Newly diagnosed patients with acute graft-versus-host disease (GvHD, grades I-IV; n = 211) were given 6-methylprednisolone (6MPred) 2 mg/kg per day for 5 consecutive days; 150 patients (71%) tapered 6MPred on day +5 and were considered responders; 61 patients (29%) could not taper their steroid dose and were considered nonresponders. The cumulative incidence of transplant-related mortality (TRM) for responders and nonresponders is, respectively, 27% and 49% (P = .009), and the 5-year survival is 53% and 35% (P = .007). Nonresponders on day +5 (n = 61) were randomized to receive 6MPred 5 mg/kg per day for 10 days alone (n = 34) or in combination with rabbit anti–thymocyte globulin (ATG, 6.25 mg/kg in 10 days; n = 27). The 2 groups were balanced for clinical and GvHD characteristics. One month after randomization, 26% had a complete response; 23%, a partial response; 33%, stable GvHD; 10%, worsened; and 8%, died. There was no significant difference in response, TRM, and survival between the non-ATG and ATG group. In conclusion, 5 days of prednisolone as first-line therapy of acute GvHD identifies patients with different risk of TRM, and second-line therapy with a combination of 6MPred + ATG does not improve patient outcome, compared with 6MPred alone.
Introduction
Acute graft-versus-host disease (GvHD) remains a major problem following allogeneic hematopoietic stem cell transplantation (HSCT) and a significant cause of death, especially in patients with advanced disease who have received an allograft from an unrelated donor.1 Treatment of acute GvHD is difficult because steroids and other immunosuppressants increase the likelihood of lethal infections, whether or not GvHD has been controlled.2 In one large study on 453 patients given prednisone 60 mg/m2 for 2 weeks as first-line therapy, followed by an 8-week taper, an overall improvement was observed in 55% of the patients, with durable (≥ 28 days) complete responses in 35%.3 The probability of survival at 1 year after initiation of therapy was 53%; favorable predictors of survival were younger age of patients, HLA-identical sibling donors, and GvHD prophylaxis other than ex vivo T-cell depletion.3 Whether a higher dose of corticosteroids would be more effective was assessed in a prospective trial of the Italian Cooperative Transplant Group (GITMO); 95 recipients of an HLA-identical bone marrow transplant (BMT) were randomized to receive intravenous 6-methylprednisolone (6MPred) 2 mg/kg per day for 5 days or 10 mg/kg per day for 5 days as initial treatment of GvHD.4 The study showed that a higher dose of 6MPred did not improve response rate or survival, nor did it reduce transplant-related mortality (TRM). One additional observation was that patients receiving 5 days of 6MPred 2 mg/kg, and who could taper their 6MPred dose on day +5 as per protocol, had a significantly lower TRM (23%) compared with patients who could not taper their dose (TRM = 46%).4 This observation led us to identify responders as patients who can follow the 6MPred tapering schedule.
We then designed a second study that would keep the low dose of 6MPred up front, and we could then test whether intensified second-line treatment would reduce TRM. In this trial, all patients received 2 mg/kg per day for 5 days at diagnosis of acute GvHD (grades I-IV); responders were followed for events and survival, whereas nonresponders were randomized for second-line 6MPred treatment with or without anti–thymocyte globulin (ATG). We are now reporting the outcome of 211 patients who entered this multicenter trial.
Patients, materials, and methods
Study design
This is a prospective multicenter trial of the Gruppo Italiano Trapianti di Midollo Osseo (GITMO). Approval for these studies was obtained from the ethics committee of the participating transplant centers. Informed consent was provided according to the Declaration of Helsinki, approved by the ethics committee, and signed by the patients.
First-line therapy
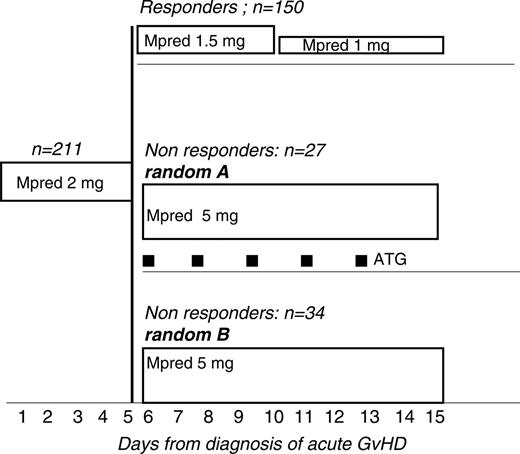
Eligibility requirements were as follows: patients with acute GvHD grades I to IV, undergoing unmanipulated HLA-identical sibling or MUD HSCT, aged 1 to 60 years, any diagnosis, receiving GvHD prophylaxis with cyclosporine (CsA, 1-3 mg/kg per day) or CsA + methotrexate (MTX), with therapeutic plasma levels of CsA (200-400 ng/mL). Patients diagnosed with aGvHD were treated, within 48 hours, with 6MPred at the dose of 2 mg/kg per day for 5 consecutive days (Figure 1). On day +5, patients showing initial improvement of acute GvHD were supposed to have their 6MPred reduced to 1.5 mg/kg per day from day 6 to day + 10 and to 1 mg/kg from day + 11 to day + 16. Failure to comply with the reduction of the dose of 6MPred on day +5 was taken as evidence of nonresponse, and patients were thus eligible for second-line therapy. Patients with acute GvHD progressing within day +5 were also eligible for randomization (Figure 1).
Response to first-line therapy
A surrogate criterion was used for GvHD response on day +5, that is, the ability to reduce the GMPred dose as per protocol. Responders were patients tapering their steroid dose, whereas failure to taper the dose was taken as evidence of nonresponse.
Randomization to second-line therapy
Exclusion criteria for randomization were as follows: patients at the end stage of disease; patients undergoing T-cell depletion or CD34+ selection as GvHD prophylaxis; patients who experienced an interval between GvHD diagnosis and randomization of more than 7 days; patients responding on day +5 to 6MPred 2 mg/kg; and patients receiving other experimental drugs for GvHD treatment. Patients were stratified at randomization for age (< or ≥ 20 years), disease status (first remission or CP/more advanced), and donor type (unrelated donors [UDs] or HLA-identical siblings [IDSs]).
Response to second-line therapy
We classified patients according to the response of GvHD. Complete response was defined as complete resolution of all signs of GvHD; partial response, reduction of GvHD to a less severe grading (ie, from grade III to grade II), but still evidence of GvHD; stable GvHD, no change; and worse, progression of GvHD to a more severe grading.
Second-line therapy
The regimen for group A was as follows: 6MPred 5 mg/kg per day (day 6 to day 10; Figure 1), then 2.5 mg/kg from day 16 to day 20, then 1.5 mg/kg from day 21 to day 30, and then 1 mg/kg from day 31 to day 35. The regimen for group B was as follows: 6MPred 5 mg/kg with the addition of rabbit anti–thymocyte globulin (ATG, thymoglobulin; Genzyme, Cambridge, MA), 1.25 mg/kg on days 6, 8, 10, 12, and 14 (total dose, 7.25 mg/kg). ATG was given as a slow (12-18 hours) intravenous infusion, with premedication with antihistamines. CsA was continued in order to maintain therapeutic plasma levels. The tapering of 6MPred in group B was the same as in group A. The design of second-line therapy, with an increase of 6Mpred from 2 mg/kg to 5 mg/kg, was justified by the fact that patients could not be offered the same dose of 6Mpred that they had already failed.
Trial design: all patients with a diagnosis of acute graft-versus-host disease (GvHD) are treated with 6MPred 2 mg/kg per day from day 1 to day 5. Responders have 6MPred tapered to 1.5 mg/kg from day 6 to day 10 and to 1 mg/kg from day 11 to day 15. Nonresponders are randomized to (A) 6MPred 5 mg/kg from day 6 to day 15, or (B) 6MPred 5 mg/kg from day 6 to day 15, and rabbit anti–thymocyte globulin (ATG, thymoglobulin) 1.25 mg/kg on days 6, 8, 10, 12, and 14.
Trial design: all patients with a diagnosis of acute graft-versus-host disease (GvHD) are treated with 6MPred 2 mg/kg per day from day 1 to day 5. Responders have 6MPred tapered to 1.5 mg/kg from day 6 to day 10 and to 1 mg/kg from day 11 to day 15. Nonresponders are randomized to (A) 6MPred 5 mg/kg from day 6 to day 15, or (B) 6MPred 5 mg/kg from day 6 to day 15, and rabbit anti–thymocyte globulin (ATG, thymoglobulin) 1.25 mg/kg on days 6, 8, 10, 12, and 14.
Supportive care
All patients received antifungal and antibacterial prophylaxis and were monitored for cytomegalovirus (CMV) as per institutional protocols. This included, in all centers, preemptive treatment of CMV, based on CMV antigenemia or CMV polymerase chain reaction (PCR), and antifungal prophylaxis with fluconazole from day – 7 to day + 70. Early systemic antifungal treatment based on Aspergillus antigenemia was used in most but not all centers.
Statistical analysis
The NCSS package (NCSS, Keysville, UT) was used for chi-square tables, actuarial survival, cumulative incidence (CI) rates, Student t test, and Mann-Whitney test.
Results
First-line therapy
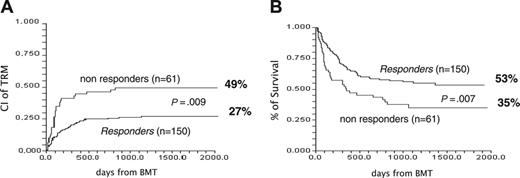
Two-hundred and eleven patients were registered as having acute GvHD grade I+ and were given first-line treatment of 2 mg/kg per day of 6MPred. On day +5, 150 patients (71%) had their dose of 6MPred tapered by 50% as per protocol and were considered responders, whereas 61 patients (29%) were considered nonresponders and were eligible for second-line therapy. We tested for differences at the time of registration between these 2 groups (Table 1). Nonresponders had higher grades of GvHD (P = .008) and a trend for more advanced disease (P = .06). The proportion of responders in patients with GvHD grades I, II, and III was 76%, 56%, and 52%, respectively (P = .01). There was no effect on day +5 response for any of the following: age, donor type, female donor/male recipient, use of radiation, or number of cells infused. The outcome of the 2 groups is outlined in Table 1. Of the responders versus the nonresponders, respectively, 56% versus 36% of patients are alive, 27% versus 49% died of transplant-related complications, and 17% versus 15% died of leukemia-related events. The CI of TRM for responders and nonresponders is, respectively, 27% and 49% (P = .009) (Figure 2A), with an actuarial 5-year survival of 53% and 35% (P = .009) (Figure 2B). The CI of TRM for patients with GvHD grade II+ on day 0 for responders versus nonresponders, is 25% versus 53%, respectively (P = .001). Causes of death in responders and nonresponders were as follows: acute GvHD, 9 and 14, respectively; chronic GvHD, 10 and 4, respectively; infections, 11 and 6, respectively; other transplant-related, 10 and 6, respectively; and leukemia, 26 and 9, respectively. Therefore GvHD (acute + chronic) was the cause of death in 19 (13%) of 150 responders and in 18 (30%) of 61 day +5 nonresponders. The CI of TRM for patients with GvHD grades I, II, and III+ is 28%, 36%, and 55%, respectively (P = .05); relapse-related death, 20%, 14%, and 5%, respectively (P = .1); and survival, 50%, 47%, and 36%, respectively (P = .1).
Clinical characteristics of patients responding or not responding to first-line treatment (univariate analysis)
Characteristic . | Responders . | Nonresponders . | P . |
|---|---|---|---|
| Donor type, no. | |||
| HLA-identical sibling | 80 | 30 | — |
| Unrelated | 70 | 31 | .5 |
| Median donor age, y (range) | 36 (2-73) | 42 (7-66) | .1 |
| Female donor/male recipient, no. (%) | 39 (26) | 10 (16) | .1 |
| Median recipient age, y (range) | 32 (6-66) | 34 (2-64) | .2 |
| Disease | |||
| Early disease, no. (%) | 73 (49) | 21 (35) | .06 |
| Conditioning with TBI, no. (%) | 96 (64) | 39 (66) | .2 |
| No. of cells infused, × 108/kg (range) | 4.6 (0.7-12.2) | 4.6 (6.0-17) | .1 |
| GvHD prophylaxis CsA MTX, no. (%) | 146 (98) | 59 (97) | .8 |
| Grade of GvHD at registration, no. | |||
| I | 91 | 24 | — |
| II | 49 | 27 | — |
| III to IV | 10 | 10 | .008 |
| Interval from Tx to GvHD, d (range) | 16 (7-42) | 17 (8-63) | .1 |
| Alive, no. (%) | 84 (56) | 22 (36) | .02 |
| Follow-up surviving patients, no. (range) | 1176 (129-1930) | 1134 (37-1969) | .8 |
| Follow-up deceased patients, no. (range) | 203 (9-1809) | 101 (15-1056) | .3 |
| Transplant-related deaths, no. (%) | 40 (27) | 30 (49) | .001 |
| Leukemia-related deaths, no. (%) | 26 (17) | 9 (15) | .4 |
Characteristic . | Responders . | Nonresponders . | P . |
|---|---|---|---|
| Donor type, no. | |||
| HLA-identical sibling | 80 | 30 | — |
| Unrelated | 70 | 31 | .5 |
| Median donor age, y (range) | 36 (2-73) | 42 (7-66) | .1 |
| Female donor/male recipient, no. (%) | 39 (26) | 10 (16) | .1 |
| Median recipient age, y (range) | 32 (6-66) | 34 (2-64) | .2 |
| Disease | |||
| Early disease, no. (%) | 73 (49) | 21 (35) | .06 |
| Conditioning with TBI, no. (%) | 96 (64) | 39 (66) | .2 |
| No. of cells infused, × 108/kg (range) | 4.6 (0.7-12.2) | 4.6 (6.0-17) | .1 |
| GvHD prophylaxis CsA MTX, no. (%) | 146 (98) | 59 (97) | .8 |
| Grade of GvHD at registration, no. | |||
| I | 91 | 24 | — |
| II | 49 | 27 | — |
| III to IV | 10 | 10 | .008 |
| Interval from Tx to GvHD, d (range) | 16 (7-42) | 17 (8-63) | .1 |
| Alive, no. (%) | 84 (56) | 22 (36) | .02 |
| Follow-up surviving patients, no. (range) | 1176 (129-1930) | 1134 (37-1969) | .8 |
| Follow-up deceased patients, no. (range) | 203 (9-1809) | 101 (15-1056) | .3 |
| Transplant-related deaths, no. (%) | 40 (27) | 30 (49) | .001 |
| Leukemia-related deaths, no. (%) | 26 (17) | 9 (15) | .4 |
For responders, n = 150 (71%); for nonresponders, n = 61 (29%).
Tx indicates transplantation; —, not applicable.
Effect of response to first-line therapy on outcome. (A) CI of TRM of day +5 responders (27%) and day +5 nonresponders (49%) (P = .009). (B) Actuarial survival of responders (53%) and nonresponders (35%) (P = .007).
Effect of response to first-line therapy on outcome. (A) CI of TRM of day +5 responders (27%) and day +5 nonresponders (49%) (P = .009). (B) Actuarial survival of responders (53%) and nonresponders (35%) (P = .007).
In multivariate COX analysis, predictors of TRM for the entire patient population (n = 211) were as follows: age older than median (> 33 years) (RR, 2.27; P = .003); acute GvHD at first-line treatment grade II+ (RR, 2.0; P = .01); lack of response on day +5 (RR, 1.76; P = .02); advanced disease phase (RR, 1.87; P = .01); and transplant from an unrelated donor (RR, 1.64; P = .05). Patients with no, one, or more than one unfavorable predictor on day +5 were scored as low risk (n = 30), intermediate risk (n = 95), and high risk (n = 86), respectively; the CI of TRM in the 3 groups is 13%, 24%, and 50% (P = .001; Figure 3). By using more than one variable, it may be possible to better predict the outcome also of day +5 responders. Indeed, of the 150 responders, 30 (20%) were low risk, 88 (59%) were intermediate risk, and 32 (21%) were high risk; conversely, of the 61 nonresponders, 7 (11%) were intermediate risk.
Identifying patients in different risk groups after first-line therapy. CI of TRM in 211 patients stratified as low, intermediate, and high risk on day +5 of treatment for acute GvHD. See multivariate COX analysis in “First-line therapy.”
Identifying patients in different risk groups after first-line therapy. CI of TRM in 211 patients stratified as low, intermediate, and high risk on day +5 of treatment for acute GvHD. See multivariate COX analysis in “First-line therapy.”
Second-line treatment
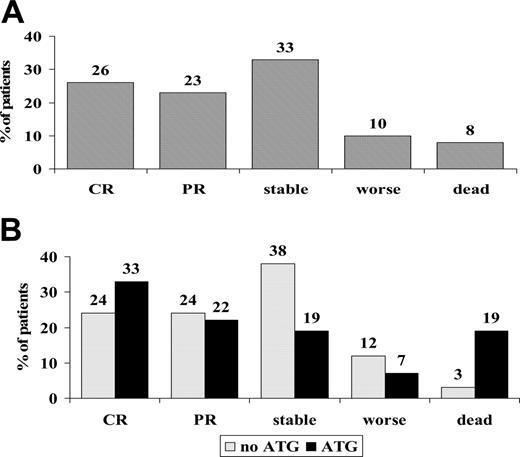
Sixty-one nonresponders were randomized on day +5 to receive 6MPred 5 mg/kg per day for 10 days, alone (n = 34) or in combination with rabbit anti–thymocyte globulin (ATG, 6.25 mg/kg in 10 days; n = 27). The 2 groups were balanced for clinical and GvHD characteristics (Table 2). One month after randomization, 26% had a complete response; 23%, partial response; 33%, stable GvHD; 10%, worsened; and 8%, died (Figure 4A). There was no significant difference in responses between non-ATG and ATG patients, as outlined in Figure 4B. The actuarial survival of non-ATG and ATG patients was 36% and 34%, respectively (P = .63; Figure 5A), and the CI of TRM was 41% and 59% (P = .17; Figure 5B). Causes of death in the non-ATG group were as follows: acute GvHD (n = 8), chronic GvHD (n = 3), cerebral hemorrhage (n = 1), infections (n = 1), multiorgan failure (MOF; n = 1), and relapse (n = 7). In the ATG group, the causes of death were as follows: acute GvHD (n = 6), chronic GvHD (n = 1), Epstein-Barr virus (EBV) lymphoma (n = 1), infections (n = 4), MOF (n = 2), hemorrhagic cystitis (n = 1), suicide (n = 1), and relapse (n = 2). Infectious complications in the ATG group included EBV (n = 1), CMV (n = 4), pneumonia (n = 1), and sepsis (n = 1). The median time from transplantation to TRM was similar in the non-ATG and the ATG groups (84 and 100 days, respectively). Platelet counts were also followed in the non-ATG and the ATG groups: median platelet counts at time of randomization were 77 × 109/L and 57 × 109/L, respectively; on day +10, 76 × 109/L and 63 × 109/L, respectively; on day +30, 49 × 109/L and 59 × 109/L, respectively; and on day +180, 115 × 109/L and 154 × 109/L, respectively. When patients were stratified for GvHD severity at the time of randomization, TRM was 36% for GvHD grade I (n = 11), 43% for grade II (n = 35), and 73% for grades III to IV (n = 15) (P = .09). TRM was 29% for patients without gut or liver involvement (n = 21), 54% for patients with either gut or liver involvement (n = 28), and 75% for patients with both gut and liver GvHD (n = 12) (P = .03). When only gut/liver GvHD patients were considered, TRM was again higher in patients receiving than those not receiving ATG (76% and 47%, respectively; P = .06).
Characteristics of patients randomized to second-line therapy
Characteristic . | Non-ATG . | ATG . | P . |
|---|---|---|---|
| Recipient sex, no. male/no. female | 17/17 | 15/12 | .6 |
| Median recipient age, y (range) | 33 (2-64) | 36 (2-63) | .4 |
| Donor type, no. | |||
| HLA-identical sibling | 16 | 14 | — |
| Unrelated | 18 | 13 | .7 |
| Diagnosis, no. | |||
| AML + ALL | 18 | 12 | — |
| CML | 12 | 7 | — |
| LY/MM | 1 | 4 | — |
| Other | 3 | 4 | .3 |
| Advanced phase | 22 (67%) | 16 (59%) | .4 |
| 14 or fewer days of first-line treatment, no. | 7 | 8 | .4 |
| Timing of GvHD, ≤ 14 d after transplantation | 7 | 7 | .4 |
| GvHD grade at randomization, no. | |||
| I | 7 | 4 | — |
| II | 19 | 16 | — |
| III | 8 | 7 | .5 |
| Skin GvHD day 5, no. | 33* | 26 | .3 |
| Liver GvHD day 5, no. | 14 | 7 | .2 |
| Gut GvHD day 5, no. | 17 | 14 | .9 |
| Response at day +30, no. (%) | |||
| Resolution of GvHD | 8 (24) | 9 (33) | — |
| Partial resolution | 8 (24) | 6 (22) | — |
| Stable GvHD | 13 (38) | 5 (19) | — |
| Worsened | 4 (12) | 2 (7) | — |
| Dead | 1 (3) | 5 (19) | — |
| Alive | 13 (38) | 9 (33) | .9 |
| Dead TRM | 14 (41) | 16 (59) | .2 |
| Dead relapse | 7 (21) | 2 (8) | .1 |
Characteristic . | Non-ATG . | ATG . | P . |
|---|---|---|---|
| Recipient sex, no. male/no. female | 17/17 | 15/12 | .6 |
| Median recipient age, y (range) | 33 (2-64) | 36 (2-63) | .4 |
| Donor type, no. | |||
| HLA-identical sibling | 16 | 14 | — |
| Unrelated | 18 | 13 | .7 |
| Diagnosis, no. | |||
| AML + ALL | 18 | 12 | — |
| CML | 12 | 7 | — |
| LY/MM | 1 | 4 | — |
| Other | 3 | 4 | .3 |
| Advanced phase | 22 (67%) | 16 (59%) | .4 |
| 14 or fewer days of first-line treatment, no. | 7 | 8 | .4 |
| Timing of GvHD, ≤ 14 d after transplantation | 7 | 7 | .4 |
| GvHD grade at randomization, no. | |||
| I | 7 | 4 | — |
| II | 19 | 16 | — |
| III | 8 | 7 | .5 |
| Skin GvHD day 5, no. | 33* | 26 | .3 |
| Liver GvHD day 5, no. | 14 | 7 | .2 |
| Gut GvHD day 5, no. | 17 | 14 | .9 |
| Response at day +30, no. (%) | |||
| Resolution of GvHD | 8 (24) | 9 (33) | — |
| Partial resolution | 8 (24) | 6 (22) | — |
| Stable GvHD | 13 (38) | 5 (19) | — |
| Worsened | 4 (12) | 2 (7) | — |
| Dead | 1 (3) | 5 (19) | — |
| Alive | 13 (38) | 9 (33) | .9 |
| Dead TRM | 14 (41) | 16 (59) | .2 |
| Dead relapse | 7 (21) | 2 (8) | .1 |
For patients not given ATG, n = 34; for patients given ATG, n = 27.
AML indicates acute myelogenous leukemia; ALL, acute lymphoblastic leukemia; CML, chronic myelogenous leukemia; LY, lymphoma; MM, multiple myeloma; —, not applicable.
Number of patients with skin, liver, or gut GvHD on day 5 (at the time of randomization).
Response rates to second-line therapy. (A) Response rates evaluated at 30 days of second-line treatment for acute GvHD in all 61 eligible patients. CR indicates complete responses; PR, partial responses; stable, stable disease; and worse, patients with progressive GvHD. (B) Responses in 61 patients according to randomization arm: no ATG (gray bars) or ATG (black bars). There is no significant difference in responses (48% vs 55% CR + PR; P = .3) in the 2 treatment groups, or in patients who worsened or died (15% vs 26%, P = .2).
Response rates to second-line therapy. (A) Response rates evaluated at 30 days of second-line treatment for acute GvHD in all 61 eligible patients. CR indicates complete responses; PR, partial responses; stable, stable disease; and worse, patients with progressive GvHD. (B) Responses in 61 patients according to randomization arm: no ATG (gray bars) or ATG (black bars). There is no significant difference in responses (48% vs 55% CR + PR; P = .3) in the 2 treatment groups, or in patients who worsened or died (15% vs 26%, P = .2).
In multivariate COX analysis, predictors of TRM, for patients receiving second-line treatment, were as follows: age older than 33 years (RR, 3.0; P = .01); the presence of gut or liver GvHD at time of randomization (RR, 3.74; P = .003); the use of ATG (RR, 2.69; P = .01); and advanced disease (RR, 2.27; P = .04).
The effect of ATG given as second-line therapy on outcome. (A) CI of TRM in 61 patients according to randomization arm: no ATG or ATG. (B) Actuarial survival in 61 patients according to randomization arm: no ATG or ATG.
The effect of ATG given as second-line therapy on outcome. (A) CI of TRM in 61 patients according to randomization arm: no ATG or ATG. (B) Actuarial survival in 61 patients according to randomization arm: no ATG or ATG.
Discussion
This study was designed primarily to test whether intensified second-line treatment of acute GvHD would reduce TRM; because all patients were registered at diagnosis of acute GvHD and received the same first-line therapy, 6MPred 2 mg/kg per day, a second objective was to follow patients responding to steroids on day +5. The results of the study can be summarized as follows: (a) 5-day treatment of GvHD is useful to identify patients with different risk of TRM; (b) second-line therapy with a combination of 6MPred + ATG does not improve patient outcome, compared with 6MPred alone.
As to first-line therapy, the major predictor of response was grading of acute GvHD at the time of first-line therapy; there were more patients with GvHD grade I in responders, and, conversely, responses were significantly lower in patients with GvHD grade II or grades III to IV. It is interesting that donor type and age were not major predictors of response on day +5.
The outcome of responders was significantly superior compared with nonresponders in terms of TRM and survival, and this confirms our previous work and other studies.4,5 This was true for the entire group of patients registered and also when excluding GvHD grade I. Of interest, TRM of day +5 responders and nonresponders (23% and 43%, respectively) in our previous study was the same as in the current study (27% and 49%, respectively), despite the fact that half of the donors are currently unrelated. Still, we are faced with TRM also in responders, and we wanted to test for other predictors; as expected, older age, advanced disease, unrelated donor, and higher GvHD grade were all independent predictors of TRM together with day +5 response. We could, therefore, build a scoring system based on these variables that identified on day +5 patients with low (13%), intermediate (24%), or high (50%) risk of TRM. This may be of some clinical relevance, especially for low-risk patients who could be eligible for trials of rapid tapering of immunosuppressive therapy; it could also be used in patients who seem to do well initially but then have flares of GvHD or additional complications. Indeed, 59% of responders were classified as intermediate-risk patients and 21% as high-risk patients. Thus, response on day +5 is important, but other clinical variables need to be taken into account for a more accurate prediction of mortality.
Second-line treatment was given to day +5 nonresponders, in a randomized fashion, to test whether the combination of 6MPred and ATG would reduce TRM compared with 6MPred alone. After 30 days from randomization, half the patients had a complete or partial response, one third had stable disease, and 20% had either progressed or died. There were no major differences in terms of response when comparing patients given 6MPred alone or the combination of 6MPred and ATG. The latter had borderline higher TRM; actually, in multivariate analysis, with patient age, disease phase, visceral GvHD, and donor type, the use of ATG significantly increased the risk of TRM (RR = 2.69) compared with patients receiving steroids alone. Again, in keeping with other studies,6 we come to the same conclusion: intensive systemic immunosuppression is not beneficial—and possibly detrimental—in patients with acute GvHD. This is true for ATG,6 CD5 immunotoxins,7 T-cell monoclonal antibodies,8-17 antibodies against TNF,18-20 and high-dose steroids.4 All of these agents are capable of inducing remission, but nonrelapse mortality remains high, despite control of GvHD, primarily due to intervening infections or related complications.2,21-23 Prophylaxis and treatment of infectious complications are rather aggressive in transplant centers, and it would seem unlikely that these policies may be further implemented and reduce infectious problems in these severely immunocompromised patients.
Where do we go from here? One question that needs to be answered is when do we start treatment of acute GvHD? Should we treat GvHD grade I or should we wait until GvHD grade II develops? Most studies call for GvHD grade II to initiate treatment, and many (but not all) centers treat only GvHD grade II.24 It should not be difficult to randomize patients with GvHD grade I to receive 6MPred 2 mg/kg per day for 5 days or be left untreated until spontaneous response or progression occurs. This would tell us if early is better than late treatment. A second question relates to tapering steroids: it is unclear whether different tapering schedules influence survival and one could ask if a rapid tapering would spare a number of side effects. Finally, we still need to test whether GvHD can be preempted; this implies identifying high-risk patients early after transplantation25 and then treating only these patients.
In conclusion, this study confirms that second-line therapy with a combination of steroids and ATG does not improve patient outcome compared with steroids alone. It also shows that patients could be assigned to different risk groups on day +5 from diagnosis of GvHD and could be eligible for different treatment strategies.
Appendix
Participating members of the Gruppo Italiano Midollo Osseo (GITMO) included A. Bacigalupo, Ospedale San Marino, Genoa; G. Milone, Ospedale Ferrarotto, Catania; G. Masera, Clinica Pediatrica, Monza; R. Scimè, Ospedale Cervello, Palermo; G. Dini, Medicina IV, Gaslini; A. Bosi, Ospedale Careggi, Florence; I. Majolino, Ospedale San Camillo, Rome; G. Leone, Università Cattolica, Rome; E. Madon, Clinica Pediatrica, Turin; A. Gallamini, Cuneo; W. Arcese, Università Tor Vergata, Rome; and B. Rotoli, Ospedale Cardarelli, Naples.
A complete list of the members of the Gruppo Italiano Trapianto Midollo Osseo appears in “Appendix.”
Supported in part by Associazione Italiana per la Ricerca sul Cancro (AIRC) Milano.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
Prepublished online as Blood First Edition Paper, January 31, 2006; DOI 10.1182/blood-2005-12-4851.



This feature is available to Subscribers Only
Sign In or Create an Account Close Modal