present in high amounts during acute viral infections. This in vitro effect of IL-2 is consistent with recently reported in vivo effects.45,46 Thus, HCV infection appears to alter the induction or expansion or both of CD4+ TRegs, possibly via induction of IL-2. The altered CD4+CD25+ TReg population may be derived either from activated effector cells or from natural or from induced TReg populations in HCV-recovered and persistently HCV-infected chimpanzees. Indeed, both regulatory and effector T cells can, in principle, be generated from the same mature T-cell precursor, depending on qualitative or quantitative differences in antigen priming47,48 and even from activated CD4+CD25– T cells.49,50
With regard to CD4+CD25+ TRegs in chronic HCV infection, our study is consistent with previous publications,26-29 but the overall enhancement of HCV-specific T-cell responses after in vitro depletion of regulatory T cells appeared to be weaker. The degree of enhancement of virus-specific T cells depends on which regulatory T-cell population is depleted. In our study, depletion of the CD25+ T cells resulted in less enhancement of IFN-γ responses than the reported depletion of the total CD4+ T-cell population, which included IL-10–producing CD4+ T cells (Tr1 cells) and TGF-β–producing CD4+ T cells (Th3 cells) in addition to CD4+CD25+ TRegs.26 In addition, weak enhancement of CD8+ T-cell responses by depletion of CD25+ cells from PBMCs of persistently infected chimpanzees can be due to the fact that TRegs sequester to liver, as suggested by the increase in intrahepatic Foxp3 mRNA levels during acute and chronic HCV infection (E-C.S. and B.R., unpublished data, August 2004). Recruitment of TRegs to the liver and suppression of CD8 T cell–mediated liver injury would be consistent with the clinically asymptomatic clinical course that is characteristic for HCV infection.
CD4+CD25+ T cells protect CD8+ T cells from AICD. (A) Apoptosis of anti-CD3–stimulated CD8+ T cells was diminished in the presence of CD4+CD25+ T cells, compared with the presence of CD4+CD25– T cells. Anti-CD3 stimulation resulted in a 57% increase of apoptosis over baseline, stimulation with PMA/ionomycin yielded similar results (not shown). (B-C) Apoptosis of HCV peptide-stimulated T-cell lines of an HCV-recovered (Ch1605) and a persistently HCV-infected chimpanzee (Ch6412) was diminished in the presence of CD4+CD25+ TRegs, compared with the presence of CD4+CD25– T cells. The results are representative for 6 experiments, using HCVcore41-, HCV-NS31444-, and HCV-NS31357-stimulated T-cell lines of recovered and persistently infected chimpanzees.
CD4+CD25+ T cells protect CD8+ T cells from AICD. (A) Apoptosis of anti-CD3–stimulated CD8+ T cells was diminished in the presence of CD4+CD25+ T cells, compared with the presence of CD4+CD25– T cells. Anti-CD3 stimulation resulted in a 57% increase of apoptosis over baseline, stimulation with PMA/ionomycin yielded similar results (not shown). (B-C) Apoptosis of HCV peptide-stimulated T-cell lines of an HCV-recovered (Ch1605) and a persistently HCV-infected chimpanzee (Ch6412) was diminished in the presence of CD4+CD25+ TRegs, compared with the presence of CD4+CD25– T cells. The results are representative for 6 experiments, using HCVcore41-, HCV-NS31444-, and HCV-NS31357-stimulated T-cell lines of recovered and persistently infected chimpanzees.
With regard to the role of CD4+CD25+ TRegs after recovery from HCV infection, our results differ considerably from previous studies, which did not detect suppression by CD4+CD25+ TRegs in most HCV-recovered patients.26,27 Importantly, we have expanded these previous studies by molecular assays, providing clear evidence of Foxp3 expression in CD4+CD25+ T cells of HCV-recovered patients, and by sensitive functional assays that reveal the effect of CD4+CD25+ TRegs on expansion (Figure 6) and AICD (Figure 7) of HCV-specific tetramer-positive T-cell populations. These results are consistent with those by Boettler et al, who studied the effect of CD4+CD25+ TRegs on proliferation of tetramer-positive HCV-specific CD8+ T cells.28 Importantly, we show that the enhancement of HCV-specific T-cell responses by depletion of CD25+ T cells is more readily detectable after recovery from repeated HCV rechallenges than after recovery from only a single HCV infection (Figure 5B), an aspect that typically is not known in patient studies.26,27
Thus, the current study demonstrates that TRegs regulate various effector functions of HCV-specific CD8+ T cells after recovery from hepatitis C. Because memory T cells have a low activation threshold and readily respond to minimal antigenic stimulation,51 regulatory T cells may protect from nonspecific memory T-cell activation and potential tissue damage. Regulatory T cells may also be responsible for the fact that the memory T-cell population has a slow proliferative turnover and reduced susceptibility to AICD (Figure 7) despite being highly susceptible to cytokines and low-affinity antigens.39,52,53 Thus, CD4+CD25+ TRegs may actively contribute to the maintenance of the memory CD8+ T-cell populations. This is consistent with a recent report that described an increased proliferative rate of CD8+ memory T cells in mice that lacked CD4+CD25+ T cells and a decreased proliferative rate of CD8+ memory T cells when CD4+CD25+ T cells were present.54
Although the investigation of the function and role of CD4+CD25+ TRegs in chimpanzees necessarily relies on in vitro experiments due to the absence of a CD25-depleting antibody for primates, the presented results relate to a recent in vivo study that was initially designed to study the relevance of memory CD4+ T cells for HCV-specific immunity. In that study, in vivo depletion of CD4+ T cells in a chimpanzee prior to HCV rechallenge resulted in the emergence of HCV escape variants with mutations in CD8+ T-cell epitopes, loss of HCV control and ultimately, persistent HCV infection.24 The authors observed strong CD8 T-cell responses in the liver of the CD4 T cell–depleted chimpanzee on rechallenge and suggest that in the absence of CD4 T-cell help, CD8 T cells might not have been able to keep pace with the evolution of viruses that rapidly accumulate escape mutations.24 Based on our results, we propose instead, that depletion of CD4+ T cells, which included the depletion of CD4+CD25+ TRegs, resulted in an enhancement of HCV-specific CD8+ T-cell responses, thereby in increased selection pressure on CD8 T-cell epitopes and subsequent HCV escape.
Collectively, our results show that functional Foxp3+-CD4+CD25+ TRegs are detectable both in persistent HCV infection and after recovery, implicating that they are part of the normal immune response to this pathogen. The study also sets the stage for experimental intervention and modulation of TReg activity in this nonhuman primate model of HCV infection.
Prepublished online as Blood First Edition Paper, February 14, 2006; DOI 10.1182/blood-2005-09-3903.
Supported by US Public Health Service grant CA85883 from the NIH (C.M.R.) and by the Intramural Research Programs of NIDDK, NIH, and CBER, FDA. C.M.R. was also supported by the Greenberg Medical Research Institute.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
We thank Dr Stephen Feinstone for support and for blood samples from the chimpanzees on campus, Drs T. Jake Liang and Harvey Alter for blood samples from chimpanzees Ch4X0132, Ch4X0190, and Ch4X0355, and Dr Christina Weiler-Normann for helpful discussion.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal