Abstract
The association of thrombosis and gestational morbidity with antiphospholipid antibodies is termed antiphospholipid syndrome (APS). Annexin 2 (A2) is a profibrinolytic endothelial cell surface receptor that binds plasminogen, its tissue activator (tPA), and β2-glycoprotein I (β2GPI), the main antigen for antiphospholipid antibodies. Here, we evaluate A2 as a target antigen in APS. Serum samples from 434 individuals (206 patients with systemic lupus erythematosus without thrombosis, 62 with APS, 21 with nonautoimmune thrombosis, and 145 healthy individuals) were analyzed by enzyme-linked immunosorbent assay (ELISA) and immunoblot for antiphospholipid and A2 antibodies. Anti-A2 antibodies (titer > 3 SDs) were significantly more prevalent in patients with APS (22.6%; venous, 17.5%; arterial, 34.3%; and mixed thrombosis, 40.4%) than in healthy individuals (2.1%, P < .001), patients with nonautoimmune thrombosis (0%, P = .017), or patients with lupus without thrombosis (6.3%, P < .001). Anti–A2 IgG enhanced the expression of tissue factor on endothelial cells (6.4-fold ± 0.13-fold SE), blocked A2-supported plasmin generation in a tPAdependent generation assay (19%-71%) independently of β2GPI, and inhibited cell surface plasmin generation on human umbilical vein endothelial cells (HUVECs) by 34% to 83%. We propose that anti-A2 antibodies contribute to the prothrombotic diathesis in antiphospholipid syndrome.
Introduction
Antiphospholipid syndrome (APS) is characterized by recurring arterial, venous, or small-vessel thrombosis at any site, as well as recurrent miscarriages in the presence of antiphospholipid antibodies.1 Thrombocytopenia and/or hemolytic anemia are accompanying features, which can be present in up to one third of patients.2-4 Other clinical features that are relatively common in these patients include livedo reticularis, heart valve lesions, epilepsy, myocardial infarction, leg ulcers, and amaurosis fugax. A large variety of other manifestations with prevalence lower than 5% has been described, including Sneddon syndrome, chorea, transverse myelopathy, adult respiratory distress syndrome, renal thrombotic microangiopathy, Addison syndrome, Budd-Chiari syndrome, nodular regenerative hyperplasia of the liver, avascular necrosis of the bone, cutaneous necrosis, and subungual splinter hemorrhages.5 APS affects mainly young individuals, most of whom require lifelong anticoagulation. In the primary syndrome there is no evidence of underlying disease,6 while the secondary syndrome exists mainly in the setting of systemic lupus erythematosus (SLE).7
Antiphospholipid antibody is a term that encompasses distinct, often coexisting antibodies including lupus anticoagulant, anticardiolipin antibodies, and antibodies against β2-glycoprotein I (β2GPI) alone. Despite the name, these antibodies are not directed against phospholipids, but, rather, target intravascular proteins, either alone or in complex with anionic phospholipids. Most pathogenic antiphospholipid antibodies, detected either as prolongation of the activated partial thromboplastin time (lupus anticoagulant) or by their ability to bind to cardiolipin-coated wells (anticardiolipins), are directed against β2GPI.8-10
Antiphospholipid antibodies are widely accepted as pathogenic11 and are believed to promote thrombosis in several ways. These antibodies are thought to interfere with normal cell surface hemostatic mechanisms by targeting coagulation factors (mainly prothrombin), natural anticoagulants, fibrinolytic proteins, other antigens such as oxidized low-density lipoproteins, and CD36.12 Of note, antibodies have also been found to develop against annexin 5, a potent anticoagulant, and their ability to expose negatively charged phospholipids and thus promote thrombosis has been elegantly described.13 In addition, thrombosis is believed to occur through antiphospholipid antibody–induced complement and cellular activation, as determined by enhanced cell surface expression of cell adhesion and procoagulant molecules, as well as secretion of proinflammatory cytokines.14-18 Vessel occlusion induced in vivo by antiphospholipid antibodies has been shown to be complement dependent and to require additional local or systemic prothrombotic stimuli.18,19 Passive transfer of human antiphospholipid antibodies into mice induces an antiphospholipid-like syndrome with enhanced injury-induced clot formation, fetal wastage, and in vivo endothelial cell activation.11,18,20,21
The classic intravascular fibrinolytic system consists of sequential proteolytic events, in which tissue plasminogen activator (tPA) cleaves and activates plasminogen, resulting in the generation of the serine protease, plasmin. Plasmin cleaves cross-linked fibrin, the major protein constituent of thrombi, leading to clot degradation.22 Emerging evidence suggests that plasma hypofibrinolysis is a risk factor for venous thrombosis in the healthy population,23 and that fibrinolysis might be impaired in APS due to increased fibrinolytic inhibitor (PAI-1) activity.24
Annexin 2 (A2) is a profibrinolytic receptor that binds both plasminogen and its activator, tPA, functioning as a cofactor for plasmin generation and localizing fibrinolytic activity to the cell surface.25 It is found on the surface membrane of endothelial cells and monocytes and on the brush-border membrane of placental syncytiotrophoblasts.26,27 Several lines of evidence indicate that A2 acts as a tPA-dependent cofactor for cell surface plasmin generation in vivo. First, homozygous A2-null mice display microvascular fibrin deposition, reduced clearance of injury-induced arterial thrombi, and markedly deficient endothelial cell surface plasmin generation.28 Second, pretreatment of rat carotid artery with A2 prevents vessel thrombosis in response to injury.29 Third, overexpression of A2 by acute promyelocytic leukemia blast cells contributes to a hyperfibrinolytic hemorrhagic state in humans.30,31 In addition, polymorphism in the annexin 2 gene is a risk factor for stroke in patients with sickle cell disease.32
We report for the first time that antibodies directed against A2 are highly prevalent in thrombosis in the setting of APS. In addition, we investigated the functional implications of A2 targeting in the pathogenesis of antiphospholipid syndrome. We found that A2 antibodies induce tissue factor expression on endothelial cells as effectively as that observed by patient anti-β2GPI antibodies. In addition, anti-A2 antibodies block the ability of placental annexin 2 to act as a cofactor for plasmin generation and consistently inhibit endothelial cell surface A2-dependent fibrinolysis.
Patients, materials, and methods
Patient populations
The study population consisted of 434 adults followed at the Instituto Nacional de Ciencias Médicas y Nutrición Salvador Zubirán, Mexico City. Consent was obtained per IRB-approved protocols. Four groups of individuals (Table 1) were studied: 145 healthy women who had no prior history of thrombosis and who met standard criteria for blood donation regardless of obstetric history33 ; 206 patients without thrombosis who fulfilled the American College of Rheumatology criteria for systemic lupus erythematosus34 ; 21 patients with at least one prior objectively documented thrombotic episode within the previous 2 to 12 months and without a history of autoimmune disease (5 with hereditary deficiency of an anticoagulant protein and 16 with acquired risk factors); and a group of 62 patients with antiphospholipid syndrome and between 1 and 4 documented venous-only (64.5%), arterial-only (27.4%), or mixed (8.1%) thrombosis. Objectively documented thrombosis was required regardless of hematocytopenias or obstetric events. Venous thrombosis was documented by compression ultrasonography, computed tomography (CT) scan, angiography, or high-probability ventilation/perfusion (V/Q) scan. Arterial thrombosis was documented by CT scan, magnetic resonance imaging (MRI), angiography, or pathologic examination. Because levels of anti-β2GPI and anticardiolipin antibodies may decrease at the time of thrombosis, thrombosis must have occurred more than 2 months prior to inclusion.35 Antiphospholipid syndrome was defined as definite (47 patients) if a positive lupus anticoagulant or moderate to high titer anticardiolipin antibodies were present on 2 or more occasions at least 6 weeks apart.1 It was defined as probable (15 patients) if lupus anticoagulant or anticardiolipin antibodies (> 3 SDs) were present on only one occasion, in conjunction with either persistently positive but low titers of anticardiolipin antibodies15 or anti-β2GPI antibodies (> 3 SDs).
Patient characteristics
. | No. . | Median age, y (range) . | Female/male . | Thrombosis . |
|---|---|---|---|---|
| Healthy controls | 145 | 36.7 (22-63) | 145/0 | No |
| Patients | ||||
| Systemic lupus without thrombosis | 206 | 29.7 (17-54) | 199/7 | No |
| Nonimmune thrombosis | 21 | 59.3 (35-88) | 15/6 | Yes |
| Antiphospholipid syndrome | 62 | 35.6 (21-66) | 49/13 | Yes |
. | No. . | Median age, y (range) . | Female/male . | Thrombosis . |
|---|---|---|---|---|
| Healthy controls | 145 | 36.7 (22-63) | 145/0 | No |
| Patients | ||||
| Systemic lupus without thrombosis | 206 | 29.7 (17-54) | 199/7 | No |
| Nonimmune thrombosis | 21 | 59.3 (35-88) | 15/6 | Yes |
| Antiphospholipid syndrome | 62 | 35.6 (21-66) | 49/13 | Yes |
Detection of anti-A2 antibodies
Sera were evaluated for anti-A2 antibodies (IgG and IgM) by enzymelinked immunosorbent assay (ELISA) using nonirradiated 96-well plates (Nunc-Immuno-plate II; Nunc, Roskilde, Denmark). Wells were coated with 50 μL of either recombinant A2 (10 μg/mL) or PBS, incubated (overnight, 4°C), and blocked (PBS containing 0.05% Tween-20, 1.5% BSA, 1.5 hours, 21°C). Positive controls included anti-A2 antibody–containing patient serum and rabbit polyclonal anti–human A2 antibodies.36 Preimmune rabbit IgG served as a negative control. Individual patient sera were diluted in blocking buffer (1:50), added to both A2- or PBS-coated wells, and incubated (1 hour, 21°C). Secondary alkaline phosphatase–conjugated antibodies, either goat anti–rabbit IgG (1:2500; ICN Pharmaceuticals, Santa Ana, CA) or goat anti–human IgG (1:8000; Sigma, St Louis, MO) or IgM (1:5000; ICN Biomedicals, Costa Mesa, CA), were incubated (1.5 hours, 21°C). Substrate was dissolved in diethanolamine buffer. Average absorbance (A405 nm) for duplicate wells was calculated by subtracting the background from wells without A2. Plates were washed (PBS–Tween-20) between steps. Titers were determined in relation to 30 individual, sex-matched, healthy donor sera, run in duplicate within each assay. A random subset of samples was rerun for sample storage and interassay control, which varied for IgG by 6.7% (1.16 ± 0.07 SE), and for IgM by 8.3% (0.15 ± 0.12 SE). Positive anti-A2 antibodies were defined as IgG or IgM values that exceeded the mean for healthy controls by 3 or more SDs.
Antiphospholipid antibody assays
Lupus anticoagulant was determined using the dilute Russel Viper Venom Time and confirmatory kits (American Diagnostica, Greenwich, CT). Anticardiolipin antibodies (IgG and IgM) were measured by ELISA.35 β2GPI purification and ELISA for detection of IgG directed against phospholipid-free β2GPI have been previously described.37
IgG purification and antigenic specificity
IgG fractions from the sera of healthy individuals or patients were purified by affinity chromatography (ImmunoPure Protein A; Pierce, Rockford, IL). Binding specificity was assessed by immunoblot using recombinant human annexin 2 (40 kDa),26 annexin 5 (33 kDa; Sigma), Glu-plasminogen (American Diagnostica), human tissue-plasminogen activator (rtPA, alte-plase; Genentech, South San Francisco, CA), actin (Sigma), enolase (Sigma), and recombinant p11 (from Escherichia coli).26,38 Primary antibodies included monoclonal mouse anti–human A2 (Transduction Laboratories, Lexington, KY), polyclonal rabbit anti–human A2,26 polyclonal rabbit anti–human A5 (BioVision, Palo Alto, CA), goat anti-β2GPI (Enzyme Research Laboratories, South Bend, IN), rabbit anti–human actin and enolase (Santa Cruz Biotechnology, Santa Cruz, CA), mouse anti–human p11 (Transduction Laboratories), and patient IgG. Secondary antibodies were alkaline-phosphatase or horseradish-peroxidase conjugated. To test whether serum-purified IgG was free of β2GPI by immunoblotting, both serum and β2GPI (48 kDa; Affinity Biologicals/ERL, Hamilton, ON) were used as positive controls.
Affinity purification of anti–A2 IgG was carried out by incubation of IgG fractions either with A2-loaded (20 μg) nitrocellulose membranes followed by elution (Elution Buffer; Pierce) or with an A2 affinity column. For the latter, 400 μg recombinant A2 was incubated batchwise with 0.5 g CnBr-activated sepharose 4B in a volume of 400 μL of 150 mM NaCl, 100 mm NaHCO3 (pH 8.3, 1.5 hours, 21°C). The beads were then washed (100 mM Tris HCl [pH 8.0], 30 minutes, 21°C) and incubated with patient IgG (1 mg/mL, 1 mL volume, 18 hours, 4°C). The supernatant was recovered, and the beads were loaded onto a column and washed with 10-column volumes of 100 mM Tris-HCl (pH 8.0), followed by 5-column volumes of 10 mm Tris-HCl (pH 8.0). The column was eluted in 400-μL fractions with 100 mM glycine (pH 2.8). Fractions were immediately neutralized with one-tenth volume 1 M Tris base (pH 8.0). Both the immunodepleted IgG and the affinity-purified IgG–containing fractions were dialyzed overnight against PBS (pH 7.2). Anti-A2 purification and depletion were verified by immunoblot analysis.
In vitro plasma clot lysis
Plasma clot lysis profiles were determined spectrophotometrically in recalcified citrated plasma, with or without added thrombin and recombinant tPA as described.39
Endothelial cell activation
Activation of endothelial cells in the presence of antiphospholipid antibodies was determined by enhanced expression of tissue factor as described.40 Briefly, confluent human umbilical vein endothelial cells (HUVECs; M199 medium, 10% fetal bovine serum) were incubated in triplicate (4 hours, 37°C, 5% CO2) with medium alone, nonimmune rabbit IgG, or polyclonal rabbit anti–human A2 IgG directed against the tPA binding tail domain,41 or patient IgG. Tumor necrosis factor α (TNF-α, 10 ng/mL; Sigma) served as positive control. Cells were fixed (2% paraformaldehyde, 10 minutes), blocked (PBS, 1% BSA, 1 hour), and analyzed by ELISA with anti–tissue factor antibody (1:500, 1 hour; American Diagnostica), followed by horseradish peroxidase–conjugated sheep anti–mouse IgG (1:500, 1 hour; Amersham Biosciences) and TMB-blue substrate solution (Dako, Glostrup, Denmark). Plates were read at 650 nm (Spectra Max Gemini XS; Molecular Devices, Sunnyvale, CA). Limulus amebocyte (LAL) assay was used for endotoxin detection (ICN Pharmaceuticals).
Plasmin generation/inhibition assays
Purified patient or control IgG fractions were preincubated (1 hour, 37°C, PBS) either alone or with human placental or recombinant A2 (250-500 nM), after which glu-plasminogen (100 nM; American Diagnostica) was added (1.5 hours, 37°C). A 4:1 molar ratio of patient IgG to purified A2 was used for all samples. A2 purification from human placenta as well as plasmin generation/inhibition assays were carried out as previously described.25 Recombinant tPA (10 nM), premixed with AFC-81 (125 μM, d-Val-Leu-Lys-aminofluoromethylcoumarin; Enzyme Systems Products, Livermore, CA), a fluorogenic plasmin substrate, was then added and the plates were read spectrofluorometrically.
For endothelial cell plasmin generation, cells in 48-well culture plates (Nuncion Surface; Nunc) were pretreated with epsilon-aminocaproic acid (EACA, 10 mM, 4°C, 15 minutes) and washed with IB-2 (PBS, 2 mg/mL BSA, 2 mM CaCl2, 1 mM MgCl2). Cells were preincubated with control or patient IgG (IB-2, 45 minutes, 37°C) or IB-2 alone. Plasminogen was then added and cells were reincubated (100 nM, 45 minutes, 37°C). Unbound plasminogen and antibodies were removed by gentle washing (IB-2). Premixed recombinant tPA and AFC-81 were then added. Plates were read spectrofluorometrically as described.25
Statistical analyses
Descriptive statistics were used to define the subjects' characteristics. Categoric variables were compared using chi-square or Fisher exact test. P value was set at less than .05, 2-tailed. Analysis was performed using the STATA 7.0 computer program (Stata Corporation, College Station, TX).
Results
Prevalence of anti-A2 antibodies
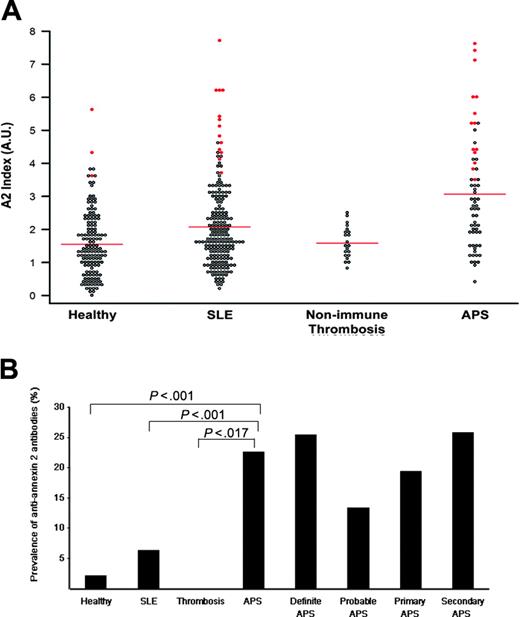
For each individual patient, the sum of A405 values for IgG and IgM from sera diluted 1:50 (Figure 1A, Annexin 2 Index) were plotted. Annexin 2 index values for patients with positive antibodies, defined as more than 3 SDs from the mean of normals, of either or both isotypes are shown in red. Among healthy individuals (Healthy), the prevalence of anti-A2 antibodies by ELISA was 3 (2.1%) of 145. This value is similar to that published for antiphospholipid antibodies in healthy populations (lupus anticoagulant: 1.2%-3.8%; anticardiolipin: 1.0%-5.6%).42 When patients with SLE, selected for not having had thrombosis, were evaluated, 13 (6.3%) of 206 patients were positive for anti-A2 antibodies. This value did not differ significantly from healthy controls (P = .07). Anti-A2 antibody prevalence by IgG or IgM isotype was 3.4% and 2.9%, respectively, in SLE patients, and 1.4% and 0.7%, respectively, in healthy controls. Anti-A2 antibodies were not detected in the group of patients with a history of thrombosis associated with hereditary natural anticoagulant deficiencies or acquired nonimmune risk factors such as cancer, surgery, or bed rest, even at low cut-off titers (> 2 SDs) (Nonimmune Thrombosis). These findings indicate that anti-A2 antibodies do not occur as a consequence of prior, clinically overt thrombosis. In patients with antiphospholipid syndrome–related thrombosis (APS), anti-A2 antibodies (> 3 SDs) were detected in 14 (22.6%) of 62 (11.3% IgG, 12.9% IgM, 2.7% concomitant IgG and IgM), a prevalence significantly greater than that in the nonimmune thrombosis group (0%, P = .017).
Prevalence of anti–A2 IgG and IgM in patients with antiphospholipid syndrome. (A) A2 Index. Sera from healthy controls (Healthy), patients with lupus without thrombosis (SLE), patients with thrombosis related to nonimmune risk factors (Nonimmune Thrombosis), and patients with antiphospholipid syndrome (APS) were analyzed for anti-A2 antibodies by ELISA as described in “Patients, materials, and methods.” For each patient, the A405 value for either IgG or IgM was expressed as a ratio to the mean A405 value for 30 simultaneously evaluated healthy control samples. The sum of the IgG and IgM ratios (A2 index) for each individual patient is represented as a single point. Shown in red are the A2 index values for patients with either IgG or IgM titers that exceed the mean control value by more than 3 SDs. Bars indicate mean values for each group. (B) Prevalence of anti-A2 antibodies (3 SDs) in patient subgroups. For each group, the percentage of patients with an anti–A2 IgG or IgM titer that exceeds the mean control value by more than 3 SDs is shown. Absolute optical density values are 1.16 for IgG and 0.15 for IgM. P values for anti-A2 antibodies in antiphospholipid syndrome (APS), compared with healthy controls (Healthy), patients with systemic lupus erythematosus without thrombosis (SLE), or individuals with nonimmune thrombosis (Thrombosis) were highly significant (P < .001, P < .001, and P < .017, respectively). Antibody prevalence is also shown for APS subgroups as defined by Sapporo Criteria.1
Prevalence of anti–A2 IgG and IgM in patients with antiphospholipid syndrome. (A) A2 Index. Sera from healthy controls (Healthy), patients with lupus without thrombosis (SLE), patients with thrombosis related to nonimmune risk factors (Nonimmune Thrombosis), and patients with antiphospholipid syndrome (APS) were analyzed for anti-A2 antibodies by ELISA as described in “Patients, materials, and methods.” For each patient, the A405 value for either IgG or IgM was expressed as a ratio to the mean A405 value for 30 simultaneously evaluated healthy control samples. The sum of the IgG and IgM ratios (A2 index) for each individual patient is represented as a single point. Shown in red are the A2 index values for patients with either IgG or IgM titers that exceed the mean control value by more than 3 SDs. Bars indicate mean values for each group. (B) Prevalence of anti-A2 antibodies (3 SDs) in patient subgroups. For each group, the percentage of patients with an anti–A2 IgG or IgM titer that exceeds the mean control value by more than 3 SDs is shown. Absolute optical density values are 1.16 for IgG and 0.15 for IgM. P values for anti-A2 antibodies in antiphospholipid syndrome (APS), compared with healthy controls (Healthy), patients with systemic lupus erythematosus without thrombosis (SLE), or individuals with nonimmune thrombosis (Thrombosis) were highly significant (P < .001, P < .001, and P < .017, respectively). Antibody prevalence is also shown for APS subgroups as defined by Sapporo Criteria.1
Overall, the prevalence of anti-A2 antibodies was highest in the patients with APS. When subgroups of patients with antiphospholipid syndrome were examined (Figure 1B), anti-A2 antibodies were seen more frequently in the 47 (25.5%) patients with definite, rather than in the 15 (13.3%) with probable, APS. Anti-A2 antibodies were detected in 7 (17.5%) of 40 patients with venous, 5 (29.4%) of 17 with arterial, and 2 (40.0%) of 5 with mixed thrombosis (P = .35), and were present at similar frequencies in the 31 (19.4%) patients with primary and the 31 (25.8%) patients with secondary syndrome, those in latter group all having SLE. Higher prevalence of anti-A2 antibodies was observed in patients with lupus associated with antiphospholipid syndrome, compared with lupus without thrombosis (25.8% vs 6.3%, P < .001). These data suggest that these antibodies may be pathogenically involved in the prothrombotic diathesis of systemic lupus.
Relation of anti–annexin 2 antibodies to other antiphospholipid antibodies and to pregnancy morbidity
In patients with SLE and APS, anti-A2 antibodies were found to be present either alone or concomitantly with other antiphospholipid antibodies. Of all patients studied, we found 11 who were positive for anti-A2 antibodies (> 3 SDs), yet negative for anti-β2GPI antibodies (> 2 SDs). Four of these patients, belonging to the SLE group, were also free of anticardiolipin antibodies and lupus anticoagulants. These data indicate that anti-A2 antibodies may occur independently of anti-β2GPI antibodies.
The prevalence of the different antiphospholipid antibodies can be compared in 178 individuals from the group of patients with SLE selected for not having had thrombosis. In single serum sample determinations, the prevalence of positive titers for anticardiolipin antibodies was 29.2% (IgG and IgM, > 2 SDs); for lupus anticoagulant, 14.6%; and for anti-β2GPI, 4.5% (> 3 SDs). The prevalence of anti-A2 antibodies in the same serum sample from these 178 patients with SLE was 13.6% using a cutoff of > 2 SDs and 6.1% using a cutoff of > 3 SDs. Therefore, in patients with SLE without thrombosis, anti-A2 was as common as either lupus anticoagulant or anti-β2GPI antibodies.
In the group of patients with antiphospholipid syndrome, the prevalence of the different antibodies cannot be directly compared, because, by definition for study entry, patients had to be persistently positive for anticardiolipin antibodies and or lupus anticoagulants. In this APS group, anti-A2 antibodies were determined once and found to be positive in 40.3% using a cutoff of > 2 SDs and 22.6% using a cutoff of > 3 SDs of patients. The prevalence of at least one positive titer of anticardiolipin (95.2%, 2 SDs), lupus anticoagulant (67.3%), and anti-β 2GPI antibodies (71.4%, > 3 SDs) was high, as determined in a given patient an average of 5.9, 2.0, and 6.1 times, respectively. Of the APS patients who were positive for anti-β2GPI antibodies in at least one determination, 69.5% did not have anti-A2 antibodies (> 3 SDs). In the group with nonimmune thrombosis, all single serum sample determinations for antiphospholipid antibodies were negative.
To determine whether anti-A2 antibodies are associated with obstetric morbidity, we studied 2 patient groups. Among both premenopausal and postmenopausal patients with APS, obstetric histories were available on 38 women. Anti-A2 antibodies were present in 37% of women with a remote (2- to 20-year) history of fetal loss and in 36% without fetal loss. Of all premenopausal women from the SLE group, 61.7% had been pregnant at least once, and 42% of these had a history of one or more spontaneous abortions. The 4 patients who had anti-A2 antibodies have not become pregnant. These latter patients are part of a prospectively followed cohort of women that will help determine whether anti–annexin 2 antibodies correlate with pregnancy-associated morbidity.
Evaluation of plasma fibrinolytic integrity
Circulating components of the fibrinolytic system include plasminogen, fibrinogen, tPA, urokinase (uPA), plasminogen activator inhibitor-1 (PAI-1), and alpha 2-plasmin inhibitor (α2-PI).22 To evaluate plasma fibrinolytic integrity, we conducted plasma clot lysis assays.39 Plasma samples from 7 patients with anti-A2 antibodies (4 IgG and 3 IgM) revealed an average half maximal lysis time that was comparable with that of healthy donor plasmas lacking anti-A2 antibodies (6.8 ± 1.7 vs 8.9 ± 2.1 minutes; mean ± SE). These data indicate that plasma components of the fibrinolytic system are not functionally impaired by antibodies to A2.
Anti–A2 IgG binding specificity
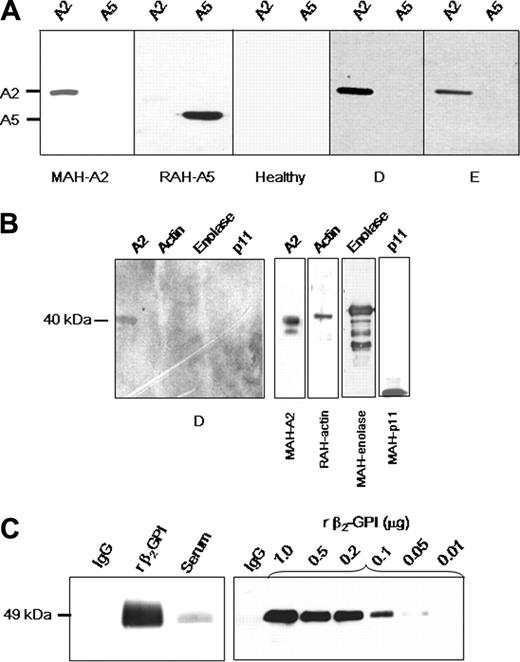
Patient IgG fractions were analyzed by immunoblot for the ability to recognize A2 (Figure 2A). Mouse anti–human A2 and rabbit anti–human A5 were used as positive controls. Nine of 9 patient IgG samples that were positive for recombinant A2 reactivity by ELISA recognized recombinant A2, but not annexin 5. These findings indicate that antibodies detected by ELISA are indeed directed against A2. IgG from healthy individuals who were negative by ELISA failed to bind to A2 in 4 of 4 cases. Anti-A2–containing IgG from 4 of 4 patients did not react to proteins in the 11-kDa range in polyacrylamide gels loaded with placental A2. This suggests that reactivity of these antibodies is directed against A2 rather than p11, the A2 heterotetramer light chain. Moreover, anti-A2–containing IgG from 2 different SLE patients did not bind to purified enolase, actin, p11, or other proposed plasminogen binding proteins (Figure 2B).
Further immunoblot analyses revealed that IgG purified from patient samples did not contain detectable β2GPI (Figure 2C). To determine the sensitivity of the assay for β2GPI, immunoblots of patient IgG (1 μg) and progressive dilutions of β2GPI (1000 to 10 ng/lane) were probed with goat anti–human β2GPI IgG. β2GPI was not detected in 1-μg per lane of patient IgG; the lower limit of β2GPI detection in this assay was 50 ng/lane. These data suggest that binding of anti–A2 IgG to purified A2 does not require the presence of β2GPI, indicating that anti–A2 IgG is likely to function independently of β2GPI. An immunoblot screen for other antifibrinolytic antibodies in patient sera15 revealed the presence of noninhibitory anti-tPA antibodies in 2 of 10 patients tested (not shown).
Activation of endothelial cells by anti–annexin 2 IgG
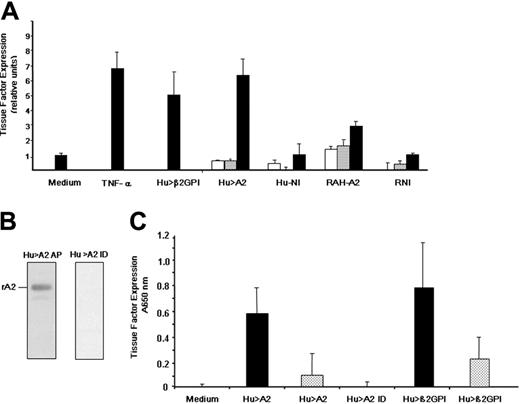
Antiphospholipid antibodies directed against β2GPI are thought to induce thrombosis, in part, by up-regulating endothelial cell tissue factor,40,43 the initiator of the coagulation cascade. The ability of murine anti–A2 IgG to enhance expression of E-selectin, as well as cell adhesion molecule (CAM)–dependent adhesion of monocytes to endothelial cells, has recently been demonstrated.44,45 However, the effect of anti-A2 antibodies on tissue factor expression by endothelial cells has not been reported. We found that tissue factor increased upon exposure to 2 known endothelial cell activators, tumor necrosis factor α (TNF-α) and patient anti-β2GPI IgG (Hu >β2GPI) by 6.9-fold ± 1.07-fold SE and 5.1-fold ± 1.4-fold SE, respectively (Figure 3A). Patient anti-A2 antibodies (Hu > A2), which were free of lupus anticoagulant, anticardiolipin, and anti-β2GPI antibodies, enhanced tissue factor expression in a concentration-dependent manner (6.4-fold ± 1.24-fold SE, at 200 μg/mL), comparable with that of anti-β2GPI. Rabbit polyclonal IgG raised against the tail domain of annexin 2 (RAH-A2) were also able to enhance tissue factor expression (2.95-fold ± 0.29-fold SE, at 200 μg/mL). However, neither patient IgG lacking anti–annexin 2 antibodies (Hu-NI) nor rabbit nonimmune IgG (RNI) up-regulated tissue factor expression. All IgG samples were free of endotoxin as assessed by the limulus amebocyte assay. Finally, IgG positive for anti-A2 from a patient lacking LA, anti-β2GPI, and ACL antibodies was applied to an A2-sepharose immunoaffinity column. Anti-A2 antibody affinity purification (Hu > A2 AP) and immunodepletion (Hu > A2 ID) were verified by immunoblot analysis (Figure 3B). While either whole anti–A2 IgG (Hu > A2) or anti-β2GPI–positive patient IgG (Hu > β2GPI) induced tissue factor expression on HUVECs in a dose-dependent manner, anti-A2–immunodepleted IgG (Hu > A2 ID) failed to do so (Figure 3C). Together, these data indicate that anti-β2GPI or anti–annexin 2 antibodies are approximately equally effective in inducing a procoagulant endothelial cell phenotype.
Characterization of anti–A2 IgG. (A) Specificity of anti–A2 IgG detected by ELISA. Lanes were loaded with either recombinant A2 (A2, 1 μg) or recombinant annexin 5 (A5, 1 μg), and IgG reactivity was analyzed by immunoblot. Positive controls were mouse monoclonal anti–human A2 IgG (MAH-A2) and rabbit polyclonal anti–human A5 (RAH-A5), respectively. Reactivity of IgG from an individual lacking anti-A2 antibodies (Healthy), and from 2 of 9 patients (D-E) positive for anti–A2 IgG is shown. Blots were developed as described in “Patients, materials, and methods” with secondary HRP-conjugated IgG. Shown are images grouped from different gels. (B) Assay of reactivity of anti-A2–containing IgG samples to other proposed plasminogen-binding proteins. SDS polyacrylamide gels were loaded with A2, actin, enolase, or p11 (1 μg/lane). Shown is reactivity of a representative patient anti-A2–containing IgG sample (patient D). Specific antibodies against the purified proteins of interest (mouse anti–human A2, rabbit anti–human actin, rabbit anti–human enolase, or mouse anti–human p11) served as positive controls (right panels). (C) Role of β2GPI. Assay of patient IgG for the presence of β2GPI (left panel). SDS polyacrylamide gels (10%) were loaded with patient purified recombinant IgG, β2GPI, or patient serum (1 μg/lane), transferred to nitrocellulose membranes, and reacted with goat anti–human β2GPI antibodies. Results indicate no detectable β2GPI in the lane containing purified IgG. Determination of assay sensitivity for β2GPI (right panel). Patient IgG (1 μg) and progressive dilutions of β2GPI (10 to 1000 ng/lane) were loaded in the same manner onto 10% SDS gels, blotted to nitrocellulose membranes, and probed with goat anti–human β2GPI antibodies. The assay was sensitive to 50 ng per lane of β2GPI. All membranes were incubated with secondary HRP-conjugated anti–goat IgG antibodies and developed.
Characterization of anti–A2 IgG. (A) Specificity of anti–A2 IgG detected by ELISA. Lanes were loaded with either recombinant A2 (A2, 1 μg) or recombinant annexin 5 (A5, 1 μg), and IgG reactivity was analyzed by immunoblot. Positive controls were mouse monoclonal anti–human A2 IgG (MAH-A2) and rabbit polyclonal anti–human A5 (RAH-A5), respectively. Reactivity of IgG from an individual lacking anti-A2 antibodies (Healthy), and from 2 of 9 patients (D-E) positive for anti–A2 IgG is shown. Blots were developed as described in “Patients, materials, and methods” with secondary HRP-conjugated IgG. Shown are images grouped from different gels. (B) Assay of reactivity of anti-A2–containing IgG samples to other proposed plasminogen-binding proteins. SDS polyacrylamide gels were loaded with A2, actin, enolase, or p11 (1 μg/lane). Shown is reactivity of a representative patient anti-A2–containing IgG sample (patient D). Specific antibodies against the purified proteins of interest (mouse anti–human A2, rabbit anti–human actin, rabbit anti–human enolase, or mouse anti–human p11) served as positive controls (right panels). (C) Role of β2GPI. Assay of patient IgG for the presence of β2GPI (left panel). SDS polyacrylamide gels (10%) were loaded with patient purified recombinant IgG, β2GPI, or patient serum (1 μg/lane), transferred to nitrocellulose membranes, and reacted with goat anti–human β2GPI antibodies. Results indicate no detectable β2GPI in the lane containing purified IgG. Determination of assay sensitivity for β2GPI (right panel). Patient IgG (1 μg) and progressive dilutions of β2GPI (10 to 1000 ng/lane) were loaded in the same manner onto 10% SDS gels, blotted to nitrocellulose membranes, and probed with goat anti–human β2GPI antibodies. The assay was sensitive to 50 ng per lane of β2GPI. All membranes were incubated with secondary HRP-conjugated anti–goat IgG antibodies and developed.
Effect of anti–annexin 2 IgG on tissue plasminogen activator (tPA)–dependent plasmin generation
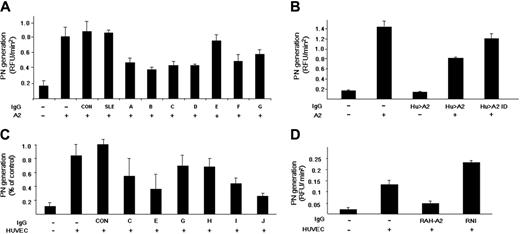
Placental A2 significantly enhances the catalytic efficiency (kcat/Km) of tPA-induced plasmin generation in a purified protein assay system.25 IgG from 3 healthy individuals (CON) and 2 patients with SLE without anti-A2 antibodies (SLE), had no effect on plasmin generation (Figure 4A). On the other hand, 7 of 7 anti-A2 patient IgG samples tested containing anti-A2 antibodies (A-G) inhibited A2-dependent, tPA-induced plasmin generation by 18.7% to 70.7%. We found, furthermore, that patient anti-A2 antibodies present in whole IgG fractions had no effect on plasmin generation in the absence of A2, while this same IgG significantly inhibited plasmin generation in the presence of A2 (Figure 4B). When the IgG was affinity depleted of anti-A2 antibodies, no significant inhibitory activity was observed (Hu > A2 ID). These data indicate that anti-A2 antibodies from patients with APS can block the ability of A2 to enhance tPA-dependent plasminogen activation in the absence of other plasma- or cell-related factors. In addition, we found no effect of anti-A2 antibodies on plasma fibrinolytic activity.
Induction of endothelial cell tissue factor expression. (A) Effect of human anti-β2GPI, human anti–A2 IgG, and rabbit anti–A2 IgG. As described in “Patients, materials, and methods,” human umbilical vein endothelial cells were incubated with either medium alone (Medium), tumor necrosis factor α (TNF-α, 10 ng/mL), or patient IgG containing anti-β2GPI (Hu > β2GPI, 200 μg/mL). Cells were also incubated with increasing concentrations of patient anti–A2 IgG that was antiphospholipid antibody- and endotoxin-free (Hu > A2), patient IgG lacking anti-A2 antiphospholipid antibodies (Hu-NI), rabbit anti–human A2 IgG (RAH-A2), or rabbit preimmune IgG (RNI; 0.2, 20, and 200 μg/mL; white, gray, and black bars, respectively). Bars represent normalized mean values (± SEM, n = 2-4). (B) Immunodepletion of patient IgG. As described in “Patients, materials, and methods,” whole IgG from a representative patient, who was positive for anti-A2 antibodies but lacked anti-β2GPI and ACL antibodies, was applied to an A2-sepharose immunoaffinity column, eluted with glycine buffer (pH 2.8), and immediately neutralized with Tris base (pH 8.0). Both affinity-purified IgG (Hu > A2 AP) and immunodepleted IgG (Hu > A2 ID) were extensively dialyzed against PBS (pH 7.2) and tested by immunoblot analysis against rA2 (250 ng/lane). (C) Effect of A2 immunodepletion on tissue factor induction. HUVECs were incubated alone (Medium), with whole anti-A2–containing IgG (Hu > A2) at 200 μg/mL (▪) or 20 μg/mL (▦), with A2-immunodepleted IgG from the same patient (Hu > A2 ID) at 200 μg/mL, or with human anti-β2GPI at 200 μg/mL (▪) or 20 μg/mL (▦). Shown are mean A650 nm values for (± SEM, n = 3).
Induction of endothelial cell tissue factor expression. (A) Effect of human anti-β2GPI, human anti–A2 IgG, and rabbit anti–A2 IgG. As described in “Patients, materials, and methods,” human umbilical vein endothelial cells were incubated with either medium alone (Medium), tumor necrosis factor α (TNF-α, 10 ng/mL), or patient IgG containing anti-β2GPI (Hu > β2GPI, 200 μg/mL). Cells were also incubated with increasing concentrations of patient anti–A2 IgG that was antiphospholipid antibody- and endotoxin-free (Hu > A2), patient IgG lacking anti-A2 antiphospholipid antibodies (Hu-NI), rabbit anti–human A2 IgG (RAH-A2), or rabbit preimmune IgG (RNI; 0.2, 20, and 200 μg/mL; white, gray, and black bars, respectively). Bars represent normalized mean values (± SEM, n = 2-4). (B) Immunodepletion of patient IgG. As described in “Patients, materials, and methods,” whole IgG from a representative patient, who was positive for anti-A2 antibodies but lacked anti-β2GPI and ACL antibodies, was applied to an A2-sepharose immunoaffinity column, eluted with glycine buffer (pH 2.8), and immediately neutralized with Tris base (pH 8.0). Both affinity-purified IgG (Hu > A2 AP) and immunodepleted IgG (Hu > A2 ID) were extensively dialyzed against PBS (pH 7.2) and tested by immunoblot analysis against rA2 (250 ng/lane). (C) Effect of A2 immunodepletion on tissue factor induction. HUVECs were incubated alone (Medium), with whole anti-A2–containing IgG (Hu > A2) at 200 μg/mL (▪) or 20 μg/mL (▦), with A2-immunodepleted IgG from the same patient (Hu > A2 ID) at 200 μg/mL, or with human anti-β2GPI at 200 μg/mL (▪) or 20 μg/mL (▦). Shown are mean A650 nm values for (± SEM, n = 3).
Inhibition of annexin 2 enhancement of plasmin generation by anti–annexin 2 IgG. (A) Plasminogen activation by tPA in a purified protein assay. Initial rates of plasmin generation were calculated as RFU/min2 . Results are shown for 3 healthy controls (CON) and 2 SLE patients lacking anti-A2 antibodies (SLE). The effect of individual patient anti-A2–containing IgG on plasmin generation is depicted (A-G). Shown are mean values ± SE (n = 3-10). (B) Effect of anti-A2–depleted IgG on plasmin generation. Plasmin generation was recorded in the presence of A2 alone, patient anti-A2–containing IgG alone (Hu > A2 IgG), both A2 and Hu > A2 IgG, and A2 in the presence of IgG depleted of anti-A2 antibodies (Hu > A2 ID). (C) Effect of patient anti–A2 IgG on HUVEC-based plasmin generation. Human umbilical vein endothelial cells (HUVECs) were preincubated with IgG lacking reactivity against A2 from 2 APS patient samples containing anti-β2GPI and anticardiolipin antibodies (CON), as well as with 6 patient IgG samples containing anti-A2 (C,E,G-J). Results are expressed in relation to the mean value for patient controls (± SEM, n = 3). (D) Effect of rabbit anti–human A2 on tPA-dependent plasmin generation. Initial rates of tPA-induced plasmin generation were recorded in the presence of HUVECs preincubated with medium alone or with rabbit polyclonal anti–human A2 (RAH-A2) or rabbit nonimmune IgG (RNI).
Inhibition of annexin 2 enhancement of plasmin generation by anti–annexin 2 IgG. (A) Plasminogen activation by tPA in a purified protein assay. Initial rates of plasmin generation were calculated as RFU/min2 . Results are shown for 3 healthy controls (CON) and 2 SLE patients lacking anti-A2 antibodies (SLE). The effect of individual patient anti-A2–containing IgG on plasmin generation is depicted (A-G). Shown are mean values ± SE (n = 3-10). (B) Effect of anti-A2–depleted IgG on plasmin generation. Plasmin generation was recorded in the presence of A2 alone, patient anti-A2–containing IgG alone (Hu > A2 IgG), both A2 and Hu > A2 IgG, and A2 in the presence of IgG depleted of anti-A2 antibodies (Hu > A2 ID). (C) Effect of patient anti–A2 IgG on HUVEC-based plasmin generation. Human umbilical vein endothelial cells (HUVECs) were preincubated with IgG lacking reactivity against A2 from 2 APS patient samples containing anti-β2GPI and anticardiolipin antibodies (CON), as well as with 6 patient IgG samples containing anti-A2 (C,E,G-J). Results are expressed in relation to the mean value for patient controls (± SEM, n = 3). (D) Effect of rabbit anti–human A2 on tPA-dependent plasmin generation. Initial rates of tPA-induced plasmin generation were recorded in the presence of HUVECs preincubated with medium alone or with rabbit polyclonal anti–human A2 (RAH-A2) or rabbit nonimmune IgG (RNI).
Plasmin generation is enhanced by A2-expressing endothelial cells and monocytes.41,46 To test the effect of patient anti–A2 IgG on cell-based fibrinolytic activity, we studied plasmin activation in the presence of HUVECs (Figure 4C). We found a mean 6.1-fold increase in the initial rate of plasmin generation when tPA and plasminogen were incubated with human umbilical vein endothelial cell monolayers, compared with cell-free controls. IgG from 2 APS patients who were anticardiolipin positive, anti-β2GPI positive, and anti-A2 negative (Figure 4C, CON) had no effect on endothelial cell surface tPA-dependent plasmin generation, while 6 of 6 patient-derived anti-A2–containing IgG samples inhibited plasmin generation by 35% to 83% (Figure 4C [C, E, G, H, I, J]). Furthermore, most of the increase in plasmin generation was explained by membrane-associated A2, because polyclonal rabbit IgG against human A2 (RAH-A2) blocked plasmin generation by 64.5%, while rabbit non–immune IgG (RNI) did not (Figure 4D). These data indicate that patient anti–A2 IgG inhibits fibrinolysis by blocking endothelial cell surface A2.
Discussion
Antiphospholipid syndrome is a systemic prothrombotic state promoted by diverse, often coexisting antibodies. Arterial and venous thrombosis at any vascular site may occur.15 Thromboses are characteristically recurrent and are a cause of significant morbidity.7 In promoting thrombosis, antiphospholipid antibodies are believed to both interfere with normal cell surface hemostatic mechanisms10,13 and to induce a proinflammatory and procoagulant cellular phenotype.14-18
In the present work, we determined the prevalence of antibodies against A2 in a population of 434 adult patients with a spectrum of autoimmune and nonautoimmune thrombotic states. We report for the first time that anti-A2 antibodies are significantly associated with thrombosis in the setting of APS. Association of antibodies against A2 with thrombosis was seen in both primary and lupus-associated APS forms, but not with thrombosis presenting in the setting of nonimmune risk factors. We also found that human as well as rabbit anti–A2 IgG induced endothelial cell expression of tissue factor, the physiologic initiator of blood coagulation. In addition, patient antibodies directed against A2 consistently blocked tPA-induced plasminogen activation both in a purified β2GPI-free protein assay as well as on human umbilical vein endothelial cells. These new data indicate that anti-A2 antibodies are highly likely to play a pathogenic role in thrombosis associated with APS.
Annexin 2, a receptor for fibrinolytic activation localized on the cell surface of endothelial cells, monocytes, and syncytiotrophoblasts, 25,44 is implicated in the pathogenesis of APS in several ways. First, we have found that antibodies to A2 can activate human endothelial cells inducing overexpression of tissue factor. In APS, endothelial cell activation is evidenced by increased circulating levels of tissue factor47 and endothelial cell microparticles.48 We found that polyclonal patient anti-A2 antibodies or anti-A2 antibodies raised in rabbits against the tPA binding domain of A2 induced endothelial cell tissue factor expression to the same degree as that observed by patient anti-β2GPI antibodies. Furthermore, human anti-A2 antibody–induced tissue factor expression occurred in the absence of antibodies directed against β2GPI. Based on our results and those of others,45,49 it appears that there are anti-β2GPI–dependent and anti-β2GPI–independent mechanisms for induction of endothelial cell activation as detected by expression of cell adhesion molecules (CAMs), E-selectin, or tissue factor.
Second, A2 serves as a coreceptor for both plasminogen and tissue plasminogen activator on the surface of endothelial cells and facilitates the generation of plasmin. The dysregulation of fibrinolytic assembly may lead to atherothrombotic disease.22 We show that anti-A2 from patients consistently inhibited tPA-dependent plasmin-generating activity in assays using both purified placental and recombinant A2, and also inhibited endothelial cell surface plasminogen activation in vitro. Both patient and mouse monoclonal anti–human A2 antibodies produced partial inhibition of fibrinolysis. The variable degree of inhibition is most likely related to individual antibody characteristics, the specifically targeted A2 epitope, the possible presence of multiple epitopes, or the possible presence of alternative endothelial cell plasminogen receptors.
Finally, high-affinity (Kd ∼ 18 nM) binding of β2GPI to human umbilical vein endothelial cells (HUVECs) is mediated by A2.44 By functioning as a receptor for β2GPI, A2 is a target not only for anti-A2 antibodies but also for anti-β2GPI antibodies.44,45 Antiphospholipid antibodies directed against β2GPI have been found to bind to and activate endothelial cells both in vitro and in vivo. Antiphospholipid antibodies directed against β2GPI have been shown to induce up-regulation of tissue factor on endothelial cells in vitro, which can be inhibited by statins.40 Anti-β2GPI antibodies have also been shown to up-regulate tissue factor expression on monocytes.50 The ability of anti-β2GPI antibodies to induce endothelial cell activation requires binding of β2GPI to the cell surface.18,51 When infused into mice, anti-β2GPI antibodies induce endothelial cell activation, fetal wastage, and enhanced injury-induced clot formation.11,21 It was recently demonstrated that murine anti-A2 antibodies directly cause endothelial cell activation of a similar magnitude and time course to that seen with anti-β2GPI antibodies, and that both antibodies require A2 for induction of cell adhesion molecule–dependent monocyte adhesion and expression of E-selectin.45 Moreover, endothelial cell activation induced by either anti-A2 or anti-β2GPI antibodies was blocked by monomeric anti-A2 Fab fragments, suggesting that A2 cross-linking is probably required.45 These data indicate that A2 constitutes a common receptor for antiphospholipid antibody induction of endothelial cell activation.
As has been shown for antiphospholipid antibodies,52,53 anti-A2 antibodies probably predate thrombosis, and are not a consequence of clinically evident vascular damage, since we did not detect them in patients with nonimmune thrombosis. This does not, however, rule out that genetically predisposed individuals may develop antibodies secondary to subclinical endothelial cell challenge from virus particles or bacterial products with sequence homology to β2GPI.54-56
Because A2 is a peripheral membrane protein that does not possess a transmembrane domain, it has been suggested that an “adaptor” protein would act as a partner to signal the cells. Meroni et al49 have shown that anti-β2GPI antibodies up-regulate mRNA expression of proinflammatory mediators through NF-kappaB translocation, activating a signaling cascade comparable with that triggered by the toll-like receptor 4 (TLR-4). This group has proposed that because of the molecular mimicry between β2GPI and viral/bacterial structures, the natural ligands for TLRs, antibodies might cross-link the molecule associated to A2 and TLR-4, eventually triggering signaling.16,57 To date, however, no adaptor molecule that mediates signaling via an annexin has been identified.
Antiphospholipid antibodies, specifically lupus anticoagulants and anticardiolipin antibodies, have been shown to be present in cancer patients. A recent study demonstrates that these antibodies are prevalent in lymphomas (27%), and that their presence enhances the rate of thromboembolic complications (5.1% patients/y) compared with patients without antiphospholipid antibodies (0.75% patients/y).58 Anti-A2 antibodies have been reported to occur in lung cancer patients (33%), yet their relation to thrombotic complications has not been studied.59 Prospective follow-up of individuals with anti-A2 antibodies and cancer is necessary to determine if anti-A2 antibodies also correlate with a higher risk of thrombosis.
Ongoing prospective studies of women with lupus without thrombosis should help elucidate the prognostic value of anti-A2 antibodies in the development of thrombosis and obstetric complications. Our findings strongly suggest a role for anti-A2 antibodies in the recurrent vascular thromboses that characterize antiphospholipid syndrome.
Prepublished online as Blood First Edition Paper, February 21, 2006; DOI 10.1182/blood-2005-07-2636.
Supported by Consejo Nacional de Ciencia y Tecnología (CONACYT) 0914P-M9506, 28733 m/1628 (G.C.-M.); CONACYT 25556-M; Wyeth-Mexico Laboratories (unconditional) (M.d.C.C.); CONACYT 3367-PM (J.S.-G.); the National Institutes of Health grants HL 42493, HL 46403, and HL 67839; and the March of Dimes grant no. 6-FY05-94 (K.A.H.).
Donato Alarcón-Segovia died on December 21, 2004.
An Inside Blood analysis of this article appears at the front of this issue.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
We thank Dr Emil Lev for expert technical assistance.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal