CD44, a transmembrane adhesion molecule involved in binding and metabolism of hyaluronan, has additional functions in inflammatory and immune responses, contributing to the ingestion and clearance of particles and apoptotic cells. Our goal was to determine the specific role of CD44 in phagocytosis and whether it functions as a primary or accessory phagocytic receptor. Using hyaluronan-coated beads and erythrocytes coated with antiCD44 antibodies as the phagocytic prey, we determined that CD44 mediates efficient phagocytosis in primary murine peritoneal macrophages and in the murine macrophage cell line RAW 264.7. In RAW cells, the phagocytic index for anti–CD44-coated erythrocytes was 25 ± 3 (mean ± SEM) compared with less than 1 for erythrocytes coated with isotype-matched control antibodies. Uptake of anti–CD44-coated erythrocytes was abrogated by pretreatment with a blocking antibody to CD44 and was absent in primary cultures of CD44-deficient murine macrophages. Down-regulation of Fc receptors by aggregated IgG-induced internalization, which blocks uptake of IgG-coated particles, had no effect on CD44-mediated particle engulfment. Using a combination of immunoprecipitation, pharmacologic inhibition, and genetic deletion, we determined that CD44-mediated phagocytosis involves Syk, Rac1, and phosphatidylinositol 3-kinase and induced activation of the phagocyte oxidase. We conclude that CD44 is a competent phagocytic receptor that efficiently mediates internalization of large particles.
Introduction
The transmembrane adhesion molecule CD44, known to be involved in binding, endocytosis, and metabolism of hyaluronan,1-3 also has additional functions in innate and adaptive immunity. CD44 encompasses a heterogeneous family of receptors with isoforms ranging from 80 to 200 kDa that are encoded by a single gene composed of 19 exons.4 This extensive heterogeneity of CD44 is attributable to a combination of alternate splicing4 and extensive N- and O-glycosylation.5-8 Purported ligands of CD44 include growth factors such as basic fibroblast growth factor and heparin-binding epidermal growth factor,5,6 fibronectin,9 collagen,10 fibrin,11,12 the proteoglycan serglycin found in lymphocytes and mast-cell granules,13 osteopontin,14 and other CD44 molecules on vicinal cells.15
Diverse functions have been attributed to CD44, including involvement in cellular adhesion and migration, lymphocyte and monocyte homing, activation and proliferation, cytocidal activity of natural killer cells, and tumor metastasis.16 Observations also point to a role for CD44 in binding, ingestion (phagocytosis), and clearance of apoptotic cells17 and microbial pathogens.18 With respect to clearance of apoptotic cells, direct antibody ligation of CD44 on macrophages19 or on airway epithelial cells20 enhanced subsequent uptake of apoptotic neutrophils and eosinophils, respectively, suggesting a costimulatory function for the receptor. However, ensuing reports have suggested that some of these effects may be attributed to the Fc portion of the macrophage-bound anti-CD44 antibody interacting with the Fcγ-receptor on the leukocytes.21 Consistent with a role in clearance of apoptotic cells, CD44–/– mice manifest diminished clearance of apoptotic cells following bleomycin-induced lung injury.17 Other studies indicate that CD44 functions primarily in the binding of apoptotic cells, whereas uptake is signaled through an associated receptor such as the phosphatidylserine (PS) receptor.22 According to this model, CD44 functions as a coreceptor via its adhesive (tethering) function, with other receptors such as the PS receptor providing the internalization signal. In concert, these observations suggest a physiologically important role for the CD44 in the binding, ingestion, and removal of apoptotic cells, but its precise role in these processes remains to be clarified.
There is also evidence that CD44 has a more general role in phagocytosis, a process that is important in development, homeostasis, and inflammatory and immune responses.23-25 For example, receptor ligation by hyaluronan or by anti-CD44 antibodies resulted in enhanced bacterial uptake and elimination by neutrophils.18 However, whether CD44 functions as a primary phagocytic receptor to directly mediate internalization of particles or whether it functions as an accessory or coreceptor is unknown.
In this study, we provide evidence that CD44 is an efficient phagocytic receptor, distinct from Fcγ receptors and CR3, which is sufficient to mediate ingestion of particles. To accomplish this, we implemented a system using biotinylated erythrocytes decorated with specific antibodies in which the function of CD44 in phagocytosis can be studied directly and in isolation from the complicating effects of other phagocytic receptors. Using a combination of pharmacologic inhibition and genetic deletion, we have characterized some of the early signaling events leading to particle internalization via CD44. Thus, CD44 should be considered as a fully functional phagocytic receptor that can trigger the uptake of large particles.
Materials and methods
Cell culture
RAW 264.7 cells (American Type Tissue Collection, Manassas, VA) were cultured in DMEM with 4 mM l-glutamine, 10% FBS, penicillin (100 U/mL), and streptomycin (100 μg/mL).
Surface expression of CD44
Macrophages were harvested, blocked with 1% BSA in PBS, and then incubated with biotinylated rat anti–mouse CD44 (clone IM7) or rat non–immune IgG2b isotype-matched antibody (BD Biosciences Pharmingen, San Diego, CA). Cells were washed and resuspended in PBS. ExtrAvidin-FITC (Sigma-Aldrich Canada, Oakville, ON) in PBS (5 μg/mL per 106 cells) was added for 15 minutes, cells were washed again, and fluorescence was quantified by flow cytometry (fluorescence-activated cell scanner [FACScan]; Becton Dickinson, Palo Alto, CA). Values are expressed as relative fluorescence index. Fluorescence microscopy was conducted as previously described.26 Nonspecific binding sites were blocked with 5% goat serum (Invitrogen Canada., Burlington, ON) and 3% BSA (Sigma-Aldrich Canada). Biotinylated rat anti–mouse CD44 (2.5 μg/mL per 106 cells; clone, IM7) or biotinylated rat non–immune IgG2b isotype matched (BD Biosciences Pharmingen) were diluted in blocking buffer (5% goat serum, 3% BSA) and incubated with the cells for 1 hour. Cells were washed with PBS, incubated with ExtrAvidin-FITC (Sigma-Aldrich Canada), washed, and mounted on glass slides. Fluorescent images were captured using a Zeiss LSM510 confocal microscope (Carl Zeiss, Jena, Germany) equipped with a 100 ×/1.4 oil-immersion objective lens, standard laser lines, and filter sets. Digital images were processed with LSM acquisition software v.3.2.
Construction of hyaluronan-coated prey and phagocytosis assay
Macrophages were cultured on 25-mm acid-washed glass coverslips and allowed to adhere overnight. The next day, 10 μL functional amino group polystyrene beads 3 μM (Spherotech, Libertyville, IL) were prepared according to the manufacturer's instructions. Soluble hyaluronan-bodipy (Invitrogen Canada.) was bound to the beads by incubating hyaluronan at a final concentration of 100 μg/mL for 10 minutes followed by washing with PBS. Hyaluronan-coating efficiency was assessed by fluorescence microscopy. Control beads were coated with soluble gelatin (1 mg/mL). Blocking of CD44 was done by preincubating macrophages with 100 μg/mL hyaluronan-bodipy (Invitrogen Canada), or 50μg/mL anti-CD44 (clone IM7; BD Biosciences Pharmingen) for 10 minutes. The phagocytosis assay was conducted in HBSS for 20 minutes at 37°C. The coverslips were washed 3 times in PBS and fixed with 4% paraformaldehyde (Canemco, Saint Laurent, Canada) for 20 minutes. The cells were permeabilized with 0.1% Triton X-100 for 20 minutes and blocked in PBS containing 3% BSA and 5% goat serum for 20 minutes. The cells were incubated with a rat anti–lysosome-associated membrane protein (LAMP1; BD Biosciences Pharmingen) and a secondary rat anti–mouse CY3-fluorescent antibody (Jackson ImmunoResearch Laboratories, West Grove, PA). Cells were mounted on a slide using DAKO mounting medium (DakoCytomation, Carpinteria, CA). Phagosomes were counted using a Leica DMRA2 fluorescent microscope equipped with a 63 ×/1.32 oil-immersion objective lens and the standard filter sets (Leica Microsystems, Jena, Germany). LAMP1 is an established marker of mature phagosomes; thus, beads surrounded by a red fluorescent ring were considered to be within phagosomes. Phagocytosis was recorded as the phagocytic index, defined as the number of internalized prey per 100 cells based on assessment of at least 100 cells.
Construction of “Ebabs” as phagocytic prey
Anti-CD44–coated prey (Figure 2A) were constructed according to the method of Hoffman et al.27 Briefly, erythrocytes were washed and resuspended in PBS, pH 8, and incubated with EZ-Link-Sulfo-NHS-LC-Biotin (Pierce Biotechnology, Rockland, IL) according to the manufacturer's instructions. The cells were washed and resuspended in PBS (pH 8), incubated with ExtrAvidin (6.6 μg/mL per 106 erythrocytes; Sigma-Aldrich Canada) for 30 minutes and washed twice in PBS. The cells were incubated with biotinylated rat anti–mouse CD44 (clone IM7; 0.6 μg/mL per 106 erythrocytes) or rat non–immune isotype-matched IgG2b (BD Biosciences Pharmingen) for 1 hour at 37°C, washed, and resuspended in HBSS. Coating of erythrocytes was confirmed by immunofluorescence using goat anti–rat IgG-TRITC–conjugated secondary antibody (Jackson ImmunoResearch Laboratories). IgG- and iC3b-coated prey were constructed in an analogous manner. For the former, 100 μL sheep erythrocytes 10% solution (Cappel, West Chester, PA) was washed twice in PBS, incubated with 2 μL rabbit anti–sheep erythrocyte IgG (INC55806; Cappel) for 1 hour, and washed twice. For iC3b-coated prey, 100 μL sheep erythrocytes (10% solution; Cappel) were washed twice and incubated with 40 μL rabbit anti–sheep erythrocyte IgM (CL9000M; Cedarlane Laboratories., Hornby, ON) for 1 hour at 37°C on a rotator. To induce complement activation, the cells were washed twice in PBS to remove the unbound IgM, incubated in 33% C5-deficient medium/PBS solution (Sigma-Aldrich Canada), and washed twice in PBS.
Phagocytosis assay of Ebabs
RAW 264.7 or primary murine macrophages were cultured in 6-ell plates on 25-mm acid-washed glass coverslips. The cells were washed twice with HBSS followed by addition of the phagocytic prey at a ratio of 20:1 and allowed to interact and bind to the macrophages for 5 minutes at 37°C. The coverslips were washed to remove unbound prey and incubated at 37°C for an additional 15 minutes to allow phagocytosis. The assays were terminated by cooling the cells by washing with ice-cold PBS without calcium and magnesium.
Phagocytosis of iC3b-coated red blood cells (RBCs) required preincubation of the phagocytes with 100 nM phorbol 12-myristate 13-acetate (PMA; Sigma-Aldrich Canada) for 10 minutes to facilitate adhesion. Following incubation, hypotonic lysis of the extracellular erythrocytes was achieved by the addition of water for 30 seconds, followed by immediate replacement with calcium and magnesium-free PBS. The coverslips were mounted on Attofluor cell chambers (Invitrogen Canada), and quantification of phagocytosis was conducted using a Leica DMIRB inverted microscope equipped with a 100 ×/1.3 oil-immersion objective lens (Leica Microsystems).
Receptor-blocking experiments
Receptor blocking was accomplished by incubating the relevant antibodies (rat anti–mouse CD44; 50 μg/mL; clone IM7) or rat non–immune isotype-matched IgG2b (50 μg/mL) or aggregated human IgG (50-100 μg/mL to block Fcγ receptors) with the macrophages for 30 minutes at room temperature. The cells were washed with HBSS, and the phagocytosis assay was conducted as described in the previous paragraph. Non–immune human IgG (Sigma-Aldrich Canada) was aggregated by heating at 62°C for 20 minutes at a concentration of 10 mg/mL in PBS, followed by centrifugation at 16 000g for 10 minutes to remove large aggregates.
Pharmacologic inhibitors
The cells were preincubated for 15 minutes with the inhibitors at the following concentrations; PP2 50 μM, piceatannol 50 μM, wortmannin 10 and 100 nM, LY-294002 10 and 100 μM (EMD Biosciences, San Diego, CA), latrunculin B 1 μM (Sigma-Aldrich Canada), PP1 50 μM (Biomol International, Plymouth Meeting, PA) and Clostridium difficile toxin B 1μg/mL (TechLab, Blacksburg, VA). The phagocytosis assay was conducted in continued presence of the inhibitors.
Murine studies
CD44-deficient mice were a gift from Dr Tak Mak (Ontario Cancer Institute, Toronto, ON, Canada). Rac1-deficient mice were generated as previously described.28 Macrophages were collected from the peritoneal cavity by lavage with PBS. The recovered cells were cultured on acid-washed glass coverslips in a 6-well plate overnight in complete DMEM. All animal studies were approved by the Animal Care Committee of the University of Toronto and the University Health Network Research Institute.
Cell transfection
Transfection with plasmid DNA was conducted as previously described.29 Fluorescent images of phagocytosing macrophages were captured using a Zeiss LSM510 confocal microscope equipped with a 100 ×/1.4 oilimmersion objective lens, standard laser lines, and filter sets. Digital images were processed with LSM 3.2 acquisition software (Carl Zeiss). Phagocytosis of the GFP-positive cells was compared with the nonexpressing (ie, GFP-negative) on the same coverslip.
Fluorescence microscopy
Fluorescent staining of the actin cytoskeleton was performed with FITC-phalloidin as previously described.29 Staining of lysosomes was accomplished using LysoTracker Red DND-99 (Invitrogen Canada) according to the manufacturer's instructions. Phosphotyrosine staining was performed with antiphosphotyrosine antibodies (clone 4G10; Upstate USA, Chicago, IL) on paraformaldehyde-fixed cells 5 minutes after initiation of phagocytosis of CD44 Ebabs. Fluorescent images were captured using a Zeiss LSM510 confocal microscope as described in “Cell transfection.”
Assessment of oxidant production by flow cytometry
For assessment of activation of the phagocyte NADPH oxidase, murine neutrophils were isolated from bone marrow as described previously30 and subsequently activated by exposure to CD44 Ebabs or IgG-opsonized erythrocytes for 10 minutes. PMA 1 μM for 20 minutes served as a positive control. The cells were then incubated with 10–5 M dihydrorhodamine (Invitrogen Canada) for an additional 5 minutes at 37°C followed by fixation with 1.6% paraformaldehyde (Canemco.). The fluorescence of the cell-associated reduction product, rhodamine 1-2-3, was evaluated by flow cytometry as a measure of oxidant production as previously described.31
Analysis of Syk phosphorylation
RAW macrophages (60-mm2 dish) were incubated at room temperature for 10 minutes with biotinylated anti-CD44 (7.5 μg/dish) or biotinylated non–immune isotype matched IgG2b (7.5 μg/dish) (both from BD Biosciences Pharmingen) and washed with PBS. The cells were cooled to 15°C before the addition of avidin (Sigma-Aldrich Canada) to crosslink the cell-bound biotinylated primary antibodies and incubated for an additional 5 minutes at 15°C. The cells were lysed in a Tris-containing buffer (50 mM Tris pH 7.5, 1% NP40, 10 mM NaPPi, 25 mM NaF, 120 mM NaCl, 200 μM Na3VO4, 1% aprotinin, 1 mM PMSF, and 5 mM DTT). The cell lysates were subject to centrifugation at 16 000g for 10 minutes at 4°C. The supernatant was precleared with Pansorbin A (Sigma-Aldrich Canada) for 10 minutes at 4°C followed by the addition of rabbit polyclonal anti-Syk antibody (1 μg/100 μg protein; C-20; Santa Cruz Biotechnology., Santa Cruz, CA) and incubation for 1 hour at 4°C. Subsequently, 50 μL prewashed protein A/G Sepharose beads (Santa Cruz Biotechnology) was added to the supernatant and incubated over night at 4°C. The beads were washed twice, and proteins were eluted by boiling in Laemmli sample buffer. Proteins were separated by sodium dodecyl sulfate–polyacrylamide gel electrophoresis (SDS-PAGE). Whole-cell lysates and Syk immunoprecipitates were blotted with anti-Syk (C-20; Santa Cruz Biotechnology) and antiphosphotyrosine (clone 4G10; Upstate USA) antibodies.
Data analysis
Data were analyzed using analysis of variance (ANOVA) with post hoc analysis by Student-Newman-Keuls using StatView (SAS Institute, Cary, NC). Statistical significance was considered for P values less than .05.
Results
CD44 mediates phagocytosis of hyaluronan-coated particles
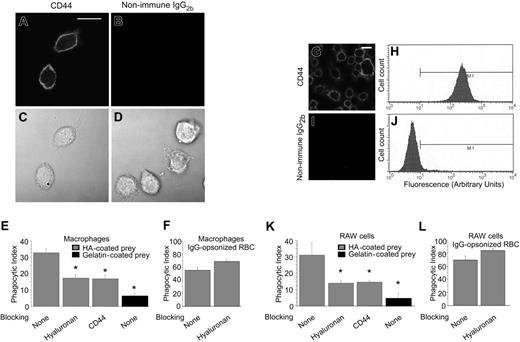
We first determined that primary murine peritoneal macrophages express high levels of CD44 on the cell surface using immunofluorescence microscopy (Figure 1A-D). To establish whether CD44 is able to mediate internalization of particles, functional amino group polystyrene beads coated with fluorescently labeled hyaluronan fragments (Mr ∼ 200 kDa) were added to the macrophages and incubated at 37°C for 20 minutes. Under these conditions, approximately one third of the macrophages ingested one or more particles (phagocytic index = 33; Figure 1E). Importantly, pretreatment of macrophages with either soluble hyaluronan or a blocking anti-CD44 antibody significantly decreased the uptake of hyaluronan-coated beads, providing additional evidence for the role of CD44 in particle uptake. Hyaluronan-coated beads were also internalized avidly by RAW 264.7 macrophages (phagocytic index = 31; Figure 1K), a murine macrophage cell line that also expresses high levels of CD44 as determined by immunofluorescence microscopy and flow cytometry (Figure 1G-J). Pretreatment of primary murine peritoneal macrophages (Figure 1F) or RAW 264.7 macrophages (Figure 1L) with soluble hyaluronan did not interfere with Fcγ-mediated phagocytosis, implying that the effects of hyaluronan are specific. Together, these data provide strong evidence that the uptake of hyaluronan-coated particles is mediated specifically via CD44.
CD44 mediates phagocytosis independently of Fcγ receptors
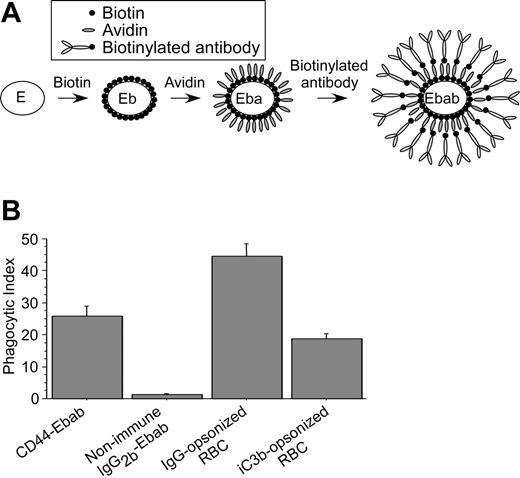
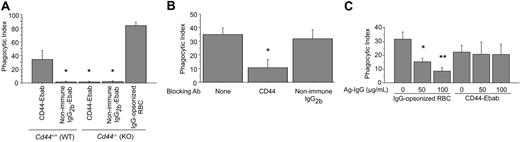
Definition of the specific role of CD44 in phagocytosis using hyaluronan-coated beads is complicated because both hyaluronan and latex are recognized by multiple receptors expressed by macrophages. To circumvent this problem, we implemented an experimental system in which anti-CD44 antibodies attached to erythrocytes are used as phagocytic prey (Figure 2A), as described previously.27,32 In this system, biotin-conjugated antibodies are bound to avidin-coated erythrocytes (hereafter termed Ebabs for erythrocyte-biotin-avidin-biotinylated-antibody). This methodology allows ligation of individual surface receptors via the antigen-binding region of specific antibodies, without the confounding effects inherent to systems such as hyaluronan-coated beads that may bind multiple receptors. We determined that anti-CD44–decorated Ebabs (CD44 Ebabs) bound to and were internalized efficiently by RAW macrophages (phagocytic index = 26) compared with Ebabs decorated with isotype-matched control antibodies (IgG2b-Ebabs; phagocytic index = 2; Figure 2B). Using a comparable density of IgG-opsonized erythrocytes, which are internalized on binding to Fcγ receptors,33,34 the phagocytic efficiency was somewhat greater (phagocytic index = 45). By comparison, internalization of iC3b-coated erythrocytes, a process mediated via complement receptor 3 (CR3), was slightly less (phagocytic index = 19) than for anti-CD44 Ebabs. Importantly, no significant uptake of CD44 Ebabs occurred in murine peritoneal macrophages from mice deficient in CD44 (Figure 3A), whereas wild-type macrophages efficiently internalized such Ebabs (phagocytic index = 36), providing strong evidence for the specific role of CD44 in this process. We also confirmed that macrophages from CD44-deficient mice can efficiently internalize IgG-opsonized erythrocytes (Figure 3A), demonstrating that these cells are otherwise competent phagocytes.
Internalization of hyaluronan (HA)–coated phagocytic prey is mediated by CD44. (A-D) Confocal fluorescence and differential interference contrast (DIC) images of murine peritoneal macrophages demonstrating the specific binding of anti-CD44 (CD44) antibody. The solid line represents 10 μm. (E) Quantitative analysis of phagocytosis of HA- and gelatin (control)–coated beads in the presence or absence of soluble HA, anti-CD44 antibody, or buffer control (none). Note that the macrophages internalize HA-coated beads and this is inhibited by soluble HA or by blocking anti-CD44 antibody. By contrast, uptake of gelatin-coated beads is substantially less (phagocytic index < 5). (F) Quantitative analysis of phagocytosis of IgG-opsonized erythrocytes (RBCs) in the presence or absence of soluble HA or buffer control (none). Note that uptake of IgG-opsonized erythrocytes (RBCs, mediated by Fcγ receptors) is not inhibited by soluble HA. (G-J) Confocal immunofluorescence images (G,I) and flow cytometric analysis (H,J) of RAW264.7 macrophages stained with anti-CD44 (G) or isotype control antibodies demonstrating high levels of expression of CD44 and confirming the specificity of the anti-CD44 antibody. (K) Quantitative analysis of phagocytosis of HA-coated beads by RAW macrophages demonstrating efficiency of internalization of HA-coated beads and inhibition by soluble HA or by blocking anti-CD44 antibody. Uptake of gelatin-coated (control) beads is comparatively very low. (L) Quantitative analysis of efficiency of phagocytosis of IgG-opsonized RBCs by RAW macrophages in the presence or absence of soluble HA or buffer control (none). Note that uptake of IgG-opsonized RBCs (mediated by Fcγ receptors) is not inhibited by soluble HA. *Difference from control (no blocking), P < .05, mean ± standard error (SE) of 4 separate experiments.
Internalization of hyaluronan (HA)–coated phagocytic prey is mediated by CD44. (A-D) Confocal fluorescence and differential interference contrast (DIC) images of murine peritoneal macrophages demonstrating the specific binding of anti-CD44 (CD44) antibody. The solid line represents 10 μm. (E) Quantitative analysis of phagocytosis of HA- and gelatin (control)–coated beads in the presence or absence of soluble HA, anti-CD44 antibody, or buffer control (none). Note that the macrophages internalize HA-coated beads and this is inhibited by soluble HA or by blocking anti-CD44 antibody. By contrast, uptake of gelatin-coated beads is substantially less (phagocytic index < 5). (F) Quantitative analysis of phagocytosis of IgG-opsonized erythrocytes (RBCs) in the presence or absence of soluble HA or buffer control (none). Note that uptake of IgG-opsonized erythrocytes (RBCs, mediated by Fcγ receptors) is not inhibited by soluble HA. (G-J) Confocal immunofluorescence images (G,I) and flow cytometric analysis (H,J) of RAW264.7 macrophages stained with anti-CD44 (G) or isotype control antibodies demonstrating high levels of expression of CD44 and confirming the specificity of the anti-CD44 antibody. (K) Quantitative analysis of phagocytosis of HA-coated beads by RAW macrophages demonstrating efficiency of internalization of HA-coated beads and inhibition by soluble HA or by blocking anti-CD44 antibody. Uptake of gelatin-coated (control) beads is comparatively very low. (L) Quantitative analysis of efficiency of phagocytosis of IgG-opsonized RBCs by RAW macrophages in the presence or absence of soluble HA or buffer control (none). Note that uptake of IgG-opsonized RBCs (mediated by Fcγ receptors) is not inhibited by soluble HA. *Difference from control (no blocking), P < .05, mean ± standard error (SE) of 4 separate experiments.
CD44 mediates phagocytosis of large particles. (A) Schematic representation of the construction of an erythrocyte-biotin-avidin-biotinylated-antibody (Ebab) beginning with the erythrocyte “E” through to the mature prey Ebab. (B) Efficiency of phagocytosis of CD44 and IgG2b-coated Ebabs as well as IgG- and iC3b-opsonized erythrocytes by RAW macrophages. Phagocytosis was recorded as the phagocytic index; mean ± SE of 4 separate experiments.
CD44 mediates phagocytosis of large particles. (A) Schematic representation of the construction of an erythrocyte-biotin-avidin-biotinylated-antibody (Ebab) beginning with the erythrocyte “E” through to the mature prey Ebab. (B) Efficiency of phagocytosis of CD44 and IgG2b-coated Ebabs as well as IgG- and iC3b-opsonized erythrocytes by RAW macrophages. Phagocytosis was recorded as the phagocytic index; mean ± SE of 4 separate experiments.
As our model system used antibodies bound to erythrocytes, 2 additional sets of control experiments were conducted to ensure that particle ingestion occurred via CD44 and not via Fcγ receptors. First, pretreatment of RAW macrophages with soluble (monomeric) anti-CD44 antibodies, but not control IgG, largely prevented subsequent uptake of CD44 Ebabs (Figure 3B). Second, to distinguish CD44-mediated events from Fcγ receptor–mediated uptake, we pretreated cells with soluble aggregated IgG. The latter binds to and induces internalization of Fcγ receptors.35 This treatment largely abrogated uptake of IgG-opsonized erythrocytes (mediated by Fcγ receptors) but had no effect on internalization of anti–CD44-coated Ebabs (Figure 3C). Considered together, these data provide strong evidence that CD44-mediated uptake of Ebabs is distinct from that mediated by Fcγ receptors.
Signaling pathways involved in CD44-mediated phagocytosis
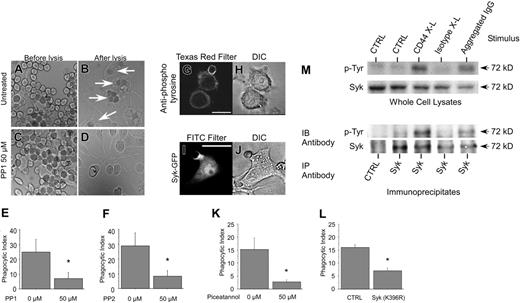
We next sought to characterize the signaling pathways involved in CD44-mediated particle internalization (phagocytosis). Because the Src-family kinases Lyn and Fyn are activated by engagement of CD4436-38 and are known to be involved in other types of phagocytosis,39,40 we studied their involvement in CD44-mediated phagocytosis using pharmacologic inhibition with PP1 and PP2, 2 specific inhibitors. Figure 4 illustrates that treatment of RAW macrophages with either of these inhibitors substantially diminished CD44-mediated phagocytosis. In this figure, panels A to D represent typical light microscopic images that illustrate binding (Figure 4A,C) and uptake (Figures 4B,D) of erythrocytes by these macrophages, underscoring the high level of both tethering and engulfment (white arrows in Figure 4B) of anti–CD44-coated Ebabs in cells treated with vehicle control (Figure 4A-B). By contrast, uptake of Ebabs was markedly diminished by preincubation of the macrophages with either PP1 (Figure 4C-E) or PP2 (Figure 4F). It is also apparent from these studies that PP1 selectively inhibits engulfment but not binding (tethering) of the phagocytic prey, thus excluding interference with particle binding (adhesion) as an explanation for diminished engulfment.
CD44 mediates phagocytosis independently of Fcγ receptors. (A) Efficiency of phagocytosis of CD44 Ebabs or IgG2b-coated Ebabs or IgG-opsonized erythrocytes by wild-type or CD44-deficient peritoneal macrophages. *Different from CD44-Ebabs/Cd44+/+ (WT); P < .05, mean ± SE of 4 separate experiments. (B) Efficiency of phagocytosis of CD44 Ebabs by RAW macrophages that have been preincubated with the vehicle control or blocking CD44 antibody or non–immune isotype control antibody. *Significant differences at the P < .05 level, mean ± SE of 4 separate experiments. (C) Efficiency of phagocytosis of IgG-opsonized erythrocytes or CD44 Ebabs by RAW cells that were preincubated with increasing concentrations of Fcγ-blocking aggregated IgG. *Different from 0 μg/mL aggregated IgG/IgG-opsonized erythrocytes; P < .05; **P < .01, mean ± SE of 4 separate experiments. Phagocytosis was recorded as the phagocytic index.
CD44 mediates phagocytosis independently of Fcγ receptors. (A) Efficiency of phagocytosis of CD44 Ebabs or IgG2b-coated Ebabs or IgG-opsonized erythrocytes by wild-type or CD44-deficient peritoneal macrophages. *Different from CD44-Ebabs/Cd44+/+ (WT); P < .05, mean ± SE of 4 separate experiments. (B) Efficiency of phagocytosis of CD44 Ebabs by RAW macrophages that have been preincubated with the vehicle control or blocking CD44 antibody or non–immune isotype control antibody. *Significant differences at the P < .05 level, mean ± SE of 4 separate experiments. (C) Efficiency of phagocytosis of IgG-opsonized erythrocytes or CD44 Ebabs by RAW cells that were preincubated with increasing concentrations of Fcγ-blocking aggregated IgG. *Different from 0 μg/mL aggregated IgG/IgG-opsonized erythrocytes; P < .05; **P < .01, mean ± SE of 4 separate experiments. Phagocytosis was recorded as the phagocytic index.
Src family kinases and Syk are involved in CD44-mediated phagocytosis. (A-D) Binding and uptake of CD44 Ebabs by macrophages was assessed quantitatively by light microscopy using a 63 ×/0.90 liquid-immersion objective lens (Leica Microsystems). Digital images were acquired through a Qimaging Retiga EX camera (Qimaging, Burnaby, Canada) and processed using OpenLab 3.4 software (Improvision, Lexington, MA). This figure illustrates the high level of both adhesion (tethering) and engulfment (white arrows) of CD44 Ebabs in the control assay (A-B) without specific inhibitor, whereas CD44 Ebabs preincubated with 50 μM PP1 Src-kinase pharmacologic inhibitor (C-D) were tethered but not engulfed. (E-F) Histograms illustrate the efficiency of phagocytosis of RAW macrophages preincubated with 0 or 50 μM PP1 or PP2 and subsequently fed CD44 Ebabs as prey. *Different from 0 μM inhibitor, P < .05, mean ± SE of 4 separate experiments. (G-H) Confocal fluorescent and bright field images of RAW macrophages labeled with antiphosphotyrosine antibodies during phagocytosis of CD44 Ebabs. The solid line represents 10 μm. (I-J) Confocal fluorescent and bright field images of RAW cells transfected with Syk-GFP and presented with CD44 Ebabs. Solid line represents 10 μm. (K) Efficiency of phagocytosis of CD44 Ebabs preincubated with 0 or 50 μM Syk inhibitor piceatannol. *Different from 0 μM piceatannol; P < .05, mean ± SE of 4 separate experiments. (L) Efficiency of phagocytosis of CD44 Ebabs by RAW cells expressing GFP-tagged Syk (K396R) dominant-negative fusions compared with nonexpressing control cells. *Different from nontransfected controls; P < .01, mean ± SE of 4 separate experiments. (M) Syk phosphorylation is increased in response to antibody crosslinking of CD44 as determined by Western blot analysis of whole-cell lysates (top) and Syk immunoprecipitates (bottom). Images are representative of 3 independent experiments. IB indicates immunoblot; IP, immunoprecipitation.
Src family kinases and Syk are involved in CD44-mediated phagocytosis. (A-D) Binding and uptake of CD44 Ebabs by macrophages was assessed quantitatively by light microscopy using a 63 ×/0.90 liquid-immersion objective lens (Leica Microsystems). Digital images were acquired through a Qimaging Retiga EX camera (Qimaging, Burnaby, Canada) and processed using OpenLab 3.4 software (Improvision, Lexington, MA). This figure illustrates the high level of both adhesion (tethering) and engulfment (white arrows) of CD44 Ebabs in the control assay (A-B) without specific inhibitor, whereas CD44 Ebabs preincubated with 50 μM PP1 Src-kinase pharmacologic inhibitor (C-D) were tethered but not engulfed. (E-F) Histograms illustrate the efficiency of phagocytosis of RAW macrophages preincubated with 0 or 50 μM PP1 or PP2 and subsequently fed CD44 Ebabs as prey. *Different from 0 μM inhibitor, P < .05, mean ± SE of 4 separate experiments. (G-H) Confocal fluorescent and bright field images of RAW macrophages labeled with antiphosphotyrosine antibodies during phagocytosis of CD44 Ebabs. The solid line represents 10 μm. (I-J) Confocal fluorescent and bright field images of RAW cells transfected with Syk-GFP and presented with CD44 Ebabs. Solid line represents 10 μm. (K) Efficiency of phagocytosis of CD44 Ebabs preincubated with 0 or 50 μM Syk inhibitor piceatannol. *Different from 0 μM piceatannol; P < .05, mean ± SE of 4 separate experiments. (L) Efficiency of phagocytosis of CD44 Ebabs by RAW cells expressing GFP-tagged Syk (K396R) dominant-negative fusions compared with nonexpressing control cells. *Different from nontransfected controls; P < .01, mean ± SE of 4 separate experiments. (M) Syk phosphorylation is increased in response to antibody crosslinking of CD44 as determined by Western blot analysis of whole-cell lysates (top) and Syk immunoprecipitates (bottom). Images are representative of 3 independent experiments. IB indicates immunoblot; IP, immunoprecipitation.
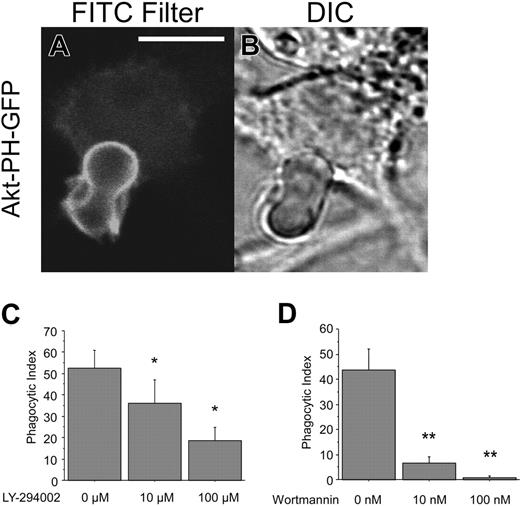
PI3 kinase is involved in CD44-mediated phagocytosis. (A-B) Confocal fluorescent and transmitted light images of RAW cells transfected with Akt-PH-GFP and presented with CD44 Ebabs. The solid line represents 5 μm. (C-D) Efficiency of phagocytosis of CD44 Ebabs by RAW cells preincubated with increasing doses of LY-294002 or wortmannin. *Different from 0 μM LY-294002; P < .05, mean ± SE of 4 separate experiments. **Different from 0 μM wortmannin; P < .05, mean ± SE of 4 separate experiments.
PI3 kinase is involved in CD44-mediated phagocytosis. (A-B) Confocal fluorescent and transmitted light images of RAW cells transfected with Akt-PH-GFP and presented with CD44 Ebabs. The solid line represents 5 μm. (C-D) Efficiency of phagocytosis of CD44 Ebabs by RAW cells preincubated with increasing doses of LY-294002 or wortmannin. *Different from 0 μM LY-294002; P < .05, mean ± SE of 4 separate experiments. **Different from 0 μM wortmannin; P < .05, mean ± SE of 4 separate experiments.
Involvement of Syk in CD44-mediated phagocytosis
During the early phases of Fcγ receptor–mediated phagocytosis, the nonreceptor tyrosine kinase Syk binds to the tyrosine-phosphorylated ITAM motif of the receptor, triggering downstream events leading to particle internalization.40 To assess the role of Syk in CD44-mediated phagocytosis, we first determined that there was selective accumulation of phosphotyrosine at the base of the phagocytic cup at early stages of phagocytosis (Figure 4G). We next investigated whether Syk is recruited to the phagocytic cup. In RAW macrophages transfected with GFP-tagged Syk, engagement of CD44 Ebabs resulted in robust and selective recruitment of Syk to the base of the phagosomal cup (Figure 4I-J). To provide more direct evidence for a role of Syk in CD44-mediated phagocytosis, we used 2 strategies. First, macrophages were treated with piceatannol, a pharmacologic inhibitor of Syk, and phagocytosis monitored. These studies revealed that CD44-mediated phagocytosis was almost completely abolished by the inhibitor (Figure 4K). Second, RAW macrophages were transiently transfected with a GFP-tagged dominant-negative mutant of Syk (Syk K396R),41 and the phagocytic efficiency of transfected and nontransfected (control) cells was monitored. As illustrated in Figure 4L, phagocytosis was substantially diminished in macrophages expressing mutant Syk compared with nonexpressing (control) cells. Finally, we determined that Syk was phosphorylated on tyrosine in a CD44-dependent manner. To accomplish this, RAW cells were activated by antibody crosslinking of CD44. Western blot analysis of whole-cell lysates with antiphosphotyrosine antibodies revealed a prominent 72-kDa band that comigrated with a band recognized by anti-Syk antibody. In addition, purification of Syk by immunoprecipitation followed by Western blot analysis of the immunoprecipitates with an antiphosphotyrosine antibody confirmed that Syk is phosphorylated on tyrosine, an event that correlates with its activation42 (Figure 4M). Together, these observations indicate an essential role for Syk in CD44-mediated phagocytosis.
Phosphatidylinositol 3-kinase (PI3K) is involved in CD44-mediated phagocytosis
PI3K lies downstream of Syk and is essential for FcγR-mediated phagocytosis.40,43,44 To determine the role of PI3K in CD44-mediated phagocytosis, RAW macrophages were transfected with a plasmid encoding a GFP-tagged fusion of the pleckstrin homology (PH) domain of Akt (Akt-PH-GFP). The PH domain of Akt binds to 3′-phosphorylated polyphosphoinositides, predominantly to phosphatidylinositol 3,4,5-trisphosphate (PIP3), the primary product of PI3K.45,46 These studies revealed robust recruitment of Akt-PH-GFP to the phagocytic cup during CD44-mediated phagocytosis (Figure 5A-B). To obtain more direct evidence for a role for PI3K in this process, we used 2 inhibitors of this kinase, LY-294002 and wortmannin.47,48 Importantly, pretreatment of RAW macrophages with either of these compounds resulted in substantial inhibition of CD44-mediated phagocytosis (Figure 5C-D). Jointly, these data provide evidence for the involvement of PI3K in this process.
Actin cytoskeletal alterations are required for CD44-mediated phagocytosis
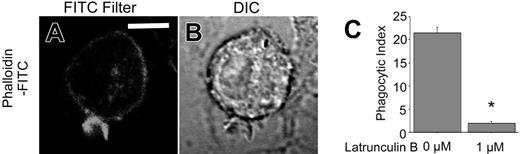
Fcγ receptor–mediated phagocytosis involves dynamic reorganization of the actin cytoskeleton that drives the formation of cell extensions around the phagocytic prey during ingestion.40 Accordingly, we sought to determine the role of the actin cytoskeleton in CD44-mediated phagocytosis. Using fluorescent staining with FITC-phalloidin, we observed accumulation of polymerized actin around the phagocytic cup (Figure 6A-B). Moreover, cells treated with latrunculin B, an inhibitor of actin polymerization by virtue of its ability to bind and sequester monomeric actin, failed to internalize CD44 Ebabs (Figure 6C). These observations provide direct evidence for the importance of assembly and reorganization of the actin cytoskeleton in CD44-mediated phagocytosis.
Rho GTPases are involved in CD44-mediated phagocytosis
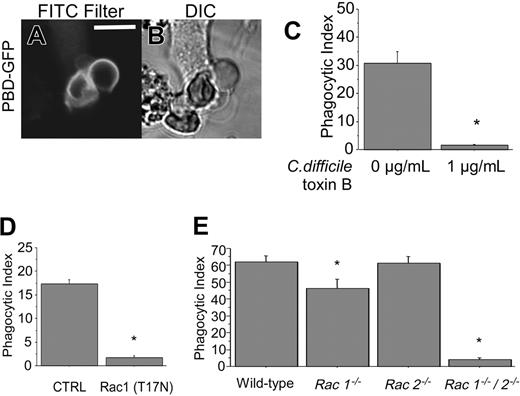
Given the profound cytoskeletal alterations documented in the previous paragraph, we next investigated the role of Rho-family GTPases, important regulators of cytoskeletal reorganization, in CD44-mediated phagocytosis. For these studies, RAW macrophages were transfected with a GFP-fusion of the Ser/Thr kinase p21-activated kinase 1 (PAK1)–binding domain (PBD) that associates with activated Rac and Cdc42.49 As is apparent from Figure 7A, GFP-PBD is recruited to the phagocytic cup of nascent phagosomes. To obtain more direct evidence of the involvement of Rho GTPases in this process, cells were treated with C difficile toxin B, an inhibitor of these GTPases. CD44-mediated phagocytosis was blocked by C difficile toxin B (Figure 7C), implying involvement of Rho-family GTPases in this process.
To determine which of the Rho-family GTPases are involved in CD44-mediated phagocytosis, 2 complementary approaches were taken. First, RAW macrophages were transfected with a GFP-tagged dominant-negative mutant of Rac1 (GFP-Rac1(T17N)). CD44-mediated phagocytosis was greatly reduced in cells expressing the inhibitory Rac1 mutant, compared with nonexpressing control cells (Figure 7D). Second, we compared phagocytosis in primary murine macrophages genetically deficient in Rac1, Rac2, or in both Rac1 and Rac 2 (double knockout) with wild-type macrophages. Because Rac1 deficiency is lethal in early embryogenesis,50 we used a conditional myeloid-specific genetic deletion strategy by mating mice with a `floxed' Rac1 allele, with transgenic mice expressing the Cre recombinase driven by the LysM promoter, as previously described.28 These studies revealed that CD44-mediated phagocytosis was significantly inhibited in Rac1-deficient cells (phagocytic index = 46) compared with wild-type controls (phagocytic index = 62), suggesting a role for Rac1 in this process (Figure 7E). Phagocytosis was not diminished in Rac2-deficient macrophages (phagocytic index = 61). In contrast, phagocytosis was severely diminished in macrophages deficient in both Rac1 and Rac2 (phagocytic index = 4). Because myeloid cells express both isoforms of Rac, which are greater than 92% identical,51 it is likely that there is redundancy between Rac1 and Rac2. We conclude that CD44-mediated phagocytosis is dependent primarily on Rac1, but Rac2 may fulfill some of the functions of Rac1 and compensate partially when Rac1 is deficient. Our data do not exclude the involvement of other GTPases such as Rho and Cdc42 in this process.
Actin cytoskeletal alterations are required for CD44-mediated phagocytosis. (A-B) Confocal fluorescent and transmitted light images of phalloidin-FITC–stained RAW cells during early stages of phagocytosis of CD44 Ebabs. The solid line represents 5 μm. (C) Efficiency of phagocytosis of CD44 Ebabs by RAW cells preincubated with latrunculin B 0 and 1 μM. *Different from 0 μM latrunculin B, P < .01, n = 4. Phagocytosis was recorded as the phagocytic index.
Actin cytoskeletal alterations are required for CD44-mediated phagocytosis. (A-B) Confocal fluorescent and transmitted light images of phalloidin-FITC–stained RAW cells during early stages of phagocytosis of CD44 Ebabs. The solid line represents 5 μm. (C) Efficiency of phagocytosis of CD44 Ebabs by RAW cells preincubated with latrunculin B 0 and 1 μM. *Different from 0 μM latrunculin B, P < .01, n = 4. Phagocytosis was recorded as the phagocytic index.
Rac1 is involved in CD44-mediated phagocytosis. (A-B) Confocal fluorescent and transmitted light images of GFP PBD-transfected RAW cells presented with CD44 Ebabs. Solid line represents 5 μm. (C) Efficiency of phagocytosis of CD44 Ebabs by RAW cells preincubated with 0 or 1 μg/mL C difficile toxin B. *Different from 0 μg/mL C difficile toxin B; P < .01, mean ± SE of 4 separate experiments. (D) Efficiency of phagocytosis of Rac1 (T17N) dominant-negative GFP-transfected RAW cells or the nontransfected controls fed with CD44 Ebabs. *Different from nontransfected controls; P < .01, n = 4. (E) Efficiency of phagocytosis of CD44 Ebabs by Rac1–/–, Rac2–/–, Rac1–/–/Rac2–/– (double knockout), and wild-type peritoneal macrophages. *Different from wild-type peritoneal macrophages; P < .01, mean ± SE of 4 separate experiments.
Rac1 is involved in CD44-mediated phagocytosis. (A-B) Confocal fluorescent and transmitted light images of GFP PBD-transfected RAW cells presented with CD44 Ebabs. Solid line represents 5 μm. (C) Efficiency of phagocytosis of CD44 Ebabs by RAW cells preincubated with 0 or 1 μg/mL C difficile toxin B. *Different from 0 μg/mL C difficile toxin B; P < .01, mean ± SE of 4 separate experiments. (D) Efficiency of phagocytosis of Rac1 (T17N) dominant-negative GFP-transfected RAW cells or the nontransfected controls fed with CD44 Ebabs. *Different from nontransfected controls; P < .01, n = 4. (E) Efficiency of phagocytosis of CD44 Ebabs by Rac1–/–, Rac2–/–, Rac1–/–/Rac2–/– (double knockout), and wild-type peritoneal macrophages. *Different from wild-type peritoneal macrophages; P < .01, mean ± SE of 4 separate experiments.
CD44 phagocytosis induces formation of a mature phagosome
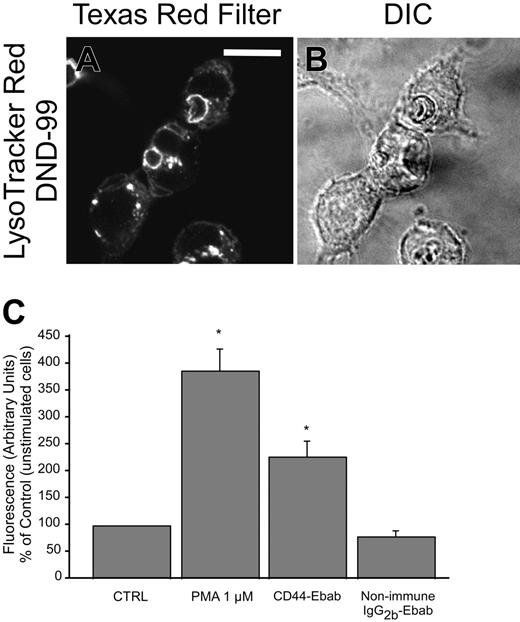
A primary purpose of phagocytosis is to sequester microbial prey in a highly specialized compartment, the phagosome, in which the pathogens can be killed and degraded. A key step in phagosome maturation is fusion with lysosomes, vesicles that contain antimicrobial proteinases in an acidic milieu.52 During maturation of the phagosome, components of the phagocyte NADPH oxidase assemble on the phagosomal membrane, leading to activation of the oxidative burst producing reactive oxygen species, also potent antimicrobial compounds.31,53 To determine whether CD44 phagocytosis leads to the formation of a competent phagosome, 2 complementary approaches were undertaken. First, phagosomelysosome fusion was assessed by fluorescent staining with Lyso-Tracker using live confocal microscopy. As illustrated in Figure 8A, CD44 phagocytosis induces robust recruitment of lysosomes to the nascent phagosome. To assess whether CD44 phagocytosis induced an oxidative burst, we assessed oxidant production by neutrophils with dihydrorhodamine using flow cytometry. These studies revealed that CD44 phagocytosis induced a vigorous oxidative burst (Figure 8B-C). Together, these experiments indicate that CD44 phagocytosis leads to the formation of a competent phagosome with concomitant activation of the phagocyte NADPH oxidase, conditions that facilitate microbial killing.
Discussion
In this manuscript, we provide evidence that CD44 is a bone fide phagocytic receptor, inasmuch as ligation and clustering of CD44 is sufficient to trigger internalization of large (> 3 μm) particles. In this regard, CD44 is comparable to Fcγ receptors and CR3, the 2 prototypic phagocytic receptors. The signaling pathways that control CD44-mediated phagocytosis involve tyrosine kinases, PI3K, Rho-family small GTPases and reorganization of the actin cytoskeleton. Although there is overlap with the pathways involved in Fcγ receptor and CR3-mediated phagocytosis, CD44-mediated particle internalization is clearly distinct in terms of signaling pathways and cytoskeletal changes. This does not however exclude the possibility that CD44 can function in conjunction with Fcγ and other phagocytic receptors in more complex systems, as discussed in the next paragraph.
As outlined in the “Introduction,” previous studies have pointed to a role for CD44 in modulation of phagocytosis,18 but to date its precise role in this process and whether CD44 is a primary or accessory phagocytic receptor has been unclear. Our studies clearly demonstrate that CD44 can function as a primary phagocytic receptor. Because pathogenic bacteria such as Pseudomonas aeruginosa,54,55 Pseudomonas multocida,56 and Group A Streptococcus57 have capsular polysaccharides that contain hyaluronic acid, ligation by CD44 may serve as a primary trigger for bacterial phagocytosis. We also provide definitive data indicating that CD44 phagocytosis induces formation of a mature phagosome as evidenced by phagosome-lysosome fusion and activation of the phagocyte NADPH oxidase with production of prodigious quantities of reactive oxygen species, potent antimicrobial compounds. Together, these observations indicate that CD44 phagocytosis promotes conditions in the phagosome that facilitate microbial killing.
CD44-mediated phagocytosis results in formation of a mature phagosome. (A-B) Confocal fluorescent and transmitted light images, respectively, of LysoTracker Red DND-99 staining during the late stages of CD44-mediated phagocytosis (30 minutes), indicating phagosome-lysosome fusion. The solid line represents 10 μm. (C) Phagocytosis of CD44 Ebabs stimulates production of reactive oxygen species by murine neutrophils. *Different from nonimmune IgG2b-Ebab; P < .05, mean ± SE of 3 separate experiments.
CD44-mediated phagocytosis results in formation of a mature phagosome. (A-B) Confocal fluorescent and transmitted light images, respectively, of LysoTracker Red DND-99 staining during the late stages of CD44-mediated phagocytosis (30 minutes), indicating phagosome-lysosome fusion. The solid line represents 10 μm. (C) Phagocytosis of CD44 Ebabs stimulates production of reactive oxygen species by murine neutrophils. *Different from nonimmune IgG2b-Ebab; P < .05, mean ± SE of 3 separate experiments.
In addition to being fully competent to elicit phagocytosis, CD44 may also operate as a phagocytic coreceptor. In this regard, it has been reported that phagocytosis of bacteria by neutrophils is enhanced by addition of anti-CD44 antibodies or of hyaluronan, consistent with a costimulatory role for CD44.18 This coreceptor function is of potential importance in more complex inflammatory environments in vivo when phagocytes encounter invading microbial pathogens. In this milieu, CD44 could be engaged when phagocytes are in contact with hyaluronan as part of the extracellular matrix or displayed on the surface of epithelial cells in the lung or gut,58-60 organs frequently subject to bacterial infections. Additionally, small hyaluronan fragments, known to be released to the extracellular fluid in inflammatory sites,17,61 could bind to CD44 on the phagocyte membrane. Thus, in an inflammatory milieu, either surface-bound hyaluronan or soluble hyaluronan fragments could ligate phagocyte CD44 resulting in enhanced phagocytosis of microbial pathogens. This notion has important implications for our understanding of the mechanisms regulating microbial phagocytosis and the host response to infection that are of fundamental importance to immune and inflammatory responses. The level of redundancy in these systems serves to thwart the potential evasive strategies adopted by microbial pathogens via alterations in their surfaces.25
CD44 has also been implicated in clearance of apoptotic cells17 and, as in bacterial phagocytosis, there is evidence that CD44 may function as a coreceptor during this process. For example, ligation of CD44 on macrophages or on airway epithelial cells increases the ensuing uptake of apoptotic neutrophils19,21 and eosinophils,20 respectively. During internalization of apoptotic cells there are several potential ligands on the surface of the apoptotic prey that could bind CD44 on the phagocyte plasma membrane, including hyaluronan and CD44 itself. During ingestion of apoptotic cells, there is evidence for cosignaling between CD44 and other receptors expressed by phagocytes, including the phosphatidyl serine receptor,62 the CD36 scavenger receptor,63 the vitronectin receptor (αvβ3),63 and calreticulin/CD91.64 Our data suggest an alternate (but not mutually exclusive) possibility that apoptotic cells engage and cluster the CD44 receptor on the phagocyte surface, thus directly signaling particle uptake. Cooperative responses initiated by these receptors acting together may help modulate uptake of apoptotic cells and the ensuing cellular responses.65
There is precedent for functional and physical interactions between other surface receptors involved in recognition of foreign or modified ligands expressed on the surface of microbial pathogens and apoptotic cells triggering particle internalization. For example, the integrin CD11b can physically associate with and modulate signals from Fcγ receptors.66-69 Conversely, ligation of Fcγ receptors induces alterations in the affinity state of CD11b and its accumulation in the phagocytic cup, processes that modulate phagocytosis.70 Recent evidence indicates that the scavenger receptor CD36 mediates binding and internalization (phagocytosis) of Gram-positive bacteria such as Staphylococcus aureus via recognition of lipotechoic acid and acts cooperatively with the Toll-like receptors TLR2 and TLR6 to initiate macrophage cytokine production in response to bacterial pathogens.25 Other examples of receptors that function cooperatively during particle internalization include the macrophage mannose receptor and DC-SIGN (reviewed in Johnson et al71 ), Dectin-1, and TLR2,72 and interacting with CD91/calreticulin and signal regulatory inhibitory protein α in collectin-dependent phagocytosis.64
In the current manuscript, we have characterized some of the signaling events involved in CD44-mediated particle internalization but many details remain unclear. Although the signal transduction pathways involved in CD44-mediated phagocytosis share some elements with those involved in Fcγ receptor and CR3-mediated phagocytosis, important differences exist. One such difference lies with the receptors themselves. Fcγ receptors (or the Fc receptor–associated γ-chain) contain an ITAM motif within their cytosolic domains, whereas CD44 has no such motif. In the paradigm of Fc receptor–mediated phagocytosis, the receptor ITAM domain is phosphorylated by Src-family kinases following receptor engagement by IgG-opsonized prey. This leads to recruitment of the Syk tyrosine kinase and of adaptor proteins such as Gab273 triggering activation of downstream pathways and functional responses involved in particle internalization (reviewed in Berton et al74 ). Because CD44 does not have an ITAM motif, how Syk is recruited to the nascent phagosome during CD44-mediated phagocytosis is unclear. It is possible that an unidentified intermediary protein such as the Fc receptor–associated γ-chain or an analogous molecule such as DAP12,74 provides a functional ITAM motif in this process. This area is the subject of our current investigations.
The data presented in the current manuscript indicate that CD44-mediated phagocytosis is dependent primarily on the Rho-family GTPase Rac1 and provide a link to the dynamic cytoskeletal alterations involved in this process. The activity of Rho GTPases is controlled by guanine nucleotide exchange factors (GEFs) that activate the rate-limiting reaction, namely the exchange of GDP for GTP.75 In this regard, 3 GEFs, including VAV2, TIAM1, and p115 RhoGEF, are known to bind to the cytosolic tail of CD44 in a ligand (hyaluronan)–dependent manner.76-78 VAV and TIAM isoforms are of particular interest because they have been implicated in Rac-dependent cytoskeletal alterations79,80 and in antimicrobial responses of phagocytes, including phagocytosis and activation of the NADPH oxidase.81,82
In addition to the binding and possible activation of GEFs, CD44 activation could also alter cytoskeletal function by other, more direct mechanisms. CD44 associates indirectly with the actin cytoskeleton through ankyrin and ERM proteins,83-85 and dynamic cytoskeletal alterations could be signaled through these actin-associated proteins. Binding of Rho to CD44 can induce activation of Rho kinase (ROK) that in turn can directly phosphorylate CD44, thereby increasing binding to ankyrin and thence to the actin cytoskeleton.86 Another potentially relevant target of activated ROK is myosin, and it is noteworthy in this respect that C3-mediated but not Fcγ-mediated phagocytosis is dependent on the Rho/ROK/myosin pathway.87 Together, these changes may facilitate actin anchorage or polymerization and consequent cytoskeletal reorganization that would facilitate closure of the phagosomal cup during CD44-mediated phagocytosis.
There is strong genetic evidence for the importance of CD44 in inflammatory and immune responses. During acute inflammation, fragments of extracellular matrix components, including hyaluronic acid, collagen, and fibronectin are released into the extracellular space where they may exert potent proinflammatory effects. CD44 mediates endocytosis and clearance of these proinflammatory fragments, thus attenuating inflammatory injury to the surrounding tissue.3,61,88,89 That CD44-deficient mice are predisposed to enhanced lung injury and are deficient in removal of apoptotic cells and hyaluronan fragments following an insult is strong evidence for the importance of this function.17 Additionally, CD44-deficient mice demonstrate enhanced susceptibility to lung injury in response to infection with Escherichia coli.90 Together, these data demonstrate the importance of CD44 in modulation of inflammatory tissue injury.
In summary, our current observations provide strong evidence that CD44 is a primary phagocytic receptor. Although the signaling pathways involved in CD44-mediated phagocytosis include some elements in common with Fcγ- and CR3-mediated phagocytosis, there are distinct differences. CD44-mediated phagocytosis may be important in clearance of apoptotic cells and of large fragments of hyaluronan generated during inflammatory responses. Additionally, CD44 may function in conjunction with the classic phagocytic receptors Fc and complement receptors and modulate phagocytosis of opsonized microbial pathogens and the ensuing cellular responses that modulate inflammation.
Supported by an operating grant from the Canadian Cystic Fibrosis Foundation (G.D. and S.G.).
E.V. designed and performed the research, analyzed the data, and wrote the first draft of the paper; R.M., J.P., V.K., and X.W. performed the research; R.W.V. contributed vital new reagents and analytic tools; M.G. contributed vital new reagents; A.K. and C.W.C. designed the research; S.G. designed the research and helped write the paper, G.P.D. designed the research, analyzed the data, and wrote the paper.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
Prepublished online as Blood First Edition Paper, February 2, 2006; DOI 10.1182/blood-2005-09-3808.
G.D. is the recipient of a Tier 1 Canada Research Chair. S.G. holds the Pidblado Chair in Cell Biology from the Hospital for Sick Children.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal